Abstract
Background: Banked donor milk (BDM) has historically been used as an alternative to formula for preterm infants. Recently, BDM has been endorsed by two national organizations for use in healthy infants. We sought to quantify utilization trends and characteristics of mothers and their healthy newborns who received BDM during their postpartum stay between 2013 and 2016 at a single academic medical center.
Materials and Methods: In this observational study, we used a clinical log to identify all infants who received BDM in the well-baby nursery between July 2013 and June 2016. From this log, we abstracted data on the numbers of babies who received BDM, the quantity of BDM provided, and indications for usage. We also collected clinical data from the medical records of a subset of corresponding mothers and infants.
Results: BDM utilization increased over time in healthy infants, with 0.04% of infants before July 2014 receiving BDM compared with 4.7% in July 2015 to June 2016. During the same periods, the number of bottles provided per infant also increased, from 0.6 bottles per infant to 4.6 bottles per infant. The most common indications for providing BDM were parent/caregiver request (19%) and excessive weight loss/dehydration (17%).
Conclusion: At our center, the use of BDM for healthy infants increased substantially over the study period. More research is urgently needed to understand the repercussions of this practice on resource utilization as well as short- and long-term breastfeeding and health outcomes.
Keywords: : healthy newborns, donor human milk, well-baby nursery
Introduction
The American Academy of Pediatrics (AAP) and the World Health Organization (WHO) recommend human milk as ideal nutrition for infants.1,2 Recently, human banked donor milk (BDM) has received increasing attention as an alternative to formula for supplementation.1 BDM is pooled and pasteurized human milk that is processed in accordance with international guidelines.3 In preterm infants, BDM use has been associated with health benefits, including a reduced risk of necrotizing enterocolitis.4 However, the impact of BDM on outcomes in healthy infants remains unknown.
Despite a lack of data on its health effects, the practice of providing BDM as supplementation to healthy infants has been endorsed by The Baby Friendly Hospital Initiative and The Academy of Breastfeeding Medicine as the recommended alternative to mother's own milk in healthy infants.5,6 The AAP has stated that “Banked human milk may be a suitable feeding alternative for infants whose mothers are unable or unwilling to provide their own milk.”7 From a regulatory standpoint, The Joint Commission indirectly supports this practice because infants who receive BDM are not distinguished from infants who receive their own mother's milk according to the definition of “exclusive breast milk feeding” in hospital perinatal quality metrics.8 It is important to note that BDM is currently not routinely covered by insurance and comes with considerable financial cost that must be borne by hospitals and/or families. The supply of BDM is dependent on donations from volunteers, so there are potential implications for resource utilization.
Given the emerging recommendation for BDM use in healthy infants on a national level, coupled with uncertainties about its benefits and substantial costs, there is an immediate need to understand trends in BDM utilization and indications for its use in healthy infants. Thus, the objective of this study was to describe trends in utilization of BDM in healthy newborns and indications for its use at an academic medical center with a high-volume maternity service over a 3-year period. Based on changes in national guidelines and recommendations, we hypothesized that there would be increased BDM utilization for the healthy newborn population over the 3-year period of this study.
Materials and Methods
Study design and population
This was a retrospective observational study conducted at a single tertiary care, academic medical center (Brigham and Women's Hospital [BWH], Boston, MA) with ∼6,600 births annually. We included all infants who received BDM and were discharged from the well-baby nursery between 2013 and 2016, identified by a detailed clinical log maintained by the lactation service to track use of BDM. This study was approved by the Partners Institutional Review Board.
Context for BDM at BWH
Before August 2014, BDM was available to infants in the BWH well-baby nursery by self-pay. In August 2014, an internal grant provided $5,000 in funding for BDM in the well-baby nursery. In November 2015, the grant was renewed and funding increased to $10,000. When these grant funds were depleted, additional funding was then provided from the nursing operations budget by the nurse director of the well-baby nursery and postpartum care. All BDM was purchased from The Mothers Milk Bank Northeast, a nonprofit milk bank located in Newton, Massachusetts.
Data collection
We used three hospital data sources for this study. First, we used a clinical log maintained by nurses and lactation consultants to identify all infants born between 2013 and 2016 who received BDM during their well-baby nursery hospitalization. Data recorded in this log included: name, medical record number, number of 100 mL bottles of BDM provided, and provider-recorded indication(s) for BDM use (2015–2016 only). Second, from a subset of infants born between June 2015 and July 2016 (after the implementation of our current electronic medical record system), we abstracted maternal and infant demographic and clinical information from the clinical record. Third, to compare mothers and infants who opted for BDM versus the overall patient population, we used the Partners Research Patient Data Registry, a clinical data repository available to investigators across the hospital, to identify maternal and infant clinical characteristics of the general delivery population during the period of June 2015 to July 2016.
Data analyses
To determine the trends in BDM utilization, the number of infants receiving BDM per month was divided by the total number of well-baby nursery discharges in the same period. To describe trends in the volume of BDM provided to each infant over time, the total number of 100 mL bottles provided per infant per month over the study period was calculated. These data were displayed by using control charts, which facilitate the visualization of a changing process over time.9 The cost of utilization was calculated based on a cost of $16 per 100 mL bottle. Lastly, to determine the indications for which BDM was utilized in this population, we abstracted data from the clinical BDM log regarding provider-recorded indications. We grouped similar indications into categories, totaled the number of times each indication was noted, and finally divided the number of times each indication was listed by the total number of times any indication was listed (if infants had multiple indications, all indications were considered.) Microsoft Excel 2010 was utilized for data management and analyses.
Results
Participants
A total of 363 infants received BDM from 2013 to 2016. Clinical characteristics for 243 mothers and 260 infants who received BDM between June 2015 and July 2016 are reported in Table 1. Overall, the demographics of mothers who opted for BDM for their infants were similar to our overall population of mothers and infants at BWH, except that mothers who opted for BDM were more likely to have a twin gestation (4.4% of the overall BWH population) or a Cesarean section delivery (32% of the overall BWH population).
Table 1.
Demographics of Infants Who Received Donor Milk and Their Mothers
| Maternal characteristics (n = 243) | |
| Age (years) | 33 ± 4.6 |
| Pregnancy complications, n (%) | |
| Gestational diabetes | 14 (5.4) |
| Preeclampsia | 16 (6.2) |
| Twin gestation | 45 (17.3) |
| Average delivery BMI (kg/m2) | 31.7 ± 6.2 |
| Vaginal delivery, n (%) | 128 (49.2) |
| Infant characteristics (n = 260) | |
| Fetal growth, n (%) | |
| SGA | 31 (11.9) |
| AGA | 213 (81.9) |
| LGA | 16 (6.2) |
| Gestational age (weeks), n (%) | |
| 35–36, 6/7 | 64 (24.6) |
| 37–39, 6/7 | 132 (50.7) |
| 40–42, 6/7 | 64 (24.6) |
| Percent weight loss during hospitalization,an (%) | |
| 0–5% | 38 (14.8) |
| 5–10% | 167 (64.9) |
| 10–15% | 52 (20.2) |
| Mean bilirubin (μmol/L) | 7.3 ± 3.2 |
| Mean hours of life at bilirubin check | 42 ± 1.2 |
| Glucose,bn (%) (n = 158) | |
| ≤20 mg/dL | 0 (0) |
| 21–30 mg/dL | 6 (3.7) |
| 31–40 mg/dL | 40 (24.8) |
| >41 mg/dL | 115 (71.4) |
Calculated using formula (birthweight − lowest weight)/birthweight × 100.
Lowest glucose measured during postpartum hospitalization.
AGA, appropriate for gestational age; BMI, body mass index; LGA, large for gestational age; SGA, small for gestational age.
The majority (82%) of infants who received BDM had appropriate fetal growth. Overall, 24.7% of donor-milk fed infants were late preterm (between 35 and 37 weeks gestation). Of all infants who received BDM, 20% had ≥10% weight loss during their postpartum hospitalization, with no infant losing >15% of their birth weight. It was observed that 3.7% of infants had a lowest blood glucose measurement of ≤30 mg/dL and 24.8% had a lowest blood glucose measurement of 31–40 mg/dL during the hospitalization.
Trends in BDM utilization
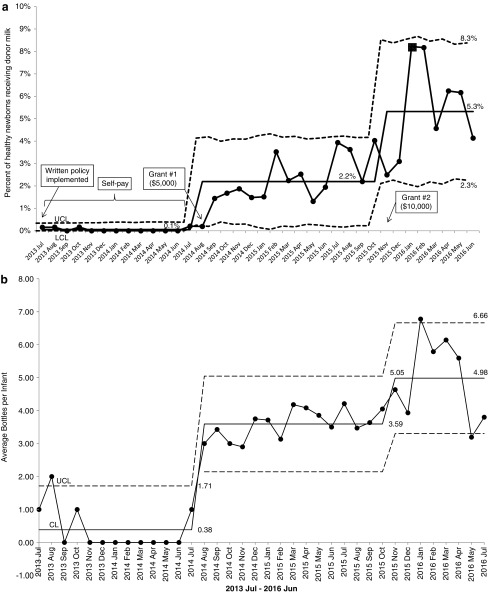
As shown in Figure 1, the prevalence of BDM utilization increased between July 2013 and June 2016. Before July 2014, 0.04% of healthy infants received BDM. Between July 2014 and July 2015, 1.6% of healthy infants received BDM. In the last year of this observation, 4.7% of the healthy newborn population received BDM. The major inflection points in utilization appear to correspond to receipt of two grants: $5,000 in August 2014 and $10,000 in November 2015, which were designated for purchasing BDM. These trends in utilization also coincided with a BDM policy change allowing all infants admitted to the neonatal intensive care unit (NICU), regardless of gestational age, to be eligible for BDM. Some of the infants who initially received BDM in the NICU were then transferred to the well-baby nursery where they continued to receive BDM during their hospital stay and were included in our cohort (Fig. 1a).
FIG. 1.
(a) Increase in donor milk use for healthy newborns since the start of the program, illustrated as a control chart with each point representing the monthly percent of total newborn nursery discharges who received donor milk. Control limits are indicated by dashed lines. Annotations show the timing of key milestones in the well-newborn donor milk program, including implementation of a written policy and the receipt of two institutional grants. Horizontal lines represent the mean percent of healthy newborns who received donor milk in relation to key milestones, increasing from 0% to 5.3% over the period of observation. (b) Increase in average number of 100 mL bottles of donor milk provided to each infant since the start of the program, illustrated as a control chart with each point representing the monthly average of bottles provided to each infant. Control limits are indicated by dashed lines. Horizontal lines represent the average number of bottles provided to each infant, increasing from 0.38 to 4.98 bottles per infant over the period of observation.
Over the 3-year period, the number of bottles provided to each BDM-supplemented newborn also increased. A mean of 0.6, 1.8, and 4.6 bottles (each 100 mL) were provided to each infant who received BDM during the periods before July 2014, between July 2014 and June 2015, and between July 2015 and June 2016, respectively (Fig. 1b). The total cost of BDM for healthy newborns (total number of bottles multiplied by cost per bottle) was $61 before July 2014, $5,309 between July 2014 and June 2015, and $19,278 between July 2015 and June 2016.
Indications for BDM use
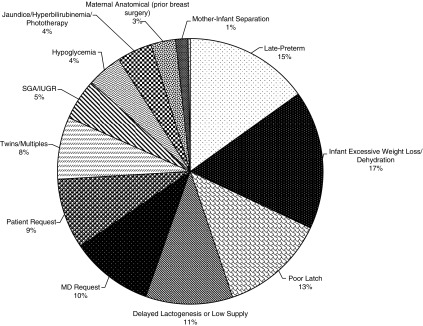
Of the 260 infants included in our 1-year study timeframe, 36 infants had no indications listed, 97 had 1 indication, and 127 had more than 1 indication listed on the BDM log. As shown in Figure 2, the most common reasons provided for BDM use were: infant excessive weight loss/dehydration (17%), late preterm birth (15%), and poor latch (13%). Approximately one in five times, clinician or parent request was listed as the indication for providing BDM to the infant.
FIG. 2.
Indications for donor milk use, 2015–2016. Out of a total of 260 infants, 36 infants had no cited indication, 97 infants had 1 indication, and 127 infants had more than 1 indication (n = 397 indications total).
Discussion
We report a substantial increase in the utilization of BDM over a 3-year period at our academic medical center in the well-baby nursery, with detailed information about the indications for use during a 1-year period. We found that BDM was provided in this setting for a variety of reasons, most commonly infant dehydration and weight loss, late preterm infant, and parental and provider request.
BDM, an alternative to formula when mother's milk is unavailable, reduces the risk of necrotizing enterocolitis, a potentially devastating intestinal disease that affects primarily preterm infants.4 Therefore, in the vulnerable preterm infant patient population, the benefits of BDM may outweigh the risks. Accordingly, the AAP specifically recommends BDM rather than formula when mother's own milk is unavailable for very low-birth-weight infants in the NICU setting. In the context of this mounting clinical evidence and AAP recommendations, BDM use has increased over the past decade. As of 2014, more than half of all NICUs in the United States offer BDM as an option for preterm infants, despite its expense.10,11
Very little is known about clinical benefits or risks of BDM supplementation in healthy infants. We could identify only one publication on the topic, a two-case series describing the use of BDM in healthy, full-term infants at a single center.12 Ongoing research includes a trial by Kair et al. in which full-term, otherwise healthy infants at high risk for excessive neonatal weight loss were randomized to either exclusive breastfeeding or 10 mL of BDM after every breastfeeding attempt.13 In a recent abstract presentation, the investigators reported no difference in breastfeeding outcomes (exclusivity and duration) over the first 3 months of life in infants who were exclusively breastfed compared with those who received this judicious amount of BDM supplementation. Although the authors concluded that supplementation with BDM does not impact subsequent breastfeeding rates, and that BDM use may be warranted for some clinical indications among populations of healthy infants, the clinical indications for which BDM is preferable to formula, as well as the optimal volume and timing of supplemental feedings, remain to be clarified and incorporated into evidence-based guidelines.
The Academy of Breastfeeding Medicine recommends BDM as the preferred supplement (over formula) with indications for supplementation such as dehydration, hypoglycemia, and hyperbilirubinemia.6 We report that BDM is being commonly utilized for these and a variety of additional reasons that mirror indications for formula supplementation. The most common reason for BDM utilization in our population was infant weight loss and dehydration, although no infant in this cohort lost >15% birth weight. Poor latch, late preterm birth, and parent and clinician request were other common reasons that BDM was used for healthy newborns in the well-baby nursery. Interestingly, we were not able to identify any studies evaluating the risks and benefits of BDM compared with formula for these indications, suggesting that BDM use is highly dependent of parent and provider perception of BDM benefit over formula.
Our findings also raise the question of whether there is an optimal volume of BDM supplementation. We report that over the 3 years of our study, the total amount of BDM provided per infant during the hospitalization increased from 60 to 460 mL. Early observational studies suggested a strong association between in-hospital formula supplementation and early breastfeeding discontinuation.14–16 In contrast, two later randomized controlled trials found that a small volume (10 mL) of formula or BDM supplementation, timed after breastfeeding attempts and given via syringe, does not negatively impact breastfeeding duration and exclusivity.13,17 This suggests that volume, timing, and route of supplementation, not necessarily type of supplementation, may be critically important in minimizing harm (i.e., breastfeeding failure) associated with supplementation practices. Our findings show that over the 3-year period of our study infants received increasing volumes of BDM. Based on the available data, there is the potential risk that this practice could interfere with the establishment of successful lactation, based on the physiology of lactation physiology, similar to large volumes of formula supplementation.18
Lastly, BDM is a potentially limited resource that was previously reserved for preterm or sick infants in the NICU. A recent study found that 81.3% of premature infants born in California were offered BDM in 2013, compared with 38.2% in 2007, requiring increased supply.19 Our results suggest that, in healthy infants, BDM supplementation is being utilized in a manner similar to formula. Given that 65% of healthy infants receive formula supplementation, the substitution of BDM for formula in even a small proportion of these infants may place a strain on available BDM for preterm infants who may benefit more than healthy infants if a shortage of BDM were to arise.20 In addition, as medical resources are limited, the documented health benefits of BDM in preterm infants (and the lack of documented benefit in healthy infants) may play a role in determining resource allocation.
Strengths of our study include the availability of detailed clinical data regarding BDM utilization since the inception of our BDM program. Using thorough records of all BDM dispensed and reasons for its use, we were able to quantify costs and discern provider-generated reasons for BDM provision. This study was conducted at a single academic center with funding for BDM in healthy infants provided by an institutional grant, in addition to funding allocated from the operational budget, potentially limiting the generalizability of findings. Future studies should examine broader trends across multiple settings and funding scenarios (e.g. insurance coverage, self pay) in BDM use for healthy newborns. We were also limited by the retrospective nature of this study, in that we were not able to obtain parental or provider perceptions pertaining to the use of BDM. In addition, we lacked data on route of BDM administration and outcomes posthospital discharge (breastfeeding and other maternal and infant outcome).
Conclusion
Here, we provide the first report of trends in BDM use in the healthy newborn population. We found that, given resources for BDM purchase, there was a large increase in BDM utilization. More infants received BDM and each infant received, on average, a higher volume of milk over the 3-year period of the study. In addition, we report that the variety of indications for which BDM was provided overlaps with common indications for formula supplementation and may be driven by the perceived benefit of BDM over formula in healthy infants. Taken together, these data suggest that further research is urgently needed to determine the benefits and risks of this practice, with a particular focus on in-hospital supplementation strategies that optimize long-term breastfeeding outcomes in healthy infants.
Acknowledgments
The authors thank the nurses and lactation consultants at BWH for their thorough record-keeping regarding BDM utilization. Funding to S.S.: K23 HD 074648.
Disclosure Statement
The authors report no disclosures or conflicts of interest.
References
- 1.Sample hospital breastfeeding policy for newborns. Available at www2.aap.org/breastfeeding/curriculum/documents/pdf/hospital%20breastfeeding%20policy_final.pdf (accessed July2017)
- 2.Global Strategy for Infant and Child Feeding. Geneva: World Health Organization Geneva, 2003, pp. 1–30 [Google Scholar]
- 3.HMBANA position paper on donor milk banking. Available at www.hmbana.org/sites/default/files/images/position-paper-donor-milk.pdf (accessed July2017)
- 4.McGuire W, Anthony MY. Donor human milk versus formula for preventing necrotizing enterocolitis in preterm infants: Systematic review. Arch Dis Child Fetal Neonatal Ed 2003;88:F11–F14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The ten steps to successful breastfeeding. Available at www.babyfriendlyusa.org/about-us/baby-friendly-hospital-initiative/the-ten-steps (accessed June2, 2017)
- 6.Kellams A, Harrel C, Omage S, et al. ABM Clinical Protocol #3: Supplementary feedings in the healthy term breastfed neonate, Revised 2017. 2017, pp. 1–10 [DOI] [PubMed]
- 7.Gartner LM, Morton J, Lawrence RA, et al. Breastfeeding and the use of human milk. Pediatrics 2005;115:496–506 [DOI] [PubMed] [Google Scholar]
- 8.Exclusive breast milk feeding. Available at https://manual.jointcommission.org/releases/TJC2015B2/DataElem0273.html (accessed July2017)
- 9.Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care 2003;12:458–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagadorn JI, Brownell EA, Lussier MM, et al. Variability of criteria for pasteurized donor human milk use: A survey of U.S. Neonatal Intensive Care Unit Medical Directors. JPEN J Parenter Enteral Nutr 2016;40:326–333 [DOI] [PubMed] [Google Scholar]
- 11.ESPGHAN Committee on Nutrition, Arslanoglu S, Corpeleijn W, et al. Donor human milk for preterm infants: Current evidence and research directions. J Pediatr Gastroenterol Nutr 2013;57:535–542 [DOI] [PubMed] [Google Scholar]
- 12.Kair LR, Colaizy TT, Hubbard D, et al. Donor milk in the newborn nursery at the University of Iowa Children's Hospital. Breastfeed Med 2014;9:547–550 [DOI] [PubMed] [Google Scholar]
- 13.Kair L, Flaherman V, Colaizy T. Rx Milk: Donor milk supplementation in the well newborn nursery, an RCT (2706312). Abstract presented at Pediatric Academic Societies, 2017 San Francisco, CA (NCT02221167) [Google Scholar]
- 14.Samuels SE, Margen S, Schoen EJ. Incidence and duration of breast-feeding in a health maintenance organization population. Am J Clin Nutr 1985;42:504–510 [DOI] [PubMed] [Google Scholar]
- 15.Wright HJ, Walker PC. Prediction of duration of breast feeding in primiparas. J Epidemiol Community Health 1983;37:89–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loughlin HH, Clapp-Channing NE, Gehlbach SH, et al. Early termination of breast-feeding: Identifying those at risk. Pediatrics 1985;75:508–513 [PubMed] [Google Scholar]
- 17.Flaherman VJ, Aby J, Burgos AE, et al. Effect of early limited formula on duration and exclusivity of breastfeeding in at-risk infants: An RCT. Pediatrics 2013;131:1059–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neville MC. Anatomy and physiology of lactation. Pediatr Clin North Am 2001;48:13–34 [DOI] [PubMed] [Google Scholar]
- 19.Kantorowska A, Wei JC, Cohen RS, et al. Impact of donor milk availability on breast milk use and necrotizing enterocolitis rates. Pediatrics 2016;137:e20153123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierro J, Abulaimoun B, Roth P, et al. Factors associated with supplemental formula feeding of breastfeeding infants during postpartum hospital stay. Breastfeed Med 2016;11:196–202 [DOI] [PubMed] [Google Scholar]