Abstract
This study explores ibuprofen (IBP) uptake and transformation in the wetland plant species Phragmites australis and the underlying mechanisms. We grew P. australis in perlite under greenhouse conditions and treated plants with 60 μg/L of IBP. Roots and rhizomes (RR), stems and leaves (SL), and liquid samples were collected during 21 days of exposure. Results show that P. australis can take up, translocate, and degrade IBP. IBP was completely removed from the liquid medium after 21 days with a half-life of 2.1 days. IBP accumulated in RR and was partly translocated to SL. Meanwhile, four intermediates were detected in the plant tissues: hydroxy-IBP, 1,2-dihydroxy-IBP, carboxy-IBP and glucopyranosyloxy-hydroxy-IBP. Cytochrome P450 monooxygenase was involved in the production of the two hydroxy intermediates. We hypothesize that transformation of IBP was first catalyzed by P450, and then by glycosyltransferase, followed by further storage or metabolism in vacuoles or cell walls. No significant phytotoxicity was observed based on relative growth of plants and stress enzyme activities. In conclusion, we demonstrated for the first time that P. australis degrades IBP from water and is therefore a suitable species for application in constructed wetlands to clean wastewater effluents containing IBP and possibly also other micropollutants.
1. Introduction
In recent years, pharmaceuticals have provoked increasing concern due to their potential risks to the environment. These ubiquitous, persistent and biologically active compounds can disturb both mammalian and nonmammalian organisms.1 Ibuprofen (IBP) is one of the most commonly used nonsteroidal anti-inflammatory pharmaceuticals with high consumption rates, for example, 4.7 mg/inhabitant/day in China.2 In addition, non-negligible levels of IBP have been found in water bodies (20 ng/L) and sediment (17–314 ng/g).3,4 In the influents of four investigated wastewater treatment plants (WWTPs) in Spain, concentration of IBP was in the range of 12–373 μg/L. Following treatment, 1–48 μg/L of IBP was measured in the effluents.1 These effluent concentrations may be concerning, considering that IBP concentrations higher than 10 μg/L could be embryotoxic to zebrafish causing decreased hatching and growth rates.5 Hence, IBP can be used as one of the guide-compounds to assess the treatability of wastewater treatment plant effluents with respect to pharmaceutical removal.6 In this study we focus on phytoremediation technologies for IBP removal.
Phytoremediation is the overarching term for a group of technologies that utilize plants and the associated rhizosphere microorganisms to remove or transform contaminants leached from soils/sediments and from used water streams.7 Plants have a pronounced ability for uptake and detoxification of many recalcitrant xenobiotics, and thus function in nature as a “green liver”.8 A highly studied and relevant field within phytoremediation is the use of constructed wetlands (CWs) for removing pharmaceuticals from wastewater treatment plant effluents.9
Some studies applied hydroponic plant microcosms as simplified CWs, in which uptake of IBP by the wetland plant species was confirmed. However, no further investigation into phytodegradation and underlying mechanisms was performed.10−12 To date, only one study reported phytodegradation of IBP in duckweed by detecting intermediates in the tissue.13 Transformation of IBP in plants may be explained by the interactions of IBP with enzymes, as plants possess a metabolic cascade capable of detoxifying xenobiotics, which resembles functions of mammalian livers.14 This “green liver” concept points out the main enzymes playing a role in the detoxification process, include cytochrome P450 monooxygenase (P450), glycosyltransferase (GT), and glutathione-S transferase (GST).15
The aim of this study is to investigate plant uptake and phytodegradation of IBP and reveal the mechanism underlying this process. Therefore, the transformation of IBP and IBP-induced enzyme defense responses were studied in Phragmites australis, which is widely applied in CWs and effectively takes up IBP, as compared with other macrophytes.11 The results presented here prove phytodegradation of IBP via enzymes present in the wetland plant species, provide insight into the transformation mechanisms, and act as a further verification of using those plant species for phytoremediation of pharmaceuticals in CWs.
2. Materials and Methods
2.1. Chemicals and Reagents
IBP sodium salt (⩾98%) was purchased from Sigma-Aldrich (U.S.). Chemical characteristics of IBP are shown in Table S1 in Supporting Information (SI). Acetonitrile, formic acid, ammonium formate, and water (Biosolve B.V., The Netherlands) used for liquid chromatography (LC) analysis were of LC grade. All other chemicals and reagents used in enzyme extraction and analysis of enzyme activities were of analytical grade or higher (SI Text S1). Deionized water from a Milli-Q system (Millipore, U.S.) was used to prepare all solutions.
2.2. Experimental Design
P. australis was obtained from a local nursery (Wasserpflanzengärtnerei Jörg Petrowsky, Eschede, Germany) and transferred to pots filled with clean perlite. Plants were cultivated under greenhouse conditions for 6 weeks: relative humidity of 60% (day) and 70% (night), temperature of 22 °C (day) and 17 °C (night), and 12 day/night hours and fed with modified Hoagland culture medium16 step by step from 10%, 30%, 50%, 80%, to 100% strength. Plants were illuminated with high pressure sodium lamps (Philips SON-TAGRO, 400 W) with a wavelength of 400–700 nm.17 After cultivation, plants were transferred to new pots with clean perlite, in order to minimize biodegradation of IBP by phytoplankton and microbes. Plants growth conditions are shown in SI Figure S1. During the exposure experiment, full strength medium was employed. Exposure concentration of IBP was 60 μg/L, which is close to the concentration of wastewater effluents shown in the introduction. 500 mL IBP solution was added to each pot with 200 g perlite. Batch experiments were conducted to investigate the sorption of IBP on perlite (SI Text S2). To compensate for water loss due to evaporation (41.4 mL/day in blank groups), 300 mL tap water was added twice to the pots resulting in exposed water surface.
Three groups of plants were used: treated plants with 60 μg/L of IBP injection into the medium (treated groups), parallel untreated plants (untreated groups) and blank control pots without plants (blank groups). The amount of perlite was the same for all these three groups. Tissue samples were collected from both the treated and untreated groups on day 0, 3, 7, 14, and 21 after IBP exposure. At each sampling time point, triplicates of treated and untreated plants were sacrificed to harvest plant tissues. Harvested tissues were divided into two sections: roots and rhizomes (RR tissue), and stems and leaves (SL tissue), prior to being frozen in liquid nitrogen and stored at −80 °C until sample processing. At the same time points, liquid samples were collected from pots of both treated and blank groups, and were filtered through 0.45 μm pore size PVDF syringe filters (Carl Roth, Germany) then stored at −20 °C until analysis. Weight of plants and water loss in pots were measured prior to sampling.
2.3. Selection of IBP Intermediates
According to the “green-liver” concept, plant detoxification of xenobiotics may show similarities with mammalian liver functions. Plants can metabolize xenobiotics via specific enzymatic reactions, namely (Table 1):
-
(A)
phase I, transformation of xenobiotics to more water-soluble compounds via P450 to allow further conversion.
-
(B)
phase II, conjugation with glycosides via GT, glutathione via GST, or amino acids to reduce toxicity and alter mobility. Amino acid conjugation was reported as a side reaction rather than a main detoxification step.
-
(C)
phase III, compartmentalization of xenobiotics and metabolites into the vacuole and/or further reaction by binding to the cell wall.18,19
Table 1. Metabolites of IBP in P. australis Were Tentatively Identified by Mass Spectrometry.
| tissues |
liquid
phase |
||||||
|---|---|---|---|---|---|---|---|
| metabolism | enzyme | selected intermediates | formula | roots and rhizomes | stem and leaves | treated groups | blank groups |
| phase I | P450 | hydroxy-IBP | C13H18O3 | √ | √ | √ | √ |
| 1,2-dihydroxy-IBP | C13H18O4 | √ | √ | √ | √ | ||
| carboxy-IBP | C13H16O4 | √ | ND | ND | ND | ||
| phase II | GT | glucopyranosyloxy-IBP | C19H28O7 | ND | ND | ND | ND |
| glucopyranosyloxy-hydroxy-IBP | C19H28O8 | √ | ND | ND | ND | ||
| glucopyranosyloxy-carboxy-IBP | C19H27O10 | ND | ND | ND | ND | ||
| GST | IBP-glutathione conjugate | C23H33N3O8S | ND | ND | ND | ND | |
Some xenobiotic conjugates from phase II might still possess unwanted properties rendering them problematic for plant cells, even though they are less toxic than parent xenobiotics. Thus, it is generally accepted that those conjugates are sequestered from susceptible organs via storage in vacuoles during phase III.20 Currently, the proposed further reactions in phase III have yet to be confirmed.21
Based on metabolism principles18,22 and detected IBP metabolites in mammal and microbial systems,23,24 we selected possible intermediates of IBP (Table 1).
2.4. Chemical Extraction
IBP and potential intermediates in RR and SL tissue were extracted from 0.5 g of frozen plant tissue. Samples were ground to a fine powder in liquid nitrogen and mixed with 1 mL of 0.1 M HCl/acetonitrile (50/50, v/v) by vortexing. The mixture was then incubated on ice and mixed on a plate shaker (Neolab, Germany) for 20 min prior to centrifugation at 12 000 rpm for 15 min under 4 °C (Eppendorf, Germany). Solid phase extraction (SPE) was performed with Oasis HLB cartridges (3 cc/60 mg, Waters, U.S.), preconditioned with 3 mL methanol and equilibrated with 3 mL deionized water. After loading 600 μL sample at a flow rate of 1 mL/min, the cartridge was washed with 6 mL deionized water and eluted with 6 mL of 25% NH4OH:MeOH (8/92, v/v). Finally, the eluate was evaporated to dryness under gentle nitrogen flow (Dri-block heater, Techne, UK). The final extract was made up to exactly 1 mL with 2% MeOH by weight. The recovery of SPE method was 110–123%. Validation of the extraction method is described in SI Text S3.
2.5. Chemical Analysis
IBP and potential intermediates were measured by high resolution accurate mass spectrometric (HRMS) detection. The used LC-HRMS system consisted of an Ultimate 3000 LC system coupled through a HESI II electrospray source to a Q-Exactive Orbitrap MS (Thermo Fisher Scientific, San Jose, CA). The LC-column used was an Atlantis HILIC Silica T3 column (3.0 × 100 mm, 3 μm) (Waters). The mobile phase consisted of: eluent A, water/acetonitrile/formic acid/ammonium formate (900/100/0.02/2); and eluent B, same components with eluent A (100/900/0.02/2). Flow rate was set at 0.4 mL/min. The step gradient was as follows: 0–0.5 min 10% B; 0.5–6 min linearly increased to 40% B; 6–7 min increased to 100% B and hold 1 min; 8–8.1 min decreased to 10% B and hold until 14 min. The column temperature was set at 60 °C and the injection volume was 50 μL. Heated electrospray ion source was used for the ionization. Detection of the compounds was performed in both full scan and targeted MS/MS approach on the Q-Exactive mass spectrometer in the negative mode. Detailed operational parameters and list of target precursor ions are shown in SI Text S4 and Table S2. In terms of quality control, the mass calibration of the mass spectrometer was checked before analysis and recalibrated if needed. External calibration was performed by calibration solution of mixed PhACs and internal calibration was performed by spiking fenoprofen as the internal standard.
2.6. Enzyme Extraction
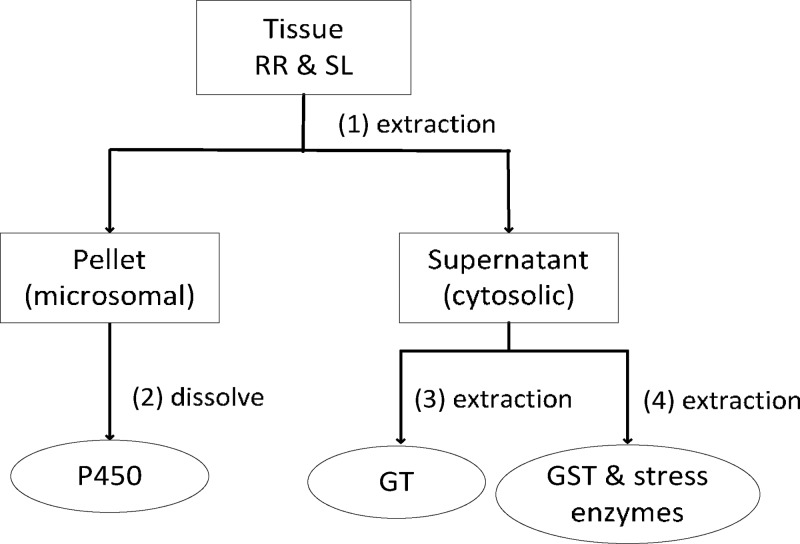
The extraction of cytochrome P450 monooxygenase (P450), glycosyltransferase (GT), glutathione-S transferase (GST), and stress enzymes including peroxidase (POX) and glutathione reductase (GR) was performed by combining methods described previously.25,26 During extraction, enzyme samples were kept on ice and the buffers used were precooled in the refrigerator before using, in order to maintain the integrity of enzymes. The extraction process for enzymes is shown in Figure 1. (1) 10 g of frozen plant tissue was ground in liquid nitrogen. The homogenized tissue was then mixed with 40 mL extraction buffer and stirred at 300 rpm for 15 min. The extraction buffer contained 250 mM of sucrose, 1 mM of ethylenediaminetetraacetic acid, 40 mM of ascorbic acid, 1 mM of fresh phenylmethanesulfonyl fluoride and 10 mM of fresh dithioerythritol (DTE) in 0.1 M of sodium phosphate buffer (pH 7.4). The mixture was filtered by miracloth (EMD Millipore) and centrifuged at 10 000g for 15 min at 4 °C (Beckman coulter avanti J-25 centrifuge, rotor JA-25.50, U.S.). The supernatant was collected into a 90 mL ultracentrifuge tube (polyallomer ultracrimp tube, Kendro, U.S.) and filled to full with buffer, which was then ultracentrifuged at 100 000g for 60 min at 4 °C (Sorvall Discovery 90 SE ultracentrifuge, rotor T-647.5, Japan). (2) After ultracentrifugation, the pellet was collected and dissolved in 1 mL of microsome buffer, which contained 1.4 mM of DTE, 20% glycerin in of 0.1 M sodium phosphate buffer (pH 7.4). The pellet solution was considered as microsomal P450 extracts and the supernatant was collected to further extract other cytosolic enzymes separately.
Figure 1.
Flowchart of enzyme extraction from plant tissues.
(3) For GT extraction, 30 mL of the supernatant was precipitated twice by adding ammonia sulfate: first time precipitation to 40% salt saturation then centrifugation and second time to 75% saturation followed by centrifugation. The centrifugation was performed at 18 500g for 30 min at 4 °C. The final pellet was redissolved in 2.5 mL of 200 mM Tris/HCl buffer (pH 7.3) and subsequently desalted with PD-10 gel filtration columns (GE Healthcare, UK). (4) For extracting GST and stress enzymes, the procedure was similar to the GT protocol with a few adjustments: precipitation was applied first to 40% and second time to 80% saturation; centrifugation was conducted at 20 000 rpm; pellet was redissolved in 2.5 mL of 25 mM Tris/HCl buffer (pH 7.8).
2.7. Determination of Enzyme Activity
P450 activity was determined by an oxygen biosensor system based on the method reported by Olry et al. (2007)27 and a commercial protocol (BD Biosciences). First, 100 μL of 100 mM Tris/HCl buffer (pH 7.5), 10 μL of 2 mM cinanamic acid in ethanol, and 100 μL of P450 extract were added into a 96-wells plate (BD falcon oxygen biosensor plate, U.S.) and incubated for 2 min at room temperature. The reaction was then started by adding 20 μL of a regenerating solution containing 6.7 mM glucose 6-phosphate (Glc-6-P), 0.4 units of Glc-6-P dehydrogenase, and 2 mM nicotinamide adenine dinucleotide phosphate (NADPH) in 100 mM Tris buffer. The biosensor plate was then incubated in a microplate reader (Gemini EM, Modula Device, U.S.) to reach 27 °C. By detecting fluorescence of the oxygen sensitive dye embedded at the bottom of wells, the consumption rate of dissolved oxygen can be monitored. The fluorescence of dye (λemission = 620 nm, λexcitation = 480 nm) was recorded for 2 h continuously in intervals of 30 s. Wells with no addition of P450 extract were set as negative controls, while wells with Na2S2O5 addition served as positive controls. P450 activity was calculated based on the rate of oxygen consumption.
GT activity was detected by high performance LC (SI Text S5) based on the method described by Meßner et al. (2003);28 POX and GR activity were measured by spectrophotometer according to methods mentioned in previous works.29,30 Concentration of proteins in the extract was determined using Bradford assay.31
Oxidative bursts might occur in plants exposed to xenobiotics.32 The oxidative burst might increase transcription of different enzyme species or/and induce the enzymatic activity to ensure that maximum protection could be maintained in the cell compartments.32,33 In this study, we used the unit nkat/mg protein to represent enzyme activities expressed in the extracted microsomal and cytosolic fractions, combining the possible increase of enzyme quantity and enzyme activity.
2.8. Statistical Analysis
Statistical differences of enzyme activities between treated and untreated groups (at the same sampling points) were established by the analysis of variance method (ANOVA, single factor) at different significance levels. Statistical difference of IBP concentration between day 3 and 7 in blank groups was calculated in the same way. Comparisons were considered significantly different for *P < 0.05, **P < 0.01, and ***P < 0.001. The non-negligible standard deviations in this study might be caused by different growth rates and transpiration rates of parallel plants.
3. Results and Discussion
3.1. Uptake, Accumulation and Translocation of IBP in Plants
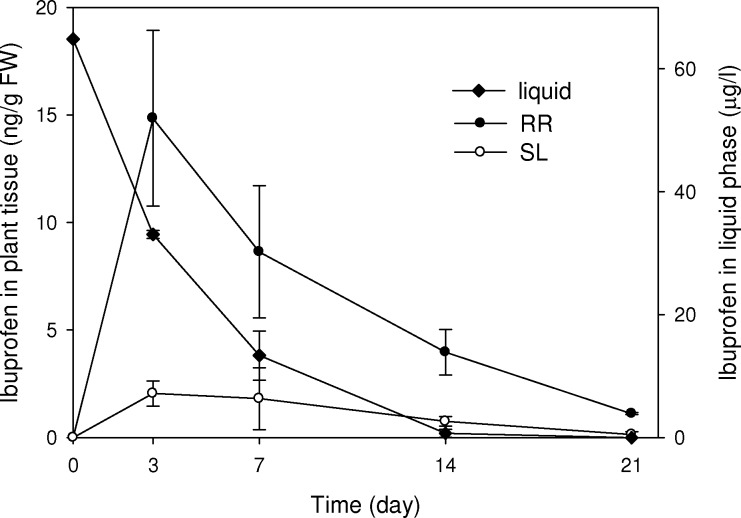
In order to evaluate the uptake, accumulation and translocation of IBP in plant tissues, we investigated IBP concentrations in liquid medium and in RR and SL tissue of treated plants. IBP in the liquid phase was completely removed after 21 days of exposure (Figure 2). Removal followed a pseudo-first order reaction with a half-life of t1/2 = 2.1 d (R2 = 0.97). IBP was present in both RR and SL tissue (Figure 2). This indicates that IBP was transported upward from medium to RR, and further translocated through RR to SL. IBP in plant tissues diminished after the initial uptake during the first 3 days (Figure 2). This may indicate phytodegradation. As plants lack excretory pathways for xenobiotics, they can only store those compounds in vacuoles or cell walls, or metabolize xenobiotics into nontoxic forms.34 Thus, P. australis could take up, translocate and possibly degrade IBP.
Figure 2.
Concentration of IBP in the liquid phase and plant tissue (RR, roots and rhizomes; SL, stems and leaves) of treated groups. Data are mean concentrations (FW, fresh weight) ± standard error (n = 3).
To further demonstrate the capacity of P. australis to take up and translocate IBP, we calculated its bioconcentration factor (BCFRR, BCFSL) and translocation factor (TF). BCFRR and BCFSL are the ratios of IBP in RR or SL, respectively, to the spiked concentration in the medium; TF is the ratio of IBP in SL to RR, all expressed as fresh weight concentration. During the whole exposure period, BCFRR, BCFSL and TF were in the range of 0.06–0.23 L/kg, 0–0.03 L/kg, and 0.01–0.21, respectively. These values are in line with IBP uptake by other aquatic plant species and vegetables in previous studies.35
Plant uptake and translocation are thought to be strongly dependent on the physicochemical characteristics of the chemical, such as the dissociation constant pKa and octanol–water partition coefficient Kow.36 The fate of neutral compounds in plant tissues has been frequently addressed. However, numerous uncertainties remain for ionic compounds such as IBP. IBP is acidic with a pKa of 4.91. It was partly dissociated in the growth medium, because plant exudates reduce the neutral medium pH to 5.2–5.7. Undissociated IBP can thus diffuse to the apoplast of root cells, and remain neutral due to the low pH of apoplasts (pH 4–6).37 Thereafter, IBP may be further transferred to the cytosol, resulting in a notable BCFRR. However, once inside a cell (pH 7–7.5 in cytosol),37 IBP was most likely ionized for more than 99% due to the elevated pH, and trapped in the root cells. The membrane of plant cells has a negative electrical potential,37 resulting in a repulsion between membrane and negatively charged IBP and creating a barrier for diffusion of ionized IBP outside of the cytosol. We used the distribution constant log Dow, as pH corrected log Kow, to describe the hydrophobicity of IBP (log Kow = 3.97). IBP is relatively hydrophobic with a log Dow of 1.88 assuming a pH 7 in a given cytosolic environment (SI Table S1). The negatively charged based repulsion and hydrophobic characteristics apparently made further transfer of IBP difficult, as observed in the low BCFSL and TF.
3.2. Fate and Transformation of IBP in the Hydroponic-Plant System
3.2.1. Metabolism of IBP in the Liquid Phase
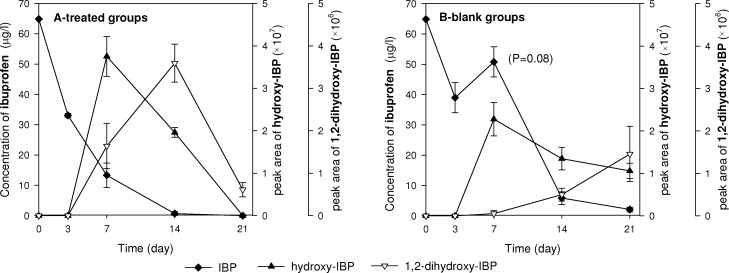
Concentrations of parent IBP and related intermediates were analyzed in the liquid medium of both treated groups with plants, and blank groups without plants. In the treated groups, an immediate exponential-wise decline of IBP concentrations was observed (Figure 3). In the blank groups, during the first 7 days a much smaller decline was observed, that is, to 45 ± 10 μg/L. This value is in line with independent experiments showing loss of IBP by sorption to the perlite of 20% (SI Figure S2). After day 7, a delayed but fast decline of the IBP concentrations occurred indicating a lag phase prior to biological degradation.
Figure 3.
Concentration of IBP and its metabolites in the liquid phase. (A) treated groups; (B) blank groups without plants. Peak area of intermediates is in unit of mAU/ml liquid. Data are mean concentrations/peak areas ± the standard error (n = 3).
Hydroxy-IBP and 1,2-dihydroxy-IBP were produced in the liquid phase of both treated and blank groups (Figure 3), but to a much lower extent in blank groups. These hydroxy intermediates can be the result of photodegradation or biodegradation in both groups. Formation of these intermediates was indeed observed in photodegradation and biodegradation in previous research.23,38 In our system, photodegradation might occur, but be less significant than biodegradation. Photodegradation of IBP was possible in the exposed water surface but most likely negligible, as our greenhouse lamps did not include UV wavelengths and the greenhouse roof filtered most sunlight UV.39 Previous studies indicate that photodegradation of IBP by visible light would be negligible without addition of photosensitizer or catalyst.40,41 In the greenhouse, fresh water phytoplankton could grow in the nutrient-rich medium, as well as heterotrophic microorganisms like bacteria, protozoa feeding on the algae and nutrients. Indeed, microbial biomasses including algae were found on the surface of RR tissue and perlite, which might contribute to IBP removal.
Metabolite production was more pronounced and proceeded at higher initial rates in the treated groups than in the blank groups (Figure 3), indicating that the presence of plants enhanced biodegradation. It is reported that roots could release exudates containing ions, inorganic, and organic acids, proteins, and enzymes. Such exuded enzymes, including peroxidases and hydrolases, can catalyze the oxidation of xenobiotics.42 Furthermore, IBP has been reported to have a relatively high biodegradablility.43 Organic acids and proteins released by roots might favor rhizosphere bacteria to degrade IBP by acting as additional carbon substrates for microbial growth. Thus, the differences in intermediate production rates between treated and blank groups may be due to a combination of enzymatic reactions and enhanced rhizosphere mediated biodegradation.
3.2.2. Metabolism of IBP in Plant Tissue
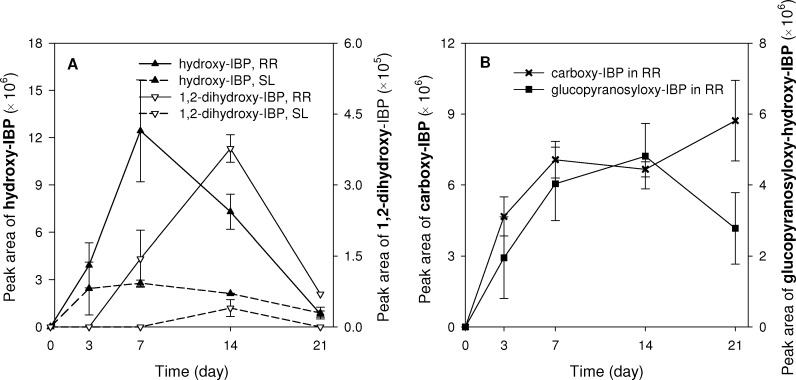
Referring to the selection in 2.3 of possible IBP intermediates formed in plant tissue (Table 1), we detected four out of the seven hypothesized intermediates. These were: hydroxy-IBP, 1,2-dihydroxy-IBP, carboxy-IBP and glucopyranosyloxy-hydroxy-IBP (IBP-glycoside conjugate) in RR and the first two intermediates in SL tissue. Mass spectra of detected compounds are shown in SI Figure S3.
The two intermediates of phase I (Table 1), hydroxy-IBP and carboxy-IBP, were reported to be formed via mammalian and microbial biodegradation of IBP. To date, this is the first study that reports production of hydroxy-IBP and carboxy-IBP in plant tissues. 1,2-dihydroxy-IBP can originate from either 1-hydroxy-IBP or 2-hydroxy-IBP.44 In our study, we observed that the production of 1,2-dihydroxy-IBP was sequential to the production of hydroxy-IBP (Figure 3), suggesting that 1,2-dihydroxy-IBP might be transformed from hydroxy-IBP. With regards to phase II intermediates, GT was thought to detoxify xenobiotics by acting on functional groups such as −OH, −NH2, −SH, and –COOH.45 However, our study only confirmed the existence of glucopyranosyloxy-hydroxy-IBP, while no glucopyranosyloxy-carboxy-IBP was detected. In addition, GST has a preference to catalyze conjugation at electrophilic double bonds or halogen functions,46 which clearly explains why the IBP-glutathione conjugate was not detected.
Overall, the detected IBP metabolites in P. australis were similar to the metabolites found in mammals and microbes. In contrast, in the first and so far only published data on IBP metabolism in the aquatic plant Lemna gibba L., a type of duckweed, Pietrini et al. (2015) detected hydroxy-IBP, and 1,2-dihydroxy-IBP as intermediates, but no carboxy-IBP.13 They suggested that the metabolic pathway of IBP in duckweed was different from mammals and microbes, but similar to fungi.
Concentrations of hydroxy-IBP and 1,2-dihydroxy-IBP were 3–4 times higher in RR than in SL tissue (Figure 4A). Those two intermediates were also produced in the liquid phase of treated groups (Figure 3A). Therefore, we could not distinguish whether their presence in tissue is a result of transport from the liquid phase or production in the plant. However, we could conclude that phytodegradation did contribute to the production of hydroxy-IBP and 1,2-dihydroxy-IBP, because IBP was transformed in both RR and SL tissue (Figure 2). The transformation tendency of IBP showed first a boost followed by a decay, which was similar to the production of two intermediates. Additionally, the production of these two intermediates occurred sequentially to the transformation of IBP. Recent research on diclofenac (DFC) metabolism in Typha latifolia detected parent DFC, hydroxy-DFC, glucopyranosyloxy-hydroxy-DFC, and DFC-glutathione conjugate in roots, and only DFC and hydroxy-DFC in leaves.15 In comparison, we also found for IBP the parent compound and hydroxy intermediates in SL, whereas in RR we did not detect IBP-glutathione conjugate but the carboxy-IBP. Overall, the results demonstrate that phytodegradation of IBP indeed occurred inside the tissues of P. australis by transforming IBP into four intermediates.
Figure 4.
Metabolism of IBP in the plant tissue (RR, roots and rhizomes; SL, stems and leaves). (A) hydroxy-IBP and 1,2-dihydroxy-IBP; (B) carboxy-IBP and glucopyranosyloxy-hydroxy-IBP. Peak area of intermediates is in unit of mAU/g tissue. Data are mean peak areas ± standard error (n = 3).
3.2.3. Enzymatic Activity
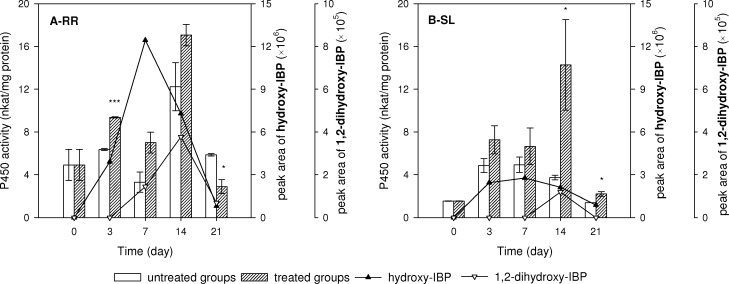
In order to understand the mechanism by which IBP is transformed, we measured activities of P450, GT, and GST in tissue of treated groups and compared with those of untreated groups. In phase I and II of plant detoxification, P450, GT, and GST normally catalyze hydrolysis, oxidation and synthesis reactions.22 P450 activity in treated RR tissue was higher than untreated tissue after 3 days of IBP exposure and the higher trend lasted until day 14 (Figure 5A). The occurrence of two hydroxy intermediates appeared to correlate with the trend of higher P450 activity. In addition, a similarly pronounced correlation was found in SL tissue: increase of P450 activity was synchronous with the detection of hydroxy-IBP (Figure 5B). P450 activity could be interpreted as an indicator of the phase I metabolism of IBP in P. australis. GT activity in RR and SL tissue showed differences compared with untreated groups, but no clear link between the alteration of enzyme activity and occurrence of glucopyranosyloxy-hydroxy-IBP was found (SI Figure S4). During exposure, GST activity increased at day 3 and 7 in RR tissue of treated groups compared with that in untreated groups (SI Figure S4). However, the activity peak disappeared afterward, and IBP-glutathione conjugate was not detected in RR tissue. Hence the increase may be attributed to some response to the oxidative burst condition of the tissue. In summary, our results show that P450 was involved in the production of two hydroxy intermediates; activity variation of GT and GST did not display a clear pattern that related with phytodegradation of IBP.
Figure 5.
P450 activity and its relationship with production of related intermediates (A) RR, roots and rhizomes (B) SL, stems and leaves. Peak area of intermediates is in unit of mAU/g tissue. Data are mean activity/peak areas ± standard error (n = 3).
3.2.4. Phytotoxicity of IBP Exposure
Phytotoxicity of pollutants can affect the phytoremediation process by making plant cells more susceptible to diseases and stress conditions.9 To determine the potential IBP-induced phytotoxicity, relative growth of plants (g/d) and activity of stress enzymes including POX and GR in RR and SL were investigated. The relative growth of P. australis showed that the biomass of all plants decreased during the first 3 days regardless of treatment, indicating plants may need an adjustment period to get used to a new growth environment and substrate (SI Figure S5). However, after 21 days exposure, relative growth of treated plants showed a similar level with that of untreated groups. Therefore, plants show resiliency during IBP exposure, as they continue to grow normally during the uptake and accumulation process and also degrade IBP with the involvement of P450.
The oxidative burst induced by xenobiotic exposure results in an overproduction of reactive oxygen species (ROS) such as superoxide radicals, which can damage plant cells.32 A stress enzyme system is one of the protective mechanisms for plants to eliminate the ROS excess.32 In our study, no obvious difference of stress enzyme activities was observed between treated and untreated groups (SI Figure S6), except for the difference found on day 3 in both RR and SL. This exception might result from the growth adaptation on day 3. The overall absence of stress enzymes might be related to the resilience of the plant species P. australis, or to the chosen IBP exposure concentration. Pawłowska et al. (2016) found that spring barley and common radish showed different sensitivity to the exposure of quaternary ammonium salts by showing different levels of stress enzyme activity.47 Different exposure concentrations also affect the variation of enzyme activity. POX activity of barley turned out to have a positive linear correlation with exposure concentrations.47 Another recent research showed that low concentrations of carbamazepine (<6 μg/L) stimulated the stress enzyme activity in two microalgae species. However, enzyme activity decreased at higher carbamazepine concentrations, because overloading stress brought functional damage to microalgae cells.48 In summary, no obvious alteration of growth rate and stress enzyme activity was observed, indicating that P. australis was resilient and resistant to IBP exposure.
3.3. IBP Transformation Pathways in Plant Tissue
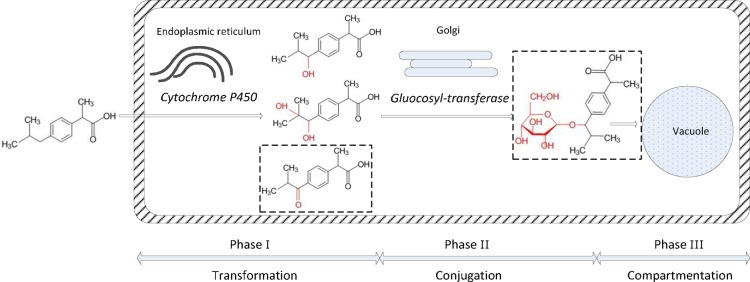
This study shows that P. australis is able to take up, accumulate, and metabolize IBP, in both RR and SL tissue without significant phytotoxicity. Based on our results, we propose the following pathway for IBP metabolism: transformation of IBP was catalyzed by P450 in the endoplasmic reticulum, then catalyzed by GT in Golgi, followed by further metabolism or storage in vacuoles or cell walls (Figure 6).
Figure 6.
Transformation of IBP in plant tissues. 1-hydroxy-IBP was used to represent hydroxy-IBP. Carboxy-IBP and glucopyranosyloxy-hydroxy-IBP (marked with dashed rectangles) were not detected in SL tissue.
3.4. Implication for Practice
Metabolism and detoxification of IBP in mammals are known processes and have been well described. The fate and transformation of IBP in aquatic macrophytes has not been investigated so far. However, aquatic macrophytes are increasingly exposed to residual pharmaceuticals in water bodies, especially when those plants are applied in phytoremediation. Therefore, it is necessary to study the fate of pharmaceuticals in macrophytes and the underlying phytoremediation mechanism. In summary, this study gives insight on the fate and transformation of IBP in P. australis, which can be applied for investigating other pharmaceuticals and forecasting their fate in other types of macrophytes. Reflecting on practice, this study proves that macrophytes have the potential to take up and degrade pharmaceuticals. The knowledge contributes to understanding and implementing phytoremediation in constructed wetlands as an effective treatment method for removing pharmaceuticals from water.
Acknowledgments
Part of this work was carried out within a Short Term Scientific Mission (STSM) of the Water2020 Cost Action ES1202: Conceiving Wastewater Treatment in 2020 | Energetic, environmental and economic challenges. Authors thanks Rudolf Harpaintner, Andres Sauvetre and Paula Haury for technical assistance. The support provided by China Scholarship Council (CSC) for the research of Yujie He at Wageningen University is kindly acknowledged.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.7b00458.
Chemicals and reagents, IBP sorption on perlite, validation of IBP extraction from plant tissue, methods for chemical analysis and GT enzyme activity, physicochemical properties of IBP, target precursor ions and mass spectra of parent ibuprofen and related metabolites, activities of GT, GST and stress enzyme activities (POX, GR) in plant tissues, relative growth rates of plants, and figure of growing plants under greenhouse conditions (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Santos J. L.; Aparicio I.; Alonso E. Occurrence and risk assessment of pharmaceutically active compounds in wastewater treatment plants. A case study: Seville city (Spain). Environ. Int. 2007, 33 (4), 596–601. 10.1016/j.envint.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Gao J.; O’Brien J.; Du P.; Li X.; Ort C.; Mueller J. F.; Thai P. K. Measuring selected PPCPs in wastewater to estimate the population in different cities in China. Sci. Total Environ. 2016, 568, 164–170. 10.1016/j.scitotenv.2016.05.216. [DOI] [PubMed] [Google Scholar]
- Matamoros V.; Arias C.; Brix H.; Bayona J. M. Preliminary screening of small-scale domestic wastewater treatment systems for removal of pharmaceutical and personal care products. Water Res. 2009, 43 (1), 55–62. 10.1016/j.watres.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Karnjanapiboonwong A.; Suski J. G.; Shah A. A.; Cai Q.; Morse A. N.; Anderson T. A. Occurrence of PPCPs at a wastewater treatment plant and in soil and groundwater at a land application site. Water, Air, Soil Pollut. 2011, 216 (1), 257–273. 10.1007/s11270-010-0532-8. [DOI] [Google Scholar]
- David A.; Pancharatna K. Developmental anomalies induced by a non-selective COX inhibitor (ibuprofen) in zebrafish (Danio rerio). Environ. Toxicol. Pharmacol. 2009, 27 (3), 390–395. 10.1016/j.etap.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Matamoros V.; Hijosa M.; Bayona J. M. Assessment of the pharmaceutical active compounds removal in wastewater treatment systems at enantiomeric level. Ibuprofen and naproxen. Chemosphere 2009, 75 (2), 200–205. 10.1016/j.chemosphere.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Susarla S.; Medina V. F.; McCutcheon S. C. Phytoremediation: An ecological solution to organic chemical contamination. Ecol. Eng. 2002, 18 (5), 647–658. 10.1016/S0925-8574(02)00026-5. [DOI] [Google Scholar]
- Vanek T.; Podlipna R.; Fialova Z.; Petrova S.; Soudek P.. Uptake of xenobiotics from polluted waters by plants. In Xenobiotics in the Urban Water Cycle: Mass Flows, Environmental Processes, Mitigation and Treatment Strategies; Fatta-Kassinos D., Bester K., Kümmerer K., Eds.; Springer Netherlands Press: Dordrecht, 2010; pp 431–444. [Google Scholar]
- Carvalho P.; Basto M. C.; Almeida C. M.; Brix H. A review of plant–pharmaceutical interactions: from uptake and effects in crop plants to phytoremediation in constructed wetlands. Environ. Sci. Pollut. Res. 2014, 21 (20), 11729–11763. 10.1007/s11356-014-2550-3. [DOI] [PubMed] [Google Scholar]
- Dordio A.; Ferro R.; Teixeira D.; Palace A. J.; Pinto A. P.; Dias C. M. B. Study on the use of Typha spp. for the phytotreatment of water contaminated with ibuprofen. Int. J. Environ. Anal. Chem. 2011, 91 (7–8), 654–667. 10.1080/03067311003782708. [DOI] [Google Scholar]
- Kotyza J.; Soudek P.; Kafka Z.; Vaněk T. Phytoremediation of pharmaceuticals—preliminary study. Int. J. Phytorem. 2010, 12 (3), 306–316. 10.1080/15226510903563900. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Lv T.; Carvalho P. N.; Arias C. A.; Chen Z.; Brix H. Removal of the pharmaceuticals ibuprofen and iohexol by four wetland plant species in hydroponic culture: plant uptake and microbial degradation. Environ. Sci. Pollut. Res. 2016, 23 (3), 2890–2898. 10.1007/s11356-015-5552-x. [DOI] [PubMed] [Google Scholar]
- Pietrini F.; Di Baccio D.; Aceña J.; Pérez S.; Barceló D.; Zacchini M. Ibuprofen exposure in Lemna gibba L.: Evaluation of growth and phytotoxic indicators, detection of ibuprofen and identification of its metabolites in plant and in the medium. J. Hazard. Mater. 2015, 300, 189–193. 10.1016/j.jhazmat.2015.06.068. [DOI] [PubMed] [Google Scholar]
- Sandermann H.Plant metabolism of organic xenobiotics. status and prospects of the ‘green liver’ concept. In Plant Biotechnology and In Vitro Biology in the 21st Century: Proceedings of the IXth International Congress of the International Association of Plant Tissue Culture and Biotechnology Jerusalem, Israel, 14–19 June 1998; Altman A.; Ziv M.; Izhar S., Eds.; Springer Netherlands Press: Dordrecht, 1999; pp 321–328. [Google Scholar]
- Bartha B.; Huber C.; Schröder P. Uptake and metabolism of diclofenac in Typha latifolia – How plants cope with human pharmaceutical pollution. Plant Sci. 2014, 227, 12–20. 10.1016/j.plantsci.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Cui H.; Hense B. A.; Müller J.; Schröder P. Short term uptake and transport process for metformin in roots of Phragmites australis and Typha latifolia. Chemosphere 2015, 134 (0), 307–312. 10.1016/j.chemosphere.2015.04.072. [DOI] [PubMed] [Google Scholar]
- Nitz G. M.; Schnitzler W. H. In Effect of PAR and UV-B Radiation on the Quality and Quantity of the Essential Oil in Sweet Basil (Ocimum basilicum L.), Acta Horticulturae, 2004; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2004; pp 375–381. [Google Scholar]
- Schröder P.Exploiting plant metabolism for the phytoremediation of organic xenobiotics. In Phytoremediation: Methods and Reviews, Willey N., Ed. Humana Press Press: Totowa, NJ, 2007; pp 251–263. [Google Scholar]
- Bartha B.; Huber C.; Harpaintner R.; Schröder P. Effects of acetaminophen in Brassica juncea L. Czern.: investigation of uptake, translocation, detoxification, and the induced defense pathways. Environ. Sci. Pollut. Res. 2010, 17 (9), 1553–1562. 10.1007/s11356-010-0342-y. [DOI] [PubMed] [Google Scholar]
- Christou A.; Antoniou C.; Christodoulou C.; Hapeshi E.; Stavrou I.; Michael C.; Fatta-Kassinos D.; Fotopoulos V. Stress-related phenomena and detoxification mechanisms induced by common pharmaceuticals in alfalfa (Medicago sativa L.) plants. Sci. Total Environ. 2016, 557–558, 652–664. 10.1016/j.scitotenv.2016.03.054. [DOI] [PubMed] [Google Scholar]
- Theodoulou F. L. Plant ABC transporters. Biochim. Biophys. Acta, Biomembr. 2000, 1465 (1–2), 79–103. 10.1016/S0005-2736(00)00132-2. [DOI] [PubMed] [Google Scholar]
- Coleman J.; Blake-Kalff M.; Davies E. Detoxification of xenobiotics by plants: chemical modification and vacuolar compartmentation. Trends Plant Sci. 1997, 2 (4), 144–151. 10.1016/S1360-1385(97)01019-4. [DOI] [Google Scholar]
- Ferrando-Climent L.; Collado N.; Buttiglieri G.; Gros M.; Rodriguez-Roda I.; Rodriguez-Mozaz S.; Barceló D. Comprehensive study of ibuprofen and its metabolites in activated sludge batch experiments and aquatic environment. Sci. Total Environ. 2012, 438, 404–413. 10.1016/j.scitotenv.2012.08.073. [DOI] [PubMed] [Google Scholar]
- Clayton E.; Taylor S.; Wright B.; Wilson I. D. The application of high performance liquid chromatography, coupled to nuclear magnetic resonance spectroscopy and mass spectrometry (HPLC-NMR-MS), to the characterisation of ibuprofen metabolites from human urine. Chromatographia 1998, 47 (5), 264–270. 10.1007/BF02466530. [DOI] [Google Scholar]
- Pflugmacher S.; Sandermann H. Cytochrome P450 monooxygenases for fatty acids and xenobiotics in marine macroalgae. Plant Physiol. 1998, 117 (1), 123–128. 10.1104/pp.117.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder P.; Maier H.; Debus R. Detoxification of herbicides in phragmites australis. Z. Naturforsch., C: J. Biosci. 2005, 60 (3–4), 317–324. 10.1515/znc-2005-3-417. [DOI] [PubMed] [Google Scholar]
- Olry A.; Schneider-Belhaddad F.; Heintz D.; Werck-Reichhart D. A medium-throughput screening assay to determine catalytic activities of oxygen-consuming enzymes: a new tool for functional characterization of cytochrome P450 and other oxygenases. Plant J. 2007, 51 (2), 331–340. 10.1111/j.1365-313X.2007.03140.x. [DOI] [PubMed] [Google Scholar]
- Meßner B.; Thulke O.; Schäffner A. R. Arabidopsis glucosyltransferases with activities toward both endogenous and xenobiotic substrates. Planta 2003, 217 (1), 138–146. [DOI] [PubMed] [Google Scholar]
- Schröder P.; Juuti S.; Roy S.; Sandermann H.; Sutinen S. Exposure to chlorinated acetic acids: responses of peroxidase and glutathione S-transferase activity in pine needles. Environ. Sci. Pollut. Res. 1997, 4 (3), 163–171. 10.1007/BF02986326. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Kirkham M. B. Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytol. 1996, 132 (3), 361–373. 10.1111/j.1469-8137.1996.tb01856.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72 (1), 248–254. 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dordio A. V.; Belo M.; Martins Teixeira D.; Palace Carvalho A. J.; Dias C. M.; Pico Y.; Pinto A. P. Evaluation of carbamazepine uptake and metabolization by Typha spp., a plant with potential use in phytotreatment. Bioresour. Technol. 2011, 102 (17), 7827–34. 10.1016/j.biortech.2011.06.050. [DOI] [PubMed] [Google Scholar]
- Vanacker H.; Carver T. L. W.; Foyer C. H. Pathogen-induced changes in the antioxidant status of the apoplast in barley leaves. Plant Physiol. 1998, 117 (3), 1103–1114. 10.1104/pp.117.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartha B.Uptake and metabolism of human pharmaceuticals in plants. Ph.D. Dissertation, Technical University of Munich, München, Germany, 2012. [Google Scholar]
- Wu X.; Dodgen L. K.; Conkle J. L.; Gan J. Plant uptake of pharmaceutical and personal care products from recycled water and biosolids: a review. Sci. Total Environ. 2015, 536, 655–666. 10.1016/j.scitotenv.2015.07.129. [DOI] [PubMed] [Google Scholar]
- Briggs G. G.; Bromilow R. H.; Evans A. A. Relationships between lipophilicity and root uptake and translocation of non-ionized chemicals by barley. Pestic. Sci. 1982, 13 (5), 495–504. 10.1002/ps.2780130506. [DOI] [Google Scholar]
- Miller E. L.; Nason S. L.; Karthikeyan K. G.; Pedersen J. A. Root uptake of pharmaceuticals and personal care product ingredients. Environ. Sci. Technol. 2015, 50, 525–541. 10.1021/acs.est.5b01546. [DOI] [PubMed] [Google Scholar]
- Illés E.; Takács E.; Dombi A.; Gajda-Schrantz K.; Rácz G.; Gonter K.; Wojnárovits L. Hydroxyl radical induced degradation of ibuprofen. Sci. Total Environ. 2013, 447, 286–292. 10.1016/j.scitotenv.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Klein R. M. Failure of supplementary ultraviolet radiation to enhance flower color under greenhouse conditions. HortScience 1990, 25 (3), 307–308. [Google Scholar]
- Bian Z.-Y.; Zhu Y.-Q.; Zhang J.-X.; Ding A.-Z.; Wang H. Visible-light driven degradation of ibuprofen using abundant metal-loaded BiVO4 photocatalysts. Chemosphere 2014, 117, 527–531. 10.1016/j.chemosphere.2014.09.017. [DOI] [PubMed] [Google Scholar]
- Jacobs L. E.; Fimmen R. L.; Chin Y.-P.; Mash H. E.; Weavers L. K. Fulvic acid mediated photolysis of ibuprofen in water. Water Res. 2011, 45 (15), 4449–4458. 10.1016/j.watres.2011.05.041. [DOI] [PubMed] [Google Scholar]
- Sandermann H.; Diesperger H.; Scheel D.. Metabolism of xenobiotics by plant cell cultures. In Plant Tissue Culture and Its Bio-technological Application: Proceedings of the First International Congress on Medicinal Plant Research, Section B, held at the University of Munich, Germany September 6–10, 1976; Barz W., Reinhard E., Zenk M. H., Eds.; Springer Berlin Heidelberg Press: Berlin, Heidelberg, 1977; pp 178–196. [Google Scholar]
- Dordio A.; Carvalho A. J. P.; Teixeira D. M.; Dias C. B.; Pinto A. P. Removal of pharmaceuticals in microcosm constructed wetlands using Typha spp. and LECA. Bioresour. Technol. 2010, 101 (3), 886–892. 10.1016/j.biortech.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Marco-Urrea E.; Pérez-Trujillo M.; Vicent T.; Caminal G. Ability of white-rot fungi to remove selected pharmaceuticals and identification of degradation products of ibuprofen by Trametes versicolor. Chemosphere 2009, 74 (6), 765–772. 10.1016/j.chemosphere.2008.10.040. [DOI] [PubMed] [Google Scholar]
- Calderón-Preciado D.; Renault Q.; Matamoros V.; Cañameras N.; Bayona J. M. Uptake of organic emergent contaminants in spath and lettuce: an in vitro experiment. J. Agric. Food Chem. 2012, 60 (8), 2000–2007. 10.1021/jf2046224. [DOI] [PubMed] [Google Scholar]
- Verkleij J. A. C.; Golan-Goldhirsh A.; Antosiewisz D. M.; Schwitzguébel J.-P.; Schröder P. Dualities in plant tolerance to pollutants and their uptake and translocation to the upper plant parts. Environ. Exp. Bot. 2009, 67 (1), 10–22. 10.1016/j.envexpbot.2009.05.009. [DOI] [Google Scholar]
- Pawłowska B.; Biczak R. Evaluation of the effect of tetraethylammonium bromide and chloride on the growth and development of terrestrial plants. Chemosphere 2016, 149, 24–33. 10.1016/j.chemosphere.2016.01.072. [DOI] [PubMed] [Google Scholar]
- Freitas R.; Almeida Â.; Pires A.; Velez C.; Calisto V.; Schneider R. J.; Esteves V. I.; Wrona F. J.; Figueira E.; Soares A. M. V. M The effects of carbamazepine on macroinvertebrate species: comparing bivalves and polychaetes biochemical responses. Water Res. 2015, 85 (85), 137–147. 10.1016/j.watres.2015.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.