Abstract
Background
Younger maternal age at birth is associated with increased risk of asthma in offspring in European descent populations, but has not been studied in Latino populations.
Objectives
We sought to examine the relationship between maternal age at birth and prevalence of asthma in a nationwide study of Latino children.
Methods
We included 3473 Latino children aged 8–21 years (1696 subjects with physician-diagnosed asthma and 1777 healthy controls) from five U.S. centers and Puerto Rico recruited from July 2008 through November 2011. We used multiple logistic regression to examine the effect of maternal age at birth on asthma in offspring overall, and in analyses stratified by ethnic subgroup (Mexican American, Puerto Rican, and Other Latino). Secondary analyses evaluated the effects of siblings, acculturation, and income on this relationship.
Results
Maternal age <20 years was significantly associated with a decreased odds of asthma in offspring, independent of other risk factors (OR = 0.73, 95% CI: 0.57–0.93). In subgroup analyses, the protective effect of younger maternal age was observed only in Mexican Americans (OR = 0.53, 95% CI: 0.36, 0.79). In Puerto Ricans, older maternal age was associated with a decreased odds of asthma (OR = 0.65, 95% CI: 0.44–0.97). In further stratified models, the protective effect of younger maternal age in Mexican Americans was seen only in children without older siblings (OR = 0.44, 95% CI: 0.23–0.81).
Conclusion and Clinical Relevance
In contrast to European descent populations, younger maternal age was associated with a decreased odds of asthma in offspring in Mexican American women. Asthma is common in urban minority populations but the factors underlying the varying prevalence among different Latino ethnicities in the U.S. is not well understood. Maternal age represents one factor which may help explain this variability.
Introduction
Asthma is one of the most common pediatric diseases, affecting 7.3% of the current US pediatric population and accounts for an annual cost of $56 billion [1, 2]. Asthma affects urban minority children with greater prevalence and disproportionately increased morbidity and mortality [3–6]. While many socioeconomic and environmental factors have been explored as a cause of this disparity, the effect of young maternal age has been understudied in urban minority populations. Younger maternal age is common in urban minority populations [7] and is associated with up to a threefold risk of asthma in offspring in studied populations [8–13]. However, most of these studies have been carried out in European descent populations. Few have included significant numbers of low-income minority populations, in whom asthma is more prevalent. Minority populations of lower socioeconomic status will also have different socio-cultural and environmental exposures in the first years of life [14–16].
Many exposures vary by race/ethnicity and have shown to be both associated with pregnancies in younger mothers and with increased risk of asthma in childhood. These include both pre/perinatal factors (such as maternal smoking during pregnancy [17], low gestational age [18], low birth weight [19], and chorioamnionitis [11, 20, 21]) and early childhood risk factors (exposure to air pollutants and tobacco smoke [14, 17], no older siblings [22], and less breastfeeding[23]). While some have suggested that maternal age at delivery could be a marker for other aspects of socioeconomic status or lifestyle [10, 13], adjusting for many of the aforementioned factors, in addition to education, income and living conditions, did not diminish the association [9, 10, 13].
It is unknown whether younger maternal age may account for increased prevalence of asthma in Latino populations which consistently have the highest birth rates in women <20 years of age compared to other racial groups [7]. We sought to determine if younger maternal age is a risk factor for asthma in Latino children. We also evaluated whether any association of maternal age with asthma was modified by ethnic subgroup, or other factors associated with young maternal age including SES, acculturation, and number of siblings.
Methods
Cohort recruitment
The Genes-environments and Admixture in Latino Americans (GALA II) is a clinic-based multicenter asthma case-control study designed to examine the genetic and environmental risk factors for asthma and asthma-related phenotypes in Latino children [24]. Asthma cases were defined as participants aged 8–21 years with a history of physician-diagnosed asthma and the presence of 2 or more symptoms of coughing, wheezing, or shortness of breath in the 2 years preceding enrollment. Healthy control subjects were recruited from the community and clinics with the same catchment area as cases. Subjects were recruited from five centers (Chicago, Illinois; Bronx, New York; Houston, Texas; the San Francisco Bay Area, California; and Puerto Rico) from July 2008 through November 2011. Control subjects were defined as participants with no reported history of asthma, lung disease, or chronic illness over their lifetime and no reported symptoms of coughing, wheezing, or shortness of breath in the last 2 years. Control subjects were frequency matched on age (within 1 year), sex, and study center. Participants were excluded if they reported any of the following: (1) 10 or more pack years of smoking; (2) any smoking within 1 year of recruitment date; (3) history of lung diseases other than asthma (cases) or chronic illness (cases and control subjects); or (4) pregnancy.
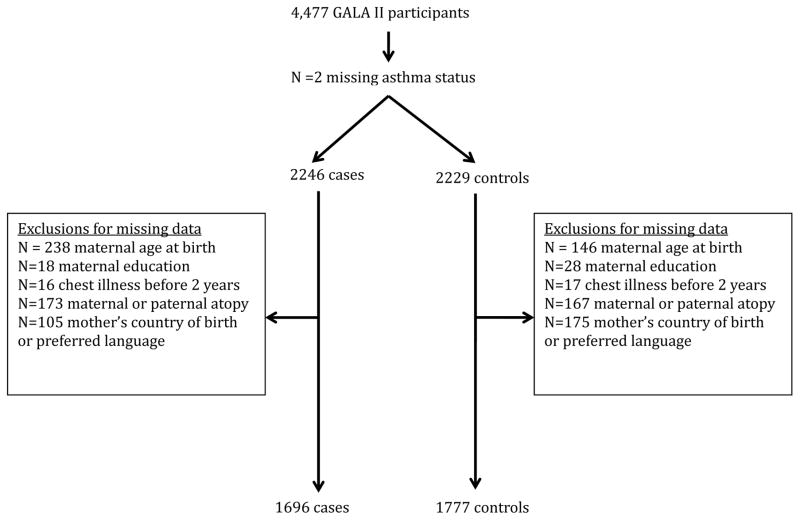
Participants (or their guardians) were administered questionnaires relating to asthma-related exposures and risk factors, underwent pulmonary function testing, and blood was obtained for DNA isolation. 4,477 subject participants were considered for inclusion in the study sample. After excluding those with missing values for key variables, the final sample included 3,473 participants (Figure 1).
Figure 1.
Final analytic sample
Determination of Key variables
Primary determinant
Maternal age – Maternal age was ascertained by the question “How old was the child’s biological mother when the child was born?” For analysis, the maternal age variable was divided into quartiles (<20, 20–24, 25–29, >29 years).
Key controlling variables
Ethnicity - Ethnicity, which was an inclusion criterion of the study, was ascertained through the question “Do you consider yourself Spanish/ Hispanic/Latino?” with a “Yes/No” choice (if yes, please specify: Mexican, Mexican American, Chicano, Spanish, Hispanic, Latino, Dominican, Cuban, etc). For our purposes, this variable was collapsed into Mexican American, Puerto Rican and Other Latino ethnicities.
Maternal education – Maternal education was ascertained through the question “What is the highest level of education that the child’s mother completed?” and is used in this report as a proxy for socioeconomic status. Responses were collapsed into the following categories: <high school, high school graduate, some college, and college graduate or graduate school.
Acculturation – Acculturation status was a 5-level variable created using the mother’s characteristics (length of time mother has been in the US, mother’s place of birth and language preference of the mother) as a proxy for the child’s home environment, as described previously [24]. Values ranged from least acculturated (acculturation = 1) to most acculturated (acculturation = 5). Due to a large number of missing values for acculturation (n missing = 1336), a separate missing category was used in analyses. Number of siblings – The number of siblings the child had was ascertained by the question “How many other children has the child’s biological mother had?” This question was further refined by asking how many are older, younger or the same as the child. For our purposes, we dichotomized the presence of older siblings (0, 1+). A separate missing category was used for older siblings (n missing = 354), given that the missing category was found to be associated with maternal age, such that younger mothers (<20) were more likely to have a missing value.
Other Covariates Evaluated
Other key variables considered for inclusion into the model based on the literature were maternal and paternal history of atopy (yes, no), age at GALA study visit (continuous), recruitment site (Chicago, Illinois; San Francisco Bay Area, California; Houston, Texas; Bronx, New York; and Puerto Rico), sex (male, female), daycare attendance (yes, no), premature birth (yes, no), breastfeeding (yes, no), maternal smoking during pregnancy (yes, no), chest illness in the first 2 years of life (yes, no), insurance status (yes, no). Premature birth was defined as being born ≥6 weeks early as reported by the participant or guardian. Both maternal and paternal atopy was defined as having a self-reported history of allergic rhinitis, hay fever, eczema, or asthma.
Statistical analysis
A bivariate analysis was performed to assess the independent relationship between each of the aforementioned variables and asthma (yes, no) and maternal age at birth (<20, 20–24, 25–29, >29) in the cohort, using a chi-square test for categorical variables and ANOVA test for continuous variables. Bivariate analyses showed that maternal education, recruitment site, presence of older siblings, breastfeeding, maternal smoking during pregnancy, premature birth, maternal atopy, paternal atopy, chest illness before 2 years, acculturation level, and ethnicity were associated with both asthma and maternal age at the p < 0.2 level. Multiple logistic regression was carried out after variable selection. Breastfeeding, maternal smoking during pregnancy, and premature birth were not significant at the α = 0.05 level after adjustment for other variables, and these variables were removed from the multivariable model. Removal of these variables did not change effect estimates for maternal age >10%. Maternal education was not significant at the α = 0.05 level but was retained in the final model as a proxy for socioeconomic status, along with age of the child and presence of older siblings.
We also evaluated the effect of ancestry (proportion African American, Native American and European) on the relationship between maternal age and asthma.
As a secondary analysis, we evaluated whether the observed associations between maternal age and asthma differed within ethnicity subgroups. We then further stratified the models in an exploratory analysis to examine whether the observed associations between maternal age and asthma within ethnicity subgroups differed by older siblings, acculturation, and family income after testing the interaction between these variables and maternal age. We also considered the effects of household size, number of children <18 years old in the household, and number of adults as covariates. Finally, we ran a sensitivity analysis using multilevel modeling to evaluate for any clustering effect among different recruitment sites. All analyses were completed using SAS v.9.3 (Cary, NC).
Each of the ten participating centers’ Institutional Review Boards reviewed and approved the study. Written informed consent was obtained by each child’s parent or legal guardian or by the subject if 18 and older. The current study sample includes data from the baseline questionnaire of children with physician-diagnosed asthma and controls recruited in GALA II through November 2011.
Results
Characteristics of the final sample with complete data (n=3,473) are shown in Table 1. Table 2 shows the characteristics of the sample by asthma status. The sample consisted of 1,696 cases with asthma and 1,777 controls. Forty-five percent were of Puerto Rican descent (n = 1,581), thirty-six percent of Mexican descent (n = 1,251) and the rest fell into the other Latino category (n = 641). The average age of asthma cases was 13.6 years versus 12.5 years for controls. 56% of asthma cases and 42.8% of controls were male. 1,002 subjects were excluded due to missing variables (Supplemental Table S1). Excluded subjects were more likely to be Puerto Rican (54.7% versus 45.5%) and less likely to be Mexican American (28.4% versus 36%) when compared to the included subjects. Excluded subjects were also somewhat more likely to have asthma (54.9% versus 48.8%).
Table 1.
Description of the sample of GALA II participants by maternal age at birtha
| Maternal Age at Birth
|
|||||
|---|---|---|---|---|---|
| Characteristicb | <20 (n = 852)c | 20–24 (n = 884)c | 25–29 (n = 923)c | >29 (n = 814)c | p-valued |
|
|
|||||
| Asthma | 371 (43.5) | 428 (48.4) | 490 (53.1) | 407 (50.0) | <0.001 |
| Mother's education | <0.001 | ||||
| <high school | 394 (46.2) | 316 (35.8) | 290 (31.4) | 282 (34.6) | |
| high school graduate | 252 (29.6) | 249 (28.2) | 235 (25.5) | 197 (24.2) | |
| some college | 141 (16.6) | 218 (24.7) | 233 (25.2) | 183 (22.5) | |
| college graduate | 65 (7.6) | 101 (11.4) | 165 (17.9) | 152 (18.7) | |
| Older siblings | <0.001 | ||||
| 0 | 369 (43.3) | 258 (29.2) | 172 (18.6) | 110 (13.5) | |
| 1+ | 285 (33.5) | 498 (56.3) | 635 (68.8) | 633 (77.8) | |
| Missing | 198 (23.2) | 128 (14.5) | 116 (12.6) | 71 (13.8) | |
| Age | 13.01±3.4 | 13.02±3.3 | 13.04±3.3 | 13.06±3.4 | 0.99 |
| Male | 412 (48.4) | 434 (49.1) | 452 (49.0) | 413 (50.7) | 0.79 |
| Ethnicity | 0.003 | ||||
| Mexican | 324 (38.0) | 354 (40.1) | 319 (34.6) | 254 (31.2) | |
| Puerto Rican | 376 (44.1) | 392 (44.3) | 418 (45.3) | 395 (48.5) | |
| Other Latino | 152 (17.8) | 138 (15.6) | 186 (20.2) | 165 (20.3) | |
| Has health insurance | 785 (93.6) | 816 (93.2) | 858 (94.2) | 768 (95.2) | 0.33 |
| Breastfed | 525 (64.0) | 606 (70.4) | 634 (70.2) | 516 (64.6) | 0.003 |
| Premature birth | 34 (5.7) | 19 (3.1) | 32 (5.0) | 31 (5.3) | 0.15 |
| Maternal smoking during pregnancy | 46 (5.4) | 21 (2.4) | 26 (2.8) | 43 (5.3) | <0.001 |
| Daycare attendance | 188 (22.5) | 197 (22.5) | 236 (26.1) | 195 (24.2) | 0.24 |
| Chest illness before 2 years | 252 (29.6) | 293 (33.1) | 323 (35.0) | 268 (32.9) | 0.11 |
| Family history of atopic disease | |||||
| maternal atopy | 243 (28.5) | 284 (32.1) | 274 (29.7) | 251 (30.8) | 0.40 |
| paternal atopy | 179 (21.0) | 190 (21.5) | 190 (20.6) | 145 (17.8) | 0.24 |
| Acculturation Level | <0.001 | ||||
| 1 | 120 (14.1) | 112 (12.7) | 92 (10.0) | 55 (6.8) | |
| 2 | 121 (14.2) | 121 (13.7) | 108 (11.7) | 62 (7.6) | |
| 3 | 141 (16.6) | 198 (22.4) | 263 (28.5) | 248 (30.5) | |
| 4 | 43 (5.1) | 31 (3.5) | 31 (3.4) | 28 (3.4) | |
| 5 | 133 (15.6) | 83 (9.4) | 74 (8.0) | 73 (9.0) | |
| Missing | 294 (34.5) | 339 (38.4) | 355 (38.5) | 348 (42.8) | |
| Recruitment Site | 0.009 | ||||
| Chicago, Illinois | 146 (17.1) | 148 (16.7) | 130 (14.1) | 147 (18.1) | |
| San Francisco, California | 165 (19.4) | 177 (20.0) | 199 (21.6) | 148 (18.2) | |
| Houston, Texas | 98 (11.5) | 87 (9.8) | 83 (9.0) | 54 (6.6) | |
| Bronx, New York | 123 (14.4) | 109 (12.3) | 128 (13.9) | 99 (12.2) | |
| Puerto Rico | 320 (37.6) | 363 (41.1) | 383 (41.5) | 366 (45.0) | |
Table values are mean ± SD for continuous variables and n (column %) for categorical variables.
Characteristic is of child unless otherwise noted.
Percentages may not sum to 100% due to rounding.
P-value is for ANOVA test (continuous variables) or χ2 test (categorical variables).
Table 2.
Description of the sample of GALA II participants by asthma statusa
| Asthma
|
|||
|---|---|---|---|
| Characteristicb | Yes (n =1696)c | No (n = 1777)c | p-valued |
|
|
|
||
| Maternal age at birth | <0.001 | ||
| <20 | 371 (21.9) | 481 (27.1) | |
| 20–24 | 428 (25.2) | 456 (25.7) | |
| 25–29 | 490 (28.9) | 433 (24.4) | |
| >29 | 407 (24.0) | 407 (22.9) | |
| Older siblings | <0.001 | ||
| 0 | 430 (25.4) | 479 (27.0) | |
| 1+ | 1056 (62.3) | 995 (66.0) | |
| Missing | 210 (12.4) | 303 (17.1) | |
| Age | 12.5 ± 3.2 | 13.6 ± 3.4 | <0.001 |
| Premature birth | 76 (6.8) | 40 (3.0) | <0.001 |
| Maternal smoking during pregnancy | 77 (4.5) | 59 (3.3) | 0.06 |
| Male | 950 (56.0) | 761 (42.8) | <0.001 |
| Ethnicity | 0.026 | ||
| Mexican | 577 (34.0) | 674 (37.9) | |
| Other Latino | 1+ | 305 (17.2) | |
| Puerto Rican | Missing | 798 (44.9) | |
| Has health insurance | 1604 (95.6) | 1623 (92.5) | <0.001 |
| Breastfed | 1081 (65.2) | 1200 (69.6) | 0.006 |
| Daycare attendance | 415 (24.8) | 401 (22.9) | 0.19 |
| Mother's education | 0.036 | ||
| <high school | 593 (35.0) | 689 (38.8) | |
| high school graduate | 465 (27.4) | 468 (26.3) | |
| some college | 408 (24.1) | 367 (20.7) | |
| college graduate | 230 (13.6) | 253 (14.2) | |
| Chest illness before 2 years | 974 (57.4) | 162 (9.1) | <0.001 |
| Family history of atopic disease | |||
| mother atopy | 711 (41.9) | 341 (19.2) | <0.001 |
| father atopy | 475 (28.0) | 229 (12.9) | <0.001 |
| Acculturation Level | <0.001 | ||
| 1 | 124 (7.3) | 255 (14.4) | |
| 2 | 206 (12.2) | 206 (11.6) | |
| 3 | 409 (24.1) | 441 (24.8) | |
| 4 | 76 (4.5) | 57 (3.2) | |
| 5 | 235 (13.9) | 128 (7.2) | |
| Missing | 646 (38.1) | 690 (38.1) | |
| Recruitment Site | 0.12 | ||
| Chicago, Illinois | 283 (16.7) | 288 (16.2) | |
| San Francisco, California | 316 (18.6) | 373 (21.0) | |
| Houston, Texas | 173 (10.2) | 149 (8.4) | |
| Bronx, New York | 236 (13.9) | 223 (12.6) | |
| Puerto Rico | 688 (40.6) | 744 (41.9) | |
Table values are mean ± SD for continuous variables and n (column %) for categorical variables.
Characteristic is of child unless otherwise noted.
Percentages may not sum to 100% due to rounding.
P-value is for t-test (continuous variables) or χ2 test (categorical variables).
The overall adjusted associations of maternal age are presented in Table 3. Maternal age <20 was associated with a significantly decreased odds of asthma (OR = 0.73, 95% CI: 0.57–0.93) compared to maternal age 25–29. This association remained significant after ancestry (data not shown) was included as a covariate, and effect estimates were not altered by the inclusion of this variable. Maternal age categories 20–24 and >29 were not significantly associated with asthma compared to maternal age 25–29. No significant clustering effects were observed in multilevel modeling (data not shown).
Table 3.
Multivariable associations of maternal age at birth and prevalence of asthma stratified by Latino subgroups a
| Unstratified model | Model Stratified by Ethnic group | |||||||
|---|---|---|---|---|---|---|---|---|
| Maternal age at birth | (n=3,473) b | p-value | Mexican Americans (n=1,251)b | p-value | Puerto Rican (n=1,581)b | p-value | Other Latino (n=641)b | p-value |
| <20 | 0.73 (0.57, 0.93) | 0.01 | 0.53 (0.36, 0.79) | 0.002 | 0.98 (0.65, 1.48) | 0.92 | 0.75 (0.42, 1.35) | 0.34 |
| 20–24 | 0.80 (0.63, 1.01) | 0.05 | 0.83 (0.58, 1.17) | 0.29 | 0.70 (0.47, 1.05) | 0.09 | 0.72 (0.42, 1.24) | 0.23 |
| 25–29 | 1.00 (ref) | - | 1.00 (ref) | - | 1.00 (ref) | - | 1.00 (ref) | - |
| >29 | 0.84 (0.67, 1.07) | 0.17 | 0.85 (0.58, 1.24) | 0.40 | 0.65 (0.44, 0.97) | 0.03 | 1.16 (0.69, 1.96) | 0.57 |
Table values are odds ratio (95% confidence interval).
Logistic regression model includes age, sex, maternal education, parental atopy, chest illness before 2 years of age, acculturation level, recruitment site and presence of older siblings as covariates.
In ethnicity subgroup analyses (Table 3), the inverse association between maternal age <20 and asthma remained significant only in Mexican Americans (OR = 0.53, 95% CI: 0.36–0.79). In Puerto Ricans, maternal age >29 was significantly associated with a decreased risk of asthma (OR = 0.65, 95% CI: 0.44, 0.97). No significant associations were observed in other Latinos.
Exploratory analyses stratified by income (dichotomized at $25,000 per year), maternal age, and the presence of older siblings were performed separately for both Mexican Americans and Puerto Ricans. We carried out these analyses after testing for interaction of maternal age with income (p for interaction = 0.02). Interaction terms approached significance for older siblings (p = 0.06), but were not significant for acculturation (p > 0.2). We still performed a stratified analysis by acculturation as well to explore additive as opposed to interactive effects.
Table 4 shows the stratified models within Mexican Americans. Within Mexican Americans, the protective effect of younger maternal age at birth (<20) was observed only in individuals with no older siblings (OR = 0.44, 95% CI: 0.23–0.81). Similarly, the association of young maternal age was only significant in those who are least acculturated (OR = 0.47, 95% CI: 0.32–0.67). Lower income had an equally protective effect in mothers <20 years of age, suggesting that this is not an explanatory factor in the effects of young maternal age. No significant associations emerged for Puerto Ricans in these stratified models (data not shown).
Table 4.
Stratified associations of maternal age at birth and prevalence of asthma within Mexican Americansa
| Maternal age at birth | Zero older siblings (n =384)b | p-value | 1+ siblings (n =703)b | p-value |
|---|---|---|---|---|
| <20 | 0.44 (0.23, 0.81) | 0.009 | 0.98 (0.56, 1.70) | 0.94 |
| 20–24 | 0.67 (0.35, 1.29) | 0.23 | 0.71 (0.47, 1.09) | 0.12 |
| 25–29 | 1.00 (ref) | - | 1.00 (ref) | - |
| >29 | 1.16 (0.48, 2.81) | 0.74 | 0.93 (0.62, 1.39) | 0.73 |
| Lowest acculturation (n = 1,061) | p-value | Acculturation > 1 (n=218) | p-value | |
|
|
||||
| <20 | 0.47 (0.32, 0.67) | <0.001 | 0.55 (0.23, 1.32) | 0.18 |
| 20–24 | 0.69 (0.49, 0.98) | 0.04 | 0.95 (0.42, 2.18) | 0.91 |
| 25–29 | 1.00 (ref) | - | 1.00 (ref) | |
| >29 | 0.82 (0.56, 1.18) | 0.28 | 1.08 (0.37, 3.12) | 0.89 |
| Income <$25,000 (n = 595) | p-value | Income $25,000 (n=572) | p-value | |
|
|
||||
| <20 | 0.46 (0.28, 0.74) | 0.001 | 0.52 (0.31, 0.88) | 0.01 |
| 20–24 | 0.49 (0.31, 0.80) | 0.004 | 1.13 (0.69, 1.87) | 0.62 |
| 25–29 | 1.00 (ref) | - | 1.00 (ref) | - |
| >29 | 0.83 (0.50, 1.36) | 0.45 | 0.96 (0.57, 1.63) | 0.88 |
Table values are odds ratio (95% confidence interval).
Logistic regression model includes age, sex, parental atopy and recruitment site.
Discussion
We examined the effect of maternal age at birth and asthma in childhood in a Latino population, and found that maternal age <20 was significantly associated with a decreased risk of asthma in offspring, independent of other risk factors. In subgroup analyses, the protective effect of younger maternal age was observed only in Mexican Americans. In Puerto Ricans, older maternal age was associated with a decreased risk of asthma, which more closely aligns with previous studies of maternal age and asthma in Whites. Indeed, except for one study, which also examined the relationship in African Americans [13], all prior studies have been in European or White populations. Many of these prior studies have demonstrated either an increased risk of asthma in offspring of young mothers [9] or a protective effect of older maternal age [8, 10–12]. Some of these studies controlled for the number of siblings [8, 10] and socioeconomic status [8–10]. In one of the only studies that included a significant minority population, young motherhood was again found to be an independent risk factor for asthma in both white and black children in the U.S. [13]. Furthermore, in addition to MD-diagnosed asthma, an increased risk of transient and later onset wheeze has also been demonstrated in the offspring of young mothers [25–28].
In contrast to what we expected, that young maternal age would be a risk factor for asthma and potentially serve as a differentially distributed risk factor, younger age was protective in Mexican American women. The reason for this protective effect remains unclear. It is possible that young mothers of Mexican descent live in a multigenerational or otherwise crowded household such that there is a protective effect of other children in the household other than biological siblings, or a protective effect from the social support of other adults in the household, thereby affecting degrees of maternal stress which is also associated with asthma in the offspring [29]. However, when we included household size, number of children under the age of 18, and number of adults in the household in the model, the protective effect of young motherhood remained significant (data not shown).
One key factor which may mitigate the effect of young maternal age is improved birth outcomes in young Mexican women. White teenage mothers have been shown to be at higher risk of having a child of low birth weight or of low gestational age compared to older mothers [30], and low birth weight is a known risk factor for childhood wheezing disorders [31]. Hispanic infants, however, have lower rates of low birth weight and mortality compared to national averages, despite being socioeconomic disadvantaged when compared to whites [33]. These favorable birth outcomes are accepted as part of the larger phenomenon of the “Hispanic Paradox”. However, these favorable birth outcomes are not equal across Latino ethnicities. Mexican American women appear to have the most advantage, while Puerto Rican women derive less benefit [34, 35].
The superior birth outcomes for Mexican immigrants have been attributed to social support, desirability of pregnancy, nutrition, and reduced substance use [32]. Mexican American women report lower consumption of alcohol, diet soda, caffeine, and tobacco during pregnancy compared to non-Hispanic Whites. Mexican American women were also more likely to report changes in their eating habits during pregnancy, and milk, meat and water intake were significantly higher as compared to non-Hispanic women [36]. Another study found that pregnant Mexican women often ate food prepared for them by their mothers [37]. Rates of cigarette smoking and alcohol use during pregnancy, known risk factors for low birth weight, are also lower among Mexican Americans compared non-Hispanic whites. Studies have shown that Mexican immigrant women are also likely to have relatives living close by, within 10 minutes of their home, which allowed for shared social and financial support [37]. It is possible that these protective social factors of Mexican American women are more common in young mothers, which could help explain why we do not see the same detrimental effect of young maternal age.
The protective effect of younger maternal age in Mexican Americans was seen only in children without older siblings or those who were least acculturated. While the association was not noted in the more acculturated groups (p = 0.16), the magnitude and direction of the effect estimate remained the same. The number of individuals in the more acculturated groups (n = 218) may have been too low to detect a significant effect. However, it remains unclear why the association might persist only in nulliparous young mothers. While maternal immunity differences by age and parity may be hypothesized to play a role, a study of mid-trimester amniotic fluid concentrations of proinflammatory cytokines IL-6, IL-8 and TNF-α showed no differences by parity [38].
A number of limitations in the current study must be considered. As a case-control study, we cannot rule out the possibility of recall bias for some of the covariates. However, our primary exposure variable – maternal age at birth – would likely not be subject to recall bias and would be remembered equally among cases and controls. Any misclassification would have biased effect estimates towards the null. We also could not directly evaluate the incidence of asthma. Rather, we examined the prevalence of existing asthma in relation to maternal age at birth. GALA II was limited to the ages of 8–21 years. Maternal age is a birth-associated factor that may be more likely associated with an early diagnosis of asthma. Diagnosis of asthma at a later age – or persistence of asthma into later years – may be affected by many other postnatal factors. Furthermore, asthma phenotypes may differ based on age at diagnosis, and early onset transient asthma may represent a different pathology than persistent or late onset asthma, with different set of risk factors [25, 27, 28]. That is, wheeze in early childhood may not necessarily develop into the clinical entity of “asthma” [25]. Finally, there may be residual confounding from unmeasured variables, including discrimination and stress in pregnancy [39, 40].
Conclusion
This was the first study to evaluate the effect of maternal age at birth and prevalence of asthma in a Latino population. Unlike in Whites, maternal age at birth <20 was found to be significantly associated with a decreased risk of asthma in offspring, independent of other risk factors. In subgroup analyses, the protective effect of younger maternal age was observed only in Mexican Americans. The mechanism underlying this finding has not yet been elucidated. The possibility of an uncontrolled socioeconomic or lifestyle confounder including social support and dietary factors cannot be ruled out. Other possible mechanisms include differential effects of age and parity on maternal immune status by ethnicity. Further study is needed to evaluate whether these ethnic differences are due to epigenetic or immunologic changes due to factors such as diet, or stress in pregnancy. The varying prevalence of asthma among different Latino ethnicities in the U.S. is not well understood. Maternal age represents one factor which may help explain this variability.
Supplementary Material
Acknowledgments
We thank the families and patients for their participation and thank the numerous health care providers and community clinics for their support and participation in GALA II and SAGE II. In particular, we thank study coordinator Sandra Salazar and the recruiters who obtained the data: Duanny Alva, MD; Gaby Ayala-Rodriguez; Ulysses Burley; Lisa Caine; Elizabeth Castellanos; Jaime Colon; Denise DeJesus; Iliana Flexas; Blanca Lopez; Brenda Lopez, MD; Louis Martos; Vivian Medina; Juana Olivo; Mario Peralta; Esther Pomares, MD; Jihan Quraishi; Johanna Rodriguez; Shahdad Saeedi; Dean Soto; and Ana Taveras.
Funding Information: Supported in part by the American Heart Association National Scientist Development Award, and by the National Institutes of Health (R01-ES015794, R01-HL088133, R01-HL078885, and R01-HL104608, R01 - HL118267, R01-AI077439, R01-CA113710); National Institute On Minority Health And Health Disparities of the National Institutes of Health under Award Number P60-MD006902; M01-RR00188 to H.J.F.; the Flight Attendant Medical Research Institute (FAMRI), the Sandler Foundation, the RWJF Amos Medical Faculty Development Award (to E.G.B.), the American Asthma Foundation (to E.G.B.); Ernest S. Bazley Grant (to PCA); General Clinical Research Center (to HF).
Abbreviations
- CI
confidence interval
- OR
odds ratio
- GALA II
Genes-environments and Admixture in Latino Americans
Footnotes
Financial Disclosures: none
Conflict of Interest: none
References
- 1.Centers for Disease Control and Prevention (CDC) Most Recent Asthma Data. 2013 [Google Scholar]
- 2.Barnett SB, Nurmagambetov TA. Costs of asthma in the United States: 2002–2007. J Allergy Clin Immunol. 2011;127:145–52. doi: 10.1016/j.jaci.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Akinbami LJ, LaFleur BJ, Schoendorf KC. Racial and income disparities in childhood asthma in the United States. Ambul Pediatr. 2002;2:382–7. doi: 10.1367/1539-4409(2002)002<0382:raidic>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123(Suppl 3):S131–45. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 5.Akinbami LJ, Rhodes JC, Lara M. Racial and ethnic differences in asthma diagnosis among children who wheeze. Pediatrics. 2005;115:1254–60. doi: 10.1542/peds.2004-0897. [DOI] [PubMed] [Google Scholar]
- 6.Lara M, Akinbami L, Flores G, Morgenstern H. Heterogeneity of childhood asthma among Hispanic children: Puerto Rican children bear a disproportionate burden. Pediatrics. 2006;117:43–53. doi: 10.1542/peds.2004-1714. [DOI] [PubMed] [Google Scholar]
- 7.Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1–68. [PubMed] [Google Scholar]
- 8.Gurka MJ, Blackman JA, Heymann PW. Risk of childhood asthma in relation to the timing of early child care exposures. J Pediatr. 2009;155:781–87. e1. doi: 10.1016/j.jpeds.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Infante-Rivard C. Young maternal age: a risk factor for childhood asthma? Epidemiology. 1995;6:178–80. [PubMed] [Google Scholar]
- 10.Laerum BN, Svanes C, Wentzel-Larsen T, Gulsvik A, Toren K, Norrman E, Gislason T, Janson C, Omenaas E. Young maternal age at delivery is associated with asthma in adult offspring. Respir Med. 2007;101:1431–8. doi: 10.1016/j.rmed.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 11.McKeever TM, Lewis SA, Smith C, Collins J, Heatlie H, Frischer M, Hubbard R. Siblings, multiple births, and the incidence of allergic disease: a birth cohort study using the West Midlands general practice research database. Thorax. 2001;56:758–62. doi: 10.1136/thorax.56.10.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metsala J, Kilkkinen A, Kaila M, Tapanainen H, Klaukka T, Gissler M, Virtanen SM. Perinatal factors and the risk of asthma in childhood--a population-based register study in Finland. Am J Epidemiol. 2008;168:170–8. doi: 10.1093/aje/kwn105. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz J, Gold D, Dockery DW, Weiss ST, Speizer FE. Predictors of asthma and persistent wheeze in a national sample of children in the United States. Association with social class, perinatal events, and race. Am Rev Respir Dis. 1990;142:555–62. doi: 10.1164/ajrccm/142.3.555. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura KK, Galanter JM, Roth LA, Oh SS, Thakur N, Nguyen EA, Thyne S, Farber HJ, Serebrisky D, Kumar R, Brigino-Buenaventura E, Davis A, LeNoir MA, Meade K, Rodriguez-Cintron W, Avila PC, Borrell LN, Bibbins-Domingo K, Rodriguez-Santana JR, Sen S, Lurmann F, Balmes JR, Burchard EG. Early-life air pollution and asthma risk in minority children. The GALA II and SAGE II studies. Am J Respir Crit Care Med. 2013;188:309–18. doi: 10.1164/rccm.201302-0264OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pacheco CM, Ciaccio CE, Nazir N, Daley CM, DiDonna A, Choi WS, Barnes CS, Rosenwasser LJ. Homes of low-income minority families with asthmatic children have increased condition issues. Allergy Asthma Proc. 2014;35:467–74. doi: 10.2500/aap.2014.35.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh SS, Tcheurekdjian H, Roth LA, Nguyen EA, Sen S, Galanter JM, Davis A, Farber HJ, Gilliland FD, Kumar R, Avila PC, Brigino-Buenaventura E, Chapela R, Ford JG, LeNoir MA, Lurmann F, Meade K, Serebrisky D, Thyne S, Rodriguez-Cintron W, Rodriguez-Santana JR, Williams LK, Borrell LN, Burchard EG. Effect of secondhand smoke on asthma control among black and Latino children. J Allergy Clin Immunol. 2012;129:1478–83. e7. doi: 10.1016/j.jaci.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burke H, Leonardi-Bee J, Hashim A, Pine-Abata H, Chen Y, Cook DG, Britton JR, McKeever TM. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129:735–44. doi: 10.1542/peds.2011-2196. [DOI] [PubMed] [Google Scholar]
- 18.Jaakkola JJ, Ahmed P, Ieromnimon A, Goepfert P, Laiou E, Quansah R, Jaakkola MS. Preterm delivery and asthma: a systematic review and meta-analysis. J Allergy Clin Immunol. 2006;118:823–30. doi: 10.1016/j.jaci.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 19.Mu M, Ye S, Bai MJ, Liu GL, Tong Y, Wang SF, Sheng J. Birth weight and subsequent risk of asthma: a systematic review and meta-analysis. Heart Lung Circ. 2014;23:511–9. doi: 10.1016/j.hlc.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Kumar R, Yu Y, Story RE, Pongracic JA, Gupta R, Pearson C, Ortiz K, Bauchner HC, Wang X. Prematurity, chorioamnionitis, and the development of recurrent wheezing: a prospective birth cohort study. J Allergy Clin Immunol. 2008;121:878–84. e6. doi: 10.1016/j.jaci.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Getahun D, Strickland D, Zeiger RS, Fassett MJ, Chen W, Rhoads GG, Jacobsen SJ. Effect of chorioamnionitis on early childhood asthma. Arch Pediatr Adolesc Med. 2010;164:187–92. doi: 10.1001/archpediatrics.2009.238. [DOI] [PubMed] [Google Scholar]
- 22.Ball TM, Castro-Rodriguez JA, Griffith KA, Holberg CJ, Martinez FD, Wright AL. Siblings, day-care attendance, and the risk of asthma and wheezing during childhood. N Engl J Med. 2000;343:538–43. doi: 10.1056/NEJM200008243430803. [DOI] [PubMed] [Google Scholar]
- 23.Dogaru CM, Nyffenegger D, Pescatore AM, Spycher BD, Kuehni CE. Breastfeeding and childhood asthma: systematic review and meta-analysis. Am J Epidemiol. 2014;179:1153–67. doi: 10.1093/aje/kwu072. [DOI] [PubMed] [Google Scholar]
- 24.Kumar R, Nguyen EA, Roth LA, Oh SS, Gignoux CR, Huntsman S, Eng C, Moreno-Estrada A, Sandoval K, Penaloza-Espinosa RI, Lopez-Lopez M, Avila PC, Farber HJ, Tcheurekdjian H, Rodriguez-Cintron W, Rodriguez-Santana JR, Serebrisky D, Thyne SM, Williams LK, Winkler C, Bustamante CD, Perez-Stable EJ, Borrell LN, Burchard EG. Factors associated with degree of atopy in Latino children in a nationwide pediatric sample: the Genes-environments and Admixture in Latino Asthmatics (GALA II) study. J Allergy Clin Immunol. 2013;132:896–905. e1. doi: 10.1016/j.jaci.2013.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caudri D, Savenije OE, Smit HA, Postma DS, Koppelman GH, Wijga AH, Kerkhof M, Gehring U, Hoekstra MO, Brunekreef B, de Jongste JC. Perinatal risk factors for wheezing phenotypes in the first 8 years of life. Clin Exp Allergy. 2013;43:1395–405. doi: 10.1111/cea.12173. [DOI] [PubMed] [Google Scholar]
- 26.Lewis S, Richards D, Bynner J, Butler N. Britton J, Prospective study of risk factors for early and persistent wheezing in childhood. Eur Respir J. 1995;8:349–56. doi: 10.1183/09031936.95.08030349. [DOI] [PubMed] [Google Scholar]
- 27.Sherriff A, Peters TJ, Henderson J, Strachan D Alspac Study Team. Risk factor associations with wheezing patterns in children followed longitudinally from birth to 3(1/2) years. Int J Epidemiol. 2001;30:1473–84. doi: 10.1093/ije/30.6.1473. [DOI] [PubMed] [Google Scholar]
- 28.Rusconi F, Galassi C, Corbo GM, Forastiere F, Biggeri A, Ciccone G, Renzoni E. Risk factors for early, persistent, and late-onset wheezing in young children. SIDRIA Collaborative Group. Am J Respir Crit Care Med. 1999;160:1617–22. doi: 10.1164/ajrccm.160.5.9811002. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto N, Nagano J. Parental stress and the onset and course of childhood asthma. Biopsychosoc Med. 2015;9:7. doi: 10.1186/s13030-015-0034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fraser AM, Brockert JE, Ward RH. Association of young maternal age with adverse reproductive outcomes. N Engl J Med. 1995;332:1113–7. doi: 10.1056/NEJM199504273321701. [DOI] [PubMed] [Google Scholar]
- 31.Mebrahtu TF, Feltbower RG. Greenwood DC, Parslow RC, Birth weight and childhood wheezing disorders: a systematic review and meta-analysis. J Epidemiol Community Health. 2015;69:500–8. doi: 10.1136/jech-2014-204783. [DOI] [PubMed] [Google Scholar]
- 32.Page RL. Positive pregnancy outcomes in Mexican immigrants: what can we learn? J Obstet Gynecol Neonatal Nurs. 2004;33:783–90. doi: 10.1177/0884217504270595. [DOI] [PubMed] [Google Scholar]
- 33.McGlade MS, Saha S, Dahlstrom ME. The Latina paradox: an opportunity for restructuring prenatal care delivery. Am J Public Health. 2004;94:2062–5. doi: 10.2105/ajph.94.12.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collins JW, Jr, Shay DK. Prevalence of low birth weight among Hispanic infants with United States-born and foreign-born mothers: the effect of urban poverty. Am J Epidemiol. 1994;139:184–92. doi: 10.1093/oxfordjournals.aje.a116980. [DOI] [PubMed] [Google Scholar]
- 35.Fuentes-Afflick E, Lurie P. Low birth weight and Latino ethnicity. Examining the epidemiologic paradox. Arch Pediatr Adolesc Med. 1997;151:665–74. doi: 10.1001/archpedi.1997.02170440027005. [DOI] [PubMed] [Google Scholar]
- 36.Guendelman S, Abrams B. Dietary intake among Mexican-American women: generational differences and a comparison with white non-Hispanic women. Am J Public Health. 1995;85:20–5. doi: 10.2105/ajph.85.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sherraden MS, Barrera RE. Maternal support and cultural influences among Mexican immigrant mothers. Families in Society: The Journal of Contemporary Human Services. 1996;77:298–313. [Google Scholar]
- 38.Bamberg C, Fotopoulou C, Linder M, Roehr CC, Dudenhausen JW, Henrich W, Kalache K. Mid-trimester amniotic fluid concentrations of the proinflammatory cytokines IL-6, IL-8, TNF-alpha, and lipopolysaccharide binding protein in normal pregnancies: a prospective evaluation according to parity, gestational age, and fetal gender. J Perinat Med. 2011;39:403–9. doi: 10.1515/jpm.2011.041. [DOI] [PubMed] [Google Scholar]
- 39.Wright RJ. Prenatal maternal stress and early caregiving experiences: implications for childhood asthma risk. Paediatr Perinat Epidemiol. 2007;21(Suppl 3):8–14. doi: 10.1111/j.1365-3016.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- 40.Wright RJ, Finn P, Contreras JP, Cohen S, Wright RO, Staudenmayer J, Wand M, Perkins D, Weiss ST, Gold DR. Chronic caregiver stress and IgE expression, allergen-induced proliferation, and cytokine profiles in a birth cohort predisposed to atopy. J Allergy Clin Immunol. 2004;113:1051–7. doi: 10.1016/j.jaci.2004.03.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.