The oldest ancestors of moths and butterflies evolved in a gymnosperm world.
Abstract
On the basis of an assemblage of fossilized wing scales recovered from latest Triassic and earliest Jurassic sediments from northern Germany, we provide the earliest evidence for Lepidoptera (moths and butterflies). The diverse scales confirm a (Late) Triassic radiation of lepidopteran lineages, including the divergence of the Glossata, the clade that comprises the vast multitude of extant moths and butterflies that have a sucking proboscis. The microfossils extend the minimum calibrated age of glossatan moths by ca. 70 million years, refuting ancestral association of the group with flowering plants. Development of the proboscis may be regarded as an adaptive innovation to sucking free liquids for maintaining the insect’s water balance under arid conditions. Pollination drops secreted by a variety of Mesozoic gymnosperms may have been non-mutualistically exploited as a high-energy liquid source. The early evolution of the Lepidoptera was probably not severely interrupted by the end-Triassic biotic crisis.
INTRODUCTION
Lepidoptera (moths and butterflies) represent one of the most admired and studied insect groups, not in the least for their remarkable associations with flowering plants. However, despite their important role in terrestrial ecosystems, the early evolutionary history of these insects remains murky and mired in an exceedingly poor fossil record (1). Current evolutionary concepts are largely based on molecular phylogenetic analyses, suggesting that Lepidoptera diverged from their sister group Trichoptera (caddisflies) during Permian (2, 3) or (Late) Triassic (4–6) times. The large discrepancies in divergence time are mainly due to competing molecular dating methods and the choice of calibration fossils for providing age constraints. However, in any case, age estimates are substantially older than the oldest known stem-group lepidopteran fossil Archaeolepis mane [Early Jurassic; Sinemurian; ca. 195 million years ago (Ma); Dorset, UK] (7) and the oldest known crown-group representative Parasabatinca aftimacrai (Early Cretaceous; Barremian; ca. 129 Ma; Lebanon) (7).
To contribute to a possible reduction of the gap between molecular and fossil dates, we explore for the first time the phylogenetic potential of dispersed lepidopteran wing scales encountered in sedimentary organic matter. Lepidoptera are characterized by, and named after, their dense covering of chitinous scales on bodies, legs, and wings. Detached scales can be transferred by wind and water action to depositional areas for burial in terrestrial or even marine sediments, from which they may be recovered by palynological methods (8, 9). Because the structure of the scales, particularly the wing scales, is taxonomically informative (10), well-preserved fossil specimens could have clade-level morphological characteristics relevant to more accurate calibration of divergence-time estimates in molecular lepidopteran phylogenies. We studied fossilized scales encountered as rare palynological elements (Fig. 1) in Triassic-Jurassic boundary sediments from the cored Schandelah-1 well, drilled in northern Germany near Braunschweig.
Fig. 1. Lepidopteran scales in palynological preparations, as seen in transmitted light.
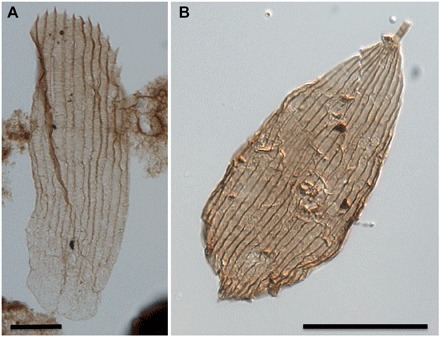
(A) Serrated scale from the Hettangian [316.70 m below surface (mbs)]. (B) Scale with a rounded apical margin from the Rhaetian (337.50 mbs). Scale bars, 20 μm.
RESULTS
The scales were found discontinuously within a 26-m stratigraphic interval embracing the Triassic-Jurassic (Rhaetian-Hettangian) transition (Fig. 2). About 70 scales and scale fragments, in various states of degradation, could be analyzed. Exceptionally well-preserved specimens were recovered from just above the palynologically defined Triassic-Jurassic boundary. Taxonomic identification of the fossil scales has been based on relevant literature data on scale morphology and structure of extant Lepidoptera and other scale-bearing hexapods, supplemented by the analysis of additional scanning electron microscopy (SEM) images (see the Supplementary Materials). Our survey of extant scale types and a compilation of the principal morphological characteristics (Table 1A) suggest that most hexapods, other than Lepidoptera, may be excluded as a source for the fossil scales (Table 1B). There is also little affinity with the scale types of the extinct neuropteran family Kalligrammatidae (11) and Tarachoptera, a recently proposed extinct order of the Amphiesmenoptera (12).
Fig. 2. Quantitative record of selected form species of gymnosperm pollen in the Schandelah-1 core and stratigraphic position of insect (wing) scales.
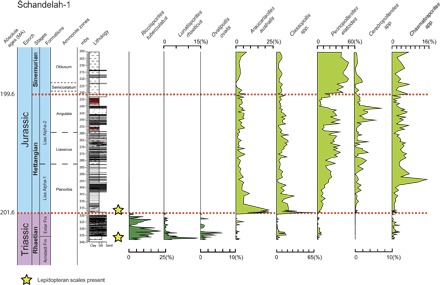
Lepidopteran and unidentified scales are present at the top of the Arnstadt Formation (Rhaetian) between 337.50 and 336.80 mbs and in the basal layers of the Lias Alpha-1 Formation (Hettangian) between 317.90 and 316.40 mbs (see Materials and Methods).
Table 1. Comparative overview of principal morphological and structural scale characteristics.
(A) Scales of scale-bearing hexapod lineages examined in this study (for details, see the Supplementary Materials). (B) Distinctive Rhaetian-Hettangian lepidopteran scale types. “-”, absence of a trait; “?”, uncertainty regarding presence/absence or interpretation of a trait.
| Group | Apical margin | Cross ridges | Microribs | Structure | Perforations | Herringbone pattern |
| A | ||||||
| Collembola | Rounded | - | - | Solid | - | - |
| Archaeognatha | Rounded | Far apart, rectangular cells | - | Hollow | Yes | - |
| Zygentoma | Rounded | Indistinct, irregular | - | Solid | - | - |
| Psocoptera | Rounded | - | - | Solid | - | - |
| Coleoptera | Rounded | - | - | Hollow | - | - |
| Trichoptera | Rounded | - | - | Hollow | Some | - |
| Culicidae | Rounded | Moderately dense | - | Solid | - | - |
| Berothidae | Rounded | ? | ? | ? | ? | ? |
| Kalligrammatidae | Rounded | ? | ? | ? | ? | ? |
| Micropterigidae | Rounded | Dense | Yes? | Solid | - | Dense |
| Eriocranidae | Rounded | Dense | Yes | Solid | - | - |
| Coelolepida | Serrated/rounded | Dense | Yes | Hollow | Yes | - |
| B | ||||||
| Herringbone | Rounded | ? | - | Solid | - | Dense–moderately dense |
| Hollow | Serrated/rounded | Dense | Present | Hollow | Small, between cross ridges | - |
Highlighting their diversity, the encountered scales could be categorized into four broad morphological groups with different overall shapes and scale margins, including triangular to rounded margins, serrated margins, elongated shapes, and fringed margins (Fig. 3, A to F). On the basis of SEM analyses and histological sections of well-preserved specimens, we identified two distinctive scale types showing decisive evidence of lepidopteran affinity. Type I scales (Fig. 4, A and B) are “solid” (fused upper and lower laminae) and have an unserrated, rounded, or slightly triangular apical margin. Areas between longitudinal ridges on the upper surface are sculptured with a faint herringbone pattern. In marked contrast, type II scales (Fig. 4, C to E) are “hollow” (upper and lower laminae connected by columnar trabeculae) and usually have serrated apical margins. The inter-ridge areas of the upper lamina contain a relatively dense covering of cross ridges, interspersed with small circular perforations. The top of the longitudinal ridges is adorned with microribs. A single type II scale also bears oblique apical crests (Fig. 4B). In addition to these lepidopteran scales, several scale types, which we have been unable to attribute to any of the modern scale-bearing hexapod groups, were encountered. The most characteristic of these unidentified types is a fringed, probably solid, scale without herringbone pattern and perforations (Figs. 3F and 4F).
Fig. 3. SEM images of scales from the basal Hettangian.
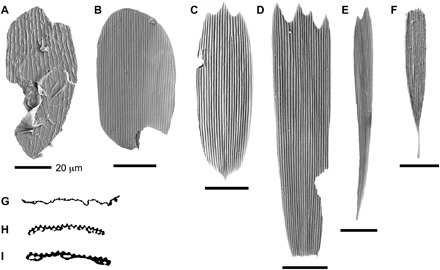
(A) Lepidopteran type I scale with solid structure and herringbone pattern. (B to E) Lepidopteran type II scales with hollow structure, cross-ridges, perforations, and in some cases (C to E) serrations of the apical margin. (F) Fringed scale of indeterminate origin. (G) Histological section of type I scale. (H and I) Histological sections of type II scales.
Fig. 4. Detailed SEM images of selected scales shown in Fig. 3.
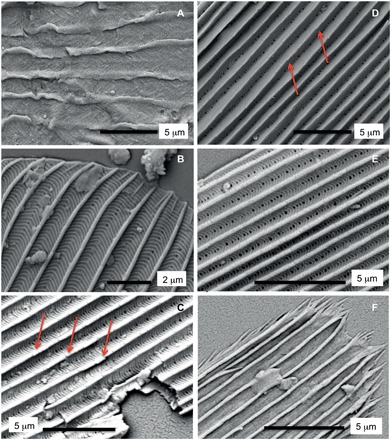
Labeling same as in Fig. 3. (A) Detail of a type I scale showing herringbone pattern. (B) Detail of a type II scale showing oblique crests at the apical margin. (C to E) Type II scales showing hollow structure, perforations, cross-ridges, and microribs (arrows in Fig. 3, C and D). (F) Detail of a fringed scale of indeterminate origin showing distinct apical margin.
The general morphology and internal structure of both type I and type II scales is consistent with scale morphologies found in the so-called non-ditrysian Lepidoptera, a paraphyletic group of extant families reflecting early lepidopteran phylogeny (13) but embracing only 1 to 2% of all lepidopteran species. The characteristic herringbone patterns, as observed in type I scales, are only present today in those lineages that diverge at the three earliest nodes in this phylogeny: Micropterigidae, Agathiphagidae, and Heterobathmiidae (10). The fossil scales share their solid structure with both Micropterigidae and Heterobathmiidae but differ from the hollow wing scales of the Agathiphagidae (10). These three primitive families represent relict lineages of small moths with mandibulate, chewing mouthparts (14). Morphologically related Jurassic fossils have been included in four extinct mandibulate families: Archaeolepidae, Eolepidopterygidae, Mesokristenseniidae, and Ascololepidopterygidae (15). Only the Archaeolepidae, with the single-wing species A. mane, are characterized by the presence of relatively well-preserved, probably solid, wing scales, but no herringbone pattern has been observed (16). The Rhaetian-Hettangian type I scales would corroborate recognition of a successful and probably diversified clade of mandibulate Lepidoptera during the Early Mesozoic.
The affinity of type II scales is clearly associated with the morphological clade Coelolepida, defined principally by hollow wing scales with perforations in the inter-ridge areas of the upper lamina, characteristic of the vast majority of extant Lepidoptera (10). Serrated apical margins are additional evidence for a coelolepidan relationship. On the basis of the number and size of the perforations and the density of cross ridges, numerous type II scales show a resemblance to scales of non-dytrisian families of the Coelolepida, such as the Acanthopteroctetoidae, Adelidae, Incurvariidae, and Cecidosidae (10). Unfortunately, morphological information on modern wing scales is still too restricted to judge whether the fossil material originates from crown- or stem-group members of the Coelolepida. Modern occurrences of oblique apical crests, noted in a single type II specimen, are limited to the non-coelolepidan Eriocraniidae (10). Nonetheless, the fossil scale is clearly hollow and has the hallmark perforations of the Coelolepida. Because the family Eriocraniidae is usually considered to be the sister group to the Coelolepida (13), a tantalizing possibility is that this scale represents a stem-group coelolepidan, still retaining a character from its non-coelolepidan ancestors.
DISCUSSION
Although their monophyly is questioned (17), coelolepidan lineages are nested in the Glossata (13), the huge clade that includes all moths and butterflies having a sucking proboscis, a sophisticated feeding device fundamentally adapted to fluid uptake from droplets and surface films (14). On the basis of an undescribed larva from Lebanese amber characterized by a spinneret, a silk-spinning organ apomorphic of Glossata (18), the minimum age of fossil representatives of this clade could thus far be constrained with any certainty as mid Early Cretaceous (Barremian; ca. 129 Ma) (1). The Rhaetian-Hettangian scale record pushes back the calibrated age by ca. 70 million years, supporting molecular estimates of a mid Late Triassic (Norian; 212 Ma) divergence of the Glossata from non-glossatan, mandibulate ancestors (4). The record falsifies an Early Cretaceous (Berriasian; ca. 141 Ma) origination of the Glossata, which followed from the comprehensive phylogenomic treatment of insects by Misof et al. (6).
The transition to exclusively feeding on liquids was most likely an evolutionary response to widespread heat and aridity during the Norian (19). When flying in dry air, the high ratio of surface area to volume inherent in the small body size of basal moths would intensify evaporative losses of body moisture (20). Because free liquid drinking is an efficient technique to replenish lost moisture and survive desiccation stress, substitution of mandibulate mouthparts by a sucking proboscis could be seen as an adaptation to adequate maintenance of body water balance of small, short-lived moths. Like in the most basal extant lineages, such as the Eriocraniidae and the Mnesarchaeidae (14, 21), short and simply composed proboscides of ancestral Glossata must have been used initially to drink from water droplets or sap from injured leaves.
In association with the rise and diversification of crown-group angiosperms during the Cretaceous (22), possession of the proboscis facilitated feeding from concealed floral nectaries, thus paving the way for the evolution of the multitude of pollination mutualisms between Lepidoptera and flowering plants. In modern non-ditrysian Glossata, long proboscides adapted to nectar feeding occur in members of the more advanced clade Eulepidoptera (14). However, preceding an angiosperm connection, early radiation of non-ditrysians evidently occurred parallel to the increasing diversity of gymnosperms during the Triassic and Jurassic. As in the majority of modern representatives (23), ovules of many Triassic-Jurassic gymnosperm species secreted pollination drops to capture airborne pollen grains and trigger their germination (24). Similar to angiosperm nectar, the sugary droplets offered a high-energy nutritional source (25), which could attract adult glossatan moths and other Mesozoic proboscate flying insects (26). To a large extent, early pollination drop exploitation must have been unidirectional, benefiting only moths over their plant hosts. Only with the evolution of bisexual reproductive structures, a prerequisite for effective cross-pollination by flying insects, gymnosperm taxa would open the prospect of reciprocal plant-pollinator benefits. Among Mesozoic gymnosperms, bisexuality has only been confidently identified in permineralized cones of the Bennettitales. The overall architecture of these structures provides strong evidence of self-pollination, whereas regular traces of beetle predation on pollen suggest fortuitous, incidental entomophily (27). Any mutualistic association between Triassic-Jurassic glossatan moths and bennettitaleans remains unlikely.
Pollinator interaction with Gnetales seems a more plausible option. Many extant members of this order produce female (ovuliferous) and bisexual (ovuliferous and polliniferous) cones on separate plants (28). The ovules on bisexual cones are usually sterile but can still secrete sugary pollination drops. Pollination is accomplished either by wind, by insects, or by a mixed wind/insect pollination mode (ambophily). A variety of flying proboscate insects has been observed feeding on the pollination drops of both fertile and sterile ovules of Gnetum (29), Ephedra (30), and Welwitschia (31). Although their organization is still imperfectly known, (probably Middle) Permian bisexual cones described as Palaeognetaleana auspicia could well be regarded as possible gnetalean stem relatives (32). In situ pollen grains correspond to the long-ranging, essentially gnetalean form genus Ephedripites. Similar ephedroid pollen is regularly detected in Triassic and Jurassic palynological records, mostly as a subordinate element. Among insects visiting modern Gnetales, the Lepidoptera are represented by glossatan moths belonging to the large ditrysian families Pyralidae and Geometridae (29, 30). If entomophily, or perhaps ambophily, is the ancestral gnetalean pollination syndrome (30), then there may have been a pollinator role for Triassic-Jurassic Glossata.
It should be noted that the wide morphological diversity of the dispersed Triassic-Jurassic pollen record strongly suggests that, perhaps, entire families or orders of seed plants have still escaped sampling in the coeval megafossil record. Some pollen types have angiosperm-like morphological characters. Although affinity to angiosperm crown-groups remains questionable (22), notably, the presence of a reticulate wall structure (33, 34) might be functionally linked to pollination by flying insects.
The early record of wing scales with unequivocal glossatan characteristics implies that not only adult moths but also their larval stages could have depended on gymnosperms to satisfy their nutritional needs. Predominant host-plant preferences of larvae of modern non-ditrysian Glossata might suggest that accommodation by woody angiosperms was a significant ancestral trait in the glossatan clade (35). However, the fundamental host shift from (Triassic-Jurassic) gymnosperms to (Cretaceous) angiosperms now challenges the underlying notion that the early radiation of proboscate Lepidoptera would have been an adaptive response to the Early Cretaceous evolution of bisexual angiosperm flowers. Most extant families of non-ditrysian Glossata include primarily leaf-mining larvae (36). Earliest mine structures are known from leaves of latest Permian peltasperms (37). In harmony with the progressive increase in insect herbivory during the Triassic (38), pre-angiospermous diversity of plant-leaf miner interactions may culminate in the Late Triassic floral record, with mines on conifers, ginkgoaleans, cycads, various orders of pteridosperms, and ferns (39, 40). Leaf-mining larvae are not restricted to the Lepidoptera; they also occur in Coleoptera, Diptera, and Hymenoptera. However, also considering the present wing-scale record, a lepidopteran affinity should not be ruled out when interpreting mine structures on Triassic and Jurassic insect-damaged leaves.
It is most noteworthy that the Rhaetian-Hettangian stratigraphic interval, in which lepidopteran scales have been detected, matches the interval where major changes in the composition of fossil spore/pollen assemblages reflect the end-Triassic ecological crisis and associated mass extinctions. As in many other Triassic-Jurassic transition sections in northwest (NW) Europe (41, 42), a marked palynofloral turnover is initiated in the latest Rhaetian by the decline or demise of distinctive form genera of gymnosperm pollen, such as Lunatisporites, Ovalipollis, and Ricciisporites, which may represent a regional vegetation predominantly composed of conifers, peltasperms, and cycads. Concomitant proliferation of fern spores assignable to the Matoniaceae and Schizaeaceae is likely to be indicative of stressed habitats (43, 44). Subsequently, a gradual return to gymnosperm woodland in the earliest Hettangian is evidenced by increasing amounts of conifer pollen, particularly Araucariacites, Perinopollenites, and Cerebropollenites (42, 45).
Similar to other insect groups (46), early Lepidoptera may have been essentially immune to the end-Triassic crisis, probably because both larvae and adult moths were thriving on long-ranging plant species. Potential host-plant availability can be inferred from those gymnosperm pollen types that cross the Triassic-Jurassic boundary in the Schandelah-1 core (Fig. 2). Most of this pollen corresponds to conifers (Araucariaceae, Araucariacites; Cheirolepidiaceae, Classopollis; Cupressaceae, Perinopollenites), cycads (Cycadales, Chasmatosporites), and pteridosperms (Caytoniales, Vitreisporites). However, among these groups, the Araucariaceae and Cheirolepidaceae were unlikely hosts for proboscate moths. Modern Araucariaceae (47) and, most probably, the extinct Cheirolepidaceae (48) are characterized by extra-ovular pollen germination, not requiring secretion of a pollination drop. Rare occurrences of Ephedripites may indicate the persistent presence of (entomophilous?) Gnetales in the regional vegetation.
By analogy with quantitative records of dispersed wing scales from young lake sediments (9), it may perhaps be hypothesized that the scale-bearing intervals of the Schandelah-1 core reflect severe outbreak of defoliating lepidopteran larvae at the time of the end-Triassic ecological crisis. It is also conceivable that the crisis presented taphonomic constraints for the preservation of the scales. Dieback of woody vegetation could have promoted increased runoff and rapid burial of organic materials under low-oxygen or even euxinic conditions, ensuring long-term preservation of delicate chitinous structures. A search for further Mesozoic wing-scale records is needed to fully assess the potential of the palynological microfossils as a useful source of evolutionary and ecological information, but it seems safe to conclude that our Rhaetian-Hettangian data already offer a new window on the timing of basal lepidopteran divergences.
MATERIALS AND METHODS
The Schandelah-1 well, covering a Late Triassic (Rhaetian) to late Early Jurassic (Toarcian) shallow-marine succession, was drilled close to the village of Schandelah in the vicinity of Braunschweig, Lower Saxony (site coordinates, 52°18′23″N/10°42′66″W; elevation, 84 m; well depth, 338 mbs). The recovered sediment core is stored at the German core repository for scientific drilling in Berlin-Spandau, managed by the German Research Centre for Geosciences (GFZ), Potsdam. The Rhaetian-Hettangian interval (Fig. 2) is composed mainly of organic-rich shales and claystones, interspersed with several massive sandstone beds. Chronostratigraphic control is provided by palynology and ammonite biostratigraphy. At 335 mbs, the presence of the dinoflagellate cyst Lunnomidinium scaniense confirms a Rhaetian age for the basal part of the studied section. Together with a marked quantitative palynofloral turnover, last occurrences of the typically Triassic pollen types Lunatisporites rhaeticus and Ovalipollis ovalis at 319.50 mbs approximate the Rhaetian-Hettangian boundary. This boundary is tentatively placed at 318.60 mbs, at the base of gray-brown sandstone beds corresponding to the Psilonoten Sandstone, a regional marker horizon. Within the Hettangian, the Planorbis, Liassicus, and Angulata chronozones of standard NW European ammonite zonation can be recognized.
Initial palynological investigation of the core revealed the presence of scales in two separate intervals: in the Rhaetian section between 337.50 and 336.80 mbs, and in the Hettangian section between 317.90 and 316.40 mbs. A single scale was noted at 312.00 mbs. For the purpose of the present study, five fractions, prepared at the palynological laboratory of the Goethe University, Frankfurt, were still available for detailed analysis; these correspond to depths of 337.50, 336.80, 317.90, 317.10, and 316.40 mbs. In addition, eight new fractions from samples of the Schandelah-1 core were prepared at the Laboratory of Palaeobotany and Palynology of Utrecht University; these preparations correspond to depths of 337.40, 336.70, 336.20, 317.70, 317.30, 316.80, 316.60, and 316.20 mbs. All fractions were chemically prepared following standard palynological protocols, including successive HCl and HF treatment and storage in a glycerol-water solution. Subsequently, individual scales were microscopically traced in uncovered preparations and transferred to separate slides using a nose hair–tipped needle. All isolated scales were photographed using light microscopy. Apart from some specimens selected for sectioning, the scales were then mounted on SEM stubs for imaging with a VEGA TESCAN TS5130LM scanning electron microscope. Both scale isolation and imaging were performed at Bonn University.
As an aid to taxonomic identification of the fossil scales, we studied SEM images showing scale morphology and structure of representatives of recent Lepidoptera and other scale-bearing hexapod groups (see the Supplementary Materials). Scales for SEM analysis were removed from specimens representing the Collembola, Archaeognatha, Zygentoma, Thysanoptera, Psocoptera, Coleoptera, Lepidoptera, and Culicidae, made available by Museum Koenig, Bonn. Imaging was performed at Bonn University. Additional images representing lepidopteran families were provided by the State Museum of Natural History, Stuttgart.
Supplementary Material
Acknowledgments
We thank N. Welters and K. Schmeling who provided valuable help with processing of the palynological samples. T. Bartolomaeus and T. Bartz are thanked for help with making the histological sections of the scales. G. Oleschinski and K. W. Schwenninger are acknowledged for their help with operating the SEM. We thank C. Labandeira for advice during the initial phase of the study, and two anonymous reviewers for their positive appraisal. Funding: This study was funded by the Department of Earth Sciences of Utrecht University. P.K.S. and B.v.d.S. acknowledge support from the German Science Foundation (DFG grant SCHOO1216/7-1), and T.W. acknowledges support from a Heisenberg Fellowship (DFG grant WA1492/8-1). Author contributions: Conceptualization: T.J.B.v.E., B.v.d.S., and P.K.S.; data acquisition: T.J.B.v.E., C.M.H.v.d.W., B.v.d.S., T.W., and H.R.; data interpretation and integration: T.J.B.v.E., B.v.d.S., and H.V.; writing: B.v.d.S. and H.V., with input and feedback by all other authors. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to the paper may be requested from the authors. Correspondence and requests should be addressed to B.v.d.S.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/1/e1701568/DC1
Supplementary Text: Comparative scale morphology
fig. S1. SEM images of scales of Collembola and Archaeognatha.
fig. S2. SEM images of scales of Zygentoma and Psocoptera.
fig. S3. SEM images of scales of non-coelolepidan Lepidoptera.
fig. S4. SEM images of scales of coelolepidan Lepidoptera.
fig. S5. SEM images of scales of Culicidae.
REFERENCES AND NOTES
- 1.Sohn J.-C., Labandeira C. C., Davis D. R., The fossil record and taphonomy of butterflies and moths (Insecta, Lepidoptera): Implications for evolutionary diversity and divergence-time estimates. BMC Evol. Biol. 15, 12 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tong K. J., Duchêne S., Ho S. Y. W., Lo N., Comment on “Phylogenomics resolves the timing and pattern of insect evolution”. Science 349, 487 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Condamine F. L., Clapham M. E., Kergoat G. J., Global patterns of insect diversification: Towards a reconciliation of fossil and molecular evidence? Sci. Rep. 6, 19208 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wahlberg N., Wheat C. W., Peña C., Timing and patterns in the taxonomic diversification of Lepidoptera (butterflies and moths). PLOS ONE 8, e80875 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malm T., Johanson K. A., Wahlberg N., The evolutionary history of Trichoptera (Insecta): A case of successful adaptation to life in freshwater. Syst. Entomol. 38, 459–473 (2013). [Google Scholar]
- 6.Misof B., Liu S., Meusemann K., Peters R. S., Donath A., Mayer C., Frandsen P. B., Ware J., Flouri T., Beutel R. G., Niehuis O., Petersen M., Izquierdo-Carrasco F., Wappler T., Rust J., Aberer A. J., Aspöck U., Aspöck H., Bartel D., Blanke A., Berger S., Böhm A., Buckley T. R., Calcott B., Chen J., Friedrich F., Fukui M., Fujita M., Greve C., Grobe P., Gu S., Huang Y., Jermiin L. S., Kawahara A. Y., Krogmann L., Kubiak M., Lanfear R., Letsch H., Li Y., Li Z., Li J., Lu H., Machida R., Mashimo Y., Kapli P., McKenna D. D., Meng G., Nakagaki Y., Navarrete-Heredia J. L., Ott M., Ou Y., Pass G., Podsiadlowski L., Pohl H., von Reumont B. M., Schütte K., Sekiya K., Shimizu S., Slipinski A., Stamatakis A., Song W., Su X., Szucsich N. U., Tan M., Tan X., Tang M., Tang J., Timelthaler G., Tomizuka S., Trautwein M., Tong X., Uchifune T., Walzl M. G., Wiegmann B. M., Wilbrandt J., Wipfler B., Wong T. K. F., Wu Q., Wu G., Xie Y., Yang S., Yang Q., Yeates D. K., Yoshizawa K., Zhang Q., Zhang R., Zhang W., Zhang Y., Zhao J., Zhou C., Zhou L., Ziesmann T., Zou S., Li Y., Xu X., Zhang Y., Yang H., Wang J., Wang J., Kjer K. M., Zhou X., Phylogenomics resolves the timing and pattern of insect evolution. Science 346, 763–767 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Wolfe J. M., Daley A. C., Legg D. A., Edgecombe G. D., Fossil calibrations for the arthropod Tree of Life. Earth-Sci. Rev. 160, 43–110 (2016). [Google Scholar]
- 8.R. V. Tyson, Sedimentary Organic Matter (Chapman & Hall, 1995). [Google Scholar]
- 9.Navarro L., Harvey A.-É., Morin H., Lepidoptera wing scales: A new paleoecological indicator for reconstructing spruce budworm abundance. Can. J. For. Res., 1–7 (2017). [Google Scholar]
- 10.Simonsen T. J., The wing vestiture of the non-ditrysian Lepidoptera (Insecta). Comparative morphology and phylogenetic implications. Acta Zool. 82, 275–298 (2001). [Google Scholar]
- 11.Labandeira C. C., Yang Q., Santiago-Blay J. A., Hotton C. L., Monteiro A., Wang Y.-J., Goreva Y., Shih C., Siljeström S., Rose T. R., Dilcher D. L., Ren D., The evolutionary convergence of mid-Mesozoic lacewings and Cenozoic butterflies. Proc. R. Soc. B Biol. Sci. 283, 20152893 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mey W., Wichard W., Müller P., Wang B., The blueprint of the Amphiesmenoptera-Tarachoptera, a new order of insects from Burmese amber (Insecta, Amphiesmenoptera). Fossil Rec. 20, 129–145 (2017). [Google Scholar]
- 13.Regier J. C., Mitter C., Kristensen N. P., Davis D. R., van Nieukerken E. J., Rota J., Simonsen T. J., Mitter K. T., Kawahara A. Y., Yen S.-H., Cummings M. P., Zwick A., A molecular phylogeny for the oldest (nonditrysian) lineages of extant Lepidoptera, with implications for classification, comparative morphology and life-history evolution. Syst. Entomol. 40, 671–704 (2015). [Google Scholar]
- 14.Krenn H. W., Feeding mechanisms of adult Lepidoptera: Structure, function, and evolution of the mouthparts. Annu. Rev. Entomol. 55, 307–327 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W., Shih C., Labandeira C. C., Sohn J.-C., Davis D. R., Santiago-Blay J. A., Flint O., Ren D., New fossil Lepidoptera (Insecta: Amphiesmenoptera) from the Middle Jurassic Jiulongshan formation of Northeastern China. PLOS ONE 8, e79500 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whalley P. E. S., The systematics and palaeogeography of the Lower Jurassic insects of Dorset, England. Bull. Br. Mus. 39, 107–189 (1985). [Google Scholar]
- 17.Mutanen M., Wahlberg N., Kaila L., Comprehensive gene and taxon coverage elucidates radiation patterns in moths and butterflies. Proc. R. Soc. B Biol. Sci. 277, 2839–2848 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D. Grimaldi, M. S. Engel, Evolution of the Insects (Cambridge Univ. Press, 2005). [Google Scholar]
- 19.Holz M., Mesozoic paleogeography and paleoclimates—A discussion of the diverse greenhouse and hothouse conditions of an alien world. J. South Am. Earth Sci. 61, 91–107 (2015). [Google Scholar]
- 20.E. B. Edney, Water Balance in Land Arthropods. Zoophysiology and Ecology, Volume 9 (Springer, 1977). [Google Scholar]
- 21.Kristensen N. P., Studies on the morphology and systematics of primitive Lepidoptera (Insecta). Steenstrupia 10, 141–191 (1984). [Google Scholar]
- 22.Herendeen P. S., Friis E. M., Pedersen K. R., Crane P. R., Palaeobotanical redux: Revisiting the age of the angiosperms. Nat. Plants 3, 17015 (2017). [DOI] [PubMed] [Google Scholar]
- 23.S. Little, N. Prior, C. Pirone, P. von Aderkas, Pollen-ovule interactions in gymnosperms, in Reproductive Biology of Plants, K. G. Ramawat, J.-M. Mérillon, K. R. Shivanna, Eds. (CRC Press, 2014), pp. 97–117. [Google Scholar]
- 24.Labandeira C. C., Kvaček J., Mostovski M. B., Pollination drops, pollen, and insect pollination of Mesozoic gymnosperms. Taxon 56, 663–695 (2007). [Google Scholar]
- 25.M. Nepi, P. von Aderkas, E. Pacini, Sugary exudates in plant pollination, in Secretions and Exudates in Biological Systems, J. M. Vivanco, F. Baluška, Eds. (Signaling and Communication in Plants 12, Springer, Berlin-Heidelberg, 2012), pp. 155–185. [Google Scholar]
- 26.Ren D., Labandeira C. C., Santiago-Blay J. A., Rasnitsyn A., Shih C., Bashkuev A., Logan M. A. V., Hotton C. L., Dilcher D., A probable pollination mode before angiosperms: Eurasian, long-proboscid scorpionflies. Science 326, 840–847 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.J. M. Osborn, M. L. Taylor, Pollen and coprolite structure in Cycadeoidea (Bennettitales): Implications for understanding pollination and mating systems in Mesozoic cycadeoids, in Plants in Deep Mesozoic Time: Morphological Innovations, Phylogeny, and Ecosystems, C. T. Gee, Ed. (Indiana Univ. Press, 2010), pp. 34–49. [Google Scholar]
- 28.Endress P. K., Structure and function of female and bisexual organ complexes in Gnetales. Int. J. Plant Sci. 157, 113–125 (1996). [Google Scholar]
- 29.Kato M., Inoue T., Nagamitsu T., Pollination biology of Gnetum (Gnetaceae) in a lowland mixed dipterocarp forest in Sarawak. Am. J. Bot. 82, 862–868 (1995). [Google Scholar]
- 30.Bolinder K., Humphreys A. M., Ehrlén J., Alexandersson R., Ickert-Bond S. M., Rydin C., From near extinction to diversification by means of a shift in pollination mechanism in the gymnosperm relict Ephedra (Ephedraceae, Gnetales). Bot. J. Linn. Soc. 180, 461–477 (2016). [Google Scholar]
- 31.Wetschnig W., Depisch B., Pollination biology of Welwitschia mirabilis Hook. f. (Welwitschia, Gnetopsida). Phyton 39, 167–183 (1999). [Google Scholar]
- 32.Wang Z.-Q., A new Permian gnetalean cone as fossil evidence for supporting current molecular phylogeny. Ann. Bot. 94, 281–288 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cornet B., Late Triassic angiosperm-like pollen from the Richmond Rift Basin of Virginia, U.S.A. Palaeontogr. Abt. B 213, 37–87 (1989). [Google Scholar]
- 34.Hochuli P. A., Feist-Burkhardt S., Angiosperm-like pollen and Afropollis from the Middle Triassic (Anisian) of the Germanic Basin (Northern Switzerland). Front. Plant Sci. 4, 344 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menken S. B. J., Boomsma J. J., van Nieukerken E. J., Large-scale evolutionary patterns of host plant associations in the Lepidoptera. Evolution 64, 1098–1119 (2010). [DOI] [PubMed] [Google Scholar]
- 36.J. A. Powell, C. Mitter, B. D. Farrell, Evolution of larval food preference in Lepidoptera, in Lepidoptera, Moths and Butterflies. Vol. 1. Evolution, Systematics, and Biogeography, N. P. Kristensen, Ed. (Handbuch der Zoologie. Vol. 4, Walter de Gruyter, Berlin–New York, 1998), pp. 403–422. [Google Scholar]
- 37.Krassilov V. A., Karasev E. V., First evidence of plant–arthropod interaction at the Permian–Triassic boundary in the Volga Basin, European Russia. Alavesia 2, 247–252 (2008). [Google Scholar]
- 38.Wappler T., Kustatscher E., Dellantonio E., Plant–insect interactions from Middle Triassic (late Ladinian) of Monte Agnello (Dolomites, N-Italy)—Initial pattern and response to abiotic environmental perturbations. PeerJ 3, e921 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott A. C., Anderson J. M., Anderson H. M., Evidence of plant–insect interactions in the Upper Triassic Molteno formation of South Africa. J. Geol. Soc. 161, 401–410 (2004). [Google Scholar]
- 40.Labandeira C. C., Anderson J. M., Insect leaf-mining in Late Triassic gymnospermous floras from the Molteno formation of South Africa. Geol. Soc. Am. 37, 15 (2005). [Google Scholar]
- 41.Lindström S., van de Schootbrugge B., Dybkjær K., Pedersen G. K., Fiebig J., Nielsen L. H., Richoz S., No causal link between terrestrial ecosystem change and methane release during the end-Triassic mass-extinction. Geology 40, 531–534 (2012). [Google Scholar]
- 42.van de Schootbrugge B., Quan T. M., Lindström S., Püttmann W., Heunisch C., Pross J., Fiebig J., Petschick R., Röhling H.-G., Richoz S., Rosenthal Y., Falkowski P. G., Floral changes across the Triassic/Jurassic boundary linked to flood basalt volcanism. Nat. Geosci. 2, 589–594 (2009). [Google Scholar]
- 43.van Konijnenburg-van Cittert J. H. A., Ecology of some Late Triassic to Early Cretaceous ferns in Eurasia. Rev. Palaeobot. Palynol. 119, 113–124 (2002). [Google Scholar]
- 44.van de Schootbrugge B., Wignall P. B., A tale of two extinctions: Converging end-Permian and end-Triassic scenarios. Geol. Mag. 153, 332–354 (2015). [Google Scholar]
- 45.Bonis N. R., Kürschner W. M., Vegetation history, diversity patterns, and climate change across the Triassic/Jurassic boundary. Paleobiology 38, 240–264 (2012). [Google Scholar]
- 46.Labandeira C. C., Johnson K. R., Wilf P., Impact of the terminal Cretaceous event on plant-insect associations. Proc. Natl. Acad. Sci. U.S.A. 99, 2061–2066 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Owens J. N., Catalano G. L., Morris S. J., Aitken-Christie J., The reproductive biology of Kauri (Agathis australis). I. Pollination and prefertilization development. Int. J. Plant Sci. 156, 257–269 (1995). [Google Scholar]
- 48.Kvaček J., Frenelopsis alata and its microsporangiate and ovuliferous reproductive structures from the Cenomanian of Bohemia (Czech Republic, central Europe). Rev. Palaebot. Palynol. 112, 51–78 (2000). [DOI] [PubMed] [Google Scholar]
- 49.Downey J. C., Allyn A. C., Wing-scale morphology and nomenclature. Bull. Allyn Mus. 31, 1–32 (1975). [Google Scholar]
- 50.Zhang F., Chen Z., Dong R.-R., Deharveng L., Stevens M. I., Huang Y.-H., Zhu C.-D., Molecular phylogeny reveals independent origins of body scales in Entomobryidae (Hexapoda: Collembola). Mol. Phylogenet. Evol. 70, 231–239 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Larink O., Entwicklung und Feinstruktur der Schuppen bei Lepismatiden und Machiliden (Insecta, Zygentoma und Archaeognatha). Zool. Jb. Anat. 95, 252–293 (1976). [Google Scholar]
- 52.Vigneron J. P., Colomer J.-F., Vigneron N., Lousse V., Natural layer-by-layer photonic structure in the squamae of Hoplia coerulea (Coleoptera). Phys. Rev. E 72, 061904 (2005). [DOI] [PubMed] [Google Scholar]
- 53.Erbey M., Candan S., Comparative morphology of scales in Cionus Clairville, 1798 (Coleoptera: Curculionidae) species. J. Entomol. Zool. Stud. 3, 246–249 (2015). [Google Scholar]
- 54.Huxley J., Barnard P. C., Wing-scales of Pseudoleptocerus chirindensis Kimmins (Trichoptera: Leptoceridae). Zool. J. Linn. Soc. 92, 285–312 (1988). [Google Scholar]
- 55.N. P. Kristensen, T. J. Simonsen, ‘Hairs’ and scales, in Lepidoptera, Moths and Butterflies, Vol. 2: Morphology, Physiology, and Development, N. P. Kristensen, Ed. (De Gruyter, 2003), pp. 9–22. [Google Scholar]
- 56.Simonsen T. J., Kristensen N. P., Agathiphaga wing vestiture revisited: Evidence for complex early evolution of lepidopteran scales (Lepidoptera: Agathiphagidae). Insect Syst. Evol. 32, 169–175 (2001). [Google Scholar]
- 57.Harbach R. E., The Culicidae (Diptera): A review of taxonomy, classification and phylogeny. Zootaxa 1668, 591–638 (2007). [Google Scholar]
- 58.Wu C. W., Kong X. Q., Wu D., Micronanostructures of the scales on a mosquito’s legs and their role in weight support. Phys. Rev. E 76, 017301 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/1/e1701568/DC1
Supplementary Text: Comparative scale morphology
fig. S1. SEM images of scales of Collembola and Archaeognatha.
fig. S2. SEM images of scales of Zygentoma and Psocoptera.
fig. S3. SEM images of scales of non-coelolepidan Lepidoptera.
fig. S4. SEM images of scales of coelolepidan Lepidoptera.
fig. S5. SEM images of scales of Culicidae.


