This review presents and discusses basic science findings and scientific evidence generated within the last 2 decades in the field of acute postoperative pain.
Keywords: Postoperative pain, Surgical incision, Sensitization, Multimodal analgesia, Ketamine, Pregabalin
Abstract
Introduction:
Pain management after surgery continues to be suboptimal; there are several reasons including lack of translation of results from basic science studies and scientific clinical evidence into clinical praxis.
Objectives:
This review presents and discusses basic science findings and scientific evidence generated within the last 2 decades in the field of acute postoperative pain.
Methods:
In the first part of the review, we give an overview about studies that have investigated the pathophysiology of postoperative pain by using rodent models of incisional pain up to July 2016. The second focus of the review lies on treatment recommendations based on guidelines and clinical evidence, eg, by using the fourth edition of the “Acute Pain Management: Scientific Evidence” of the Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine.
Results:
Preclinical studies in rodent models characterized responses of primary afferent nociceptors and dorsal horn neurons as one neural basis for pain behavior including resting pain, hyperalgesia, movement-evoked pain or anxiety- and depression-like behaviors after surgery. Furthermore, the role of certain receptors, mediators, and neurotransmitters involved in peripheral and central sensitization after incision were identified; many of these are very specific, relate to some modalities only, and are unique for incisional pain. Future treatment should focus on these targets to develop therapeutic agents that are effective for the treatment of postoperative pain as well as have few side effects. Furthermore, basic science findings translate well into results from clinical studies. Scientific evidence is able to point towards useful (and less useful) elements of multimodal analgesia able to reduce opioid consumption, improve pain management, and enhance recovery.
Conclusion:
Understanding basic mechanisms of postoperative pain to identify effective treatment strategies may improve patients' outcome after surgery.
1. Introduction
More than 230 million people undergo surgery each year worldwide and the number is increasing annually.183 Surgery causes commonly postoperative pain that should be alleviated as soon and as effective as possible to reduce suffering, to promote the healing process and rehabilitation and to prevent complications. However, clinical pain management after surgery is far from being successful despite dramatically increased scientific evidence in this area. Many patients suffer from severe pain after surgery55,104; even less well recognized, many develop chronic pain after surgery which might be, at least in part, a result of undertreated acute postoperative pain.50 One reason for this undertreatment is the limited translation of basic and clinical scientific findings into clinical practice. For example, pain after surgery is a very specific entity; it is neither the result of an inflammatory process alone nor only the result of isolated injury to nerves. Although inflammation and neural tissue damage occur, the pathophysiology of postoperative pain is unique and the consequences are specific. However, treatment strategies used in the “real world” are still not based on these findings. Furthermore, analgesics and techniques with limited adverse effects and/or with benefits targeting specific aspects of postoperative pain (eg, movement-evoked pain) are lacking. It is necessary to gain new insights into the mechanisms of postoperative pain in experimental and clinical settings to develop therapeutic options with greater efficacy and less risk of adverse effects than those available today. Finally, comprehensive evidence based on results from clinical studies enhances knowledge, but needs to be implemented into clinical practice as well. Here we will present and discuss results from basic science studies and clinical scientific evidence generated within the last 2 decades to improve knowledge and enable translation of these findings into clinical practice more rapidly.
2. Animal-experimental basic research
2.1. Animal models of postoperative pain: an overview
To identify the mechanisms inherent in incision-induced postoperative pain, a specific surgery-related animal model in rats was developed in the 90s of the last century.19,197 By using this plantar incision model (and other animal models developed thereafter investigating specific aspects of pain after surgical trauma), we have learnt much about the underlying neurophysiology of incisional pain (comprehensive reviews are found here).18,20,141,189: In general, it ensued that many of those mechanisms inherent in pure inflammatory, antigen-induced or neuropathic pain are not relevant for incisional pain and vice versa. In the following, we will summarize some aspects of incisional pain, which were identified by using the plantar incision model. Because of the high number of published studies during the last 2 to 3 decades (PubMed entry key words: “Incision” and “animals” and “postoperative pain” revealed 397 hits, November 9, 2016), we are not able to report all findings. Those up to 2007 are mainly summarized in the reviews quoted here.20,141 We will provide a selective overview of topics, which are relevant to the pain field in general and specifically interesting to those dealing with postoperative pain in the clinical setting.
2.1.1. Brief description of the models
The original plantar incision model was developed in 1996 by Brennan et al.19 Briefly, under general anesthesia, a 1-cm longitudinal incision is performed through the glabrous skin, fascia, and plantar muscle of the rat hind paw. The skin is surgically sutured, then the animals recover from anesthesia; pain behavior can be measured from one hour thereafter. The combination of transsection of the skin, fascia, and retracted muscle compares well to the tissue trauma of patients undergoing surgery.18 Similar to patients after surgery, rats develop short-lasting nonevoked guarding pain behavior (for a short time period of approximately 2 days after incision) and longer lasting evoked pain-related behavior to punctate mechanical stimuli (von Frey hairs); these pain behaviors are seen as a surrogate for nonevoked resting pain (lasting some days) and evoked pain (lasting several days till weeks) after surgery, respectively, and are therefore relevant to postoperative pain in patients.35,166
Mechanical hyperalgesia occurs at the side of the incision (primary hyperalgesia) and in an area surrounding the injury (secondary hyperalgesia) for several days after the incision.196 Furthermore, heat hyperalgesia, but not cold hyperalgesia,156 is prominent at the site of the incision lasting up to approximately 7 days.196 Secondary heat hyperalgesia (usually measured as withdrawal thresholds to radiant heat) does not occur after this incision. Additionally, an incision model within the hairy skin hind paw of rats was developed for better investigation of secondary mechanical hyperalgesia in animals (gastrocnemius incision).134
More recently, anxiety- and depression-like behaviors were investigated after plantar incision; for example, rats show an increase in anxiety-like avoidance behaviors in the light/dark box and an increase in depression-like behavior (sucrose preference test) for some days after incision.86,168 Other studies assessing anxiety-like behavior after incision in rats gave similar results by using slightly different approaches and study designs.36,97 Interestingly, the anxiety-like behavior lasted longer than hyperalgesia in 2 (but not one) of these studies. As anxiety and other psychological factors such as depression, catastrophizing, and stress enhance acute and promote long-lasting pain after surgery, investigation of appropriate assays in animals might be relevant.
In 2003, the rat plantar incision model has been transferred from the rat to the mouse with some modification (5 mm incision, one mattress suture, modulated assessment of mechanical, and heat hyperalgesia), with very similar results in behavioral experiments.136 To investigate prolonged pain after a surgical incision more specifically, a skin and muscle retraction injury model (SMIR) was introduced.48 Here, a 1.5 to 2-cm incision is made in the skin of the medial inner thigh, 4 mm medial to the saphenous vein. The superficial (gracilis) muscle layer is then incised (7–10 mm) approximately 4 mm medial to the saphenous nerve and retracted with a microdissecting retractor for one hour. The tissue is sutured with silk. Mechanical hyperalgesia in the SMIR model lasts for up to 3 weeks and therefore much longer than after plantar incision, but heat hyperalgesia was not observed. Interestingly, the SMIR model has been transferred to a porcine model of prolonged incisional pain,28 which may have some very interesting advantages: For instance, pigs have a greater phylogenetic proximity with humans like a similar metabolism and a comparable skin anatomy with great homology in wound healing compared to rodents.33 These advantages could be deployed for validating new topical and localized treatment options for postoperative, incisional pain in human. Many other pain models related to surgical and trauma injuries have been developed and a comprehensive overview can be found in the review quoted here.189 One interesting model, for instance, is the back hairy skin incision model that represents another clinically relevant model for pain behavior after an incision in hairy skin.43,125 However, because of space limitations and because of the fact that the plantar (and more recently the SMIR incision) is used most frequently for studying mechanisms of incisional pain, we will mainly focus on these 2 models. Finally, an incision model in humans is also available and may provide an important link between animal studies and patients (eg, see 23,47,77,139). However, we will mainly focus on results from animal studies for this review.
2.2. Mechanisms of pain caused by a surgical incision
The patterns of pain behavior after surgical incisions in rodents indicate that peripheral and central sensitization occur. In neurophysiological experiments, activation and sensitization of peripheral nociceptors62,133 and spinal dorsal horn neurons196 were identified, and their specific mechanisms were investigated further.
2.2.1. Spinal sensitization after surgical incision
It is very intriguing that spinal administration of many substances that are able to prevent central sensitization in other pain models failed to produce a significant effect after incision; the first studies in the plantar incision model indicated this using spinal N-methyl-d-aspartate (NMDA) receptor antagonists which basically failed to produce any effect.49,137,196 These effects (or rather failure of effects) led to the assumption that an incision causes a different “form” of spinal sensitization compared to other pain entities. Additional pharmacological support for a unique spinal sensitization process after incision came from pharmacological studies showing that non-NMDA/α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptor antagonists are involved in the spinal transmission of pain behavior after incision.195,198,199 This again differs from many other pain models and was somehow striking by firstly questioning the general “rule” of a NMDA receptor-dependent central sensitization process relevant for certain types of pain, eg, after an incision and secondly “promoting” another rather new player in the game, namely the non-NMDA receptor group. The first issue was elaborated further by studies investigating the role of preemptive analgesia for postoperative pain, where NMDA receptors had earlier been identified as key molecules.140 Spinal sensitization after the plantar incision is maintained (at least initially) by the afferent barrage of sensitized nociceptors133 and the non-NMDA/AMPA receptor group is maintaining this process which is responsible for nonevoked pain and hyperalgesia after incision.198 More recent studies further elucidated mechanisms of AMPA-receptor mediated pain and hyperalgesia after incision and found that phosphorylation of the AMPA receptor GluR1 subunit at Serine-831 via phospho kinase C gamma (PKCγ), but not other conventional PKC's isoforms (PKCα, βI and βII), leads to an increased trafficking of Ca2+ permeable AMPA receptors in the neuronal plasma membrane and might play a role for incisional pain.181 Interestingly, AMPA receptor phosphorylation was enhanced under (social defeat) stress after incision96; at the same time, incision-induced punctate mechanical hyperalgesia was prolonged. Besides phosphorylation of AMPA receptors, one subunit, GluR1, but not GluR2, is upregulated in the spinal cord ipsilateral to an incision. The regulation of the surface delivery of this spinal AMPA receptor subunit is caused by stargazin (member of the AMPA receptor regulatory protein family), and selective down-regulation of stargazin was able to reduce synaptic targeting of GluR1 subunit and nonevoked pain behavior and mechanical hyperalgesia caused by plantar incision60 (Fig. 1). These spinal AMPA receptor subunits and the mechanisms regulating them after incision are therefore useful targets for drugs to treat pain after surgery in the future. However, spinal NMDA-receptor blockade might still have some indications. For example, remifentanil-induced enhanced mechanical hyperalgesia after incision in rats is regulated via phosphorylation of spinal NR2B at Tyr1472 which was prevented by ketamine.59 This has clinical implications as discussed below.
Figure 1.
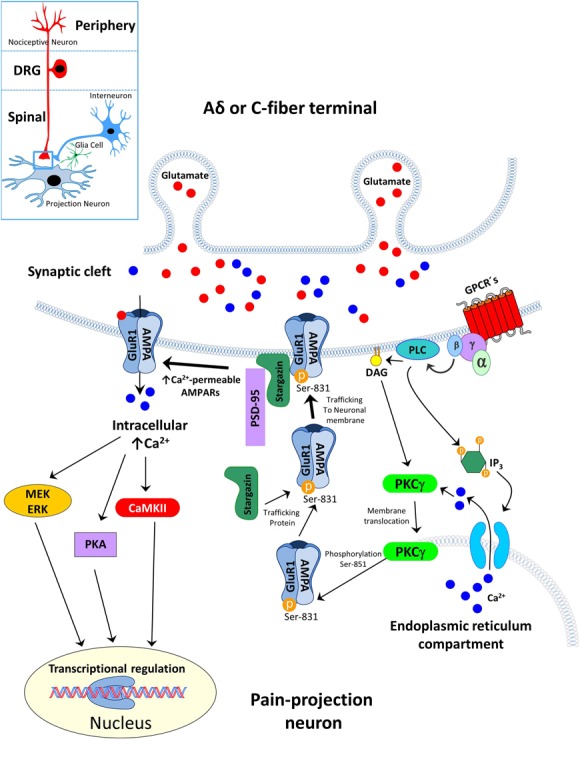
Postoperative pain is associated with increased trafficking of the GluR1 subunit of AMPA-receptors by phosphorylation of Ser-831. Surgical plantar incision enhances the membrane translocation of PKCγ, but not other PKC isoforms, and induces the phosphorylation the Ser-831 site of the GluR1 subunit from AMPA-receptors. Stargazin interacts with the phosphorylated subunit in the endoplasmic reticulum compartment and trafficking into the neuronal membrane.60,181 The enhanced phosphorylation of GluR1 subunit and interaction of stargazin increased insertion of Ca2+ -permeable AMAPA receptors in the postsynaptic density (via PSD-95) that enhanced spinal nociceptive transmission. AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; Ca, calcium; CaMKII, Ca2+/calmodulin-dependent protein; DAG, diacylglycerin kinase II; ERK, extracellular-signal Regulated Kinase; GluR, AMPA receptor subunit; GPCR´s, G-Protein-coupled recetors; IP3, inosit-1,4,5-trisphosphat; MEK, mitogen-activated protein kinase kinase; P, phosphat; PKA, phospho kinase A; PKCγphospho kinase C gamma; PLC, phospholipase C; PSD-95, postsynaptic density-95; Ser, serine.
Studies showing different effects of spinal substances on different pain modalities are very interesting. Two examples are an effect on mechanical hyperalgesia, but not on nonevoked guarding pain and vice versa; and the activation of spinal aminobutyric acid (GABA)-receptors, especially GABAA and GABAB, attenuated the generation of mechanical/heat hyperalgesia but not nonevoked pain after plantar incision.143 Similarly, the selective inhibition of spinal pERK1/2 before incision exclusively altered mechanical hyperalgesia after incision but not nonevoked pain.160,177 A comparable differentiation has been shown by Reichl et al.145; the inhibition of glutamate transporter (GluT) upregulation via mitogen-activated protein kinase p38 enhanced acute nonevoked pain after plantar incision, but did not alter mechanical or heat hyperalgesia. Interestingly, the effect of GluT inhibition extended the acute phase and lasted for approximately 2 weeks after incision. Thus, development of prolonged pain after surgery may be a result of impaired GLuT upregulation via p38 indicating a role spinal GluT in the prevention of chronic pain after incision. In the same way, prolonged pain behavior after the plantar incision was reported by inhibition of spinal cannabinoid receptors (CB1 and CB2) and dysregulation of mitogen-activated protein kinase phosphatase-3.3,152 Mitogen-activated protein kinase phosphatase-3 knockout mice showed a persistent mechanical hyperalgesia up to 21 days after surgical incision; this correlated with persistent phosphorylation of spinal p38 and extracellular signal-regulated kinases (ERKs)-1/2 in neurons and microglia on postoperative day 12.
Together, these studies indicate the relevance of assessing the effect of various compounds after plantar incision in different pain behavior assays (nonevoked pain behavior, mechanical hyperalgesia), in particular to determine potential clinical relevance (resting pain vs movement evoked pain) after surgery.35,166 Secondly, these studies identify factors relevant for prolongation of incisional pain; this may translate to mechanisms relevant for the development of chronic pain after surgery, another important clinical issue.39,80
Many other studies were undertaken to investigate pharmacological options to influence pain and hyperalgesia after incision by spinal modulation. Because of space limitations, we refer to Table 1 and to a review of studies published earlier than 2007.141
Table 1.
Pharmacological modulation of incision-induced pain behavior.
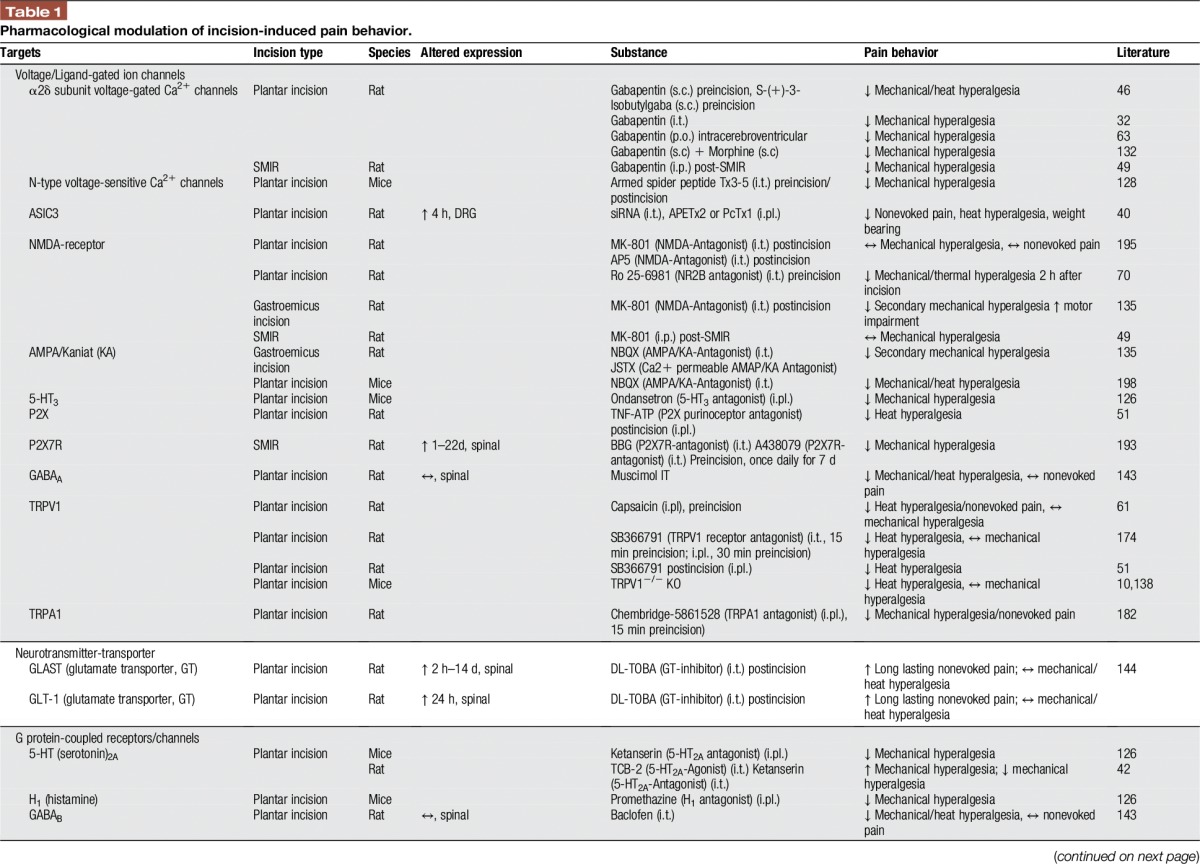
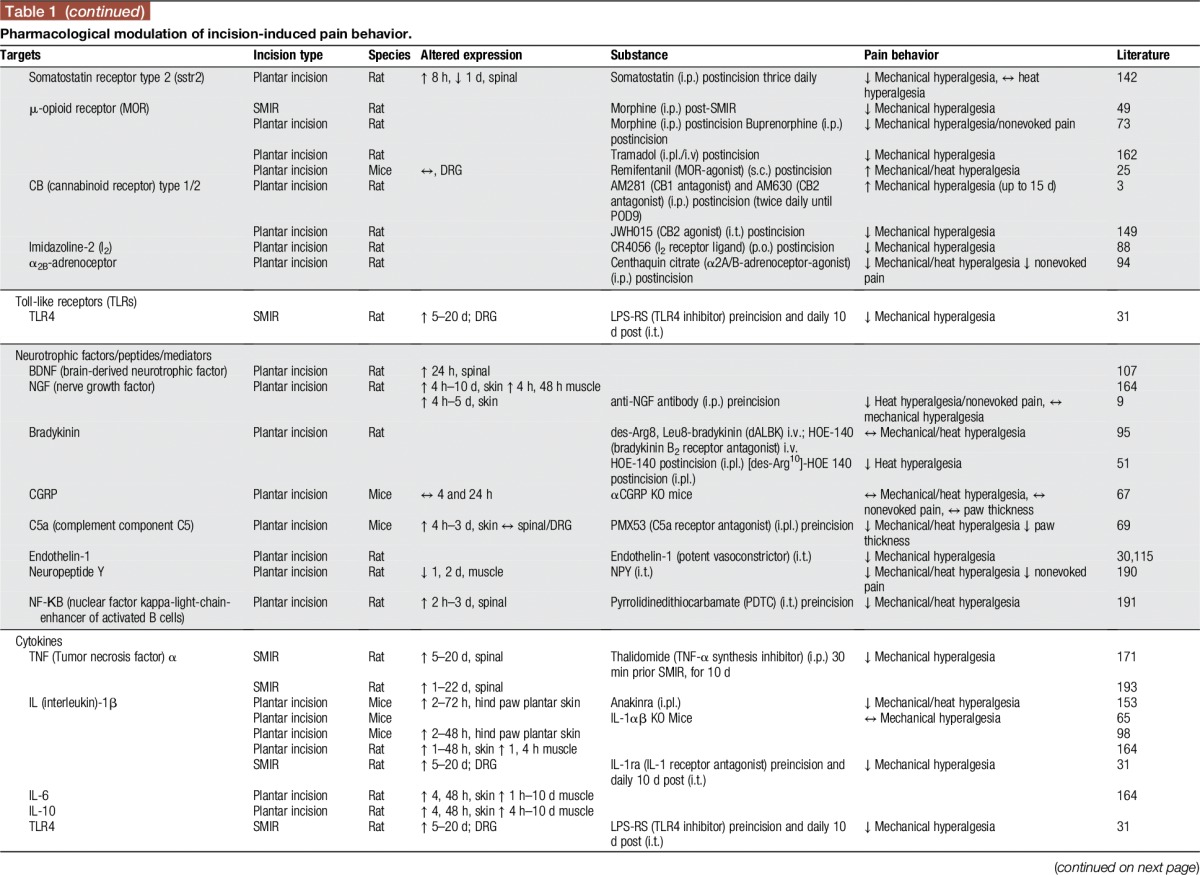
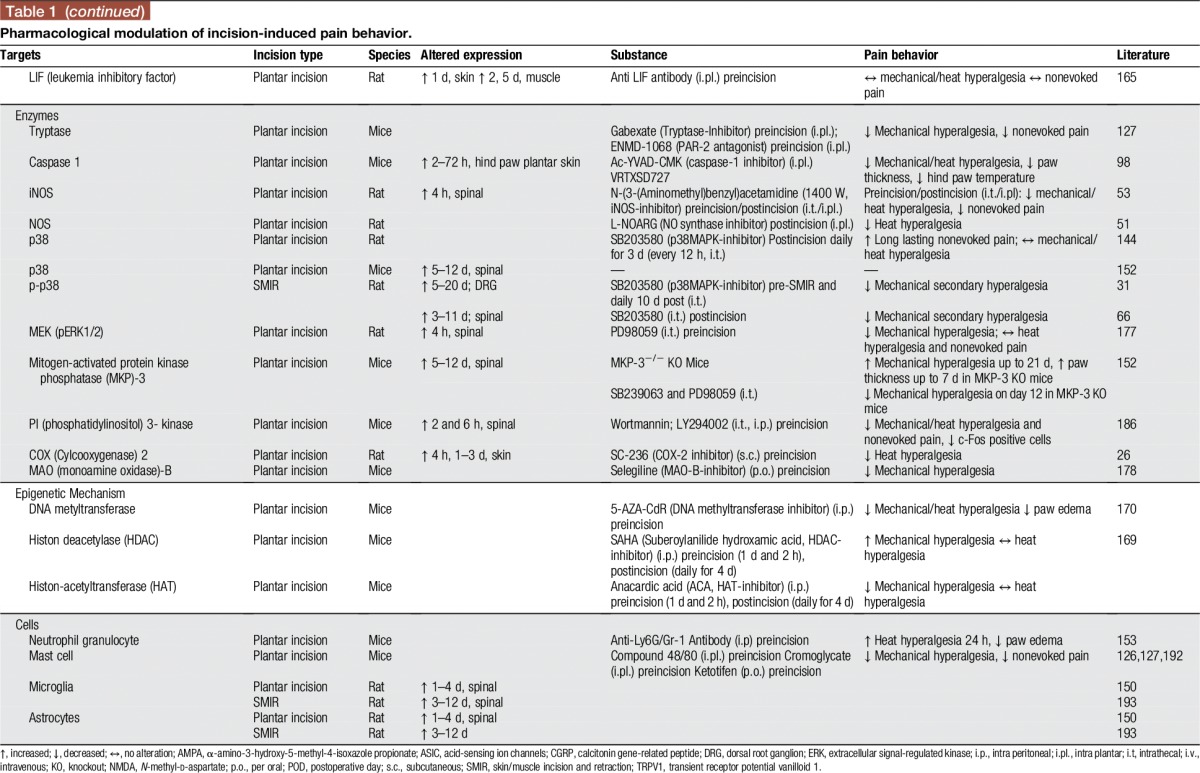
However, we assess here some studies investigating the role of multimodal analgesia for postoperative pain, highly relevant to clinical practice. For example, subcutaneous co-administration of morphine and gabapentin generates a dose-dependent antihyperalgesic synergistic effect.132 Similarly, intrathecal administration of gabapentin together with diclofenac, in doses not affecting nociception, reduced secondary hyperalgesia after incision. Thus, diclofenac augments the antihyperalgesic effects of gabapentin through spinal action.120 These findings highlight that certain combinations of medicines might offer benefits in the treatment of postsurgical pain and need to be assessed in future clinical studies.
2.2.2. Peripheral sensitization after incision
After plantar incision, peripheral C- and Aδ-fibers are sensitized contributing to nonevoked pain and heat and mechanical hyperalgesia in the early days after incision.8,62,133 Some of the underlying mechanisms were investigated within the last 10 years. Combined behavioral and neurophysiological experiments revealed that muscle nociceptors play a central role in the genesis of nonevoked guarding behavior after incision; the mechanism seems to be the spontaneous activity of C-fibers sensitized after incision.18,187,188 In contrast, a skin incision without a muscle tissue injury seems to be responsible for inducing mechanical hyperalgesia after incision; muscle injury seems to be not required.18,187,188 A series of studies revealed mechanisms inherent in (muscle) fiber sensitization after incision. They basically found a decrease in pH (pH ∼6.8) and an increase in lactate concentration (6 mM) that correlated well with a peak in pain behaviors at 1 to 2 days after incision.83,184 Similarly, a decrease of oxygen tension in both skeletal muscle and skin was detected immediately after incision for several days74; together with an increased lactate and decrease pH (see above), these ischemic-like conditions in the incisional wound are likely to contribute to peripheral sensitization (eg, muscle C-fibers, see81) and pain behavior (eg, nonevoked pain) after incision.74 Furthermore, a response of muscle C-fibers to antagonists to acid-sensing ion channels (ASICs) in cultured dorsal root ganglion (DRG) neurons points towards the role of tissue acidity in nonevoked pain behavior after incision.81 Interestingly, the ASIC3 channel is upregulated early after incision in muscle tissue innervating DRG neurons and seems to be important for nonevoked incisional pain, weight-bearing, and also partially for heat hyperalgesia. The role of ASIC3 for heat hyperalgesia is being controversially discussed in the literature with regard to different pain models. Whereas in vivo knockdown or specific inhibition via toxin APETx2a leads to complete remission of heat hyperalgesia after Complete Freund's Adjuvant (CFA) injection,41 mechanical but not heat hyperalgesia after carrageenan injection into the muscle seems to be ASIC3 mediated.161 Together, the role of ASIC (eg, ASIC3) channel blocker for pain in general and specifically for incisional pain needs further investigation.
The role of specific peripheral mechanisms contributing to hyperalgesia after the incision has been investigated as well. There are 2 important aspects, which will be highlighted here: Firstly there are different nociceptors that seem to be responsible for heat vs mechanical hyperalgesia after incision. Secondly, the molecular mechanisms of the fiber sensitization process responsible for mechanical and heat hyperalgesia after incision differ in many ways to other pain entities. Hints for the first aspect came from early studies using pretreatment of the incision site with a low dose of capsaicin (transient receptor potential vanilloid 1 receptor agonist); this reduced the heat hyperalgesia (and nonevoked pain) but not mechanical hyperalgesia after incision.61 Consistent with other pain models, there is an upregulation of peripheral (and spinal) transient receptor potential vanilloid 1, which contributes to the development of heat (but not mechanical) hyperalgesia after incision.10,138,174 There are many other examples differentiating mechanical and heat hyperalgesia after incision, which we will not address in detail but which are represented in Table 1.
One example for the second aspect, a differential sensitization process relevant for hyperalgesia after incision compared to inflammation or neuropathic pain, is the important role of calcitonin gene-related peptide in inflammation-related pain (CFA), which is not involved in spontaneous pain or mechanical and heat hyperalgesia after incision in mice.67 Further confirmation for an unique peripheral sensitization process after incision came from a study investigating 84 mRNAs from the neurotrophins and inflammatory cytokines families in skin, muscle, and DRG after plantar incision.164 As demonstrated, most alterations in the mRNA expression are present in incised skin and muscle, less in the DRG. They occur within the first 48 hours after incision when the mechanical and heat hyperalgesia, as well as guarding pain, is most obvious. In particular, genes for wound healing, re-innervation, and the immune response are differently expressed in comparison to other pain models.164 More examples supporting this are shown in Table 1.
Only recently peripheral inflammatory cell responses were investigated after incision injury in animals. For instance, migration of neutrophilic granulocytes (NGs) into tissue traumatized by incision occurs shortly after surgery, reaching a maximum at 24 hours and declining rapidly to baseline within 3 days.27,45,153 Neutrophilic granulocytess release many well-known proinflammatory mediators and contain endogenous opioid peptides (met-enkephalin and β-endorphin).146 Sahbaie and colleagues demonstrated that the systemic depletion of NGs (with Gr-1 antibody) reduced the paw edema and the interleukin-1β (IL-1β) concentration, but increased significantly the heat hyperalgesia for 24 hours and did not alter the mechanical hyperalgesia after plantar incision in mice.153 This suggests a role of endogenous opioids (released by NGs) for incisional pain similar to that shown in inflammatory pain models.147 However, another study from Carreira et al.27 used the same method to deplete NGs but showed attenuation of mechanical hyperalgesia after incision; they suggested the role of CXCL-1-CXCR1/2 recruitment of NGs after incision. Presumably, proinflammatory mediators from NGs (IL-1β and C5a) may play a role for hyperalgesia after incision.69,99,153 Thus, as the local concentration of C5a after the incision is increased, this complement factor may provide a novel target for analgesic drug development.69,99 However, the exact role of NGs in postoperative pain is currently unclear because of the contradictory results of both NG-depletion studies.27,153
The prevention of mast cell degranulation (mast cell membrane stabilization with Cromoglycate) or depletion of mast cell mediators (with Compound 48/80 prior to incision), thus inhibiting the effect of histamine, 5-HT, and tryptase (a serine protease localized exclusively in mast cells) reduce the mechanical hyperalgesia and nonevoked pain in mice.127,192 Similar effects are observed by administration of tryptase-binding receptor antagonist (protease-activated receptor 2, PAR2).127 These results suggest a role of mast cells contributing to hypersensitization after incision. However, it should be noted that the mast cell degranulation alone seems insufficient to promote pain (eg, allergies, some drug administration).
2.2.3. Neuroplastic changes in the brain after incision
Our understanding of the processing of pain in the human brain has improved significantly105; however, activity and neuroplasticity in the pain matrix after incision contributing to pain-related behavior remains poorly understood. A recent study in animals indicates directly how the brain reacts to an incision compared to inflammation by using functional magnetic resonance imaging to assess oxygenation levels of the blood as an indirect measure of neural activity and functional magnetic resonance spectroscopy.5 Mechanical stimulation of the incised hind paw showed blood oxygen level dependent (BOLD) signals, which differed significantly in quantity and quality to BOLD signals related to mechanical stimulation of the hind paw after CFA inflammation. Similarly, BOLD signals after electrical stimulation in both animal models differed to mechanical stimulation.5 However, GABA levels (measured with functional magnetic resonance spectroscopy) increased in both pain models within the thalamus, during rest and during mechanical stimulation.5 Thus, the thalamus might play a central role for hyperalgesia regardless of the pain entity and GABA neurotransmission might be involved. Further imaging studies investigated central neuroplastic changes relevant for the development of more chronic pain after incision. Human functional magnetic resonance imaging studies confirmed the role the thalamus139 and indicated a lack of descending inhibition in enhanced pain responses of patients with chronic pain after incision.22,23 By using the positron emission tomography-method, Romero et al.148 demonstrated long-lasting changes in glucose metabolism in central pain-related areas and opioid-related pathways up to 21 days after incision. The metabolic changes in the pain matrix were positively correlated with hypersensitivity caused by naloxone injection in rats which received remifentanil anesthesia earlier.148 This suggests long-lasting neuroplastic adaptations in central opioid circuits possibly contributing to chronic pain after incision.
Systemic administration of gabapentin, or inhibition of ERK within the anterior cingulate cortex (ACC) early after surgery, but not systemic morphine, reduced incision-induced anxiety.36,86,97 Interestingly, ERK-inhibition reduced anxiety-like behavior and mechanical hyperalgesia early after surgery (1 hour), but (different to inflammatory and neuropathic pain) in the later phase (6 hour), it exclusively reduced anxiety.
A recent study showed that presurgical or postsurgical exposure to stress factors like immobilization and force swimming test does not change the basal pain perception to different stimuli, such as mechanical, hot and cold, but prolongs the duration of incision-induced hyperalgesia after incision.26 By blocking spinal glucocorticoid receptors or removing the adrenal glands, stress-induced prolongation of incision-induced hyperalgesia was abrogated. These results indicate a direct connection between the activation of the hypothalamic-pituitary-adrenal axis through presurgery and postsurgery stress and duration of incision-induced hypersensitivities. Maternal adversity in the form of perinatal stress and depression may also activate the hypothalamic-pituitary-adrenal system and increased incisional pain in adult rats.85 Thus, acute stress might be—similar to other psychological factors—relevant for the transformation of acute into chronic pain after surgery.26
2.3. Epigenetic modulation after incision
In recent years, a growing body of publications has examined the potential of the epigenetic modulation, such as DNA methylation, histone acetylation and noncoding RNA, for chronic pain conditions.90,131 Some epigenetic results are now available for acute postoperative incisional pain in animals.154,169,170 An incision seems to induce changes in global DNA methylation, which leads to increased incision-induced hyperalgesia. Peripheral and spinal inhibition of a DNA methyltransferase via 5-Aza-2´-deoxycytidine led to attenuation of the mechanical and heat hyperalgesia and reduced hind paw swelling.170 Furthermore, epigenetic modulation of spinal Bdnf− (brain-derived neurotropic factor) and Pdyn− (prodynorphin) genes via acetylated Histone H3K9 in mice under chronic opioid exposure seems to be involved in opioid tolerance after incision.154 Notably, different histone deacetylase inhibitors, such as suberoylanilide hydroxamic acid or trichostatin A, attenuated heat hyperalgesia7 or mechanical hyperalgesia159 in an inflammatory (CFA) and in a neuropathic pain model, but exacerbated mechanical hyperalgesia after incision in mice.169 Taken together, these first epigenetic results suggest that peripheral and spinal epigenetic modulation are involved in increased postoperative nociceptive sensitization (Fig. 2). The additional influence of epigenetic regulation by drugs (eg, opioids) or environmental input could induce long-lasting changes in the pain system, one possible cause for a transformation from acute to chronic conditions.
Figure 2.
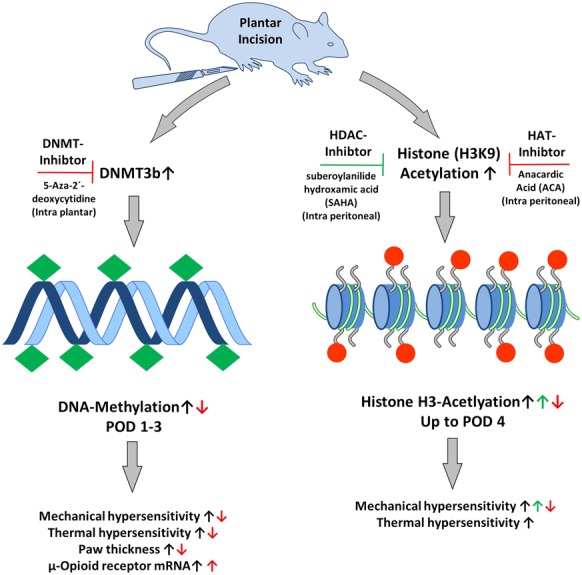
Epigenetic mechanisms modulate nociceptive sensitization after incision. Intra plantar (i.pl.) application of DNA-methyltransferase (DNMT) inhibitor (5-Aza-2´-deoxycytindine) reduced DNA-methylation and attenuated mechanical/heat hyperalgesia (↓), paw thickness (↓), and reinforced peripheral µ-opioid receptor mRNA expression (↑).170 The inhibition of Histon-deacetylase (HDAC) with suberoylanilide hydroxamic acid (SAHA, i.p.) reinforced mechanical hyperalgesia (↑). However, treatment of histon acetyltransferase inhibitor anacardic acid (ACA, i.p.) attenuated mechanical hyperalgesia (↓).169
2.4. New drugs in the pipeline
In recent years, nonclassical active pharmaceutical ingredients from venoms of spiders128,163 or from other sources66,82,84,100,106,122,155,180,200,201 have been tested for their potential to reduce mechanical/heat hyperalgesia and/or nonevoked pain or gait abnormalities after incision. Some substances act directly at receptors, such as the vitexin, a C-glycosylated flavone present in several medicinal herbs, which binds to GABAA and opioid receptors.200 Some more recent studies report that curcumin (diferuloylmethane), a phenolic constituent of turmeric, reduces incisional inflammation, nociceptive hypersensitivity,201 spontaneous pain, and functional gait abnormalities by increasing the level of TGF-β in incisional skin.155 Other substances block spinal N-type voltage-sensitive Ca2+ channels and reduce mechanical hyperalgesia after incision without altering the normal nociceptive sensitivity, eg, venom of the Brazilian armed spider Phoneutria nigriventer.128 These nonclassical active pharmaceutical substances have characteristics making them suitable as potential candidates for the development of new analgesics for postoperative pain.
2.5. Challenges in the translation of animal studies to man
The translation of findings from animals to patients (and back) is one of the greatest challenges in modern (pain) research. Previous studies have shown that the direct translation of results from rodent experiments is difficult and should be performed and interpreted with caution.111 One major disadvantage of many animal pain models is that they are not representing the pain etiology or pain entity they are translated to.111,112 The development of more sophisticated animal models, mimicking human pain conditions to improve bench-to-bedside translation, is part of the current discussion.24,34,111 The same, in fact, relates to human experimental pain models and their translation to patients and needs attention as well.103 Furthermore, the portfolio of behavioral pain measurements in animals does not represent well clinically relevant pain aspects in humans. For several years, we and others assess spontaneous pain behavior in rats after incision representative of pain at rest in patients.19,133,143,144 Hyperalgesia to pinprick stimuli are assessed in rats frequently; interestingly, the same stimuli are used to assess hyperalgesia around a surgical wound in patients,37,167 the mechanisms behind this might be relevant for central sensitization and prolongation of pain after surgery and are therefore useful to study.44,93 More recently, movement-evoked pain and pain-related anxiety and depression are explored in animals after incision; presumably, these might be other translatable pain-related behaviors and should be focused on in the future.111,112,176 Together, adequate animal pain models (eg, incisions for surgical pain,18,19) and relevant pain behavior assessed in these models combined with experimental human studies139 will pave the way for a refined and more applicable bench-to-bedside translation in postoperative pain.
3. Evidence for clinical management of postoperative pain
The preceding part of this article has outlined quite clearly that postoperative pain as a manifestation of acute pain is markedly more complex than originally thought. The complexity of postoperative pain requires, therefore, considerably more than simply applying opioids as required. It is, therefore, not surprising that there is now a large scientific evidence basis for the management of postoperative pain. This has been summarized recently in the fourth edition of the document “Acute Pain Management: Scientific Evidence”, published by the Australian and New Zealand College of Anaesthetists and its Faculty of Pain Medicine.157 The sheer size of this document reflects the complexity quite well; the document has nearly 650 pages, assesses over 8500 references, and condenses the evidence-based information in 669 key messages. It is obvious that it would be impossible to summarize that entire document in this article. The article will, therefore, concentrate on overarching key strategies and specific treatment options as far as they are of more general importance.
3.1. Multimodal analgesia
The concept of multimodal (“balanced”) analgesia has been introduced into the management of postoperative pain more than 20 years now.79 The concept suggests that it is superior to combine analgesics with different modes or sites of action, as such combinations will improve analgesia, reduce opioid requirements (so-called “opioid-sparing” effect), and thereby reduce the adverse effects of opioids.194 This concept is in line with the observations in basic science models that combinations of, for example, peripherally and centrally acting analgesic compounds are of value here. In addition, such multimodal approaches show further benefits with regard to other postoperative outcomes. Just to mention a few examples here, after total knee joint replacement, multimodal analgesia increases patient satisfaction scores and permits earlier achievement of milestones of physical therapy.87 Similarly, after spinal surgery, use of multimodal analgesia improves postoperative mobilization.108 In view of data showing that increased opioid use, with resulting opioid adverse effects (in particular nausea, vomiting, and constipation), delays recovery after surgery and thereby leads to extended hospital stay with increased costs,124 multimodal analgesia would reduce such complications, speed up recovery, and possibly even reduce hospital costs. This is nicely reflected in the fact that more or less all approaches using enhanced recovery after surgery protocols include multimodal analgesia concepts as one component.78 However, it has to be acknowledged that multimodal analgesia by itself does not result in early rehabilitation or enhanced recovery after surgery. To achieve these goals, multimodal analgesia needs to be integrated into a holistic and multidisciplinary approach to the postoperative period.119 In particular, again the importance seems to be the opioid-sparing effect, as avoidance of oral opioids in the postoperative period after colorectal surgery reduces the length of stay.2 This is confirmed by other data that show that opioid-sparing analgesic techniques reduce postoperative ileus.11
In this context, it is interesting to look in more detail at which compounds are actually useful components of multimodal analgesia and should thereby be combined with opioids.
3.2. Reduction of peripheral sensitisation due to inflammation
3.2.1. Nonsteroidal anti-inflammatory drugs
As outlined before, peripheral sensitisation of nociceptors leading to primary hyperalgesia is an important contributor to postoperative pain. It is, therefore, not surprising that in clinical reality drugs which are reducing peripheral prostaglandin concentration and thereby leading to reduced peripheral sensitisation are a useful component of multimodal analgesia. In randomized controlled trials52 and their meta-analyses, these drugs fulfill all 3 requirements on multimodal analgesia, ie, improved analgesia, reduced opioid requirements, and reduced adverse effects of opioids.109 A reduction of postoperative nausea and vomiting, one of the most disturbing adverse effects of opioids in the early postoperative period, is reported. COX-2 selective nonsteroidal anti-inflammatory drugs (NSAIDs) (coxibs) have similar efficacy to nonselective NSAIDs.113 However, they are superior in the postoperative setting because of reduced adverse events.
With regard to bleeding complications, coxibs lack platelet inhibition116 and therefore cause less postoperative blood loss than nonselective NSAIDs64 and are comparable to placebo.101 Furthermore, these compounds show a gastric ulceration rate similar to placebo and significantly lower than nonselective NSAIDs in high-risk patients, even for short-term use.56 Coxibs do not cause bronchospasm in patients with NSAID-exacerbated respiratory disease, a complication, which can occur with nonselective NSAIDs.114 Concerns about cardiovascular complications of coxibs, identified with rofecoxib and leading to its withdrawal,21 have not eventuated with short-term use of parecoxib158 or even long-term use of celecoxib.123
The effect of NSAIDs may be enhanced by the addition of paracetamol as the combination of paracetamol and NSAIDs is more effective than either compound alone.129
3.2.2. Corticosteroids
Dexamethasone as an anti-inflammatory corticosteroid is widely used in anesthetic practice to prevent nausea and vomiting.38 Other effects include an improvement of the quality of recovery and reduced fatigue.117 In addition, dexamethasone in therapeutic doses reduces postoperative pain scores and opioid consumption.179 However, these effects are small and only statistically significant and might not be of clinical relevance. In addition, there is still an ongoing debate about potential risks of perioperative steroid administration with regard to induction of hyperglycemia, increasing risk of infection and bleeding and possibly malignancy recurrence.173 A large randomized controlled trial currently underway will try to address these unresolved questions (https://www.paddi.org.au).
3.3. Reduction of secondary hyperalgesia due to central sensitisation
As outlined in detail in the preceding part of the article, it is obvious that central sensitisation plays a much more relevant role in the development of postoperative pain than previously thought. Findings in this setting illustrate that contrary to common beliefs, central sensitisation can occur within a very short time span and can significantly contribute to the overall picture of a postoperative pain state. It is therefore not surprising that there is increasing interest in the use of medications, which are attenuating such states of secondary hyperalgesia due to central sensitisation. A number of these compounds have become components of clinically useful multimodal analgesia. These include the NMDA receptor ketamine, the alpha-2-delta ligands pregabalin and gabapentin and the alpha-2-adrenergic agonist's clonidine and dexmedetomidine.
3.3.1. Ketamine
Ketamine is a noncompetitive antagonist of the NMDA receptor when used in subanaesthetic doses. Meta-analyses support the use of perioperative IV infusions of low-dose ketamine (in the range of around 0.1 mg·kg−1·h−1) with resulting improved analgesia, an opioid-sparing effect and reduction of opioid side effects such as postoperative nausea and vomiting.89 The benefits of ketamine are, in particular, seen in patients after major surgeries that are suffering severe pain (VAS >7/10). This explains why these benefits have been shown after thoracic and upper abdominal and major orthopedic surgery. In addition, not only in laboratory settings but also in the clinical settings, NMDA receptor antagonists such as ketamine are reducing the development of opioid-induced hyperalgesia, for example after remifentanil use.185 It is, therefore, not surprising that ketamine is also a useful analgesic in the settings of patients with established opioid tolerance13,175 and preoperative high opioid use.102 Similar findings with regard to opioid-sparing and improvement of analgesia have also been found with a perioperative infusion of magnesium which has to be regarded as another NMDA-receptor antagonist.118 Last, not least, there are data supporting the effect of perioperative ketamine in reducing the incidence of chronic postsurgical pain (see preventive analgesia).29
3.3.2. Alpha-2-delta ligands
The alpha-2-delta ligands pregabalin and gabapentin, which were developed for the treatment of neuropathic pain where they find their most relevant indication, have also been shown to have an effect on central sensitisation and are, therefore, for example, indicated in the treatment of fibromyalgia with FDA approval. In this context, it is, therefore, not surprising that for both compounds there is evidence from meta-analyses supporting their role as a component of multimodal analgesia.110,172 Data show reduced pain scores as well as reduced opioid consumption and thereby reduced adverse effects of opioids. This beneficial effect can be achieved with a single preoperative dose. In addition, the anxiolytic effect of these drugs should be taken into consideration and might be an additional beneficial factor,130 again in analogy to the basic science findings.
3.3.3. Alpha-2-adrenergic agonists
Perioperative systemic use of alpha-2 agonists such as clonidine and dexmedetomidine also fulfills the criteria for successful multimodal analgesia resulting in reduced pain intensity, opioid consumption, and nausea.15 However, with their use, potential adverse effects such as hypotension and bradycardia and possibly dose-dependent sedation need to be considered.
In conclusion, the concept of multimodal analgesia is supported by a large clinical data set, which shows that addressing both peripheral and central sensitisation after surgical incision leads to improved analgesia with reduced opioid requirements and thereby opioid side effects. Current data do not permit a decision on which combinations of how many components may comprise multimodal analgesia after what kind of surgical incision. However, from a practical point of view it seems to become increasingly routine in the setting of acute pain services to use an NSAID (best seems to be a COX-2 selective one) routinely, paracetamol and (eg, before major procedures, in healthy patients) an alpha-2-delta ligand as standard components of multimodal analgesia with rescue opioid being available on top of this. Other components such as ketamine and alpha-2 agonists are used in specific indications. The role of corticosteroids is not yet fully established in this setting and requires further investigation.
3.4. Procedure-specific postoperative pain management
The basic science data presented above suggest that depending on the type and location of the incisional model, different pain states result. Again this is confirmed by clinical data which show that analgesics may have different efficacies in different surgical settings.57 This is true even for a simple analgesic like paracetamol, which is significantly less effective after orthopedic surgery (relative risk reduction 1.87) than after dental extraction (relative risk reduction 3.77). Current large meta-analyses used to calculate the number needed to treat of analgesic agents might pool data from different postoperative pain states and thereby ignore the specific effects of a specific analgesic in a specific postoperative pain state.57 In addition, it has to be acknowledged that different surgical procedures do not only cause different pain states but also pain states of different severities in different locations. These observations and the support by basic science have led to the development of the concept of procedure-specific postoperative pain management. Treatment pathways for the management of postoperative pain after different surgical procedures can be developed in an evidence-based fashion by analyzing the literature specific to the respective procedure.72 Guidelines for a number of surgical procedures of different types have been developed by the PROSPECT initiative with the consideration of primarily procedure-specific evidence (www.postoppain.org). The guidelines can be found at the website of this initiative, and most of the guidelines have been accompanied by publications in the peer-reviewed literature with regard to these specific procedures.
3.5. Acute postoperative neuropathic pain
Neuropathic pain is still widely considered a chronic pain state. However, clinical experience and clinical data, as well as the animal data provided above, are showing that neuropathic pain can occur acutely and can be a component of postoperative pain. The literature shows that for example following sternotomy 50% of patients presented with dysaesthesia in the early postoperative period as a manifestation of acute neuropathic pain.4 As in other chronic neuropathic pain states, a manifestation of neuropathic pain is accompanied by increased pain severity. Similarly, after cancer surgery, use of a screening tool in a prospective setting found acute neuropathic pain in the first week postoperatively in around 10% of the cases.68 A case series in a general surgical population identified an incidence in the range of 3% to 4%.151 It is, therefore, relevant for clinicians looking after postoperative patients (and even more so after post-traumatic patients) to identify a neuropathic pain component, which might then require appropriate treatment, for example, with alpha-2-delta ligands or ketamine on top of commonly used opioids.
3.6. Preventive analgesia
Nerve injury leading to acute neuropathic pain is one of the major risk factors for the progression of acute to chronic pain.1 Chronic postsurgical pain is much more common than usually thought and the estimated incidence of chronic severe pain with an intensity of more than 5/10 occurs in 2% to 10% of patients after surgery.157 Besides nerve injury as a risk factor, other risk factors include preexisting preoperative pain, preoperative anxiety, catastrophizing as well as genetic predisposition. Postoperative factors are again severe acute postoperative pain and psychosocial risk factors similar to those in the preoperative setting. This is in line with some of the observations with regard to behavioral changes observed in animal studies. In view of nerve injury as a risk factor, it is not surprising that a large percentage of chronic postsurgical pain has features of neuropathic pain.71
With regard to the prevention of such pain states, there has been a significant change in concepts away from the previously supported pre-emptive analgesia approach to a preventive analgesia approach.91 Pre-emptive analgesia is defined as a preoperative treatment, which is more effective than the identical treatment administered after the incision. The key difference is the timing of the administration. It has become increasingly obvious that preventive analgesia, ie, an analgesic effect beyond its expected duration is a more useful approach. For practical terms, this has been defined as analgesia which persists beyond 5.5 half-lives of a medicine.76 In the context of prevention of chronic surgical pain, it is also important to maximize the benefits of any analgesic strategy by continuing the treatment into the postoperative period as long as the sensitising stimulus persists. With regard to the preventive effect on chronic postsurgical pain, best data are available for the use of regional or neuraxial analgesia. A meta-analysis supports the use of epidural analgesia after thoracotomy and the use of paravertebral blocks after breast cancer surgery.6 Data at lower levels of evidence support the use of spinal anesthesia over general anesthesia for Caesarean section121 and hysterectomy,17 as well as the use of epidural analgesia after major abdominal surgery92 and for amputations with regard to the reduction of phantom limb pain.54 Interesting specifically for phantom limb pain is the idea of decreasing the already existing preoperative pain to reduce phantom limb pain after surgery; here, both epidural analgesia and systemic opioids seem to be effective.75 Other data support the use of perioperative local anesthetics for wound infiltration.14,16 However, it is important in this context to realize that intravenous lignocaine also has preventive effects on acute postoperative pain12 and in one small study reduced chronic postsurgical pain.58
Further evidence supports the use of ketamine as a preventive treatment for chronic postsurgical pain. A meta-analysis of 14 rather small randomized controlled trials shows a reduction of chronic postsurgical pain at 3 and 6 months, in particular if ketamine is administered for more than 24 hours perioperatively.29 With regard to the alpha-2-delta ligands gabapentin and pregabalin, there may be an effect on preventing chronic postsurgical pain; however, the data are currently rather contradictory and looking at only a few usually small studies with a large degree of heterogeneity so that uncertainty continues here.29,33
In conclusion, the current evidence base for the management of acute postoperative pain is significant. Much of the clinical data, which support certain approaches are in line with findings in the experimental setting. However, there are a number of discrepancies here and it will be interesting to see if the development of new compounds based on basic science studies will further help to improve the management of acute postoperative pain. It remains important to realize that postoperative pain management is not only a humanitarian task to reduce patient suffering and improve patient satisfaction but that treatment of acute postoperative pain has the potential to reduce morbidity possibly even mortality after surgery and in parallel enhance recovery, improve rehabilitation, reduce hospital stay and thereby overall hospital cost.
Disclosures
Stephan Schug: The Anesthesiology Unit of the University of Western Australia, but not Stephan Schug personally, has received research, travel funding, and speaking and consulting honoraria from bioCSL/Seqirus/Grunenthal, iXBiopharma, Mundipharma, and Pfizer within the last 2 years. Esther Pogatzki-Zahn received travel funding, and speaking and consulting honoraria from Grünenthal, Mundipharma, MSD, Fresenius Kabi, Janssen Cilag, The Medicine Company and ArcelRx and research support from Mundipharma (paid to the hospital).
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Aasvang EK, Brandsborg B, Christensen B, Jensen TS, Kehlet H. Neurophysiological characterization of postherniotomy pain. PAIN 2008;137:173–81. [DOI] [PubMed] [Google Scholar]
- [2].Ahmed J, Lim M, Khan S, McNaught C, Macfie J. Predictors of length of stay in patients having elective colorectal surgery within an enhanced recovery protocol. Int J Surg 2010;8:628–32. [DOI] [PubMed] [Google Scholar]
- [3].Alkaitis MS, Solorzano C, Landry RP, Piomelli D, DeLeo JA, Romero-Sandoval EA. Evidence for a role of endocannabinoids, astrocytes and p38 phosphorylation in the resolution of postoperative pain. PLoS One 2010;5:e10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Alston RP, Pechon P. Dysaesthesia associated with sternotomy for heart surgery. Br J Anaesth 2005;95:153–8. [DOI] [PubMed] [Google Scholar]
- [5].Amirmohseni S, Segelcke D, Reichl S, Wachsmuth L, Görlich D, Faber C, Pogatzki-Zahn E. Characterization of incisional and inflammatory pain in rats using functional tools of MRI. NeuroImage 2016;127:110–22. [DOI] [PubMed] [Google Scholar]
- [6].Andreae MH, Andreae DA. Regional anaesthesia to prevent chronic pain after surgery: a cochrane systematic review and meta-analysis. Br J Anaesth 2013;111:711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bai G, Wei D, Zou S, Ren K, Dubner R. Inhibition of class II histone deacetylases in the spinal cord attenuates inflammatory hyperalgesia. Mol Pain 2010;6:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Banik RK, Brennan TJ. Sensitization of primary afferents to mechanical and heat stimuli after incision in a novel in vitro mouse glabrous skin-nerve preparation. PAIN 2008;138:380–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Banik RK, Subieta AR, Wu C, Brennan TJ. Increased nerve growth factor after rat plantar incision contributes to guarding behavior and heat hyperalgesia. PAIN 2005;117:68–76. [DOI] [PubMed] [Google Scholar]
- [10].Barabas ME, Stucky CL. TRPV1, but not TRPA1, in primary sensory neurons contributes to cutaneous incision-mediated hypersensitivity. Mol Pain 2013;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Barletta JF, Asgeirsson T, Senagore AJ. Influence of intravenous opioid dose on postoperative ileus. Ann Pharmacother 2011;45:916–23. [DOI] [PubMed] [Google Scholar]
- [12].Barreveld A, Witte J, Chahal H, Durieux ME, Strichartz G. Preventive analgesia by local anesthetics: the reduction of postoperative pain by peripheral nerve blocks and intravenous drugs. Anesth Analg 2013;116:1141–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Barreveld AM, Correll DJ, Liu X, Max B, McGowan JA, Shovel L, Wasan AD, Nedeljkovic SS. Ketamine decreases postoperative pain scores in patients taking opioids for chronic pain: results of a prospective, randomized, double-blind study. Pain Med 2013;14:925–34. [DOI] [PubMed] [Google Scholar]
- [14].Batoz H, Verdonck O, Pellerin C, Roux G, Maurette P. The analgesic properties of scalp infiltrations with ropivacaine after intracranial tumoral resection. Anesth Analg 2009;109:240–4. [DOI] [PubMed] [Google Scholar]
- [15].Blaudszun G, Lysakowski C, Elia N, Tramer MR. Effect of perioperative systemic alpha2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta-analysis of randomized controlled trials. Anesthesiology 2012;116:1312–22. [DOI] [PubMed] [Google Scholar]
- [16].Blumenthal S, Dullenkopf A, Rentsch K, Borgeat A. Continuous infusion of ropivacaine for pain relief after iliac crest bone grafting for shoulder surgery. Anesthesiology 2005;102:392–7. [DOI] [PubMed] [Google Scholar]
- [17].Brandsborg B. Pain following hysterectomy: epidemiological and clinical aspects. Dan Med J 2012;59:B4374. [PubMed] [Google Scholar]
- [18].Brennan TJ. Pathophysiology of postoperative pain. PAIN 2011;152:S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. PAIN 1996;64:493–501. [DOI] [PubMed] [Google Scholar]
- [20].Brennan TJ, Zahn PK, Pogatzki-Zahn EM. Mechanisms of incisional pain. Anesthesiol Clin North America 2005;23:1–20. [DOI] [PubMed] [Google Scholar]
- [21].Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A, Konstam MA, Baron JA. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 2005;352:1092–102. [DOI] [PubMed] [Google Scholar]
- [22].Burgmer M, Pfleiderer B, Maihöfner C, Gaubitz M, Wessolleck E, Heuft G, Pogatzki-Zahn E. Cerebral mechanisms of experimental hyperalgesia in fibromyalgia. Eur J Pain 2012;16:636–47. [DOI] [PubMed] [Google Scholar]
- [23].Burgmer M, Pogatzki-Zahn E, Gaubitz M, Wessoleck E, Heuft G, Pfleiderer B. Altered brain activity during pain processing in fibromyalgia. NeuroImage 2009;44:502–8. [DOI] [PubMed] [Google Scholar]
- [24].Burma NE, Leduc-Pessah H, Fan CY, Trang T. Animal models of chronic pain: advances and challenges for clinical translation. J Neurosci Res 2016. 10.1002/jnr.23768. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [25].Cabañero D, Célérier E, García-Nogales P, Mata M, Roques BP, Maldonado R, Puig MM. The pro-nociceptive effects of remifentanil or surgical injury in mice are associated with a decrease in delta-opioid receptor mRNA levels: prevention of the nociceptive response by on-site delivery of enkephalins. PAIN 2009;141:88–96. [DOI] [PubMed] [Google Scholar]
- [26].Cao J, Wang P, Tiwari V, Liang L, Lutz BM, Shieh K, Zang W, Kaufman AG, Bekker A, Gao X, Tao Y. Short-term pre- and post-operative stress prolongs incision-induced pain hypersensitivity without changing basal pain perception. Mol Pain 2015;11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Carreira EU, Carregaro V, Teixeira MM, Moriconi A, Aramini A, Verri WA, Ferreira SH, Cunha FQ, Cunha TM. Neutrophils recruited by CXCR1/2 signalling mediate post-incisional pain. Eur J Pain 2013;17:654–63. [DOI] [PubMed] [Google Scholar]
- [28].Castel D, Willentz E, Doron O, Brenner O, Meilin S. Characterization of a porcine model of post-operative pain. Eur J Pain 2014;18:496–505. [DOI] [PubMed] [Google Scholar]
- [29].Chaparro LE, Smith SA, Moore RA, Wiffen PJ, Gilron I. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database Syst Rev 2013;7:CD008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chen G, Tanabe K, Yanagidate F, Kawasaki Y, Zhang L, Dohi S, Iida H. Intrathecal endothelin-1 has antinociceptive effects in rat model of postoperative pain. Eur J Pharmacol 2012;697:40–6. [DOI] [PubMed] [Google Scholar]
- [31].Chen H, Jiang Y, Sun Y, Xiong Y. p38 and interleukin-1 beta pathway via toll-like receptor 4 contributed to the skin and muscle incision and retraction-induced allodynia. J Surg Res 2015;197:339–47. [DOI] [PubMed] [Google Scholar]
- [32].Cheng JK, Pan HL, Eisenach JC. Antiallodynic effect of intrathecal gabapentin and its interaction with clonidine in a rat model of postoperative pain. Anesthesiology 2000;92:1126–31. [DOI] [PubMed] [Google Scholar]
- [33].Clarke H, Bonin RP, Orser BA, Englesakis M, Wijeysundera DN, Katz J. The prevention of chronic postsurgical pain using gabapentin and pregabalin: a combined systematic review and meta-analysis. Anesth Analg 2012;115:428–42. [DOI] [PubMed] [Google Scholar]
- [34].Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ. Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. PAIN 2012;153:876–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cooper SA, Desjardins PJ, Turk DC, Dworkin RH, Katz NP, Kehlet H, Ballantyne JC, Burke LB, Carragee E, Cowan P, Croll S, Dionne RA, Farrar JT, Gilron I, Gordon DB, Iyengar S, Jay GW, Kalso EA, Kerns RD, McDermott MP, Raja SN, Rappaport BA, Rauschkolb C, Royal MA, Segerdahl M, Stauffer JW, Todd KH, Vanhove GF, Wallace MS, West C, White RE, Wu C. Research design considerations for single-dose analgesic clinical trials in acute pain: IMMPACT recommendations. PAIN 2016;157:288–301. [DOI] [PubMed] [Google Scholar]
- [36].Dai R, Li C, Zhang J, Li F, Shi X, Zhang J, Zhou X. Biphasic activation of extracellular signal-regulated kinase in anterior cingulate cortex distinctly regulates the development of pain-related anxiety and mechanical hypersensitivity in rats after incision. Anesthesiology 2011;115:604–13. [DOI] [PubMed] [Google Scholar]
- [37].De Kock MF, Lavand'homme PM. The clinical role of NMDA receptor antagonists for the treatment of postoperative pain. Best Pract Res Clin Anaesthesiol 2007;21:85–98. [DOI] [PubMed] [Google Scholar]
- [38].De Oliveira GS, Jr, Castro-Alves LJ, Ahmad S, Kendall MC, McCarthy RJ. Dexamethasone to prevent postoperative nausea and vomiting: an updated meta-analysis of randomized controlled trials. Anesth Analg 2013;116:58–74. [DOI] [PubMed] [Google Scholar]
- [39].Deumens R, Steyaert A, Forget P, Schubert M, Lavand'homme P, Hermans E, Kock M de. Prevention of chronic postoperative pain: cellular, molecular, and clinical insights for mechanism-based treatment approaches. Prog Neurobiol 2013;104:1–37. [DOI] [PubMed] [Google Scholar]
- [40].Deval E, Noël J, Gasull X, Delaunay A, Alloui A, Friend V, Eschalier A, Lazdunski M, Lingueglia E. Acid-sensing ion channels in postoperative pain. J Neurosci 2011;31:6059–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Deval E, Noël J, Lay N, Alloui A, Diochot S, Friend V, Jodar M, Lazdunski M, Lingueglia E. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J 2008;27:3047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Dong R, Yu B, Chen L, Yu W. The 5-HT2A receptor potassium-chloride cotransporter 2 signaling pathway in a rat incision pain model. Exp Ther Med 2016;12:3583–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Duarte AM, Pospisilova E, Reilly E, Mujenda F, Hamaya Y, Strichartz GR. Reduction of postincisional allodynia by subcutaneous bupivacaine: findings with a new model in the hairy skin of the rat. Anesthesiology 2005;103:113–25. [DOI] [PubMed] [Google Scholar]
- [44].Eisenach JC. Preventing chronic pain after surgery: who, how, and when? Reg Anesth Pain Med 2006;31:1–3. [DOI] [PubMed] [Google Scholar]
- [45].Engelhardt E, Toksoy A, Goebeler M, Debus S, Bröcker EB, Gillitzer R. Chemokines IL-8, GROalpha, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am J Pathol 1998;153:1849–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Field MJ, Holloman EF, McCleary S, Hughes J, Singh L. Evaluation of gabapentin and S-(+)-3-isobutylgaba in a rat model of postoperative pain. J Pharmacol Exp Ther 1997;282:1242–6. [PubMed] [Google Scholar]
- [47].Fimer I, Klein T, Magerl W, Treede R, Zahn PK, Pogatzki-Zahn EM. Modality-specific somatosensory changes in a human surrogate model of postoperative pain. Anesthesiology 2011;115:387–97. [DOI] [PubMed] [Google Scholar]
- [48].Flatters SJ. Characterization of a model of persistent postoperative pain evoked by skin/muscle incision and retraction (SMIR). PAIN 2008;135:119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Flatters SJ. Effect of analgesic standards on persistent postoperative pain evoked by skin/muscle incision and retraction (SMIR). Neurosci Lett 2010;477:43–7. [DOI] [PubMed] [Google Scholar]
- [50].Fletcher D, Stamer UM, Pogatzki-Zahn E, Zaslansky R, Tanase NV, Perruchoud C, Kranke P, Komann M, Lehman T, Meissner W. Chronic postsurgical pain in Europe: an observational study. Eur J Anaesthesiology 2015;32:725–34. [DOI] [PubMed] [Google Scholar]
- [51].Füredi R, Bölcskei K, Szolcsányi J, Petho G. Comparison of the peripheral mediator background of heat injury- and plantar incision-induced drop of the noxious heat threshold in the rat. Life Sci 2010;86:244–50. [DOI] [PubMed] [Google Scholar]
- [52].Gan TJ, Joshi GP, Zhao SZ, Hanna DB, Cheung RY, Chen C. Presurgical intravenous parecoxib sodium and follow-up oral valdecoxib for pain management after laparoscopic cholecystectomy surgery reduces opioid requirements and opioid-related adverse effects. Acta Anaesthesiol Scand 2004;48:1194–207. [DOI] [PubMed] [Google Scholar]
- [53].Gautam M, Kumar R, Prasoon P, Ray SB. Antinociceptive effect of 1400 W, an inhibitor of inducible nitric oxide synthase, following hind paw incision in rats. Nitric Oxide 2015;50:98–104. [DOI] [PubMed] [Google Scholar]
- [54].Gehling M, Tryba M. Prophylaxis of phantom pain: is regional analgesia ineffective? Schmerz 2003;17:11–9. [DOI] [PubMed] [Google Scholar]
- [55].Gerbershagen HJ, Pogatzki-Zahn E, Aduckathil S, Peelen LM, Kappen TH, van Wijck AJ, Kalkman CJ, Meissner W. Procedure-specific risk factor analysis for the development of severe postoperative pain. Anesthesiology 2014;120:1237–45. [DOI] [PubMed] [Google Scholar]
- [56].Goldstein JL, Kivitz AJ, Verburg KM, Recker DP, Palmer RC, Kent JD. A comparison of the upper gastrointestinal mucosal effects of valdecoxib, naproxen and placebo in healthy elderly subjects. Aliment Pharmacol Ther 2003;18:125–32. [DOI] [PubMed] [Google Scholar]
- [57].Gray A, Kehlet H, Bonnet F, Rawal N. Predicting postoperative analgesia outcomes: NNT league tables or procedure-specific evidence? Br J Anaesth 2005;94:710–4. [DOI] [PubMed] [Google Scholar]
- [58].Grigoras A, Lee P, Sattar F, Shorten G. Perioperative intravenous lidocaine decreases the incidence of persistent pain after breast surgery. Clin J Pain 2012;28:567–72. [DOI] [PubMed] [Google Scholar]
- [59].Gu X, Wu X, Liu Y, Cui S, Ma Z. Tyrosine phosphorylation of the N-Methyl-D-Aspartate receptor 2B subunit in spinal cord contributes to remifentanil-induced postoperative hyperalgesia: the preventive effect of ketamine. Mol Pain 2009;5:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Guo R, Zhao Y, Zhang M, Wang Y, Shi R, Liu Y, Xu J, Wu A, Yue Y, Wu J, Guan Y, Wang Y. Down-regulation of stargazin inhibits the enhanced surface delivery of α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor GluR1 subunit in rat dorsal horn and ameliorates postoperative pain. Anesthesiology 2014;121:609–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hamalainen MM, Subieta A, Arpey C, Brennan TJ. Differential effect of capsaicin treatment on pain-related behaviors after plantar incision. J Pain 2009;10:637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hämäläinen MM, Gebhart GF, Brennan TJ. Acute effect of an incision on mechanosensitive afferents in the plantar rat hindpaw. J Neurophysiol 2002;87:712–20. [DOI] [PubMed] [Google Scholar]
- [63].Hayashida K, DeGoes S, Curry R, Eisenach JC. Gabapentin activates spinal noradrenergic activity in rats and humans and reduces hypersensitivity after surgery. Anesthesiology 2007;106:557–62. [DOI] [PubMed] [Google Scholar]
- [64].Hegi TR, Bombeli T, Seifert B, Baumann PC, Haller U, Zalunardo MP, Pasch T, Spahn DR. Effect of rofecoxib on platelet aggregation and blood loss in gynaecological and breast surgery compared with diclofenac. Br J Anaesth 2004;92:523–31. [DOI] [PubMed] [Google Scholar]
- [65].Honore P, Wade CL, Zhong C, Harris RR, Wu C, Ghayur T, Iwakura Y, Decker MW, Faltynek C, Sullivan J, Jarvis MF. Interleukin-1alphabeta gene-deficient mice show reduced nociceptive sensitivity in models of inflammatory and neuropathic pain but not post-operative pain. Behav Brain Res 2006;167:355–64. [DOI] [PubMed] [Google Scholar]
- [66].Huang L, Wang C, Serhan CN, Strichartz G. Enduring prevention and transient reduction of postoperative pain by intrathecal resolvin D1. PAIN 2011;152:557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ishida K, Kawamata T, Tanaka S, Shindo T, Kawamata M. Calcitonin gene-related peptide is involved in inflammatory pain but not in postoperative pain. Anesthesiology 2014;121:1068–79. [DOI] [PubMed] [Google Scholar]
- [68].Jain P, Padole D, Bakshi S. Prevalence of acute neuropathic pain after cancer surgery: a Prospective Study. Indian J Anaesth 2014;58:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Jang JH, Liang D, Kido K, Sun Y, Clark DJ, Brennan TJ. Increased local concentration of complement C5a contributes to incisional pain in mice. J Neuroinflammation 2011;8:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Jiang M, Zhang W, Ma Z, Gu X. Antinociception and prevention of hyperalgesia by intrathecal administration of Ro 25-6981, a highly selective antagonist of the 2B subunit of N-methyl-D-aspartate receptor. Pharmacol Biochem Behav 2013;112:56–63. [DOI] [PubMed] [Google Scholar]
- [71].Johansen A, Schirmer H, Stubhaug A, Nielsen CS. Persistent post-surgical pain and experimental pain sensitivity in the Tromso study: comorbid pain matters. PAIN 2014;155:341–8. [DOI] [PubMed] [Google Scholar]
- [72].Joshi GP, Schug SA, Kehlet H. Procedure-specific pain management and outcome strategies. Best practice & research. Clin Anaesthesiology 2014;28:191–201. [DOI] [PubMed] [Google Scholar]
- [73].Kabadi R, Kouya F, Cohen HW, Banik RK. Spontaneous pain-like behaviors are more sensitive to morphine and buprenorphine than mechanically evoked behaviors in a rat model of acute postoperative pain. Anesth Analg 2015;120:472–8. [DOI] [PubMed] [Google Scholar]
- [74].Kang S, Lee D, Theusch BE, Arpey CJ, Brennan TJ. Wound hypoxia in deep tissue after incision in rats. Wound Repair Regen 2013;21:730–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Karanikolas M, Aretha D, Tsolakis I, Monantera G, Kiekkas P, Papadoulas S, Swarm RA, Filos KS. Optimized perioperative analgesia reduces chronic phantom limb pain intensity, prevalence, and frequency: a prospective, randomized, clinical trial. Anesthesiology 2011;114:1144–54. [DOI] [PubMed] [Google Scholar]
- [76].Katz J, Clarke H, Seltzer Z. Review article: preventive analgesia: quo vadimus? Anesth Analg 2011;113:1242–53. [DOI] [PubMed] [Google Scholar]
- [77].Kawamata M, Takahashi T, Kozuka Y, Nawa Y, Nishikawa K, Narimatsu E, Watanabe H, Namiki A. Experimental incision-induced pain in human skin: effects of systemic lidocaine on flare formation and hyperalgesia. PAIN 2002;100:77–89. [DOI] [PubMed] [Google Scholar]
- [78].Kehlet H. Fast-track surgery-an update on physiological care principles to enhance recovery. Langenbecks Arch Surg 2011;396:585–90. [DOI] [PubMed] [Google Scholar]
- [79].Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg 1993;77:1048–56. [DOI] [PubMed] [Google Scholar]
- [80].Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618–25. [DOI] [PubMed] [Google Scholar]
- [81].Kido K, Gautam M, Benson CJ, Gu H, Brennan TJ. Effect of deep tissue incision on pH responses of afferent fibers and dorsal root ganglia innervating muscle. Anesthesiology 2013;119:1186–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kim JG, Lim DW, Cho S, Han D, Kim YT. The edible brown seaweed Ecklonia cava reduces hypersensitivity in postoperative and neuropathic pain models in rats. Molecules 2014;19:7669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kim TJ, Freml L, Park SS, Brennan TJ. Lactate concentrations in incisions indicate ischemic-like conditions may contribute to postoperative pain. J Pain 2007;8:59–66. [DOI] [PubMed] [Google Scholar]
- [84].Kim WJ, Kang H, Choi GJ, Shin HY, Baek CW, Jung YH, Woo YC, Kim JY, Yon JH. Antihyperalgesic effects of ginseng total saponins in a rat model of incisional pain. J Surg Res 2014;187:169–75. [DOI] [PubMed] [Google Scholar]
- [85].Knaepen L, Rayen I, Charlier TD, Fillet M, Houbart V, van Kleef M, Steinbusch HW, Patijn J, Tibboel D, Joosten EA, Pawluski JL. Developmental fluoxetine exposure normalizes the long-term effects of maternal stress on post-operative pain in Sprague-Dawley rat offspring. PLoS One 2013;8:e57608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kouya F, Iqbal Z, Charen D, Shah M, Banik RK. Evaluation of anxiety-like behaviour in a rat model of acute postoperative pain. Eur J Anaesthesiol 2015;32:242–7. [DOI] [PubMed] [Google Scholar]
- [87].Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty 2014;29:329–34. [DOI] [PubMed] [Google Scholar]
- [88].Lanza M, Ferrari F, Menghetti I, Tremolada D, Caselli G. Modulation of imidazoline I2 binding sites by CR4056 relieves postoperative hyperalgesia in male and female rats. Br J Pharmacol 2014;171:3693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Laskowski K, Stirling A, McKay WP, Lim HJ. A systematic review of intravenous ketamine for postoperative analgesia. Can J Anaesth 2011;58:911–23. [DOI] [PubMed] [Google Scholar]
- [90].Laumet G, Garriga J, Chen S, Zhang Y, Li D, Smith TM, Dong Y, Jelinek J, Cesaroni M, Issa J, Pan H. G9a is essential for epigenetic silencing of K(+) channel genes in acute-to-chronic pain transition. Nat Neurosci 2015;18:1746–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Lavand'homme P. From preemptive to preventive analgesia: time to reconsider the role of perioperative peripheral nerve blocks? Reg Anesth Pain Med 2011;36:4–6. [DOI] [PubMed] [Google Scholar]
- [92].Lavand'homme P, De Kock M, Waterloos H. Intraoperative epidural analgesia combined with ketamine provides effective preventive analgesia in patients undergoing major digestive surgery. Anesthesiology 2005;103:813–20. [DOI] [PubMed] [Google Scholar]
- [93].Lavand'homme P. Perioperative pain. Curr Opin Anaesthesiol 2006;19:556–61. [DOI] [PubMed] [Google Scholar]
- [94].Leonard MG, Jung S, Andurkar SV, Gulati A. Centhaquin attenuates hyperalgesia and non-evoked guarding in a rat model of postoperative pain primarily through α2B-adrenoceptors. Eur J Pharmacol 2016;789:81–7. [DOI] [PubMed] [Google Scholar]
- [95].Leonard PA, Arunkumar R, Brennan TJ. Bradykinin antagonists have no analgesic effect on incisional pain. Anesth Analg 2004;99:1166–72; table of contents. [DOI] [PubMed] [Google Scholar]
- [96].Li C, Yang Y, Liu S, Fang H, Zhang Y, Furmanski O, Skinner J, Xing Y, Johns RA, Huganir RL, Tao F. Stress induces pain transition by potentiation of AMPA receptor phosphorylation. J Neurosci 2014;34:13737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Li C, Zhang J, Dai R, Wang J, Luo X, Zhou X. Surgical incision induces anxiety-like behavior and amygdala sensitization: effects of morphine and gabapentin. Pain Res Treat 2010;2010:705874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Liang D, Li X, Li W, Fiorino D, Qiao Y, Sahbaie P, Yeomans DC, Clark JD. Caspase-1 modulates incisional sensitization and inflammation. Anesthesiology 2010;113:945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Liang D, Li X, Shi X, Sun Y, Sahbaie P, Li W, Clark JD. The complement component C5a receptor mediates pain and inflammation in a postsurgical pain model. PAIN 2012;153:366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Lim D, Kim J, Han D, Kim Y. Analgesic effect of harpagophytum procumbens on postoperative and neuropathic pain in rats. Molecules 2014;19:1060–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Lin J, Zhang L, Yang H. Perioperative administration of selective cyclooxygenase-2 inhibitors for postoperative pain management in patients after total knee arthroplasty. J Arthroplasty 2013;28:207–13. e2. [DOI] [PubMed] [Google Scholar]
- [102].Loftus RW, Yeager MP, Clark JA, Brown JR, Abdu WA, Sengupta DK, Beach ML. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology 2010;113:639–46. [DOI] [PubMed] [Google Scholar]
- [103].Magerl W, Klein T. Chapter 33 experimental human models of neuropathic pain. Handb Clin Neurol 2006;81:503–16. [DOI] [PubMed] [Google Scholar]
- [104].Maier C, Nestler N, Richter H, Hardinghaus W, Pogatzki-Zahn E, Zenz M, Osterbrink J. The quality of pain management in German hospitals. Dtsch Arztebl Int 2010;107:607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Maihöfner C, Seifert F, Decol R. Activation of central sympathetic networks during innocuous and noxious somatosensory stimulation. Neuroimage 2011;55:216–24. [DOI] [PubMed] [Google Scholar]
- [106].Martins DF, Emer AA, Batisti A, Donatello N, Carlesso MG, Mazzardo-Martins L, Venzke D, Micke GA, Pizzolatti MG, Piovezan A, dos Santos A. Inhalation of cedrus atlantica essential oil alleviates pain behavior through activation of descending pain modulation pathways in a mouse model of postoperative pain. J Ethnopharmacol 2015;175:30–8. [DOI] [PubMed] [Google Scholar]
- [107].Masaki E, Mizuta K, Ohtani N, Kido K. Early postoperative nociceptive threshold and production of brain-derived neurotrophic factor induced by plantar incision are not influenced with minocycline in a rat: role of spinal microglia. Neurosignals 2016;24:15–24. [DOI] [PubMed] [Google Scholar]
- [108].Mathiesen O, Dahl B, Thomsen BA, Kitter B, Sonne N, Dahl JB, Kehlet H. A comprehensive multimodal pain treatment reduces opioid consumption after multilevel spine surgery. Eur Spine J 2013;22:2089–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Maund E, McDaid C, Rice S, Wright K, Jenkins B, Woolacott N. Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs for the reduction in morphine-related side-effects after major surgery: a systematic review. Br J Anaesth 2011;106:292–7. [DOI] [PubMed] [Google Scholar]
- [110].Mishriky BM, Waldron NH, Habib AS. Impact of pregabalin on acute and persistent postoperative pain: a systematic review and meta-analysis. Br J Anaesth 2015;114:10–31. [DOI] [PubMed] [Google Scholar]
- [111].Mogil JS. Animal models of pain: progress and challenges. Nature reviews. Neuroscience 2009;10:283–94. [DOI] [PubMed] [Google Scholar]
- [112].Mogil JS, Davis KD, Derbyshire SW. The necessity of animal models in pain research. PAIN 2010;151:12–7. [DOI] [PubMed] [Google Scholar]
- [113].Moore RA, Derry S, McQuay HJ, Wiffen PJ. Single dose oral analgesics for acute postoperative pain in adults. Cochrane Database Syst Rev 2011;9:CD008659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Morales DR, Lipworth BJ, Guthrie B, Jackson C, Donnan PT, Santiago VH. Safety risks for patients with aspirin-exacerbated respiratory disease after acute exposure to selective nonsteroidal anti-inflammatory drugs and COX-2 inhibitors: meta-analysis of controlled clinical trials. J Allergy Clin Immunol 2014;134:40–5. [DOI] [PubMed] [Google Scholar]
- [115].Mujenda FH, Duarte AM, Reilly EK, Strichartz GR. Cutaneous endothelin-A receptors elevate post-incisional pain. PAIN 2007;133:161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Munsterhjelm E, Niemi TT, Ylikorkala O, Neuvonen PJ, Rosenberg PH. Influence on platelet aggregation of i.v. parecoxib and acetaminophen in healthy volunteers. Br J Anaesth 2006;97:226–31. [DOI] [PubMed] [Google Scholar]
- [117].Murphy GS, Szokol JW, Greenberg SB, Avram MJ, Vender JS, Nisman M, Vaughn J. Preoperative dexamethasone enhances quality of recovery after laparoscopic cholecystectomy: effect on in-hospital and postdischarge recovery outcomes. Anesthesiology 2011;114:882–90. [DOI] [PubMed] [Google Scholar]
- [118].Murphy JD, Paskaradevan J, Eisler LL, Ouanes JP, Tomas VA, Freck EA, Wu CL. Analgesic efficacy of continuous intravenous magnesium infusion as an adjuvant to morphine for postoperative analgesia: a systematic review and meta-analysis. Middle East J Anesthesiol 2013;22:11–20. [PubMed] [Google Scholar]
- [119].Nanavati AJ, Prabhakar S. Fast-track surgery: toward comprehensive peri-operative care. Anesth Essays Res 2014;8:127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Narai Y, Imamachi N, Saito Y. Gabapentin augments the antihyperalgesic effects of diclofenac sodium through spinal action in a rat postoperative pain model. Anesth Analg 2012;115:189–93. [DOI] [PubMed] [Google Scholar]
- [121].Nikolajsen L, Sorensen HC, Jensen TS, Kehlet H. Chronic pain following caesarean section. Acta Anaesthesiol Scand 2004;48:111–6. [DOI] [PubMed] [Google Scholar]
- [122].Nishijima CM, Ganev EG, Mazzardo-Martins L, Martins DF, Rocha LR, Santos AR, Hiruma-Lima CA. Citral: a monoterpene with prophylactic and therapeutic anti-nociceptive effects in experimental models of acute and chronic pain. Eur J Pharmacol 2014;736:16–25. [DOI] [PubMed] [Google Scholar]
- [123].Nissen SE, Yeomans ND, Solomon DH, Lüscher TF, Libby P, Husni ME, Graham DY, Borer JS, Wisniewski LM, Wolski KE, Wang Q, Menon V, Ruschitzka F, Gaffney M, Beckerman B, Berger MF, Bao W, Lincoff AM. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med 2016;375:2519–29. [DOI] [PubMed] [Google Scholar]
- [124].Oderda GM, Evans RS, Lloyd J, Lipman A, Chen C, Ashburn M, Burke J, Samore M. Cost of opioid-related adverse drug events in surgical patients. J Pain Symptom Manage 2003;25:276–83. [DOI] [PubMed] [Google Scholar]
- [125].Ohri R, Wang JC, Blaskovich PD, Pham LN, Costa DS, Nichols GA, Hildebrand WP, Scarborough NL, Herman CJ, Strichartz GR. Inhibition by local bupivacaine-releasing Microspheres of acute postoperative pain from hairy skin incision. Anesth Analg 2013;117:717–30. [DOI] [PubMed] [Google Scholar]
- [126].Oliveira SM, Drewes CC, Silva CR, Trevisan G, Boschen SL, Moreira CG, de Almeida Cabrini D, Da Cunha C, Ferreira J. Involvement of mast cells in a mouse model of postoperative pain. Eur J Pharmacol 2011;672:88–95. [DOI] [PubMed] [Google Scholar]
- [127].Oliveira SM, Silva CR, Ferreira J. Critical role of protease-activated receptor 2 activation by mast cell tryptase in the development of postoperative pain. Anesthesiology 2013;118:679–90. [DOI] [PubMed] [Google Scholar]
- [128].Oliveira SM, Silva CR, Trevisan G, Villarinho JG, Cordeiro MN, Richardson M, Borges MH, Castro CJ, Gomez MV, Ferreira J. Antinociceptive effect of a novel armed spider peptide Tx3-5 in pathological pain models in mice. Pflugers Arch 2016;468:881–94. [DOI] [PubMed] [Google Scholar]
- [129].Ong CK, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg 2010;110:1170–9. [DOI] [PubMed] [Google Scholar]
- [130].Owen RT. Pregabalin: its efficacy, safety and tolerability profile in generalized anxiety. Drugs Today (Barc) 2007;43:601–10. [DOI] [PubMed] [Google Scholar]
- [131].Pan Z, Zhu L, Li Y, Hao L, Yin C, Yang J, Guo Y, Zhang S, Hua L, Xue Z, Zhang H, Cao J. Epigenetic modification of spinal miR-219 expression regulates chronic inflammation pain by targeting CaMKIIγ. J Neurosci 2014;34:9476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Papathanasiou T, Juul RV, Heegaard A, Kreilgaard M, Lund TM. Co-administration of morphine and gabapentin leads to dose dependent synergistic effects in a rat model of postoperative pain. Eur J Pharm Sci 2016;82:97–105. [DOI] [PubMed] [Google Scholar]
- [133].Pogatzki EM, Gebhart GF, Brennan TJ. Characterization of adelta- and C-fibers innervating the plantar rat hindpaw one day after an incision. J Neurophysiol 2002;87:721–31. [DOI] [PubMed] [Google Scholar]
- [134].Pogatzki EM, Niemeier JS, Brennan TJ. Persistent secondary hyperalgesia after gastrocnemius incision in the rat. Eur J Pain 2002;6:295–305. [DOI] [PubMed] [Google Scholar]
- [135].Pogatzki EM, Niemeier JS, Sorkin LS, Brennan TJ. Spinal glutamate receptor antagonists differentiate primary and secondary mechanical hyperalgesia caused by incision. PAIN 2003;105:97–107. [DOI] [PubMed] [Google Scholar]
- [136].Pogatzki EM, Raja SN. A mouse model of incisional pain. Anesthesiology 2003;99:1023–7. [DOI] [PubMed] [Google Scholar]
- [137].Pogatzki EM, Zahn PK, Brennan TJ. Effect of pretreatment with intrathecal excitatory amino acid receptor antagonists on the development of pain behavior caused by plantar incision. Anesthesiology 2000;93:489–96. [DOI] [PubMed] [Google Scholar]
- [138].Pogatzki-Zahn EM, Shimizu I, Caterina M, Raja SN. Heat hyperalgesia after incision requires TRPV1 and is distinct from pure inflammatory pain. PAIN 2005;115:296–307. [DOI] [PubMed] [Google Scholar]
- [139].Pogatzki-Zahn EM, Wagner C, Meinhardt-Renner A, Burgmer M, Beste C, Zahn PK, Pfleiderer B. Coding of incisional pain in the brain: a functional magnetic resonance imaging study in human volunteers. Anesthesiology 2010;112:406–17. [DOI] [PubMed] [Google Scholar]
- [140].Pogatzki-Zahn EM, Zahn PK. From preemptive to preventive analgesia. Curr Opin Anaesthesiology 2006;19:551–5. [DOI] [PubMed] [Google Scholar]
- [141].Pogatzki-Zahn EM, Zahn PK, Brennan TJ. Postoperative pain–clinical implications of basic research. Best practice & research. Clin Anaesthesiology 2007;21:3–13. [DOI] [PubMed] [Google Scholar]
- [142].Prasoon P, Kumar R, Gautam M, Sebastian EK, Reeta KH, Ray SB. Role of somatostatin and somatostatin receptor type 2 in postincisional nociception in rats. Neuropeptides 2015;49:47–54. [DOI] [PubMed] [Google Scholar]
- [143].Reichl S, Augustin M, Zahn PK, Pogatzki-Zahn EM. Peripheral and spinal GABAergic regulation of incisional pain in rats. PAIN 2012;153:129–41. [DOI] [PubMed] [Google Scholar]
- [144].Reichl S, Segelcke D, Keller V, Jonas R, Boecker A, Wenk M, Evers D, Zahn PK, Pogatzki-Zahn EM. Activation of glial glutamate transporter via MAPK p38 prevents enhanced and long-lasting non-evoked resting pain after surgical incision in rats. Neuropharmacology 2016;105:607–17. [DOI] [PubMed] [Google Scholar]
- [145].Reichl S, Segelcke D, Keller V, Jonas R, Boecker A, Wenk M, Evers D, Zahn PK, Pogatzki-Zahn EM. Activation of glial Glutamate transporter via MAPK p38 prevents enhanced and long-lasting non-evoked resting pain after surgical incision in rats. Neuropharmacology 2016;105:607–17. [DOI] [PubMed] [Google Scholar]
- [146].Rittner HL, Machelska H, Stein C. Leukocytes in the regulation of pain and analgesia. J Leukoc Biol 2005;78:1215–22. [DOI] [PubMed] [Google Scholar]
- [147].Rittner HL, Mousa SA, Labuz D, Beschmann K, Schäfer M, Stein C, Brack A. Selective local PMN recruitment by CXCL1 or CXCL2/3 injection does not cause inflammatory pain. J Leukoc Biol 2006;79:1022–32. [DOI] [PubMed] [Google Scholar]
- [148].Romero A, Rojas S, Cabañero D, Gispert JD, Herance JR, Campillo A, Puig MM. A 18F-fluorodeoxyglucose MicroPET imaging study to assess changes in brain glucose metabolism in a rat model of surgery-induced latent pain sensitization. Anesthesiology 2011;115:1072–83. [DOI] [PubMed] [Google Scholar]
- [149].Romero-Sandoval A, Eisenach JC. Spinal cannabinoid receptor type 2 activation reduces hypersensitivity and spinal cord glial activation after paw incision. Anesthesiology 2007;106:787–94. [DOI] [PubMed] [Google Scholar]
- [150].Romero-Sandoval A, Nutile-McMenemy N, DeLeo JA. Spinal microglial and perivascular cell cannabinoid receptor type 2 activation reduces behavioral hypersensitivity without tolerance after peripheral nerve injury. Anesthesiology 2008;108:722–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Sadler A, Wilson J, Colvin L. Acute and chronic neuropathic pain in the hospital setting: use of screening tools. Clin J Pain 2013;29:507–11. [DOI] [PubMed] [Google Scholar]
- [152].Saha M, Skopelja S, Martinez E, Alvarez DL, Liponis BS, Romero-Sandoval EA. Spinal mitogen-activated protein kinase phosphatase-3 (MKP-3) is necessary for the normal resolution of mechanical allodynia in a mouse model of acute postoperative pain. J Neurosci 2013;33:17182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Sahbaie P, Li X, Shi X, Clark JD. Roles of Gr-1+ leukocytes in postincisional nociceptive sensitization and inflammation. Anesthesiology 2012;117:602–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Sahbaie P, Liang D, Shi X, Sun Y, Clark JD. Epigenetic regulation of spinal cord gene expression contributes to enhanced postoperative pain and analgesic tolerance subsequent to continuous opioid exposure. Mol Pain 2016;12;pii:1744806916641950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Sahbaie P, Sun Y, Liang D, Shi X, Clark JD. Curcumin treatment attenuates pain and enhances functional recovery after incision. Anesth Analg 2014;118:1336–44. [DOI] [PubMed] [Google Scholar]
- [156].Scherer M, Reichl SU, Augustin M, Pogatzki-Zahn EM, Zahn PK. The assessment of cold hyperalgesia after an incision. Anesth Analg 2010;110:222–7. [DOI] [PubMed] [Google Scholar]
- [157].Schug S, Palmer G, Scott D, Halliwell R, Trinca J. Acute pain management: scientific evidence. 4th ed Melbourne: ANZCA & FPM, 2015. [DOI] [PubMed] [Google Scholar]
- [158].Schug SA, Joshi GP, Camu F, Pan S, Cheung R. Cardiovascular safety of the cyclooxygenase-2 selective inhibitors parecoxib and valdecoxib in the postoperative setting: an analysis of integrated data. Anesth Analg 2009;108:299–307. [DOI] [PubMed] [Google Scholar]
- [159].Shen X, Liu Y, Xu S, Zhao Q, Wu H, Guo X, Shen R, Wang F. Menin regulates spinal glutamate-GABA balance through GAD65 contributing to neuropathic pain. Pharmacol Rep 2014;66:49–55. [DOI] [PubMed] [Google Scholar]
- [160].Shi X, Di Fu, Xu J, Zhang Y, Dai R. Activation of spinal ERK1/2 contributes to mechanical allodynia in a rat model of postoperative pain. Mol Med Rep 2013;7:1661–5. [DOI] [PubMed] [Google Scholar]
- [161].Sluka KA, Radhakrishnan R, Benson CJ, Eshcol JO, Price MP, Babinski K, Audette KM, Yeomans DC, Wilson SP. ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. PAIN 2007;129:102–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Sousa AM, Ashmawi HA. Local analgesic effect of tramadol is not mediated by opioid receptors in early postoperative pain in rats. Rev Bras Anestesiol 2015;65:186–90. [DOI] [PubMed] [Google Scholar]
- [163].de Souza AH, Lima MC, Drewes CC, da Silva JF, Torres KC, Pereira EM, de Castro CJ, Jr, Vieira LB, Cordeiro MN, Richardson M, Gomez RS, Romano-Silva MA, Ferreira J, Gomez MV. Antiallodynic effect and side effects of Phα1β, a neurotoxin from the spider Phoneutria nigriventer: comparison with ω-conotoxin MVIIA and morphine. Toxicon 2011;58:626–33. [DOI] [PubMed] [Google Scholar]
- [164].Spofford CM, Brennan TJ. Gene expression in skin, muscle, and dorsal root ganglion after plantar incision in the rat. Anesthesiology 2012;117:161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Spofford CM, Mohan S, Kang S, Jang JH, Brennan TJ. Evaluation of leukemia inhibitory factor (LIF) in a rat model of postoperative pain. J Pain 2011;12:819–32. [DOI] [PubMed] [Google Scholar]
- [166].Srikandarajah S, Gilron I. Systematic review of movement-evoked pain versus pain at rest in postsurgical clinical trials and meta-analyses: a fundamental distinction requiring standardized measurement. PAIN 2011;152:1734–9. [DOI] [PubMed] [Google Scholar]
- [167].Stubhaug A, Breivik H, Eide PK, Kreunen M, Foss A. Mapping of punctuate hyperalgesia around a surgical incision demonstrates that ketamine is a powerful suppressor of central sensitization to pain following surgery. Acta Anaesthesiol Scand 1997;41:1124–32. [DOI] [PubMed] [Google Scholar]
- [168].Su C, D'amour J, Lee M, Lin H, Manders T, Xu D, Eberle SE, Goffer Y, Zou AH, Rahman M, Ziff E, Froemke RC, Huang D, Wang J. Persistent pain alters AMPA receptor subunit levels in the nucleus accumbens. Mol Brain 2015;8:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Sun Y, Sahbaie P, Liang D, Li W, Li X, Shi X, Clark JD. Epigenetic regulation of spinal CXCR2 signaling in incisional hypersensitivity in mice. Anesthesiology 2013;119:1198–208. [DOI] [PubMed] [Google Scholar]
- [170].Sun Y, Sahbaie P, Liang D, Li W, Shi X, Kingery P, Clark JD, Taylor B. DNA methylation modulates nociceptive sensitization after incision. PLoS One 2015;10: e0142046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Sun Y, Yang M, Tang H, Ma Z, Liang Y, Li Z. The over-production of TNF-α via Toll-like receptor 4 in spinal dorsal horn contributes to the chronic postsurgical pain in rat. J Anesth 2015;29:734–40. [DOI] [PubMed] [Google Scholar]
- [172].Tiippana EM, Hamunen K, Kontinen VK, Kalso E. Do surgical patients benefit from perioperative gabapentin/pregabalin?: a systematic review of efficacy and safety. Anesth Analg 2007;104:1545–56; table of contents. [DOI] [PubMed] [Google Scholar]
- [173].Turan A, Sessler DI. Steroids to ameliorate postoperative pain. Anesthesiology 2011;115:457–9. [DOI] [PubMed] [Google Scholar]
- [174].Uchytilova E, Spicarova D, Palecek J. TRPV1 antagonist attenuates postoperative hypersensitivity by central and peripheral mechanisms. Mol Pain 2014;10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Urban MK, Ya Deau JT, Wukovits B, Lipnitsky JY. Ketamine as an adjunct to postoperative pain management in opioid tolerant patients after spinal fusions: a prospective randomized trial. HSS J 2008;4:62–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Urban R, Scherrer G, Goulding EH, Tecott LH, Basbaum AI. Behavioral indices of ongoing pain are largely unchanged in male mice with tissue or nerve injury-induced mechanical hypersensitivity. PAIN 2011;152:990–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].van den Heuvel I, Reichl S, Segelcke D, Zahn PK, Pogatzki-Zahn EM. Selective prevention of mechanical hyperalgesia after incision by spinal ERK1/2 inhibition. Eur J Pain 2015;19:225–35. [DOI] [PubMed] [Google Scholar]
- [178].Villarinho JG, Oliveira SM, Silva CR, Cabreira TN, Ferreira J. Involvement of monoamine oxidase B on models of postoperative and neuropathic pain in mice. Eur J Pharmacol 2012;690:107–14. [DOI] [PubMed] [Google Scholar]
- [179].Waldron NH, Jones CA, Gan TJ, Allen TK, Habib AS. Impact of perioperative dexamethasone on postoperative analgesia and side-effects: systematic review and meta-analysis. Br J Anaesth 2013;110:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Walker CI, Trevisan G, Rossato MF, Silva CR, Pinheiro FV, Franciscato C, Tatsch E, Moretto MB, Silva MD, Manfron MP, Noal Moresco R, Santos AR, Pereira ME, Ferreira J. Antinociceptive effect of Mirabilis jalapa on acute and chronic pain models in mice. J Ethnopharmacology 2013;149:685–93. [DOI] [PubMed] [Google Scholar]
- [181].Wang Y, Wu J, Guo R, Zhao Y, Zhang M, Chen Z, Wu A, Yue Y. Surgical incision induces phosphorylation of AMPA receptor GluR1 subunits at Serine-831 sites and GluR1 trafficking in spinal cord dorsal horn via a protein kinase Cγ-dependent mechanism. Neuroscience 2013;240:361–70. [DOI] [PubMed] [Google Scholar]
- [182].Wei H, Karimaa M, Korjamo T, Koivisto A, Pertovaara A. Transient receptor potential ankyrin 1 ion channel contributes to guarding pain and mechanical hypersensitivity in a rat model of postoperative pain. Anesthesiology 2012;117:137–48. [DOI] [PubMed] [Google Scholar]
- [183].Weiser TG, Regenbogen SE, Thompson KD, Haynes AB, Lipsitz SR, Berry WR, Gawande AA. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 2008;372:139–44. [DOI] [PubMed] [Google Scholar]
- [184].Woo YC, Park SS, Subieta AR, Brennan TJ. Changes in tissue pH and temperature after incision indicate acidosis may contribute to postoperative pain. Anesthesiology 2004;101:468–75. [DOI] [PubMed] [Google Scholar]
- [185].Wu L, Huang X, Sun L. The efficacy of N-methyl-D-aspartate receptor antagonists on improving the postoperative pain intensity and satisfaction after remifentanil-based anesthesia in adults: a meta-analysis. J Clin Anesth 2015;27:311–24. [DOI] [PubMed] [Google Scholar]
- [186].Xu B, Guan X, Yu J, Lv J, Zhang H, Fu Q, Xiang H, Bu H, Shi D, Shu B, Qin L, Manyande A, Tian Y. Activation of spinal phosphatidylinositol 3-kinase/protein kinase B mediates pain behavior induced by plantar incision in mice. Exp Neurol 2014;255:71–82. [DOI] [PubMed] [Google Scholar]
- [187].Xu J, Brennan TJ. Comparison of skin incision vs. skin plus deep tissue incision on ongoing pain and spontaneous activity in dorsal horn neurons. PAIN 2009;144:329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Xu J, Brennan TJ. Guarding pain and spontaneous activity of nociceptors after skin versus skin plus deep tissue incision. Anesthesiology 2010;112:153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Xu J, Brennan TJ. The pathophysiology of acute pain: animal models. Curr Opin Anaesthesiol 2011;24:508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Yalamuri SM, Brennan TJ, Spofford CM. Neuropeptide Y is analgesic in rats after plantar incision. Eur J Pharmacol 2013;698:206–12. [DOI] [PubMed] [Google Scholar]
- [191].Yang T, Yang P, Jiang L, Zhou R. Activation of spinal NF-КB mediates pain behavior induced by plantar incision. Int J Clin Exp Med 2015;8:9149–55. [PMC free article] [PubMed] [Google Scholar]
- [192].Yasuda M, Kido K, Ohtani N, Masaki E. Mast cell stabilization promotes antinociceptive effects in a mouse model of postoperative pain. J Pain Res 2013;6:161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Ying Y, Wei X, Xu X, She S, Zhou L, Lv J, Li D, Zheng B, Liu X. Over-expression of P2X7 receptors in spinal glial cells contributes to the development of chronic postsurgical pain induced by skin/muscle incision and retraction (SMIR) in rats. Exp Neurol 2014;261:836–43. [DOI] [PubMed] [Google Scholar]
- [194].Young A, Buvanendran A. Recent advances in multimodal analgesia. Anesthesiol Clin 2012;30:91–100. [DOI] [PubMed] [Google Scholar]
- [195].Zahn PK, Brennan TJ. Lack of effect of intrathecally administered N-methyl-D-aspartate receptor antagonists in a rat model for postoperative pain. Anesthesiology 1998;88:143–56. [DOI] [PubMed] [Google Scholar]
- [196].Zahn PK, Brennan TJ. Primary and secondary hyperalgesia in a rat model for human postoperative pain. Anesthesiology 1999;90:863–72. [DOI] [PubMed] [Google Scholar]
- [197].Zahn PK, Pogatzki EM, Brennan TJ. Mechanisms for pain caused by incisions. Reg Anesth Pain Med 2002;27:514–6. [DOI] [PubMed] [Google Scholar]
- [198].Zahn PK, Pogatzki-Zahn EM, Brennan TJ. Spinal administration of MK-801 and NBQX demonstrates NMDA-independent dorsal horn sensitization in incisional pain. PAIN 2005;114:499–510. [DOI] [PubMed] [Google Scholar]
- [199].Zahn PK, Umali E, Brennan TJ. Intrathecal non-NMDA excitatory amino acid receptor antagonists inhibit pain behaviors in a rat model of postoperative pain. PAIN 1998;74:213–23. [DOI] [PubMed] [Google Scholar]
- [200].Zhu Q, Mao L, Liu C, Sun Y, Jiang B, Zhang W, Li J. Antinociceptive effects of vitexin in a mouse model of postoperative pain. Sci Rep 2016;6:19266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Zhu Q, Sun Y, Yun X, Ou Y, Zhang W, Li J. Antinociceptive effects of curcumin in a rat model of postoperative pain. Sci Rep 2014;4:4932. [DOI] [PMC free article] [PubMed] [Google Scholar]


