Abstract
Purpose
To determine whether Low Luminance Questionnaire (LLQ) scores are associated with objective measures of visual function in early and intermediate age-related macular degeneration (AMD).
Methods
Cross-sectional study of subjects with early AMD Age-Related Eye Disease Study (AREDS) stage 2, N = 33), intermediate AMD (AREDS stage 3, N = 47), and age-matched healthy controls (N = 21). Subjects were interviewed with the LLQ. Psychophysical tests performed included best-corrected visual acuity (BCVA), mesopic microperimetry, dark adaptometry (DA), low luminance visual acuity (LLVA), and cone contrast test (CCT). Low luminance deficit (LLD) was the difference in the number of letters read under photopic versus low luminance settings. The relationship between LLQ and visual function test scores was assessed with linear regression.
Results
Subjects with intermediate AMD had significantly lower LLQ composite scores (mean = 75.8 ± 16.7; median = 76, range [29, 97]) compared with early AMD (mean = 85.3 ± 13.3; median = 88, range [50, 100], P = 0.007) or controls (mean = 91.4 ± 6.5; median = 94, range [79, 99], P < 0.001) in the overall cohort. LLQ composite scores were associated with computerized BCVA (β = 0.516), computerized LLVA at two background luminance (1.3 cd/m2, β = 0.660; 0.5 cd/m2, β = 0.489) along with their respective computerized LLDs (β = −0.531 and −0.467), rod intercept (β = −0.312), and CCT green (β = 0.183) (all P < 0.05). Only the computerized LLVAs and computerized LLDs remained statistically significant after adjusting for AMD versus control status (P < 0.05). Among AMD subjects, LLQ composite scores were significantly associated with the computerized LLVAs (β = 0.622 and 0.441) and LLDs (β = −0.795 and −0.477) at both the 1.3 and 0.5 cd/m2 luminance levels, respectively, and these associations remained significant after adjusting for AMD severity (P < 0.05).
Conclusions
Among subjects with early and intermediate AMD, LLQ scores were significantly associated with computerized LLVA and LLD. LLQ is a useful patient-centered functional measure of visual impairment in early and intermediate AMD.
Keywords: age-related macular degeneration, low luminance questionnaire, patient-centered outcomes, functional metrics, low luminance visual acuity, low luminance deficit
Age-related macular degeneration (AMD) is the most common cause of permanent central acuity loss in older adults in the United States.1 It affects more than 1.75 million individuals in the United States, and is expected to impact more than 3 million people by 2020.2 While loss of central vision has been a primary outcome of concern in a majority of studies on AMD, recent studies have shown that patients with early AMD demonstrate poor visual performance in low luminance settings, even before the onset of central vision loss.3–8 Patients with a normal fundus exam but high-risk genotypes for AMD, such as carrying a mutation in Complement Factor H and LOC 387715/ARMS2/HRTA1, have impaired mesopic visual function impacting cones and rods but not cones alone.9 Histopathologic studies of eyes with AMD have also documented that degeneration of rod photoreceptors precede that of cones.10–12 Such findings may help explain why patients with early AMD often report impaired visual function in low lighting settings.4,6,8 Assessing the visual function of AMD patients under mesopic and scotopic conditions is important because it may help us to not only better understand the functional impairment of the disease, but also to more effectively screen for the onset of AMD in an aging population.
The goal of secondary public health prevention in chronic disease management is early detection such that treatment can be instituted to either slow down or halt the progression of disease. In the case of AMD, the only Food and Drug Administration (FDA)-approved treatments for intermediate AMD are the Age-Related Eye Disease Study (AREDS) vitamin formulations that have been shown to slow the progression to advanced AMD.13 Although there are currently no FDA-approved treatments for early AMD, clinical trials are in the planning stages or currently underway to test new potential treatments for both early and intermediate AMD. Sensitive screening tests are thus needed to diagnose early and intermediate AMD both objectively and subjectively, so that these subjects can be identified and treated early before the onset of central vision loss from advanced disease.
In terms of clinical endpoints, trials for early and intermediate AMD will need to assess visual function not only via psychophysical tests but also more subjective patient-reported outcomes (PROs). PROs usually employ questionnaires to solicit the patient's perspective on their illness through subjective self-reporting on their experience of visual health and function as well as quality of life. PROs not only provide complementary correlates to psychophysical tests, but may also be more widely accessible to the general medical and ophthalmologic community due to ease of administration and lower cost. Moreover, regulators have increasingly mandated PROs in order to confirm the clinical relevance of psychophysical measures of visual function as endpoints for clinical trials. As a result, there has been a notable emphasis in the recent literature on the importance of such measures across the fields of medicine, particularly in ophthalmology.4,14–18 Currently, the National Eye Institute's Visual Function Questionnaire-25 (NEI VFQ-25) is the most widely used PRO for measuring vision-related quality of life.16 However, a majority of its questions refer to daytime activities.16,17 Several studies have suggested that visual function in early AMD may be impaired under suboptimal lighting conditions even when standard visual acuity is normal.4,6,7,9,17,19,20 In response to an identified need for low luminance patient-centered measures, Owsley et al.4 developed and validated the 32-item Low Luminance Questionnaire (LLQ) for use in older adults with either normal aging retinas or AMD pathology. In the original study, they demonstrated that LLQ scores significantly decreased in advanced disease. Moreover, LLQ scores were associated with rod-mediated but not cone-mediated parameters.4 A manuscript recently published by Yazdanie et al.,21 similarly demonstrated that prolonged dark adaptation (DA) testing was associated with lower scores on the LLQ in a sample of subjects with a range of AMD pathology, including those with late-stage disease.
Compared with the work by Yazdanie et al.,21 which showed a relationship between LLQ and DA as AMD severity increased, we were interested in determining the utility of the LLQ in the assessment of visual impairment in AMD before the onset of advanced disease. It is important to identify tests that can help us better understand visual dysfunction in early and intermediate AMD, as such knowledge will influence our ability to accurately diagnose and better manage and monitor the disease. Subjects with lower stages of AMD may also stand to benefit the most from future interventions aimed at preventing disease progression and resulting vision loss. To date, all clinical trials for geographic atrophy from severe AMD have failed to restore or preserve visual function. This may be due to the timing of the therapeutic intervention after the retina has already suffered extensive, irreversible damage to the photoreceptors and retinal pigment epithelium.
The primary aim of our study was to evaluate whether scores on the LLQ are significantly associated with a range of psychophysical measures of visual function in patients with early and intermediate AMD in order to provide a more comprehensive understanding of visual dysfunction at earlier stages of AMD where interventions for secondary prevention may be most effective. We hypothesized that scotopic and mesopic, but not photopic, tests of visual function would be associated with LLQ scores in this cohort. Also, in addition to using the standard psychophysical tests, we are the first to evaluate the relationship between the LLQ and computerized visual acuity and low luminance tests. The rationale for this was two-fold. First, computer testing can improve efficiency and minimize operator-dependent variability.22 Second, the computer interface allowed us to evaluate patient performance at a lower background luminance setting from the standard protocol, which we hypothesized would prove more sensitive for subjects with early to intermediate disease.
Methods
Study Participants
This single center, exploratory prospective cohort study (NCT01822873) was approved by the institutional review board of Duke University Medical Center and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice according to the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) and applicable international regulatory authority laws, regulations, and guidelines. This paper presents the baseline cross-sectional results relating the outcomes of psychophysical tests of visual function to performance on the LLQ. Analysis of the relationship of the different psychophysical tests to each other have been presented and are under consideration for publication in a separate manuscript (Cocce K, et al. IOVS 2017;58:ARVO E-Abstract 3765).
Study subjects with early and intermediate AMD were recruited from the Duke Eye Center's optometry and ophthalmology clinics. Control subjects were recruited from the optometry clinics and also from the spouses and friends of subjects with AMD. In order to be enrolled in the study, subjects needed to have the capacity to provide written informed consent.
AMD subjects were enrolled if they met the following inclusion criteria: diagnosis of early (AREDS category 2) or intermediate (AREDS category 3) AMD,13 age 50- to 90-years old, Snellen best-corrected visual acuity (BCVA) of at least 20/50 (logarithm of the minimum angle of resolution, 0.40). Subjects were eligible to enroll if they had drusenoid pigment epithelial detachments. Exclusion criteria consisted of ocular pathology other than early or intermediate AMD, visually significant cataracts (>1 + nuclear sclerosis), choroidal neovascularization, geographic atrophy in the study eye, or inability to complete the testing.
Control subjects were enrolled if they met the same visual acuity and age criteria, but had no evidence of AMD in either eye and had no more than 10 small drusen (not exceeding 63 μm).13 Age-matched recruitment was done by enrolling controls whose age was within 5 years of the age of the subject with AMD. Subjects with reticular pseudodrusen were not eligible to serve as controls.
If both eyes met inclusion criteria, the eye with better visual acuity was selected as the study eye. When both eyes had the same visual acuity and met inclusion criteria, an algorithm was used to assign the study eye: odd birth month-right eye, and even birth month-left eye.
Functional Testing
All psychophysical testing performed at the baseline visit was monocular, with subjects wearing their best correction over the study eye. In order to prevent bleaching of the retina, these tests were performed prior to fundus imaging.
Subjects first read a standard Early Treatment in Diabetic Retinopathy Study (ETDRS) chart (85 cd/m2; Good-Lite, Elgin, IL, USA) to assess BCVA. They then read the same chart through a 2.0-log neutral density filter that reduced the background luminance by 100-fold to determine the standard low luminance visual acuity (LLVA).23 The standard low luminance deficit (LLD) was the calculated difference between the standard BCVA and LLVA in EDTRS letters.
Next, subjects performed computerized monocular tests at 4 m (Innova Systems, Burr Ridge, IL, USA) for the computerized BCVA, LLVA, and cone contrast test (CCT). The computerized BCVA and LLVA tests presented five letter lines of decreasing size on a personal computer screen (Dell Optiplex 9010; Dell, Plano, TX, USA). The initial background luminance was 100 cd/m2 to generate the computerized BCVA. A different set of Snellen lines was then presented on one of two lower background luminance levels. For computerized LLVA1, the luminance was set at 1.3 cd/m2, which was comparable to the 100-fold reduction in luminance that occurs during testing with the standard 2-0 log neutral density filter. For computerized LLVA2, the luminance was set at 0.5 cd/m2. This second luminance setting was based on the levels studied by Fujita and coworkers24 in central serous retinopathy; the darkest level that differentiated between central serous retinopathy and controls was chosen. Snellen acuities were converted to ETDRS letters.25 The computerized low luminance deficits (LLD1 and LLD2) were computed by calculating the difference between the computerized versions of BCVA and the LLVA1 or LLVA2, respectively.
The CCT was then used to quantify the severity of deficits in cone color (green, blue, or red) discrimination at the photoreceptor level in the study eye.22,26 This computer-based test was performed in a dark room, and consisted of the presentation of a series of random letters visible to a single cone class (long [L], medium [M], or short [S] wavelength cones), while successively decreasing the cone contrast to determine a threshold. The cone scores were normalized to a 100-point logarithmic scale. We demonstrated in a prior pilot study that the CCT has high test-retest reliability in subjects with early and intermediate AMD.22
The pupils were then dilated with 1% tropicamide and 2.5% phenylephrine. Dark adaptation (DA; AdaptDx dark adaptometer; MacuLogix, Hummelstone, PA, USA) was tested on the dilated study eye while occluding the contralateral eye. The DA protocol had been modified for intermediate AMD as previously described.11 A 505-nm stimulus light equivalent to 76% bleaching level for rods was used to bleach the study eye in a 2° circular test spot located 5° on the inferior visual meridian. After DA, the number of invalid thresholds divided by total number of thresholds (i.e., fixation rate) was calculated. The valid thresholds were used to determine the rod intercept, or the amount of time required for sensitivity recovery to reach criterion sensitivity of 5 × 10−3 scot cd/m2.11
Microperimetry testing was then performed on the dilated study eye using a microperimeter with eye tracking (Macular Integrity Assessment [MAIA]; CenterVue, San Jose, CA, USA). Retinal sensitivity was estimated as the percent-reduced threshold (PRT) and the average threshold.18 The standard 37-10 degree MAIA grid was employed. The PRT was the percentage of measured thresholds below 25 dB, and the average threshold was the average of retinal sensitivity values from all tested loci.
Imaging and Clinical Examination
Dilated fundus examinations were performed by retinal specialists (EML, SC, LV) and a comprehensive ophthalmologist (AH). Drusen size and extent of pigmentary change on stereo color fundus photographs (Zeiss FF 450 Plus IR; Carl Zeiss Meditec, Inc., Dublin, CA, USA) were also graded by a medical retina specialist (EML). The AREDS criteria were used to categorize subjects as follows: (1) early AMD subjects met criteria for AREDS category 2 (retinal pigment abnormalities, many small drusen <63 μm in diameter, and/or few intermediate drusen 63–125 μm in diameter), (2) intermediate AMD subjects met criteria for AREDS category 3 (at least 1 large drusen >125 μm in diameter and extensive intermediate drusen), and (3) eyes with fewer than 10 small drusen were classified as controls.27 Spectral domain optical coherence tomography (Spectralis 6-mode; Heidelberg Engineering US, Carlsbad, CA, USA) and fundus autofluorescence (Spectralis 3-mode; Heidelberg Engineering US) images were used to confirm the grading on color fundus images (Fig. 1).
Figure 1.
Images of control, early AMD and intermediate AMD. (A) Control right eye, (B) early AMD left eye, and (C) intermediate AMD left eye fundus photography, fundus autofluorescence, infrared, and optical coherence tomography from left to right.
Low Luminance Questionnaire
During the study visit, subjects were interviewed using the LLQ. The LLQ is a 32-item questionnaire with six subscales related to low luminance settings: driving, mobility, extreme lighting, general dim lighting, and peripheral vision. Each question is scored on a scale ranging from 0, or maximal difficulty, to 100, or no difficulty in low luminance settings. The questions are assigned to different subscales and are averaged to generate one score per subscale. After weighting each subscale for the number of questions, the weighted subscales are averaged to generate a composite LLQ score.
The LLQ can be accessed in the public domain at: https://www.uab.edu/medicine/ophthalmology/images/research/Low_Luminance.pdf
This questionnaire has been shown to be a valid and reliable test for patient-centered assessment of visual function in a low luminance, or mesopic setting.4,17,20
Data Management
The data collected from case report forms were double-entered into a Research Electronic Data Capture (REDCap) database by certified data entry analysts from the Duke Office of Clinical Research. Data quality assurance checks were performed in SAS 9.3 (SAS Institute, Inc., Cary, NC, USA).
Statistical Analysis
Descriptive statistics were performed to assess baseline demographic variables for both the AMD and control groups. The nonparametric Wilcoxon rank sum test was carried out to compare controls, early AMD, and intermediate AMD subjects on their composite and subscale scores on the LLQ. Univariate linear regression was used to assess the relationship between the composite score on the LLQ and each of the demographic variables. In the overall cohort, separate univariate linear regression models against the LLQ composite score were performed with each of the psychophysical tests. If the relationship between a psychophysical test and the LLQ composite score reached a P value less than 0.05 in univariate analysis, multivariate regression was used to control for whether or not the study eye had AMD. A subanalysis was performed in AMD subjects to assess the association between LLQ scores and the psychophysical tests that had shown any potentially significant associations (P < 0.10) in the overall cohort; multivariate analysis was used to control for early versus intermediate AMD severity. Statistical analyses were completed in STATA 12.1 (STATACorp, College Station, TX, USA).
Results
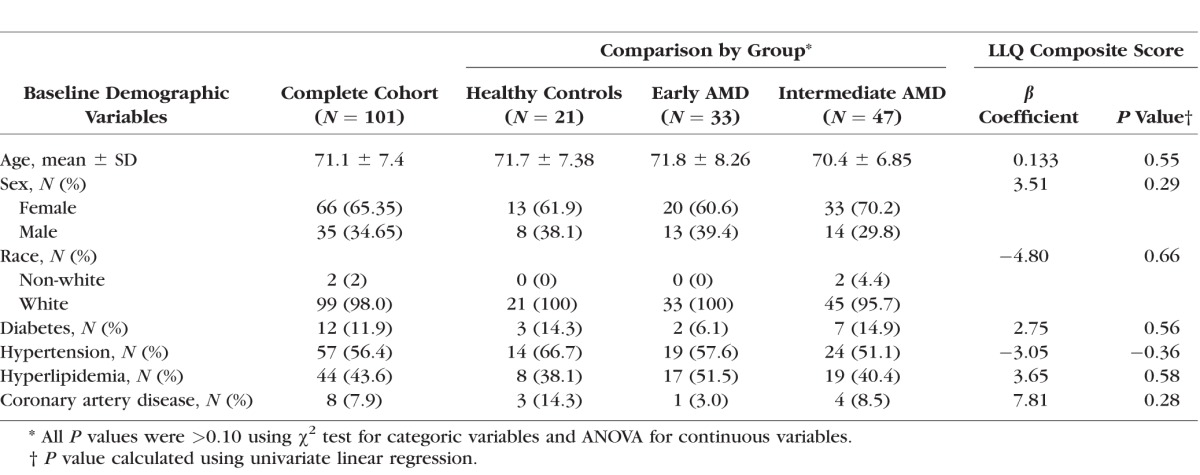
A total of 101 subjects (33 early AMD, 47 intermediate AMD, and 21 age-matched healthy controls) were enrolled in this study. These groups did not significantly differ by age, race, sex, or medical co-morbidities (all P > 0.10). Also, none of the baseline demographic variables were significantly associated with the LLQ composite score in univariate linear regression analysis (all P > 0.10; Table 1).
Table 1.
Bivariate Analysis of Baseline Demographics Stratified by Disease Group and Univariate Regression Analysis of Composite Score on LLQ
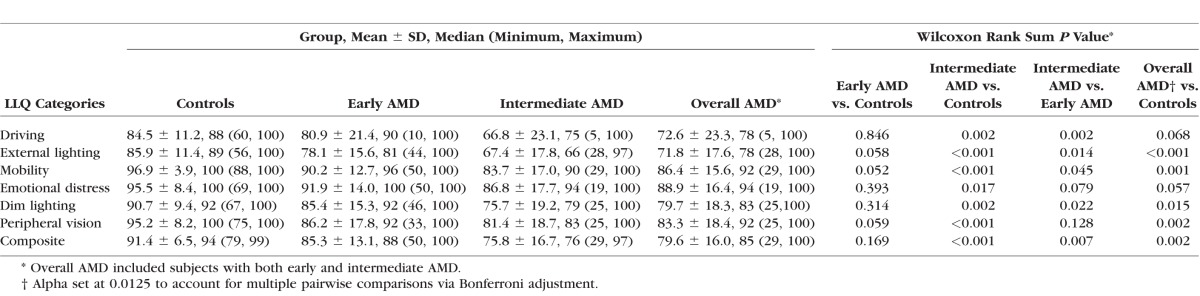
When subjects with early and intermediate AMD were grouped together, they had significantly lower mean and median LLQ composite scores than controls (P ≤ 0.002). Subjects with intermediate AMD had significantly lower LLQ composite scores compared with those with early AMD (P = 0.007) and controls (P = 0.001; Table 2, Fig. 2). Several of the subscales on the LLQ, such as the mobility and dim lighting scores, were also significantly lower for those with intermediate versus early AMD or controls (Table 2).
Table 2.
Baseline LLQ Subscale and Composite Scores and Comparison Between Subjects With AMD and Controls
Figure 2.
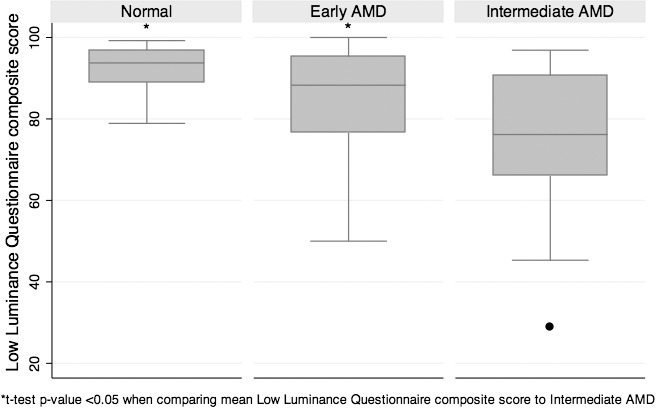
Comparison of LLQ composite score by disease group.
In univariate linear regression, the LLQ composite score was positively associated with computerized BCVA (P = 0.042), computerized LLVA1 (1.3 cd/m2) and LLVA2 (0.5 cd/m2, P < 0.001), and CCT green (P = 0.024), but inversely associated with both computerized LLD1 (P = 0.05) and LLD2 (P = 0.003) and rod intercept (P = 0.042) in the overall cohort (Table 3). Compared with the computerized LLVA2, the computerized LLVA1 showed a slightly stronger relationship with the LLQ in the overall cohort. For example, a 15-word (or 3-line) loss in computerized LLVA1 corresponded to a 10-point worsening in the LLQ composite score, whereas a 15-word loss in computerized LLVA2 corresponded to a 7-point worsening in the composite LLQ. The LLQ composite score was not associated with standard BCVA (P = 0.60), and the P value only reached borderline significance for standard LLVA and LLD (P = 0.07) in the overall cohort. Among AMD subjects, LLQ composite scores were similarly associated with computerized LLVA1 and LLVA2 (P = 0.005, P = 0.003, respectively) as well as computerized LLD1 and LLD2 (P = 0.013, P = 0.007, respectively).
Table 3.
Linear Regression of Psychophysical Tests Against LLQ Composite Score in Overall Cohort and in Subjects With AMD
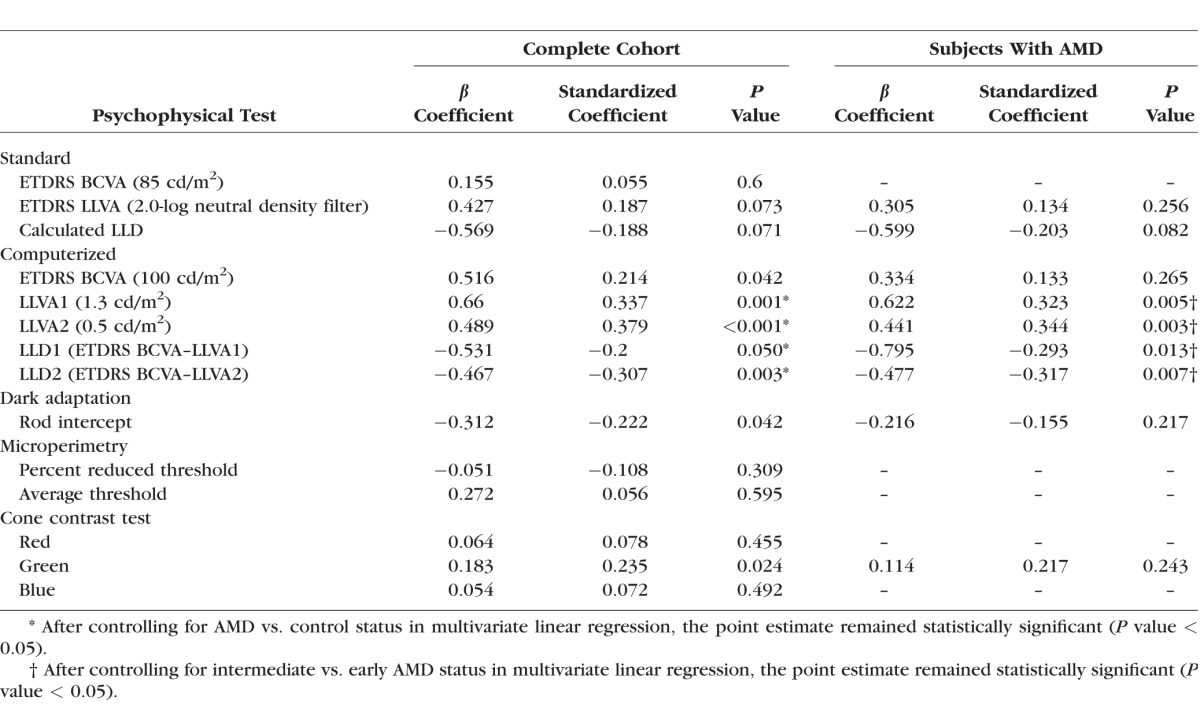
In multivariate modeling, the relationship between LLQ and each of the following computerized psychophysical tests—LLVA1, LLVA2, LLD1, and LLD2—was independent of AMD versus control status in the overall cohort and independent of disease severity in the subanalysis of AMD subjects (all P < 0.05; Table 3). No other demographic variables were entered into the multivariate models since they were not significantly associated with the LLQ composite score in univariate analysis.
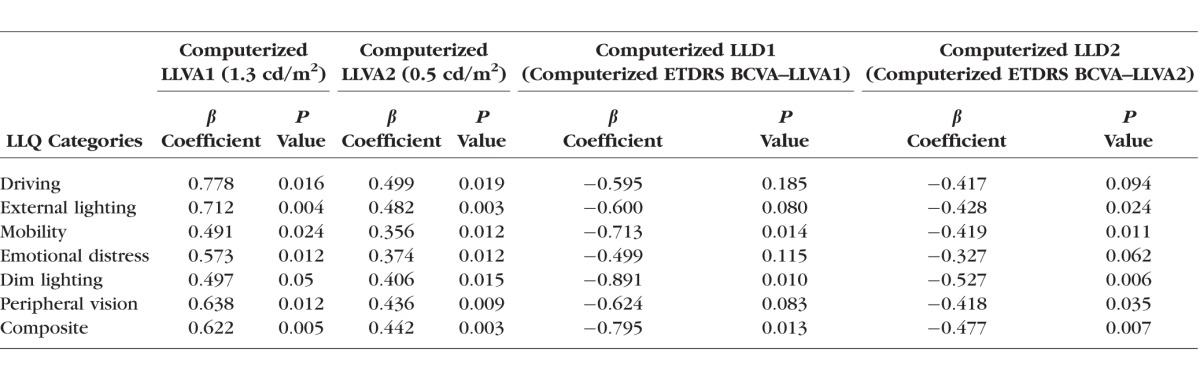
Among AMD subjects, multiple LLQ subscales were significantly related to the computerized LLVA1, LLVA2, LLD1, and LLD2 (P < 0.05; Table 4).
Table 4.
Linear Regression Analysis of Computerized Low Luminance Psychophysical Tests Against LLQ Subscales and Composite Score in Subjects With AMD
Discussion
We present baseline study results documenting the relationship between the LLQ and a range of psychophysical measures of visual function in a cohort of subjects with early and intermediate AMD. To our knowledge, this is the largest published cohort of early and intermediate AMD subjects in which the utility of the LLQ has been evaluated. We also administered a larger number of psychophysical tests than prior studies that administered the LLQ to patients with AMD.4,21 PROs that assess visual function, such as the LLQ, are of increasing interest in the field of ophthalmology.4,8,16,17,20 Validated questionnaire-based metrics are quick, accessible, and affordable tools for assessing the degree to which visual impairment impacts patient quality of life. We demonstrated that LLQ scores were lower for those with intermediate compared with early or no AMD. This finding of an inverse relationship between disease severity and LLQ score is similar to what others have reported in cohorts that included subjects with advanced AMD.4,21 However, we are the first to show that computerized LLVA and LLD tests are associated with the LLQ composite score in early and intermediate AMD. This is of special significance because poor low luminance acuity has been shown to be a risk factor for incident early AMD as well as vision loss from disease progression.23,28 Moreover, Sunness et al.23 previously demonstrated that among eyes with good baseline visual acuity, worse LLD is associated with a 3-fold greater risk of losing three lines of vision from geographic atrophy within 2 years. The LLQ may thus prove clinically useful if it can help identify subjects with visual dysfunction in low luminance settings who are at risk for progression. The use of computerized testing also allowed us to modulate the luminance intensity and compare the utility of these tests to the standard protocols. Our findings suggest that the computerized, rather than the standard, LLVA and LLD may more closely parallel patient experience of visual impairment from early and intermediate AMD in low luminance settings as described by the LLQ. We hope this work will further our understanding of visual function deficits experienced by patients with early and intermediate AMD, and help contribute to the development of alternative clinical trial endpoints, including PROs, for interventions aimed at preventing the progression of AMD before irreversible retinal damage.
In the overall cohort, which included early and intermediate AMD subjects with controls, we found that a lower computerized LLVA, or higher computerized LLD, was associated with a decrease in LLQ composite score independent of disease severity. Also, the computerized versions of LLVA and LLD performed better than the standard versions of LLVA and LLD. Under standard testing conditions, the association between the composite LLQ score and either LLVA or LLD was only borderline significant in the overall cohort (P = 0.07), and did not reach statistical significance among subjects with AMD. Both computerized tests for LLVA had a stronger association with the LLQ. For example, loss of 10 letters (or 2 lines) in the standard LLVA (2.0-log neutral density filter) corresponded to a 4.3-point loss on the LLQ. Even though the computerized LLVA1 (1.3 cd/m2) was measured at a background luminance that is comparable to the 100-fold reduction in luminance of the standard test, a 10-word loss in computerized LLVA1 corresponded to a greater change on the LLQ (6.6 points). After standardization of the coefficients, the magnitude of the strength of the association for the LLQ was greater for the computerized LLD2 (standardized coefficient [SC] = −0.307) than either computerized LLD1 (SC = −0.200) or standard LLD (SC = −0.188). Moreover, among AMD patients, the computerized LLVAs and LLDs were significantly associated with the LLQ, independent of whether they had early or intermediate AMD. LLQ subscales of mobility and dim lighting were significantly associated with each of the computerized tests for LLVA1, LLVA2, LLD1, and LLD2, and may highlight situations or settings during which visual impairment is noticed by patients with early or intermediate AMD. Similarly, the computerized but not the standard ETDRS BCVA was associated with LLQ in the overall cohort. The fact that the LLQ was more consistently associated with computerized rather than standard testing for LLVA, LLD, and BCVA may suggest that the computerized metrics are superior at detecting subtle functional changes in low- and high-luminance settings that more closely mirror patient experience. The reasons for this may be varied. For example, computerized testing has the advantage of providing a more standardized testing experience, and thus may afford more reliable and reproducible test results that are not mitigated by as many operator- and patient-related factors.22 The computerized tests for LLVA also have the advantage of providing different low luminance settings of increasing difficulty, and may be more sensitive than the standard tests.
Although we found an association between the LLQ and standard and computerized LLD or LLVA in those with early to intermediate AMD, we did not find any association with microperimetry measures such as average threshold, mean sensitivity, or percent reduced threshold. This is similar to what has been reported by Wu and colleagues.8 Their group evaluated subjects with bilateral intermediate AMD using a shorter 10-item Night Vision Questionnaire (NVQ-10) that was derived from four items on the NEI-VFQ-25.8 Because the NVQ presumably refers to urban settings that may provide some light at night, they compared the NVQ scores with several mesopic tests such as low luminance visual acuity (using a standard 2.0-log neutral density filter), standard LLD, microperimetric mean sensitivity (equivalent to average threshold in our study), and central sensitivity. While their standard LLD scores were associated with NVQ scores, they did not find any association with BCVA, standard LLVA, and mean or central sensitivity. Our study suggests that low luminance vision is a clinically relevant functional outcome for patients with early and intermediate AMD. Additional studies are needed to further investigate the significance of the LLQ versus microperimetry,8 as the latter may be more representative of photopic, cone-mediated function.
LLQ scores were also associated with two computerized photopic tests in the overall cohort—the computerized BCVA and the CCT green. However, this effect was eliminated after controlling for control versus AMD status, and it was not present when only examining the AMD subjects. In a pilot study, we previously suggested that red cone contrast deficits may develop in early and intermediate AMD,22 while other groups have suggested deficiencies in blue color vision29 or the dysfunction of all three cone classes.30 Given such heterogeneous findings related to cone dysfunction in prior studies on AMD, additional research is needed to determine whether cone-specific contrast and computerized visual acuity tests are substantially and consistently compromised with disease progression.
In our study, the rod intercept was modestly associated with LLQ scores in the overall cohort but not in the macular degeneration subcohort. This differs slightly from what several others have reported. Owsley et al.,4 for example, has previously shown that LLQ subscale scores are associated with rod-mediated parameters of dark adaptation (P < 0.03), but not with cone-mediated parameters. Yazdanie and colleagues21 have recently found that a lower score on LLQ is associated with prolonged dark adaptation testing in a sample of subjects with a range of AMD pathology, including those with late-stage disease. However, the authors did not adjust for disease severity when comparing rod intercept time with the LLQ, even though they previously found that the rod intercept time correlated with AMD severity. Two other groups have similarly shown that impairment in rod function and dark adaptation correlate with greater disease severity in AMD.3,31 Thus, the most plausible explanation for why our analysis did not find a strong, significant association between rod intercept and the LLQ in AMD subjects, is that it evaluated patients with early-intermediate AMD and not those with advanced disease. The use of a shorter dark adaptation protocol that had been modified for intermediate AMD as described by Jackson et al.,11 may have also attenuated our results and led to differences from prior publications.4,21
This study has several limitations. Among the AMD subjects enrolled in our study, a majority were intermediate AMD, which may have the potential to bias the results toward this stage of the disease. In addition, our sample size may have limited our ability to assess subtle differences between groups, especially between early AMD and controls. However, it is important to point out that the sample size reflects the current challenges in the standard of care for diagnosis and staging of AMD. Due to diagnostic limitations, patients are often not diagnosed in the early stage of AMD, and a substantial percentage of AMD patients may not be identified until they have advanced disease. Thus, while the results of our study do not find significant differences between controls and early AMD, this may be a reflection of the known measurements available in the standard of care of AMD diagnosis and management. Due to current limitations in early AMD diagnosis and management, a much larger sample size and more sensitive psychophysical tests or protocols may be needed to robustly detect small but consistent differences between controls and those with early AMD. Moreover, 20% of eyes had fewer than 10 small drusen and were classified as controls, but may have been in the process of developing early AMD. This percentage does not differ from the normal aging population as described in the Beaver Dam Study.32 However, because the disease from normal aging to early AMD is on a continuum, it is possible that the emergence of pigmentary changes and small drusen may predispose to the conversion to AMD, and thus may explain some of the mild visual dysfunction in these patients. The existence of small drusen may explain why the LLQ scores were not significantly different between the early AMD and control group. The results of this study may not be generalizable to non-white racial and ethnic groups. Finally, these are baseline cross-sectional results of the relationship between psychophysical tests and the LLQ composite score. Longitudinal studies are currently ongoing to evaluate the relationship between the LLQ and visual function tests over time and will provide additional important insights into the natural course of AMD and visual impairment.
Conclusions
Among subjects with early and intermediate AMD, LLQ scores are significantly associated with objective computerized psychophysical measures of mesopic function, such as LLVA and LLD, but are not associated with microperimetry, dark adaptometry, BCVA, or CCT. Our findings suggest that LLQ is an important PRO in early and intermediate AMD. Future larger studies are needed to determine whether the LLQ may be a useful screening tool for diagnosing the onset of AMD, predicting disease outcomes, and detecting a response to future therapeutic interventions. Clinical trials should incorporate PROs such as the LLQ when assessing the impact of interventions to treat or prevent macular degeneration.
Acknowledgments
Supported by grants from the National Eye Institute (NEI) Clinical Scientist Development award National Institutes of Health/NEI 5K12 EY016333-08 (EL; Bethesda, MD, USA). The parent clinical study was funded by Hoffmann- La Roche Ltd. (Basel, Switzerland) through a research agreement with Duke University (Durham, NC, USA).
Disclosure: A.C. Thompson, None; U.F.O. Luhmann, Roche (E); S.S. Stinnett, None; L. Vajzovic, Alcon (F), Janssen (R), Knights Templar Eye Foundation (R), PDCs ENABLE (R), Roche (F), Second Sight (F), F. Hoffmann-La Roche Ltd (E); A. Horne, None; C.A. Toth, Alcon (R), P; S.W. Cousins, Alimera (C), Bausch&Lomb (C, F), PanOptica (C), Stealth (C, F), Therakine (C); E.M. Lad, Hoffman (F), Roche (F), Apellis (F), Janssen Research (C), Genentech (C)
References
- 1. Bressler NM, Bressler SB. . Preventative ophthalmology. Age-related macular degeneration. Ophthalmology. 1995; 102: 1206– 1211. [DOI] [PubMed] [Google Scholar]
- 2. Friedman DS, O'Colmain BJ, Munoz B,et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004; 122: 564– 572. [DOI] [PubMed] [Google Scholar]
- 3. Owsley C, McGwin G Jr, Jackson GR, Kallies K, Clark M. . Cone- and rod-mediated dark adaptation impairment in age-related maculopathy. Ophthalmology. 2007; 114: 1728– 1735. [DOI] [PubMed] [Google Scholar]
- 4. Owsley C, McGwin G Jr, Scilley K, Kallies K. . Development of a questionnaire to assess vision problems under low luminance in age-related maculopathy. Invest Ophthalmol Vis Sci. 2006; 47: 528– 535. [DOI] [PubMed] [Google Scholar]
- 5. Puell MC, Barrio AR, Palomo-Alvarez C, Gomez-Sanz FJ, Clement-Corral A, Perez-Carrasco MJ. . Impaired mesopic visual acuity in eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012; 53: 7310– 7314. [DOI] [PubMed] [Google Scholar]
- 6. Scilley K, Jackson GR, Cideciyan AV, Maguire MG, Jacobson SG, Owsley C. . Early age-related maculopathy and self-reported visual difficulty in daily life. Ophthalmology. 2002; 109: 1235– 1242. [DOI] [PubMed] [Google Scholar]
- 7. Steinmetz RL, Haimovici R, Jubb C, Fitzke FW, Bird AC. . Symptomatic abnormalities of dark adaptation in patients with age-related Bruch's membrane change. Br J Ophthalmol. 1993; 77: 549– 554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu Z, Guymer RH, Finger RP. . Low luminance deficit and night vision symptoms in intermediate age-related macular degeneration. Br J Ophthalmol. 2016; 100: 395– 398. [DOI] [PubMed] [Google Scholar]
- 9. Feigl B, Cao D, Morris CP, Zele AJ. . Persons with age-related maculopathy risk genotypes and clinically normal eyes have reduced mesopic vision. Invest Ophthalmol Vis Sci. 2011; 52: 1145– 1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Curcio CA, Medeiros NE, Millican CL. . Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996; 37: 1236– 1249. [PubMed] [Google Scholar]
- 11. Jackson GR, Scott IU, Kim IK, Quillen DA, Iannaccone A, Edwards JG. . Diagnostic sensitivity and specificity of dark adaptometry for detection of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014; 55: 1427– 1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Medeiros NE, Curcio CA. . Preservation of ganglion cell layer neurons in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2001; 42: 795– 803. [PubMed] [Google Scholar]
- 13. Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001; 119: 1417– 1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ellwein LB, Fletcher A, Negrel AD, Thulasiraj RD. . Quality of life assessment in blindness prevention interventions. Int Ophthalmol. 1994; 18: 263– 268. [DOI] [PubMed] [Google Scholar]
- 15. Mangione CM, Gutierrez PR, Lowe G, Orav EJ, Seddon JM. . Influence of age-related maculopathy on visual functioning and health-related quality of life. Am J Ophthalmol. 1999; 128: 45– 53. [DOI] [PubMed] [Google Scholar]
- 16. Mangione CM, Lee PP, Gutierrez PR,et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001; 119: 1050– 1058. [DOI] [PubMed] [Google Scholar]
- 17. Owsley C, McGwin G Jr.. Vision-targeted health related quality of life in older adults: patient-reported visibility problems in low luminance activities are more likely to decline than daytime activities. BMC Ophthalmol. 2016; 16: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu Z, Ayton LN, Guymer RH, Luu CD. . Low-luminance visual acuity and microperimetry in age-related macular degeneration. Ophthalmology. 2014; 121: 1612– 1619. [DOI] [PubMed] [Google Scholar]
- 19. Brabyn J, Schneck M, Haegerstrom-Portnoy G, Lott L. . The Smith-Kettlewell Institute (SKI) longitudinal study of vision function and its impact among the elderly: an overview. Optom Vis Sci. 2001; 78: 264– 269. [DOI] [PubMed] [Google Scholar]
- 20. Finger RP, Fenwick E, Owsley C, Holz FG, Lamoureux EL. . Visual functioning and quality of life under low luminance: evaluation of the German Low Luminance Questionnaire. Invest Ophthalmol Vis Sci. 2011; 52: 8241– 8249. [DOI] [PubMed] [Google Scholar]
- 21. Yazdanie M, Alvarez J, Agron E,et al. Decreased visual function scores on a low luminance questionnaire is associated with impaired dark adaptation. Ophthalmology. 2017; 124: 1332– 1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chandramohan A, Stinnett SS, Petrowski JT,et al. Visual function measures in early and intermediate age-related macular degeneration. Retina. 2016; 36: 1021– 1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sunness JS, Rubin GS, Broman A, Applegate CA, Bressler NM, Hawkins BS. . Low luminance visual dysfunction as a predictor of subsequent visual acuity loss from geographic atrophy in age-related macular degeneration. Ophthalmology. 2008; 115: 1480– 1488.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fujita K, Shinoda K, Matsumoto CS,et al. Low luminance visual acuity in patients with central serous chorioretinopathy. Clin Exp Optom. 2013; 96: 100– 105. [DOI] [PubMed] [Google Scholar]
- 25. Gregori NZ, Feuer W, Rosenfeld PJ. . Novel method for analyzing snellen visual acuity measurements. Retina. 2010; 30: 1046– 1050. [DOI] [PubMed] [Google Scholar]
- 26. Rabin J, Gooch J, Ivan D. . Rapid quantification of color vision: the cone contrast test. Invest Ophthalmol Vis Sci. 2011; 52: 816– 820. [DOI] [PubMed] [Google Scholar]
- 27. The Age-Related Eye Disease Study Research Group The Age-Related Eye Disease Study (AREDS) system for classifying age-related macular degeneration from stereoscopic color fundus photographs: AREDS Report No. 6. Am J Ophthalmol. 2001; 132: 668– 681. [DOI] [PubMed] [Google Scholar]
- 28. Owsley C, Clark ME, Huisingh CE, Curcio CA, McGwin G Jr.. Visual function in older eyes in normal macular health: association with incident early age-related macular degeneration 3 years later. Invest Ophthalmol Vis Sci. 2016; 57: 1782– 1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Holz FG, Gross-Jendroska M, Eckstein A, Hogg CR, Arden GB, Bird AC. . Colour contrast sensitivity in patients with age-related Bruch's membrane changes. Ger J Ophthalmol. 1995; 4: 336– 341. [PubMed] [Google Scholar]
- 30. Neelam K, Nolan J, Chakravarthy U, Beatty S. . Psychophysical function in age-related maculopathy. Surv Ophthalmol. 2009; 54: 167– 210. [DOI] [PubMed] [Google Scholar]
- 31. Flamendorf J, Agron E, Wong WT,et al. Impairments in dark adaptation are associated with age-related macular degeneration severity and reticular pseudodrusen. Ophthalmology. 2015; 122: 2053– 2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Klein R, Klein BE, Linton KL. . Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992; 99: 933– 943. [DOI] [PubMed] [Google Scholar]