Abstract
Purpose
The potential consequences of confounding due to drug formulary restrictions in pharmacoepidemiologic research remain incompletely understood. Our objective was to illustrate this potential bias using the example of fluticasone/salmeterol combination therapy, an oral inhaler used to treat asthma and chronic obstructive pulmonary disease, whose use is restricted in the province of Quebec, Canada.
Methods
We identified all new users of fluticasone/salmeterol in Quebec’s administrative databases and classified those who received their initial dispensing of fluticasone/salmeterol between September 1, 1999 and September 30, 2003 as users from the liberal period and those who received it between January 1, 2004 and October 31, 2006 as users from the restricted period. The primary outcome was time to first hospitalization for respiratory causes within 12 months of cohort entry.
Results
Our cohort included 72,154 new users from the liberal period and 5,058 from the restricted period. Compared with use during the liberal period, use during the restricted period was associated with an increased rate of hospitalization for respiratory causes (crude hazard ratio [HR] = 1.41, 95% confidence interval [CI] = 1.32, 1.51). Subsequent adjustment for age, sex, and hospitalization for respiratory causes in the previous year attenuated the association (HR = 1.05, 95% CI = 0.98, 1.12). Further adjustment for other potential confounders resulted in a lower rate during the restricted period (HR = 0.78, 95% CI = 0.73, 0.83).
Conclusions
Formulary restrictions can result in substantial and unexpected confounding and should be considered during the design and analysis of pharmacoepidemiologic studies.
Keywords: Confounding, Bias, Drug Formulary Restrictions, Pharmacoepidemiology
INTRODUCTION
Confounding by indication is well established as a threat to the internal validity of pharmacoepidemiologic investigations1,2. Previous studies examining this issue have focused on the characteristics of patients and their treating physicians as markers for unmeasured confounders responsible for this bias, with study design approaches such as restriction and statistical approaches such as propensity scores and instrumental variable analyses developed to reduce its effects3–6. While patients and physicians undoubtedly contribute to the presence of confounding by indication, the role of drug formulary restrictions also warrants consideration. Recent analyses have revealed that formulary restrictions can introduce important exposure misclassification in administrative databases due to incomplete data capture7,8. However, the confounding by indication due to formulary restrictions remains poorly understood. Our objective was to illustrate the confounding by indication that can occur due to drug formulary restrictions using the example of fluticasone/salmeterol combination therapy (Advair©), an oral inhaler used for the treatment of asthma and chronic obstructive pulmonary disease (COPD), whose use is restricted in the province of Quebec, Canada.
METHODS
Study Population
We conducted a retrospective cohort study of new users of respiratory medications using data extracted from Quebec’s administrative databases. These linked databases included anonymized administrative health records for hospitalizations (Maintenance et exploitation des données pour l’étude de la clientèle hospitalière [MED-ECHO]), physician visits (Régie de l’assurance maladie du Québec [RAMQ]), and vital statistics for Quebec residents as well as drugs dispensed for those who participate in the provincial drug plan (RAMQ). Data were restricted to those with prescription drug coverage, which includes individuals aged ≥ 65 years, those of lower social economic status, and those who are self-employed and do not have other prescription drug coverage.
The cohort that served as the source population for the present study has been described in detail elsewhere9,10. Briefly, we obtained data for 1,410,211 individuals who were dispensed a respiratory medication (any bronchodilator, inhaled corticosteroid, cromone, or anti-leukotriene) between January 1st, 1990 and December 31st, 2005. These data included the administrative health records from one year prior to the dispensing date of this index respiratory drug through the end of the study period (March 31st, 2007). From this source cohort, we identified all new users of fluticasone/salmeterol combination therapy, defined as those with no previous dispensing of fluticasone/salmeterol in the prior year. We excluded all those aged < 18 years and those with < one year of continuous drug coverage (defined as no gap > seven days) before the dispensing date for fluticasone/salmeterol. We also excluded patients who were dispensed fluticasone and salmeterol as two separate drugs on the same day in the year before cohort entry to ensure that study was restricted to new users11 and to avoid the inclusion of patients who were switching formulations. We did not exclude patients who were dispensed either fluticasone or salmeterol separately during the prior year or those who were dispensed both as two separate drugs but on different days. Given the progressive nature of respiratory disease and its treatment (where one class is added to another when symptoms are uncontrolled), restriction to patients who have not used the components would result in a restriction to patients less likely to have respiratory disease or more likely to have the drugs prescribed inappropriately. The dispensing date of the first fluticasone/salmeterol prescription to meet the inclusion criteria was used to define the cohort entry date; with fluticasone/salmeterol combination therapy entering the market in September 1999, the earliest possible date of cohort entry was September 1, 1999. Patients were followed until an event (defined below) or censoring due to death (for hospitalization endpoints), departure from the database, the end of the 12-month follow-up, or the end of the study period (March 31st, 2007), whichever occurred first.
Approval was obtained from the Access to Information Commission of Quebec (Commission d’accès à l’information du Québec) and the Research Ethics Board of the Jewish General Hospital in Montreal, Quebec.
Exposure Definition
The fluticasone/salmeterol oral inhaler was placed on the Quebec drug formulary without restriction in September 1999 and restrictions were first introduced in October 2003. As a result of these listing changes, fluticasone/salmeterol combination therapy became restricted to patients with 1) asthma or other reversible obstructive diseases who remained poorly controlled despite their use of an inhaled corticosteroid; or 2) patients with moderate or severe COPD with an exacerbation in the last year despite regular use of a long-acting bronchodilator12.
We classified all new users who received their initial dispensing of fluticasone/salmeterol between September 1st, 1999 and September 30th, 2003 as users from the liberal or unrestricted use period and those who received their initial dispensing between January 1st, 2004 and October 31st, 2006 as users from the restricted period. Patients who initiated use between these two periods were excluded to minimize misclassification. In addition, all new users who entered the cohort during the liberal period were censored at the end of this period to ensure that each patient only contributed to one period of use.
Outcome Definition
The primary endpoint for this study was time to hospitalization for respiratory causes, which was defined as a first hospitalization with a primary diagnosis of asthma, COPD, or pneumonia13 during follow-up (see Online Appendix 1 for ICD-9 and ICD-10 codes). We restricted to diagnostic codes listed as primary diagnoses in the discharge abstract as all included patients had known respiratory disease at the time of cohort entry. Hospitalizations in which admission and discharge occurred on the same date (i.e., length of stay = 0 days) were not considered events to ensure that outpatient visits were not considered. A composite endpoint of respiratory hospitalization was used as respiratory conditions such as asthma, COPD, and pneumonia are often misdiagnosed as each other. In secondary analyses, we examined the time to hospitalization for any cause to examine differences in the overall health of users of fluticasone/salmeterol during the liberal and restricted use periods. Finally, we examined the time to all-cause mortality in these two groups.
Potential Confounders
To illustrate that the confounding by formulary restriction goes beyond traditional patient-level confounding, we adjusted for a number of potential confounders. These potential confounders included age, sex, a history of asthma, COPD, or pneumonia in the year before cohort entry, hospitalization for respiratory causes in the year before cohort entry. We also adjusted for the number of hospitalizations for any cause, number of physician visits, and number of distinct prescription drugs dispensed, all measured in the year before cohort entry, as well as the Romano version of the Charlson comorbidity index14,15, as the use of multiple comorbidity scores has been shown to reduce potential confounding16. In addition, we adjusted for respiratory medications and medications thought to be associated with the risk of hospitalization for respiratory diseases in the year before cohort entry, including bronchodilators, long-acting beta-agonists, long-acting beta-agonists with inhaled corticosteroid, short-acting beta-agonists, short-acting beta-agonists with antichoinergic combination therapy, cromoglycates, inhaled corticosteroids, leukotrienes, xanthines, narcotics, non-steroidal anti-inflammatory drugs (NSAIDs), non-topical antibiotics, oral corticosteroids, and statins. Finally, we also adjusted for prescribing-physician specialty (i.e., respirologist, general practitioner, or other specialist) and dispensing during the summer months (April to September).
Statistical Analyses
We first examined the effect of the formulary restrictions on the rate of fluticasone/salmeterol prescription and the rate of new use via interrupted time-series analyses. The outcomes of these fitted regression models included the rate of fluticasone/salmeterol prescription per month and the rate of new use of fluticasone/salmeterol per month, respectively. To calculate the denominator for these rates, we included all patients with a recorded dispensing of a respiratory medication between September 1st, 1999 and March 31st, 2007 in the RAMQ database who were aged ≥ 18 years with ≥ 1 year of continuous coverage, with follow-up starting at their first respiratory prescription and ending at death or administrative censoring due to end of coverage or end of follow-up, whichever came first. Both segmented regression models adjusted for time in months from the start of the observation period, with an indicator variable used to identify the restricted period and a continuous variable for time in months after restriction. Durbin-Watson statistic reported significant serial autocorrelation of orders 1, 2, 3, 5, and 12 for the prescription rate analysis and of orders 1, 2, 3, 11, and 12 for the new user analysis. Therefore, to account for non-independent errors, we also estimated the above mentioned autocorrelation parameters and included them in our models.
We then descriptively compared the characteristics of new users of fluticasone/salmeterol in the liberal use and restricted use periods. Dichotomous data are presented as counts and percentages, and continuous data are presented as mean (standard deviation). Differences between groups were estimated and are presented with 95% confidence intervals (CI).
We calculated crude and age- and sex-adjusted rates of hospitalization for respiratory causes for each group using Poisson regression. Rates were estimated at the mean age (62 years) and are expressed as events per 100 person-years. We then compared rates between groups using four Cox proportional hazards models with increasing level of statistical adjustment. The first model was unadjusted. The second model was age- and sex-adjusted. The third model was also adjusted for hospitalization for asthma, COPD, or pneumonia in the year before cohort entry. The final, fully-adjusted model included further adjustment for comorbidities and non-respiratory medications listed above, use of other respiratory medications in the year before cohort entry, the specialty of the prescribing physician, and dispensing during summer. We then repeated analyses for the outcomes of any hospitalization and all-cause mortality. We also conducted two sensitivity analyses to examine the effect of changes in indication for fluticasone/salmeterol that occurred during the study period (COPD was introduced as an indication in 2003). In the first, we excluded all patients with a diagnosis or hospitalization for COPD in the year before cohort entry, and in the second, we excluded all patients aged >40 years. All analyses were conducted using SAS version 9.3 (The SAS Institute, Cary, North Carolina).
RESULTS
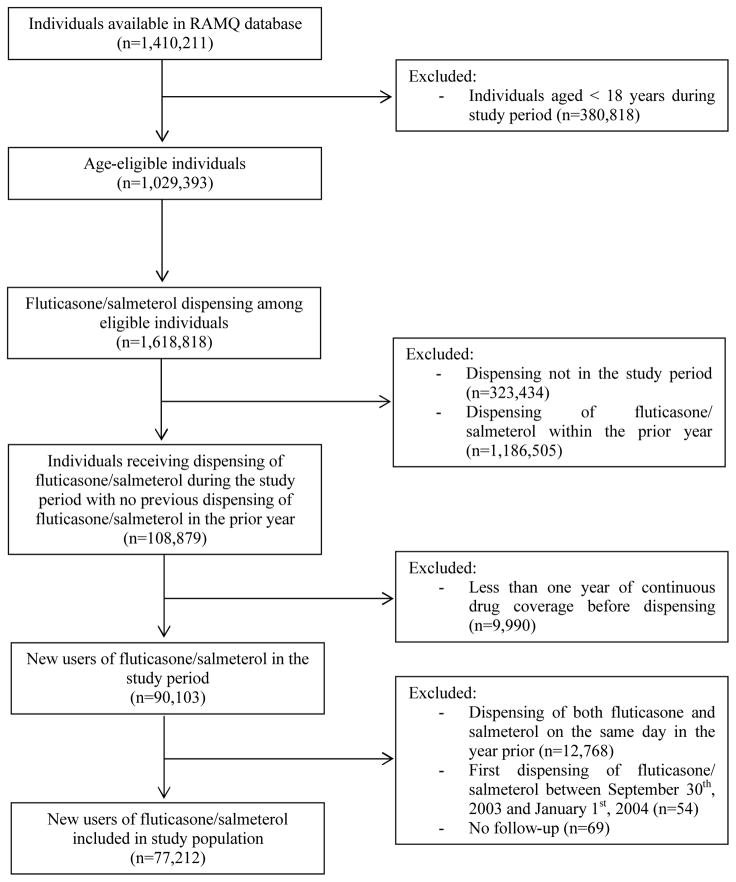
In our initial cohort of 1,410,211 patients who received a respiratory medication, 1,029,393 patients were aged ≥ 18 years and thus eligible for inclusion in our study (Figure 1). Following the application of inclusion and exclusion criteria, a total of 77,212 new users of fluticasone/salmeterol were included in our study, including 72,154 new users from the liberal period and 5,058 from the restricted period.
Figure 1.
Description of study cohort construction
Trend Analysis
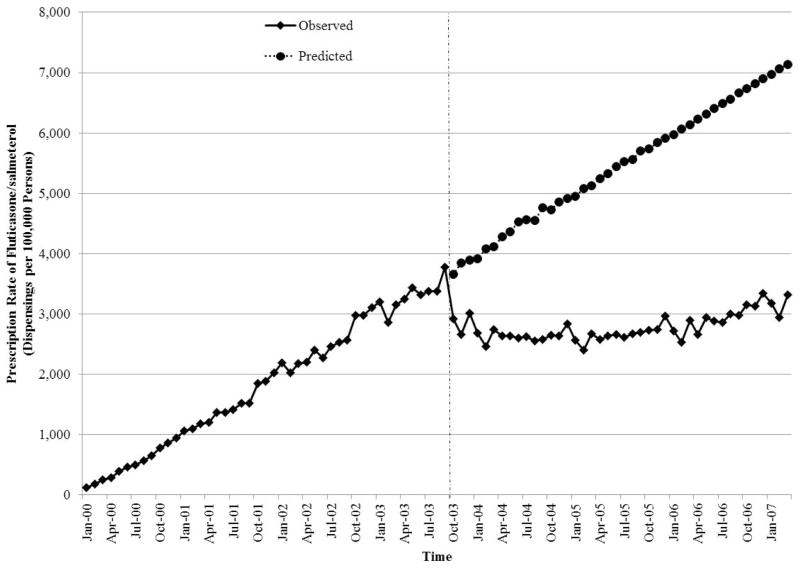
The initiation of formulary restrictions for fluticasone/salmeterol resulted in a substantial decrease in its prescription rate (Figure 2, Online Appendix 2). At the time of the restrictions (September 30, 2003), the prescription rate was 3,776 prescriptions per 100,000 persons. By January 2007, the prescription rate had decreased to 3,311 prescriptions per 100,000 persons. In contrast, the predicted prescription rate in the absence of this regulatory change was 7,133 prescriptions per 100,000.
Figure 2.
Interrupted time-series analysis of the impact of the formulary restriction on the rate of prescription of fluticasone/salmeterol among patients receiving respiratory medications in the province of Quebec, Canada.
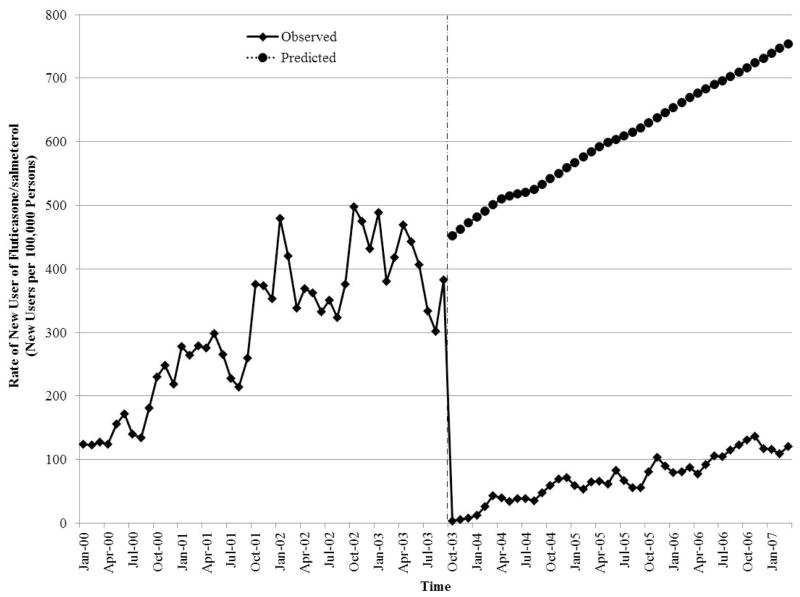
The formulary changes also resulted in a substantial decrease in the rate of new use of fluticasone/salmeterol (Figure 3, Online Appendix 2). Just prior to the onset of the restrictions, the rate was 382 new users per 100,000 persons. The rate dropped dramatically immediately following implementation of the restrictions before slowly beginning to increase over time. By January 2007, the observed rate was 120 new users of fluticasone/salmeterol per 100,000 persons whereas the predicted rate in the absence of the formulary restrictions was 754 new users per 100,000 persons.
Figure 3.
Interrupted time-series analysis of the impact of the formulary restriction on the rate of new use of fluticasone/salmeterol among patients receiving respiratory medications in the province of Quebec, Canada.
Patient Characteristics
There were substantial differences in the characteristics of new users of fluticasone/salmeterol before and after the implementation of formulary restrictions (Table 1). Compared with new users from the liberal period (n=72,154), new users in the restricted period (n=5,058) were more likely to be older, had a more pronounced history of respiratory illness, and a greater burden of disease. In addition, they had greater use of respiratory medications and were more likely to have had their fluticasone/salmeterol prescribed by a respirologist. While they were more likely to use oral corticosteroids and statins, they were less likely to use NSAIDs and non-topical antibiotics.
Table 1.
Characteristics of new users of fluticasone/salmeterol before and after the introduction of formulary restrictions *†.
| Characteristics | Liberal (n = 72,154) | Restricted (n = 5,058) | Difference (95% CI) |
|---|---|---|---|
| Demographic | |||
| Age, years | 62.1 (17) | 64.0 (17) | 1.9 (1.4, 2.3) |
| Male | 29,647 (41) | 2,139 (42) | 1 (0, 3) |
| Respiratory History | |||
| Medical History in the Prior Year | |||
| Asthma | 23,666 (33) | 2,475 (49) | 16 (15, 18) |
| COPD | 31,747 (44) | 2,476 (49) | 5 (4, 6) |
| Pneumonia | 9,863 (14) | 717 (14) | 1 (−1, 2) |
| Hospitalization in the Prior Year for Respiratory | |||
| Cause | |||
| Asthma | 2,961 (4) | 397 (8) | 4 (3, 5) |
| COPD | 8,078 (11) | 1,103 (22) | 11 (9, 12) |
| Pneumonia | 2,273 (3) | 316 (6) | 3 (2, 4) |
| Measures of Disease Burden | |||
| Hospitalization for Any Cause in the Prior Year | 14,937 (21) | 1,523 (30) | 9 (8, 11) |
| Physician Visits in the Prior Year | |||
| 0–5 | 16,125 (22) | 877 (17) | −5 (−6, −4) |
| 6–10 | 18,617 (26) | 1,241 (25) | −1 (−3, 0) |
| 11–15 | 13,520 (19) | 935 (18) | 0 (−1, 1) |
| 16+ | 23,892 (33) | 2,005 (40) | 7 (5, 8) |
| Charlson Comorbidity Index | |||
| 0 | 17,985 (25) | 739 (15) | −10 (−11,−9) |
| 1 | 34,662 (48) | 2,575 (51) | 3 (1, 4) |
| 2 | 8,616 (12) | 650 (13) | 1 (0, 2) |
| ≥3 | 10,891 (15) | 1,094 (22) | 7 (5, 8) |
| Prescribing Physician Specialty | |||
| General Practitioner | 59,101 (82) | 3,488 (69) | −13 (−14, −12) |
| Respirologist | 10,173 (14) | 1,361 (27) | 13 (12, 14) |
| Other | 2,880 (4) | 209 (4) | 0 (0, 1) |
| Prior Medication Use | |||
| Number of Unique Prescription Drugs | 9.3 (5) | 11.6 (5) | 2.3 (2.1, 2.4) |
| Respiratory Medications | |||
| Bronchodilators | 8,256 (11) | 1,513 (30) | 19 (17, 20) |
| Cromoglycates | 117 (0) | 5 (0) | 0 (0, 0) |
| Inhaled corticosteroids | 31,339 (43) | 3,581 (71) | 27 (26, 29) |
| Leukotrienes | 3,522 (5) | 432 (9) | 4 (3, 4) |
| Beta-agonists | |||
| LABA | 5,827 (8) | 1,198 (24) | 16 (14, 17) |
| LABA with Inhaled Corticosteroid | 168 (0) | 237 (5) | 5 (4, 5) |
| SABA | 35,198 (49) | 3,716 (73) | 25 (23, 26) |
| SABA/Anticholinergic Combined | 13,451 (19) | 1,828 (36) | 18 (16, 19) |
| Xanthines | 4,915 (7) | 325 (6) | 0 (−1, 0) |
| Non-Respiratory Medications | |||
| Narcotics | 14,463 (20) | 1,175 (23) | 3 (2, 4) |
| NSAIDs | 27,339 (38) | 1,411 (28) | −10 (−11, −9) |
| Non-topical Antibiotics | 50,767 (70) | 3,420 (68) | −3 (−4, −1) |
| Oral Corticosteroids | 19,353 (27) | 2,366 (47) | 20 (19, 21) |
| Statins | 16,380 (23) | 1,636 (32) | 10 (8, 11) |
Abbreviations: LABA = long-acting beta-agonist; NSAIDs = non-steroidal anti-inflammatory drugs; SABA = short-acting beta-agonist;
Data are presented as mean ± standard deviation or n (%).
The liberal use period was defined as September 1st, 1999 to September 30th, 2003, and restricted use was defined as January 1st, 2004 to October 31st, 2006.
Hospitalizations and All-Cause Mortality
There were substantial differences in the rate of hospitalization for respiratory causes among new users of fluticasone/salmeterol before and after the implementation of formulary restrictions (Table 2). The crude rate during the liberal period was 18.7 events per 100 person-years (95% CI = 18.3, 19.1) but was 26.2 events per 100 person-years (95% CI = 24.7, 27.9) during the restricted period (crude hazards ratio [HR] = 1.41, 95% = 1.32, 1.51; Table 3). Subsequent adjustment for age, sex, and hospitalization for respiratory causes in the previous year attenuated the association (HR = 1.05, 95% CI = 0.98, 1.12). Further adjustment for other potential confounders resulted in a significantly lower rate during the restricted period (HR = 0.78, 95% CI = 0.73, 0.83).
Table 2.
Rates of hospitalization for respiratory causes, hospitalization for any cause, and all-cause mortality among new users of fluticasone/salmeterol before and after the introduction of formulary restrictions in Quebec, Canada.
| Period* | Number of Events | Number of Person-Years | Rate (95% CI)†
|
|
|---|---|---|---|---|
| Crude | Age- and Sex Adjusted‡ | |||
| Hospitalizations for Respiratory Causes: | ||||
| Restricted | 1,020 | 3,889 | 26.2 (24.7, 27.9) | 25.1 (23.5, 26.8) |
| Liberal | 10,001 | 53,537 | 18.7 (18.3, 19.1) | 18.9 (18.4, 19.5) |
| Hospitalizations for Any Cause: | ||||
| Restricted | 1,248 | 3,783 | 33.0 (31.2, 34.9) | 32.1 (30.3, 34.1) |
| Liberal | 14,378 | 51,490 | 27.9 (27.5, 28.4) | 28.5 (27.8, 29.2) |
| All-Cause Mortality: | ||||
| Restricted | 274 | 4,359 | 6.3 (5.6, 7.1) | 5.1 (4.5, 5.8) |
| Liberal | 2,610 | 58,126 | 4.5 (4.3, 4.7) | 4.0 (3.7, 4.2) |
Abbreviations: CI: confidence interval.
We classified all new users who received their initial dispensing of fluticasone/salmeterol between September 1st, 1999 and September 30th, 2003 as users from the liberal or unrestricted use period and those who received their initial dispensing between January 1st, 2004 and October 31st, 2006 as users from the restricted period.
Rates are expressed as events per 100 person-years.
Estimated for a male at the mean age of 62 years.
Table 3.
Hazard ratios of hospitalization for respiratory causes, hospitalization for any cause, and all-cause mortality among new users of fluticasone/salmeterol before and after the introduction of formulary restrictions in Quebec, Canada.
| Period* | Number of Events | Number of Person-Years | HR (95% CI)
|
|||
|---|---|---|---|---|---|---|
| Crude | Age- and Sex Adjusted | Partially-Adjusted Model† | Fully-Adjusted‡ | |||
| Hospitalizations for Respiratory Causes: | ||||||
| Restricted | 1,020 | 3,889 | 1.41 (1.32, 1.51) | 1.33 (1.25, 1.42) | 1.05 (0.98, 1.12) | 0.78 (0.73, 0.83) |
| Liberal | 10,001 | 53,537 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Hospitalizations for Any Cause: | ||||||
| Restricted | 1,248 | 3,783 | 1.19 (1.12, 1.26) | 1.13 (1.07, 1.20) | 0.96 (0.90, 1.02) | 0.82 (0.77, 0.87) |
| Liberal | 14,378 | 51,490 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| All-Cause Mortality: | ||||||
| Restricted | 274 | 4,359 | 1.40 (1.24, 1.59) | 1.28 (1.13, 1.45) | 1.10 (0.97, 1.25) | 0.97 (0.84, 1.11) |
| Liberal | 2,610 | 58,126 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
Abbreviations: CI: confidence interval; HR: hazards ratio.
We classified all new users who received their initial dispensing of fluticasone/salmeterol between September 1st, 1999 and September 30th, 2003 as users from the liberal or unrestricted use period and those who received their initial dispensing between January 1st, 2004 and October 31st, 2006 as users from the restricted period.
Partially-adjusted model 1 = adjusted for age, sex, hospitalization for asthma, COPD, or pneumonia in the year before cohort entry.
Fully-adjusted model = adjusted for covariates included in partially-adjusted model + number of hospitalizations for any cause in the year before cohort entry, number of physician visits in the year before cohort entry, number of prescription drugs dispensed in the prior year, Charlson comorbidity index, and a history of asthma, COPD, or pneumonia in the prior year, narcotics, non-steroidal anti-inflammatory drugs (NSAIDs), non-topical antibiotics, oral corticosteroids, statins, bronchodilators, short-acting beta-agonists, long-acting beta-agonists, long-acting beta-agonists with inhaled corticosteroid, short-acting beta-agonists with anticholinergic combination, cromoglycates, inhaled corticosteroids, inhaled corticosteroid/bronchodilator, leukotrienes, and xanthines, prescribing physician specialty (respirologist, general practitioner, and other), dispensing during the summer months (April – September).
Similar trends were observed for hospitalization for any cause with the crude rate increasing from 27.9 events per 100 person-years (95% CI = 27.5, 28.4) during the liberal period to 33.0 events per 100 person-years (95% CI = 31.2, 34.9)(Table 2). Statistical adjustment decreased the HR from 1.19 (95% CI = 1.12, 1.26) in our crude model to 0.82 (95% CI = 0.77, 0.87) in our fully adjusted model (Table 3).
Similar trends were also observed in our analysis of all-cause mortality but with the fully-adjusted model resulting in a null association (HR = 0.97, 95% CI = 0.84, 1.11)(Tables 2–3).
Sensitivity Analyses
Our sensitivity analyses that excluded patients with a history of COPD in the year before cohort entry produced estimates that were consistent with those of our primary analyses (Online Appendix 3). The exclusion of patients aged >40 years produced similar estimates but with wider 95% CIs (data not shown).
DISCUSSION
Our study was designed to examine the impact of drug formulary restrictions on the validity of pharmacoepidemiologic studies using the example of fluticasone/salmeterol combination therapy. We found that the implementation of these restrictions had a profound effect on drug utilization, with the policy resulting in an important decrease in the rates of prescription and of new use of fluticasone/salmeterol. These prescription changes resulted in channeling and confounding by indication, with new users of fluticasone/salmeterol having a significantly higher crude rate of hospitalization for respiratory causes during the restricted period (crude HR = 1.41, 95% CI = 1.32, 1.51) due to the presence of more severe underlying respiratory disease. Adjustment for potential confounders attenuated and reversed the association, with new users during the restricted period having a significantly lower rate of hospitalization for respiratory causes compared with those during the liberal period (fully adjusted HR = 0.78, 95% CI = 0.73, 0.83). These results suggest that drug formulary restrictions can result in substantial and unexpected confounding by indication that threatens the validity of study results. These results also suggest that adjusting for patient demographic and clinical characteristics is insufficient to account for channeling due to formulary restrictions. Consequently, such restrictions must be considered in the design and analysis of pharmacoepidemiologic studies.
Our study was designed to illustrate the potential bias at play and not to evaluate the impact of the formulary restrictions on clinical outcomes. While our study found that adverse outcomes were significantly lower during the restricted period, these findings should not be interpreted as evidence that the formulary restrictions improved outcomes as our study did not consider outcomes in patients who would have received the medication in the absence of the restrictions. If future studies are able to demonstrate that such formulary restrictions do not impact clinical outcomes, the formulary policy has the potential itself to be used as an instrumental variable to reduce confounding.
The impact of formulary restrictions on estimates of risk in pharmacoepidemiologic studies has been examined previously. Guertin and colleagues examined the cardiovascular risk of Attention Deficit Hyperactivity Disorder (ADHD) drugs among children using Quebec’s administrative health data17. Importantly, formulary restrictions were in place during the study period that limited access to amphetamines and atomoxetine to those whose symptoms persisted or experienced side effects with other ADHD medications. To minimize the effects of detection and susceptibility bias, the authors conducted secondary analyses involving sub-cohorts of patients that excluded patients with prior cardiovascular events. In doing so, the authors demonstrated that the association differed for both amphetamines and atomoxetine in the full and sub-cohorts, suggesting that the differing results were due to the formulary restrictions. While the restrictions may have played a role, the heterogeneity of results may also be a reflection of different underlying risks in patients with and without a history of cardiovascular events.
The potential impact of formulary restrictions was also well illustrated in a recent study conducted by the Canadian Network for Observational Drug Effect Studies (CNODES)18. In this study, we used a distributed protocol approach with meta-analysis to examine the effect of proton pump inhibitors on the risk of hospitalization for community-acquired pneumonia in eight databases. To avoid the confounding by indication and protopathic bias present in previous studies of this issue, we restricted inclusion to new users of NSAIDs, with proton pump inhibitor users receiving them for prophylaxis. Null results were obtained in all jurisdictions except Nova Scotia, where formulary restrictions were in place and resulted in substantial confounding (adjusted odds ratio in Nova Scotia = 3.73, 95% CI = 1.12, 12.36; adjusted odds ratio in all other jurisdictions = 1.03, 95% CI = 0.87, 1.22).
Our study has several strengths. First, by comparing the effect of the same combination drug on the same outcomes before and after the implementation of formulary restrictions, the true effect measure in the absence of bias is known (i.e., HR should equal 1.0 in the absence of confounding or non-comparability of patients across periods). Second, we included an interrupted time-series analysis to better understand the impact of the formulary restrictions on prescribing practices and the subsequent channeling that occurred. Finally, our restriction to new users avoided the biases associated with the study of prevalent users11, which would have been particularly problematic when comparing use before and after the implementation of these formulary restrictions.
Our study also has some potential limitations. First, the approved indications for fluticasone/salmeterol combination therapy were expanded during the study period to also include COPD. For this reason, we conducted sensitivity analyses excluding patients hospitalized for COPD in the year prior to cohort entry; these analyses produced estimates that were consistent with those of our primary analysis. Second, smoking status was not available in our database and may be responsible for some residual confounding. Third, the restricted period contained a relatively small number of new users of fluticasone/salmeterol. While the 95% CIs obtained in our analysis of hospitalization outcomes were relatively precise, those of our all-cause mortality analysis had modest precision. Fourth, it is possible that other formulary or administrative changes that occurred around the same time may have affected our results. However, to our knowledge, no other changes that would have impacted our results occurred at the same time as the fluticasone/salmeterol formulary restrictions. Finally, while this study was conducted to illustrate the potential confounding that can occur with drug formulary restrictions, its generalizability to other formulary restrictions or other jurisdictions is unclear.
CONCLUSIONS
Drug formulary restrictions can result in substantial and unexpected confounding. In the case of fluticasone/salmeterol, crude estimates indicating an increased risk of hospitalizations for respiratory causes during the liberal period relative to the restricted period but with a statistically significant decreased risk during the restricted period following statistical adjustment. To ensure that study results are valid, formulary restrictions should be considered during the design and analysis of pharmacoepidemiologic studies.
Supplementary Material
Take Home Messages/Key Points.
The potential consequences of confounding due to drug formulary restrictions in pharmacoepidemiologic research remain incompletely understood.
Using the example of fluticasone/salmeterol combination therapy, whose use is restricted in the province of Quebec, Canada, we compared the rate of hospitalization for respiratory causes among new users of this oral inhaler before and after the introduction of formulary restrictions.
Compared with use during the liberal period, use during the restricted period was associated with an increased rate of hospitalization for respiratory causes (crude HR = 1.41, 95% CI = 1.32, 1.51). Adjustment for potential confounders resulted in a lower rate during the restricted period (HR = 0.78, 95% CI = 0.73, 0.83).
Formulary restrictions can result in substantial and unexpected confounding and should be considered during the design and analysis of pharmacoepidemiologic studies.
Acknowledgments
Dr. Filion is supported by a Canadian Institutes of Health Research (CIHR) New Investigator Award and a Young Investigator Establishment Award from the Fonds de Recherche du Québec – Santé [Quebec Foundation for Research – Health]. This study was supported by the CIHR (funding reference number 89709).
Footnotes
Conflict of Interest Statement
ME is currently an employee at Algorithme Pharma. KBF and PE have no relationships to disclose.
This study was presented as a poster at the Society of Epidemiologic Research annual meeting in Denver, Colorado in 2015 and as an oral presentation at the International Conference on Pharmacoepidemiology in Boston, Massachusetts in August 2015.
AUTHORS CONTRIBUTIONS
KBF and PE were involved in study conception and design. ME conducted the statistical analyses, and all authors were involved in the interpretation of data. KBF drafted the manuscript, and all authors critically reviewed the manuscript for important intellectual content.
References
- 1.Blais L, Ernst P, Suissa S. Confounding by indication and channeling over time: the risks of beta 2-agonists. Am J Epidemiol. 1996;144(12):1161–1169. doi: 10.1093/oxfordjournals.aje.a008895. [DOI] [PubMed] [Google Scholar]
- 2.Joffe MM. Confounding by indication: the case of calcium channel blockers. Pharmacoepidemiol Drug Saf. 2000;9(1):37–41. doi: 10.1002/(SICI)1099-1557(200001/02)9:1<37::AID-PDS471>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 3.Huybrechts KF, Brookhart MA, Rothman KJ, et al. Comparison of different approaches to confounding adjustment in a study on the association of antipsychotic medication with mortality in older nursing home patients. Am J Epidemiol. 2011;174(9):1089–1099. doi: 10.1093/aje/kwr213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiol. 2009;20(4):512–522. doi: 10.1097/EDE.0b013e3181a663cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klungel OH, Martens EP, Psaty BM, et al. Methods to assess intended effects of drug treatment in observational studies are reviewed. J Clin Epidemiol. 2004;57(12):1223–1231. doi: 10.1016/j.jclinepi.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 6.McMahon AD. Approaches to combat with confounding by indication in observational studies of intended drug effects. Pharmacoepidemiol Drug Saf. 2003;12(7):551–558. doi: 10.1002/pds.883. [DOI] [PubMed] [Google Scholar]
- 7.Gamble JM, McAlister FA, Johnson JA, Eurich DT. Restrictive drug coverage policies can induce substantial drug exposure misclassification in pharmacoepidemiologic studies. Clin Ther. 2012;34(6):1379–1386. doi: 10.1016/j.clinthera.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Gamble JM, McAlister FA, Johnson JA, Eurich DT. Quantifying the impact of drug exposure misclassification due to restrictive drug coverage in administrative databases: a simulation cohort study. Value Health. 2012;15(1):191–197. doi: 10.1016/j.jval.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Saad N, Camus P, Suissa S, Ernst P. Statins and the risk of interstitial lung disease: a cohort study. Thorax. 2013;68(4):361–364. doi: 10.1136/thoraxjnl-2012-201823. [DOI] [PubMed] [Google Scholar]
- 10.Lapi F, Kezouh A, Suissa S, Ernst P. The use of inhaled corticosteroids and the risk of adrenal insufficiency. Eur Respir J. 2013;42(1):79–86. doi: 10.1183/09031936.00080912. [DOI] [PubMed] [Google Scholar]
- 11.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 12.Régie de l’assurance maladie du Québec. [Accessed 03/03/2015];Systeme respiratoire. 2015 http://www.ramq.gouv.qc.ca/SiteCollectionDocuments/professionnels/medicaments/codes-medicaments-exception/RE.pdf.
- 13.Skull SA, Andrews RM, Byrnes GB, et al. ICD-10 codes are a valid tool for identification of pneumonia in hospitalized patients aged > or = 65 years. Epidemiol Infect. 2008;136(2):232–240. doi: 10.1017/S0950268807008564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075–1079. doi: 10.1016/0895-4356(93)90103-8. [DOI] [PubMed] [Google Scholar]
- 15.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 16.Schneeweiss S, Seeger JD, Maclure M, Wang PS, Avorn J, Glynn RJ. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol. 2001;154(9):854–864. doi: 10.1093/aje/154.9.854. [DOI] [PubMed] [Google Scholar]
- 17.Guertin J, LeLorier J, Durand M, Gow R, Holbrook A, Levine M. Impact of a restrictive drug access program on the risk of cardiovascular encounters in children exposed to ADHD medications. J Popul Ther Clin Pharmacol. 2014;21(3):e357–369. [PubMed] [Google Scholar]
- 18.Filion KB, Chateau D, Targownik LE, et al. Proton pump inhibitors and the risk of hospitalisation for community-acquired pneumonia: replicated cohort studies with meta-analysis. Gut. 2014;63(4):552–558. doi: 10.1136/gutjnl-2013-304738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.