Abstract
Sphingosine 1-phosphate (S1P) is a potent lipid mediator that works on five kinds of S1P receptors located on the cell membrane. In the circulation, S1P is distributed to HDL, followed by albumin. Since S1P and HDL share several bioactivities, S1P is believed to be responsible for the pleiotropic effects of HDL. Plasma S1P levels are reportedly lower in subjects with coronary artery disease, suggesting that S1P might be deeply involved in the pathogenesis of atherosclerosis. In basic experiments, however, S1P appears to possess both pro-atherosclerotic and anti-atherosclerotic properties; for example, S1P possesses anti-apoptosis, anti-inflammation, and vaso-relaxation properties and maintains the barrier function of endothelial cells, while S1P also promotes the egress and activation of lymphocytes and exhibits pro-thrombotic properties. Recently, the mechanism for the biased distribution of S1P on HDL has been elucidated; apolipoprotein M (apoM) carries S1P on HDL. ApoM is also a modulator of S1P, and the metabolism of apoM-containing lipoproteins largely affects the plasma S1P level. Moreover, apoM modulates the biological properties of S1P. S1P bound to albumin exerts both beneficial and harmful effects in the pathogenesis of atherosclerosis, while S1P bound to apoM strengthens anti-atherosclerotic properties and might weaken the pro-atherosclerotic properties of S1P. Although the detailed mechanisms remain to be elucidated, apoM and S1P might be novel targets for the alleviation of atherosclerotic diseases in the future.
Keywords: Sphingosine 1-phosphate, HDL, Apolipoprotein M, Atherosclerosis
Introduction
Sphingosine 1-phosphate (S1P) is a potent lipid mediator composed of one long hydrophobic chain and one phosphoric acid group. S1P exerts potent physiological effects through five S1P receptors (S1P1–5) located on cell membranes, while some reports have also demonstrated potential roles of S1P inside cells. The physiological activities of S1P are various: S1P promotes cell proliferation, prevents apoptosis1), preserves the endothelial barrier2), attracts lymphocytes3), and so on. Therefore, S1P is thought to be involved in various diseases including atherosclerosis, cancer4), diabetes5), congenital disorders6), kidney diseases7), and immunological diseases8). Among them, the association between S1P and atherosclerosis has been investigated for a long time.
A unique characteristic distribution of S1P in the circulation is that about two-thirds of S1P in plasma is carried on HDL, followed by albumin9). Importantly, Christoffersen et al. revealed that apolipoprotein M (apoM), a minor apolipoprotein on HDL10), is a carrier of S1P on HDL11). Since HDL possesses several pleiotropic properties, such as anti-inflammation, anti-oxidation, anti-thrombosis, and vasorelaxation12), and S1P shares many of these effects, S1P is believed to contribute to many of the cardioprotective properties of HDL13). In addition to the properties of apoM as a carrier of S1P, we demonstrated that apoM is not a mere carrier of S1P, but also a modulator of plasma and cellular S1P levels and that the homeostasis of apoM-containing lipoproteins can largely affect the plasma S1P levels. In addition, as described in this review, recent studies have elucidated that S1P bound to apoM/HDL possesses different properties from those of S1P bound to albumin, which may at least partly explain the dual nature of S1P in the fields of atherosclerosis.
Keeping these backgrounds in mind, the present review will provide an overview of recent findings on the association between S1P and atherosclerosis, focusing mainly on apoM.
Biosynthesis and Homeostasis of S1P
S1P is derived from ceramide inside cells, which is formed de novo or from the breakdown of membrane-resident sphingomyelin. Ceramide is converted to sphingosine by ceramidase, and then sphingosine is phosphorylated into S1P by sphingosine kinase (SphK) 1 or 2, which are the enzymes responsible for producing S1P14). In cells, S1P can be reversibly or irreversibly degraded to sphingosine or hexadecenal and phosphoethanolamine by S1P-specific phosphatase or S1P lyase (Fig. 1A). The main sources of S1P in the circulation are erythrocytes15), platelets16), and the endothelium17), while many kinds of cells express SphKs and produce S1P de novo. Although how S1P is exported from cells remains to be fully elucidated, several transporters have been demonstrated to be involved in this process. S1P secretion from platelets has been speculated to occur via a vesicle-mediated manner18) or via some unknown S1P transporters19), and ATP binding cassette subfamilies20) and Band321) might be involved in the excretion of S1P from erythrocytes, while spinster homolog 2 has been shown to function as an S1P transporter in endothelium cells22). After S1P is secreted from cells, S1P can work as an agonist for S1P receptors located on cell membrane and can be rapidly degraded by lipid phosphate phosphatases23). In circulation, about two-thirds of S1P is distributed to HDL, followed by albumin and other lipoproteins9) (Fig. 1B). It remains to be elucidated, however, how exported S1P is bound to these carriers, although a phospholipid transfer protein is reportedly required to maintain the S1P content on HDL24).
Fig. 1.
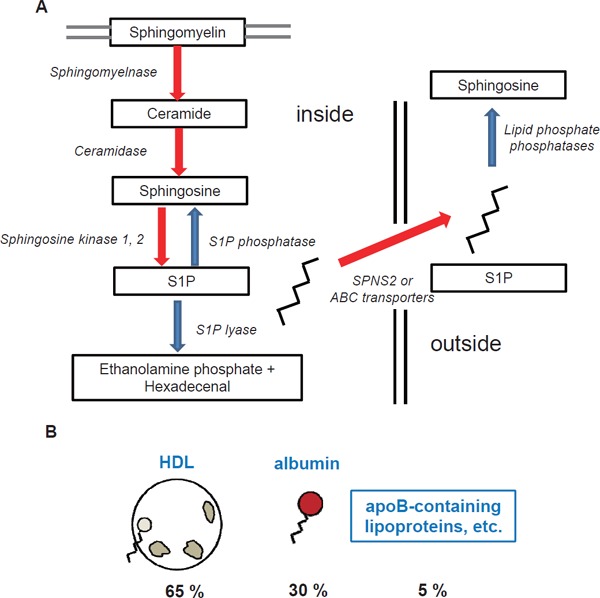
Homeostasis of sphingosine 1-phosphate
The schema show the biosynthesis of sphingosine 1-phosphate (S1P) and its distribution in the circulation. (A) Biosynthesis, efflux, and degradation of S1P. (B) Distribution of S1P in plasma.
Dual Nature of S1P in the Pathogenesis of Atherosclerosis
The roles of S1P in the fields of atherosclerosis have been demonstrated in many elegant reports; in general, S1P possesses protective roles in atherosclerosis, but it can also exert harmful effects during the pathogenesis of atherosclerosis (Fig. 2).
Fig. 2.
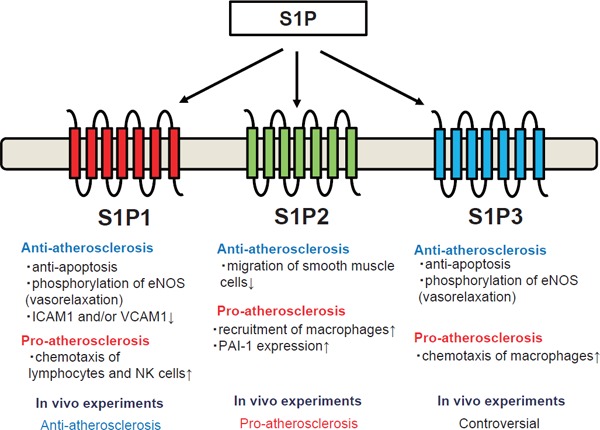
Dual nature of sphingosine 1-phosphate in the pathogenesis of atherosclerosis The biological properties exerted by S1P through each S1P receptor are shown.
Regarding the protective properties of S1P, HDL-associated S1P promotes the survival and prevents the apoptosis of endothelial cells mainly through S1P1 and S1P325–27), as is the case in macrophages28). HDL-associated S1P also induces the phosphorylation of eNOS though S1P1 and S1P3, thereby promoting the relaxation of vessels29, 30) and accounting, at least in part, for the statin-induced activation of eNOS31, 32). S1P also preserves the endothelial barrier function through the spreading of endothelial cells33), the stabilization of endothelial cell-cell junctions34), and the NO-mediated suppression of endothelial cell contractility35) via the S1P1/PI3K pathway. S1P suppresses the attachment of blood cells, which is an initial step in the formation of atherosclerotic lesions, by inhibiting the expression of VCAM1 and/or ICAM1 on endothelial cells30, 36). While only limited reports are presently available, S1P might also attenuate inflammatory responses in monocytes37) and prevent atherosclerosis by inhibiting the migration of smooth muscle cells through S1P238). As well as its protective effects on vessels, S1P can also protect the heart from damage, such as ischemia. S1P protects cardiomyocytes from apoptosis mainly though S1P1 and S1P339, 40).
Regarding the harmful properties of S1P, S1P is reported to induce inflammation and thrombosis. The most famous role of S1P in inflammation is its ability to act as a chemoattractant for lymphocytes, and this role is the target of a novel immunosuppressant, fingolimod41); the S1P gradient facilitates the egress of lymphocytes from lymphoid organs into the circulation and the recruitment of lymphocytes to sites of inflammation42, 43). S1P is also believed to facilitate inflammation by activating other immune cells; S1P induces intracellular calcium signaling as a second messenger44), activates NF-κB44), promotes chemotaxis, and stimulates the production of TNF-α in macrophages and/or monocytes45, 46), and S1P is required for NK cells to egress from lymphoid tissue and bone marrow through S1P1 and S1P547, 48). A recent study also elucidated that the blockade of S1P2 signaling augments B1 lymphocytes49), which are deemed to be atheroprotective cells. Another possible harmful aspect of S1P is that S1P or its receptors has been reported to be associated with coagulation factors. S1P has been shown to augment the thrombin-induced expression of tissue factor in endothelial cells50), and S1P has also been proposed to induce the expression of PAI-1 in adipocytes51, 52), hepatocytes53), and glioblastoma cells54), suggesting that S1P has a pro-thrombotic property. SphK1 is also reported to be involved in the Factor-Xa-induced migration of smooth muscle cells55). A recent study also demonstrated the possible involvement of S1P in platelet activation; when SphK2, which is an enzyme that produces S1P in platelets, is deleted in mice, the mice showed resistance to arterial thrombosis56). In addition, although S1P preserves the barrier functions of endothelial cells, it can contrarily inhibit barrier functions via the S1P2/Rho/ROCK pathway57).
Actually, several reports have investigated the physiological roles of S1P and S1P receptors in the pathogenesis of atherosclerosis using animal experiments. For example, S1P3 knockout mice exhibited resistance to the protective properties of HDL or S1P in a model of coronary infarction58), while one report showed the promotion of monocyte/macrophage recruitment and neointima formation caused by carotid artery ligation in S1P3 knockout mice45). S1P2 knockout mice developed atherosclerotic lesions to a lesser degree when they had a background of apoE deficiency with reduced macrophage recruitment59). Regarding S1P1, although S1P1 knockout mice are embryonic lethal60), pharmacological experiments using an S1P1 agonist showed that the S1P1 agonist protected LDL receptor knockout mice from atherosclerosis61). Together, these reports suggest that S1P1 and S1P3 mainly exert anti-atherosclerotic properties, while S1P2 exerts pro-atherosclerotic properties. Further studies are needed to investigate the involvement of S1P receptors in the pathogenesis of atherosclerosis. Regarding the effects of S1P levels, a sphingosine kinase inhibitor has been demonstrated to suppress atherosclerosis lesions in LDL receptor knockout mice fed a high cholesterol diet, but not in mice fed normal chow62). Contrary to the possible anti-atherosclerotic properties of lowering S1P using a SphK inhibitor, the overexpression of apoM in LDLr knockout mice and apoE knockout mice protected the mice against atherosclerosis, although the association with S1P was not mentioned63). These results suggest that S1P bound to apoM might possess distinct properties from S1P bound to albumin in the pathogenesis of atherosclerosis.
ApoM Modulates the Homeostasis of Plasma S1P Levels
As described above, the distribution of S1P in plasma is unique compared with those of other lysophospholipids; about two-thirds of S1P in plasma is carried on HDL. For a long time, the reason for this distribution of S1P remained unknown. In 2011, however, Christoffersen et al. reported that apoM serves as a carrier of S1P on HDL11). In addition to the role of apoM as a vehicle of S1P, we have demonstrated that apoM increased S1P levels in the whole body; when we overexpressed apoM in HepG2 cells or murine livers, we observed that not only did the S1P levels in the supernatant or plasma increase, but the S1P contents in the cells or liver also increased. The mechanism for the apoM-mediated modulation of S1P metabolism has now been fully elucidated, and we reported the possibility that apoM might retard the degradation of S1P levels64). Although still uncertain, apoM possesses a lipophilic pocket, which might bind S1P11); therefore, enzymes that degrade S1P, such as lipid phosphate phosphatase, cannot physically access S1P. Considering the same modulation of plasma S1P levels reported in several papers65, 66), the net amount of S1P in the total body might be affected by apoM. Of note, however, human plasma S1P levels were not or were only weakly correlated with serum apoM levels67) (data not shown in our previous article68)). The reasons might be that S1P is also distributed to albumin as well as HDL and/or that apoM can bind to lipids other than S1P, such as retinol69).
Since apoM rides on lipoproteins, especially HDL10), the apoM and S1P levels are affected by lipoprotein homeostasis (Fig. 3). Interestingly, when we investigated the correlation between S1P levels and lipoproteins, we observed the significant correlation between S1P levels and LDL cholesterol levels70). Therefore, we investigated the modulation of plasma S1P levels by the LDL receptor, which largely affects the LDL cholesterol levels. When we overexpressed the LDL receptor in the livers of wild-type mice, we observed a marked decrease in the plasma apoM and S1P levels, especially the S1P levels on HDL. When we overexpressed the LDL receptor in the livers of apoE-deficient mice, however, no modulations of the plasma apoM and S1P levels were seen71). Considering that both apoE and apoB act as ligands for the LDL receptor, we concluded that the LDL receptor cleared S1P on apoM-containing lipoproteins, using apoE as a ligand. Actually, in a human study, statin was reported to decrease the serum apoM levels, which agrees with this conclusion72, 73). In addition to the LDL receptor, we also investigated the modulation of plasma apoM and S1P levels using cholesteryl ester transfer protein (CETP), since CETP largely affects the HDL-cholesterol levels and has attracted attention as a possible target for overcoming hypo-HDL cholesterolemia74). When we overexpressed CETP in murine livers, we observed no modulation of the plasma apoM or S1P levels. Regarding the distribution of apoM and S1P, however, we found that the apoM and S1P contents shifted from HDL to apoB-containing lipoproteins. Interestingly, we also observed that S1P bound to apoB-containing lipoproteins possessed rather stronger properties in the phosphorylation of eNOS in HUVECs and the secretion of insulin from MIN6 cells. Therefore, CETP might augment the physiological properties of S1P in the plasma by shifting the distribution of S1P and apoM from HDL to apoB-containing lipoproteins, on which S1P might exert more potent bioactivities75).
Fig. 3.
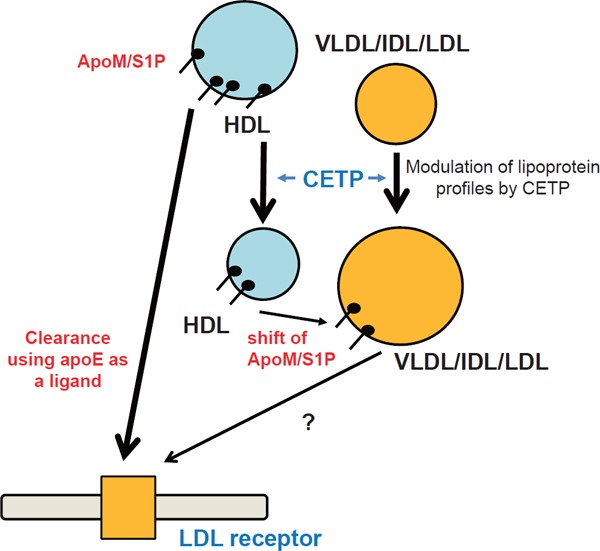
Modulation of plasma sphingosine 1-phoshate levels by homeostasis of apolipoprotein M-containing lipoproteins
Apolipoprotein M (ApoM)-containing lipoproteins are cleared through the LDL receptor using apolipoprotein E (apoE) as a ligand, and modulation of the lipid profile by cholesteryl ester transfer protein (CETP) (i.e., a decrease in HDL cholesterol and an increase in VLDL/IDL/LDL cholesterol) shifts sphingosine 1-phosphate (S1P) bound to apoM-containing HDL to VLDL/IDL/LDL.
In agreement with this idea, we and others have demonstrated that the modulation of apoM by some reagents or conditions affects the plasma S1P levels both inside and outside of cells. Regarding reagents, we have recently revealed that resveratrol modulates S1P levels by affecting apoM levels76). Resveratrol increases the cellular expression of apoM in HepG2 cells and therefore increases cellular S1P levels; regarding medium levels of apoM and S1P, although resveratrol increased the medium apoM and S1P levels when administered at a concentration up to 2 µM, it decreased the medium apoM and S1P levels when administered at a concentration of 20 µM by augmenting the LDL receptor-mediated clearance of apoM and S1P, as described above71). In human subjects, however, considering that a moderate intake of resveratrol did not increase the resveratrol concentrations in vivo to a concentration of 20 µM and that a decrease in the apoM and S1P levels was not observed in the medium of human primary hepatocyte cultures, resveratrol, which has been proposed as a supplement to prevent atherosclerosis77), might exert its anti-atherosclerotic properties, at least in part, by increasing the plasma apoM and S1P levels. Another reagent deeply involved in the regulation of apoM and S1P is insulin. Insulin treatment decreased the expression of apoM levels in murine plasma, livers, and kidneys, as well as in HepG2 cells78). The physiological regulation of apoM and S1P levels by insulin, however, remains to be elucidated. While an elevation in plasma and hepatic apoM and S1P levels was observed in streptozotocin-induced diabetic mice78), another group reported that the apoM levels were down-regulated by insulin in an alloxan-diabetic mouse model79), which is a model of insulin deficiency similar to streptozotocin-induced diabetic mice. Although the involvement of apoM has not been determined, S1P levels ar e reportedly modulated by several other reagents, such as propofol80), intralipid81), and rosiglitazone82). Further studies are necessary to elucidate the involvement of the regulation of apoM by these reagents, considering the emerging importance of apoM in the functions of S1P as described below.
As well as reagents, several pathological conditions have been reported to affect plasma S1P levels by regulating apoM. For example, apoM and/or S1P levels were reduced in subjects with severe infectious diseases83, 84) and septic animal models85). In sepsis, the plasma apoM and S1P levels are reduced. Considering the vasoprotective properties of S1P, this decrement in apoM during sepsis might somehow explain the organ injuries and disturbed vascular barrier function86). As expected from the modulation of apoM by insulin, diabetes, which is one of the major risk factors for atherosclerosis, might be associated with plasma apoM/S1P levels; S1P bound to HDL has been demonstrated to be higher in subjects with type 2 diabetes87), and although the concentration of apoM and S1P was not modulated, apoM and S1P were shifted toward light HDL particles in type 1 diabetes88). In addition, several reports have demonstrated an association between SNPs of APOM and diabetes89, 90).
Modulation of S1P Functions by apoM
In addition to the modulation of S1P metabolism by apoM, apoM might possibly influence the biological functions of S1P. S1P bound to apoM exerts more potent activities on S1P1 in endothelial cells; S1P bound to apoM containing HDL activates the ERK and Akt signals through S1P1 to a greater degree than S1P bound to albumin11), and S1P bound to apoM containing HDL activated Gi signaling to a greater degree than S1P bound to albumin and attenuated the TNFα-induced activation of NF-κB and the expression of ICAM-191). Along with endothelial cells, S1P bound to apoM augmented insulin secretion from MIN6 cells, a cell line of pancreatic β-cells, through S1P1 and/or S1P392). The reason why apoM augments the biological properties of S1P remains to be elucidated, but several possibilities exist. One possibility is that apoM maintains the S1P concentration in the medium by inhibiting S1P from degrading as a result of lipid phosphate phosphatases92). Another possibility is that S1P bound to apoM-containing HDL might access S1P1 located on the cell membrane through HDL receptors, such as SR-BI, more easily30).
Interestingly, some reports demonstrated that apoM did not only increment S1P activities, but that it might attenuate some aspects of S1P bioactivities. Unlike S1P bound to albumin, which promotes the egress of lymphocytes, S1P bound to apoM does not promote lymphocyte trafficking and instead inhibits lymphopoiesis through S1P193). Therefore, the inflammatory properties of S1P, regarding the activation of lymphocytes as described above42, 43, 94), might be limited only to S1P bound to albumin, and not to S1P bound to apoM. Regarding the pro-thrombotic properties of S1P, the carrier might also influence the physiological roles of S1P. As described above, S1P is thought to induce PAI-1, which causes thrombotic conditions51–54). However, the abilities of S1P to increase the expression of PAI-1 have not been compared between S1P bound to albumin and S1P bound to apoM. Recently, we measured the plasma S1P levels and the apoM levels and compared them with the active PAI-1 concentrations. A significant correlation was observed only between PAI-1 and S1P, and not between PAI-1 and apoM. Therefore, we compared the ability of S1P bound to albumin and that of S1P bound to apoM to induce PAI-1 expression in 3T3L1 adipocytes and found that only S1P bound to albumin, and not S1P bound to apoM, increased the PAI-1 expression. Moreover, when we performed similar experiments using HUVECs, we observed that S1P bound to apoM decreased the expression of PAI-168). Since the induction of PAI-1 by S1P can be ascribed to S1P2 signaling68), we speculated that S1P bound to apoM might not or only weakly work as an agonist for S1P2.
Considering these reports on the modulation of S1P functions by apoM, apoM appears to strengthen the agonist properties of S1P for S1P1 and/or S1P3, while it weakens the agonist properties for S1P2 (Fig. 4). Moreover, although the involvement of apoM was not investigated, S1P bound to HDL has been reported to possess mainly beneficial effects, as described above, supporting this idea. Further studies are necessary to prove this hypothesis.
Fig. 4.
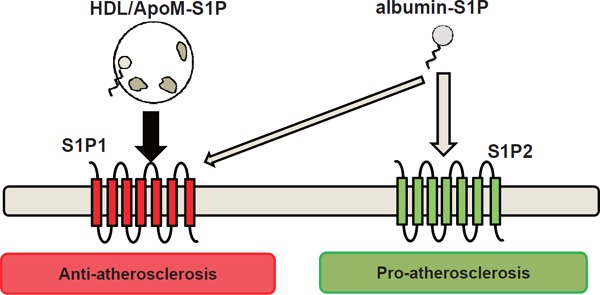
Modulation of the functions of sphingosine 1-phosphate by apoM
Apolipoprotein M (ApoM) strengthens the agonist properties of sphingosine 1-phosphate (S1P) for S1P1 (anti-atherosclerotic S1P receptor), while it weakens the agonist properties for S1P2 (proatherosclerotic S1P receptor).
S1P and apoM in Atherosclerosis in Human Samples
Despite the possible involvement of S1P in the pathogenesis of atherosclerosis, clinical evidence of the involvement of S1P in atherosclerosis has remained insufficient, possibly because plasma S1P levels should be measured in samples collected under specific conditions70) and because it would be better to investigate the association between S1P and atherosclerosis by separating S1P bound to HDL from S1P bound to albumin. At present, only four studies have shown an association between plasma S1P levels and atherosclerotic diseases95–98). Among them, Sattler et al. reported that S1P bound to HDL was less prevalent, while S1P uncoupled from HDL was more prevalent, in subjects with myocardial infarction and stable angina96), supporting the idea that S1P bound to apoM possesses anti-atherosclerotic properties while S1P bound to albumin might be somehow involved in the harmful effects of S1P (Fig. 4). Other clinical studies have also demonstrated that the plasma S1P levels were lower in patients with myocardial infarction95, 97) and that S1P bound to HDL might predict the severity of coronary heart disease98).
Regarding apoM, although an association between the total apoM level and atherosclerosis has not been shown68), APOM polymorphism has been reported to be associated with atherosclerosis99, 100). Further studies are needed to elucidate the involvement of apoM in human atherosclerosis.
Conclusions
The results of basic studies suggest that S1P is involved in the pathogenesis of atherosclerosis. However, a dual nature, i.e., both anti-atherosclerotic and pro-atherosclerotic properties, has been reported. Recently, apoM has been shown to act as a carrier and a modulator of S1P. ApoM largely affects the homeostasis of S1P, and importantly, S1P bound to apoM might possess only anti-atherosclerotic effects. Although further studies are needed, the emerging importance of apoM suggests that apoM might be useful for laboratory testing and/or medical therapy to realize the pleiotropic effects of HDL in clinical practice.
Acknowledgements
This work was supported by CREST from the JST/AMED, a Grant-in-Aid for Scientific Research on Innovative Areas 15H05906 (Y.Y.), JSPS KAK-ENHI Grant Numbers 1 16H06236 (M.K.), the Takeda Science Foundation, and the MSD Life Science Foundation, Public Interest Incorporated Foundation (M.K.).
Conflicts of Interest
None.
References
- 1). Goetzl EJ: Pleiotypic mechanisms of cellular responses to biologically active lysophospholipids. Prostaglandins, 2001; 64: 11-20 [DOI] [PubMed] [Google Scholar]
- 2). Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D: Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest, 2001; 108: 689-701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H: Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science, 2002; 296: 346-349 [DOI] [PubMed] [Google Scholar]
- 4). Pyne NJ, Pyne S: Sphingosine 1-phosphate and cancer. Nat Rev Cancer, 2010; 10: 489-503 [DOI] [PubMed] [Google Scholar]
- 5). Ng ML, Wadham C, Sukocheva OA: The role of sphingolipid signalling in diabetesassociated pathologies (Review). Int J Mol Med, 2017; 39: 243-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N: The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science, 2009; 323: 524-527 [DOI] [PubMed] [Google Scholar]
- 7). Koch A, Pfeilschifter J, Huwiler A: Sphingosine 1-phosphate in renal diseases. Cell Physiol Biochem, 2013; 31: 745-760 [DOI] [PubMed] [Google Scholar]
- 8). Mao-Draayer Y, Sarazin J, Fox D, Schiopu E: The sphingosine-1-phosphate receptor: A novel therapeutic target for multiple sclerosis and other autoimmune diseases. Clin Immunol, 2017; 175: 10-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Okajima F: Plasma lipoproteins behave as carriers of extracellular sphingosine 1-phosphate: is this an atherogenic mediator or an anti-atherogenic mediator? Biochim Biophys Acta, 2002; 1582: 132-137 [DOI] [PubMed] [Google Scholar]
- 10). Xu N, Dahlback B: A novel human apolipoprotein (apoM). J Biol Chem, 1999; 274: 31286-31290 [DOI] [PubMed] [Google Scholar]
- 11). Christoffersen C, Obinata H, Kumaraswamy SB, Galvani S, Ahnstrom J, Sevvana M, Egerer-Sieber C, Muller YA, Hla T, Nielsen LB, Dahlback B: Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc Natl Acad Sci U S A, 2011; 108: 9613-9618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Brewer HB, Jr.: Clinical review: The evolving role of HDL in the treatment of high-risk patients with cardiovascular disease. J Clin Endocrinol Metab, 2011; 96: 1246-1257 [DOI] [PubMed] [Google Scholar]
- 13). Levkau B: HDL-S1P: cardiovascular functions, disease-associated alterations, and therapeutic applications. Front Pharmacol, 2015; 6: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Le Stunff H, Milstien S, Spiegel S: Generation and metabolism of bioactive sphingosine-1-phosphate. J Cell Biochem, 2004; 92: 882-899 [DOI] [PubMed] [Google Scholar]
- 15). Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR: Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science, 2007; 316: 295-298 [DOI] [PubMed] [Google Scholar]
- 16). Yatomi Y, Ruan F, Hakomori S, Igarashi Y: Sphingosine-1-phosphate: a platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood, 1995; 86: 193-202 [PubMed] [Google Scholar]
- 17). Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T: Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res, 2008; 102: 669-676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Jonnalagadda D, Sunkara M, Morris AJ, Whiteheart SW: Granule-mediated release of sphingosine-1-phosphate by activated platelets. Biochim Biophys Acta, 2014; 1841: 1581-1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Kobayashi N, Nishi T, Hirata T, Kihara A, Sano T, Igarashi Y, Yamaguchi A: Sphingosine 1-phosphate is released from the cytosol of rat platelets in a carrier-mediated manner. J Lipid Res, 2006; 47: 614-621 [DOI] [PubMed] [Google Scholar]
- 20). Kobayashi N, Yamaguchi A, Nishi T: Characterization of the ATP-dependent sphingosine 1-phosphate transporter in rat erythrocytes. J Biol Chem, 2009; 284: 21192-21200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Kurano M, Nishikawa M, Kuma H, Jona M, Yatomi Y: Involvement of Band3 in the efflux of sphingosine 1-phosphate from erythrocytes. PLoS One, 2017; 12: e0177543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Fukuhara S, Simmons S, Kawamura S, Inoue A, Orba Y, Tokudome T, Sunden Y, Arai Y, Moriwaki K, Ishida J, Uemura A, Kiyonari H, Abe T, Fukamizu A, Hirashima M, Sawa H, Aoki J, Ishii M, Mochizuki N: The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J Clin Invest, 2012; 122: 1416-1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Sciorra VA, Morris AJ: Roles for lipid phosphate phosphatases in regulation of cellular signaling. Biochim Biophys Acta, 2002; 1582: 45-51 [DOI] [PubMed] [Google Scholar]
- 24). Yu Y, Guo S, Feng Y, Feng L, Cui Y, Song G, Luo T, Zhang K, Wang Y, Jiang XC, Qin S: Phospholipid transfer protein deficiency decreases the content of S1P in HDL via the loss of its transfer capability. Lipids, 2014; 49: 183-190 [DOI] [PubMed] [Google Scholar]
- 25). Kimura T, Sato K, Kuwabara A, Tomura H, Ishiwara M, Kobayashi I, Ui M, Okajima F: Sphingosine 1-phosphate may be a major component of plasma lipoproteins responsible for the cytoprotective actions in human umbilical vein endothelial cells. J Biol Chem, 2001; 276: 31780-31785 [DOI] [PubMed] [Google Scholar]
- 26). Nofer JR, Levkau B, Wolinska I, Junker R, Fobker M, von Eckardstein A, Seedorf U, Assmann G: Suppression of endothelial cell apoptosis by high density lipoproteins (HDL) and HDL-associated lysosphingolipids. J Biol Chem, 2001; 276: 34480-34485 [DOI] [PubMed] [Google Scholar]
- 27). Kimura T, Sato K, Malchinkhuu E, Tomura H, Tamama K, Kuwabara A, Murakami M, Okajima F: High-density lipoprotein stimulates endothelial cell migration and survival through sphingosine 1-phosphate and its receptors. Arterioscler Thromb Vasc Biol, 2003; 23: 1283-1288 [DOI] [PubMed] [Google Scholar]
- 28). Feuerborn R, Becker S, Poti F, Nagel P, Brodde M, Schmidt H, Christoffersen C, Ceglarek U, Burkhardt R, Nofer JR: High density lipoprotein (HDL)-associated sphingosine 1-phosphate (S1P) inhibits macrophage apoptosis by stimulating STAT3 activity and survivin expression. Atherosclerosis, 2017; 257: 29-37 [DOI] [PubMed] [Google Scholar]
- 29). Nofer JR, van der Giet M, Tolle M, Wolinska I, von Wnuck Lipinski K, Baba HA, Tietge UJ, Godecke A, Ishii I, Kleuser B, Schafers M, Fobker M, Zidek W, Assmann G, Chun J, Levkau B: HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest, 2004; 113: 569-581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Kimura T, Tomura H, Mogi C, Kuwabara A, Damirin A, Ishizuka T, Sekiguchi A, Ishiwara M, Im DS, Sato K, Murakami M, Okajima F: Role of scavenger receptor class B type I and sphingosine 1-phosphate receptors in high density lipoprotein-induced inhibition of adhesion molecule expression in endothelial cells. J Biol Chem, 2006; 281: 37457-37467 [DOI] [PubMed] [Google Scholar]
- 31). Igarashi J, Miyoshi M, Hashimoto T, Kubota Y, Kosaka H: Statins induce S1P1 receptors and enhance endothelial nitric oxide production in response to high-density lipoproteins. Br J Pharmacol, 2007; 150: 470-479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Kimura T, Mogi C, Tomura H, Kuwabara A, Im DS, Sato K, Kurose H, Murakami M, Okajima F: Induction of scavenger receptor class B type I is critical for simvastatin enhancement of high-density lipoprotein-induced anti-inflammatory actions in endothelial cells. J Immunol, 2008; 181: 7332-7340 [DOI] [PubMed] [Google Scholar]
- 33). Xu M, Waters CL, Hu C, Wysolmerski RB, Vincent PA, Minnear FL: Sphingosine 1-phosphate rapidly increases endothelial barrier function independently of VE-cadherin but requires cell spreading and Rho kinase. Am J Physiol Cell Physiol, 2007; 293: C1309-1318 [DOI] [PubMed] [Google Scholar]
- 34). Argraves KM, Gazzolo PJ, Groh EM, Wilkerson BA, Matsuura BS, Twal WO, Hammad SM, Argraves WS: High density lipoprotein-associated sphingosine 1-phosphate promotes endothelial barrier function. J Biol Chem, 2008; 283: 25074-25081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Filep JG, Foldes-Filep E, Sirois P: Nitric oxide modulates vascular permeability in the rat coronary circulation. Br J Pharmacol, 1993; 108: 323-326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Ruiz M, Frej C, Holmer A, Guo LJ, Tran S, Dahlback B: High-Density Lipoprotein-Associated Apolipoprotein M Limits Endothelial Inflammation by Delivering Sphingosine-1-Phosphate to the Sphingosine-1-Phosphate Receptor 1. Arterioscler Thromb Vasc Biol, 2017; 37: 118-129 [DOI] [PubMed] [Google Scholar]
- 37). Duenas AI, Aceves M, Fernandez-Pisonero I, Gomez C, Orduna A, Crespo MS, Garcia-Rodriguez C: Selective attenuation of Toll-like receptor 2 signalling may explain the atheroprotective effect of sphingosine 1-phosphate. Cardiovasc Res, 2008; 79: 537-544 [DOI] [PubMed] [Google Scholar]
- 38). Tamama K, Tomura H, Sato K, Malchinkhuu E, Damirin A, Kimura T, Kuwabara A, Murakami M, Okajima F: High-density lipoprotein inhibits migration of vascular smooth muscle cells through its sphingosine 1-phosphate component. Atherosclerosis, 2005; 178: 19-23 [DOI] [PubMed] [Google Scholar]
- 39). Frias MA, James RW, Gerber-Wicht C, Lang U: Native and reconstituted HDL activate Stat3 in ventricular cardiomyocytes via ERK1/2: role of sphingosine-1-phosphate. Cardiovasc Res, 2009; 82: 313-323 [DOI] [PubMed] [Google Scholar]
- 40). Tao R, Hoover HE, Honbo N, Kalinowski M, Alano CC, Karliner JS, Raffai R: High-density lipoprotein determines adult mouse cardiomyocyte fate after hypoxia-reoxygenation through lipoprotein-associated sphingosine 1-phosphate. Am J Physiol Heart Circ Physiol, 2010; 298: H1022-1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Pelletier D, Hafler DA: Fingolimod for multiple sclerosis. N Engl J Med, 2012; 366: 339-347 [DOI] [PubMed] [Google Scholar]
- 42). Goetzl EJ, Graler MH: Sphingosine 1-phosphate and its type 1 G protein-coupled receptor: trophic support and functional regulation of T lymphocytes. J Leukoc Biol, 2004; 76: 30-35 [DOI] [PubMed] [Google Scholar]
- 43). Swan DJ, Kirby JA, Ali S: Vascular biology: the role of sphingosine 1-phosphate in both the resting state and inflammation. J Cell Mol Med, 2010; 14: 2211-2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44). Shatrov VA, Lehmann V, Chouaib S: Sphingosine-1-phosphate mobilizes intracellular calcium and activates transcription factor NF-kappa B in U937 cells. Biochem Biophys Res Commun, 1997; 234: 121-124 [DOI] [PubMed] [Google Scholar]
- 45). Keul P, Lucke S, von Wnuck Lipinski K, Bode C, Graler M, Heusch G, Levkau B: Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis. Circ Res, 2011; 108: 314-323 [DOI] [PubMed] [Google Scholar]
- 46). Lewis ND, Haxhinasto SA, Anderson SM, Stefanopoulos DE, Fogal SE, Adusumalli P, Desai SN, Patnaude LA, Lukas SM, Ryan KR, Slavin AJ, Brown ML, Modis LK: Circulating monocytes are reduced by sphingosine-1-phosphate receptor modulators independently of S1P3. J Immunol, 2013; 190: 3533-3540 [DOI] [PubMed] [Google Scholar]
- 47). Allende ML, Zhou D, Kalkofen DN, Benhamed S, Tuymetova G, Borowski C, Bendelac A, Proia RL: S1P1 receptor expression regulates emergence of NKT cells in peripheral tissues. FASEB J, 2008; 22: 307-315 [DOI] [PubMed] [Google Scholar]
- 48). Jenne CN, Enders A, Rivera R, Watson SR, Bankovich AJ, Pereira JP, Xu Y, Roots CM, Beilke JN, Banerjee A, Reiner SL, Miller SA, Weinmann AS, Goodnow CC, Lanier LL, Cyster JG, Chun J: T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J Exp Med, 2009; 206: 2469-2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49). Grimm M, Tischner D, Troidl K, Albarran Juarez J, Sivaraj KK, Ferreiros Bouzas N, Geisslinger G, Binder CJ, Wettschureck N: S1P2/G12/13 Signaling Negatively Regulates Macrophage Activation and Indirectly Shapes the Atheroprotective B1-Cell Population. Arterioscler Thromb Vasc Biol, 2016; 36: 37-48 [DOI] [PubMed] [Google Scholar]
- 50). Takeya H, Gabazza EC, Aoki S, Ueno H, Suzuki K: Synergistic effect of sphingosine 1-phosphate on thrombin-induced tissue factor expression in endothelial cells. Blood, 2003; 102: 1693-1700 [DOI] [PubMed] [Google Scholar]
- 51). Lee MH, Hammad SM, Semler AJ, Luttrell LM, Lopes-Virella MF, Klein RL: HDL3, but not HDL2, stimulates plasminogen activator inhibitor-1 release from adipocytes: the role of sphingosine-1-phosphate. J Lipid Res, 2010; 51: 2619-2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52). Ito S, Iwaki S, Koike K, Yuda Y, Nagasaki A, Ohkawa R, Yatomi Y, Furumoto T, Tsutsui H, Sobel BE, Fujii S: Increased plasma sphingosine-1-phosphate in obese individuals and its capacity to increase the expression of plasminogen activator inhibitor-1 in adipocytes. Coron Artery Dis, 2013; 24: 642-650 [DOI] [PubMed] [Google Scholar]
- 53). Iwaki S, Yamamura S, Asai M, Sobel BE, Fujii S: Posttranscriptional regulation of expression of plasminogen activator inhibitor type-1 by sphingosine 1-phosphate in HepG2 liver cells. Biochim Biophys Acta, 2012; 1819: 1132-1141 [DOI] [PubMed] [Google Scholar]
- 54). Bryan L, Paugh BS, Kapitonov D, Wilczynska KM, Alvarez SM, Singh SK, Milstien S, Spiegel S, Kordula T: Sphingosine-1-phosphate and interleukin-1 independently regulate plasminogen activator inhibitor-1 and urokinase-type plasminogen activator receptor expression in glioblastoma cells: implications for invasiveness. Mol Cancer Res, 2008; 6: 1469-1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55). Bohm A, Flosser A, Ermler S, Fender AC, Luth A, Kleuser B, Schror K, Rauch BH: Factor-Xa-induced mitogenesis and migration require sphingosine kinase activity and S1P formation in human vascular smooth muscle cells. Cardiovasc Res, 2013; 99: 505-513 [DOI] [PubMed] [Google Scholar]
- 56). Urtz N, Gaertner F, von Bruehl ML, Chandraratne S, Rahimi F, Zhang L, Orban M, Barocke V, Beil J, Schubert I, Lorenz M, Legate KR, Huwiler A, Pfeilschifter JM, Beerli C, Ledieu D, Persohn E, Billich A, Baumruker T, Mederos y Schnitzler M, Massberg S: Sphingosine 1-Phosphate Produced by Sphingosine Kinase 2 Intrinsically Controls Platelet Aggregation In Vitro and In Vivo. Circ Res, 2015; 117: 376-387 [DOI] [PubMed] [Google Scholar]
- 57). Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T: Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol, 2007; 27: 1312-1318 [DOI] [PubMed] [Google Scholar]
- 58). Theilmeier G, Schmidt C, Herrmann J, Keul P, Schafers M, Herrgott I, Mersmann J, Larmann J, Hermann S, Stypmann J, Schober O, Hildebrand R, Schulz R, Heusch G, Haude M, von Wnuck Lipinski K, Herzog C, Schmitz M, Erbel R, Chun J, Levkau B: High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation, 2006; 114: 1403-1409 [DOI] [PubMed] [Google Scholar]
- 59). Skoura A, Michaud J, Im DS, Thangada S, Xiong Y, Smith JD, Hla T: Sphingosine-1-phosphate receptor-2 function in myeloid cells regulates vascular inflammation and atherosclerosis. Arterioscler Thromb Vasc Biol, 2011; 31: 81-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60). Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL: Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest, 2000; 106: 951-961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61). Poti F, Gualtieri F, Sacchi S, Weissen-Plenz G, Varga G, Brodde M, Weber C, Simoni M, Nofer JR: KRP-203, sphingosine 1-phosphate receptor type 1 agonist, ameliorates atherosclerosis in LDL-R-/- mice. Arterioscler Thromb Vasc Biol, 2013; 33: 1505-1512 [DOI] [PubMed] [Google Scholar]
- 62). Poti F, Ceglarek U, Burkhardt R, Simoni M, Nofer JR: SKI-II--a sphingosine kinase 1 inhibitor--exacerbates atherosclerosis in low-density lipoprotein receptor-deficient (LDL-R-/-) mice on high cholesterol diet. Atherosclerosis, 2015; 240: 212-215 [DOI] [PubMed] [Google Scholar]
- 63). Wolfrum C, Poy MN, Stoffel M: Apolipoprotein M is required for prebeta-HDL formation and cholesterol efflux to HDL and protects against atherosclerosis. Nat Med, 2005; 11: 418-422 [DOI] [PubMed] [Google Scholar]
- 64). Kurano M, Tsukamoto K, Ohkawa R, Hara M, Iino J, Kageyama Y, Ikeda H, Yatomi Y: Liver involvement in sphingosine 1-phosphate dynamism revealed by adenoviral hepatic overexpression of apolipoprotein M. Atherosclerosis, 2013; 229: 102-109 [DOI] [PubMed] [Google Scholar]
- 65). Liu M, Seo J, Allegood J, Bi X, Zhu X, Boudyguina E, Gebre AK, Avni D, Shah D, Sorci-Thomas MG, Thomas MJ, Shelness GS, Spiegel S, Parks JS: Hepatic apolipoprotein M (apoM) overexpression stimulates formation of larger apoM/sphingosine 1-phosphate-enriched plasma high density lipoprotein. J Biol Chem, 2014; 289: 2801-2814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66). Liu M, Allegood J, Zhu X, Seo J, Gebre AK, Boudyguina E, Cheng D, Chuang CC, Shelness GS, Spiegel S, Parks JS: Uncleaved ApoM signal peptide is required for formation of large ApoM/sphingosine 1-phosphate (S1P)-enriched HDL particles. J Biol Chem, 2015; 290: 7861-7870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67). Karuna R, Park R, Othman A, Holleboom AG, Motazacker MM, Sutter I, Kuivenhoven JA, Rohrer L, Matile H, Hornemann T, Stoffel M, Rentsch KM, von Eckardstein A: Plasma levels of sphingosine-1-phosphate and apolipoprotein M in patients with monogenic disorders of HDL metabolism. Atherosclerosis, 2011; 219: 855-863 [DOI] [PubMed] [Google Scholar]
- 68). Takahashi C, Kurano M, Nishikawa M, Kano K, Dohi T, Miyauchi K, Daida H, Shimizu T, Aoki J, Yatomi Y: Vehicle-dependent Effects of Sphingosine 1-phosphate on Plasminogen Activator Inhibitor-1 Expression. J Atheroscler Thromb, 2017; 24: 954-969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69). Sevvana M, Ahnstrom J, Egerer-Sieber C, Lange HA, Dahlback B, Muller YA: Serendipitous fatty acid binding reveals the structural determinants for ligand recognition in apolipoprotein M. J Mol Biol, 2009; 393: 920-936 [DOI] [PubMed] [Google Scholar]
- 70). Ohkawa R, Nakamura K, Okubo S, Hosogaya S, Ozaki Y, Tozuka M, Osima N, Yokota H, Ikeda H, Yatomi Y: Plasma sphingosine-1-phosphate measurement in healthy subjects: close correlation with red blood cell parameters. Ann Clin Biochem, 2008; 45: 356-363 [DOI] [PubMed] [Google Scholar]
- 71). Kurano M, Tsukamoto K, Hara M, Ohkawa R, Ikeda H, Yatomi Y: LDL receptor and ApoE are involved in the clearance of ApoM-associated sphingosine 1-phosphate. J Biol Chem, 2015; 290: 2477-2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72). Kappelle PJ, Ahnstrom J, Dikkeschei BD, de Vries R, Sluiter WJ, Wolffenbuttel BH, van Tol A, Nielsen LB, Dahlback B, Dullaart RP: Plasma apolipoprotein M responses to statin and fibrate administration in type 2 diabetes mellitus. Atherosclerosis, 2010; 213: 247-250 [DOI] [PubMed] [Google Scholar]
- 73). Thongtang N, Diffenderfer MR, Ooi EM, Barrett PH, Turner SM, Le NA, Brown WV, Schaefer EJ: Metabolism and proteomics of large and small dense LDL in combined hyperlipidemia. Effects of rosuvastatin. J Lipid Res, 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74). Rader DJ, deGoma EM: Future of cholesteryl ester transfer protein inhibitors. Annu Rev Med, 2014; 65: 385-403 [DOI] [PubMed] [Google Scholar]
- 75). Kurano M, Hara M, Ikeda H, Tsukamoto K, Yatomi Y: Involvement of CETP (Cholesteryl Ester Transfer Protein) in the Shift of Sphingosine-1-Phosphate Among Lipoproteins and in the Modulation of its Functions. Arterioscler Thromb Vasc Biol, 2017; 37: 506-514 [DOI] [PubMed] [Google Scholar]
- 76). Kurano M, Hara M, Nojiri T, Ikeda H, Tsukamoto K, Yatomi Y: Resveratrol exerts a biphasic effect on apolipo-protein M. Br J Pharmacol, 2016; 173: 222-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77). Wu JM, Wang ZR, Hsieh TC, Bruder JL, Zou JG, Huang YZ: Mechanism of cardioprotection by resveratrol, a phenolic antioxidant present in red wine (Review). Int J Mol Med, 2001; 8: 3-17 [DOI] [PubMed] [Google Scholar]
- 78). Nojiri T, Kurano M, Tokuhara Y, Ohkubo S, Hara M, Ikeda H, Tsukamoto K, Yatomi Y: Modulation of sphingosine-1-phosphate and apolipoprotein M levels in the plasma, liver and kidneys in streptozotocin-induced diabetic mice. J Diabetes Investig, 2014; 5: 639-648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79). Xu N, Nilsson-Ehle P, Ahren B: Suppression of apolipo protein M expression and secretion in alloxan-diabetic mouse: Partial reversal by insulin. Biochem Biophys Res Commun, 2006; 342: 1174-1177 [DOI] [PubMed] [Google Scholar]
- 80). Ma X, Zhao JY, Zhao ZL, Ye J, Li SF, Fang HH, Gu MN, Hu YW, Qin ZS: Propofol Attenuates Lipopolysaccharide-Induced Monocyte Chemoattractant Protein-1 Production Through Enhancing apoM and foxa2 Expression in HepG2 Cells. Inflammation, 2015; 38: 1329-1336 [DOI] [PubMed] [Google Scholar]
- 81). Zheng L, Feng Y, Shi Y, Zhang J, Mu Q, Qin L, Berggren-Soderlund M, Nilsson-Ehle P, Zhang X, Luo G, Xu N: Intralipid decreases apolipoprotein M levels and insulin sensitivity in rats. PLoS One, 2014; 9: e105681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82). Luo G, Feng Y, Zhang J, Mu Q, Shi Y, Qin L, Zheng L, Berggren-Soderlund M, Nilsson-Ehle P, Zhang X, Xu N: Rosiglitazone enhances apolipoprotein M (Apom) expression in rat's liver. Int J Med Sci, 2014; 11: 1015-1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83). Kumaraswamy SB, Linder A, Akesson P, Dahlback B: Decreased plasma concentrations of apolipoprotein M in sepsis and systemic inflammatory response syndromes. Crit Care, 2012; 16: R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84). Michels M, Japtok L, Alisjahbana B, Wisaksana R, Sumardi U, Puspita M, Kleuser B, de Mast Q, van der Ven AJ: Decreased plasma levels of the endothelial protective sphingosine-1-phosphate are associated with dengue-induced plasma leakage. J Infect, 2015; 71: 480-487 [DOI] [PubMed] [Google Scholar]
- 85). Palmiere C, Bonsignore A, Augsburger M: Measurement of apolipoprotein M in sepsis-related deaths. Clin Chem Lab Med, 2015; 53: e93-96 [DOI] [PubMed] [Google Scholar]
- 86). Christoffersen C, Nielsen LB: Apolipoprotein M--a new biomarker in sepsis. Crit Care, 2012; 16: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87). Tong X, Peng H, Liu D, Ji L, Niu C, Ren J, Pan B, Hu J, Zheng L, Huang Y: High-density lipoprotein of patients with type 2 diabetes mellitus upregulates cyclooxgenase-2 expression and prostacyclin I-2 release in endothelial cells: relationship with HDL-associated sphingosine-1-phosphate. Cardiovasc Diabetol, 2013; 12: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88). Frej C, Mendez AJ, Ruiz M, Castillo M, Hughes TA, Dahlback B, Goldberg RB: A Shift in ApoM/S1P Between HDL-Particles in Women With Type 1 Diabetes Mellitus Is Associated With Impaired Anti-Inflammatory Effects of the ApoM/S1P Complex. Arterioscler Thromb Vasc Biol, 2017; 37: 1194-1205 [DOI] [PubMed] [Google Scholar]
- 89). Niu N, Zhu X, Liu Y, Du T, Wang X, Chen D, Sun B, Gu HF: Single nucleotide polymorphisms in the proximal promoter region of apolipoprotein M gene (apoM) confer the susceptibility to development of type 2 diabetes in Han Chinese. Diabetes Metab Res Rev, 2007; 23: 21-25 [DOI] [PubMed] [Google Scholar]
- 90). Zhou JW, Tsui SK, Ng MC, Geng H, Li SK, So WY, Ma RC, Wang Y, Tao Q, Chen ZY, Chan JC, Ho YY: Apolipoprotein M gene (APOM) polymorphism modifies metabolic and disease traits in type 2 diabetes. PLoS One, 2011; 6: e17324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91). Galvani S, Sanson M, Blaho VA, Swendeman SL, Conger H, Dahlback B, Kono M, Proia RL, Smith JD, Hla T: HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci Signal, 2015; 8: ra79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92). Kurano M, Hara M, Tsuneyama K, Sakoda H, Shimizu T, Tsukamoto K, Ikeda H, Yatomi Y: Induction of insulin secretion by apolipoprotein M, a carrier for sphingosine 1-phosphate. Biochim Biophys Acta, 2014; 1841:1217-1226 [DOI] [PubMed] [Google Scholar]
- 93). Blaho VA, Galvani S, Engelbrecht E, Liu C, Swendeman SL, Kono M, Proia RL, Steinman L, Han MH, Hla T: HDL-bound sphingosine-1-phosphate restrains lymphopoiesis and neuroinflammation. Nature, 2015; 523: 342-346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94). Rivera J, Proia RL, Olivera A: The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol, 2008; 8: 753-763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95). Knapp M, Baranowski M, Czarnowski D, Lisowska A, Zabielski P, Gorski J, Musial W: Plasma sphingosine-1-phosphate concentration is reduced in patients with myocardial infarction. Med Sci Monit, 2009; 15: CR490-493 [PubMed] [Google Scholar]
- 96). Sattler KJ, Elbasan S, Keul P, Elter-Schulz M, Bode C, Graler MH, Brocker-Preuss M, Budde T, Erbel R, Heusch G, Levkau B: Sphingosine 1-phosphate levels in plasma and HDL are altered in coronary artery disease. Basic Res Cardiol, 2010; 105: 821-832 [DOI] [PubMed] [Google Scholar]
- 97). Knapp M, Lisowska A, Zabielski P, Musial W, Baranowski M: Sustained decrease in plasma sphingosine-1-phosphate concentration and its accumulation in blood cells in acute myocardial infarction. Prostaglandins Other Lipid Mediat, 2013; 106: 53-61 [DOI] [PubMed] [Google Scholar]
- 98). Sattler K, Lehmann I, Graler M, Brocker-Preuss M, Erbel R, Heusch G, Levkau B: HDL-bound sphingosine 1-phosphate (S1P) predicts the severity of coronary artery atherosclerosis. Cell Physiol Biochem, 2014; 34: 172-184 [DOI] [PubMed] [Google Scholar]
- 99). Jiao GQ, Yuan ZX, Xue YS, Yang CJ, Lu CB, Lu ZQ, Xiao MD: A prospective evaluation of apolipoprotein M gene T-778C polymorphism in relation to coronary artery disease in Han Chinese. Clin Biochem, 2007; 40: 1108-1112 [DOI] [PubMed] [Google Scholar]
- 100). Xu WW, Zhang Y, Tang YB, Xu YL, Zhu HZ, Ferro A, Ji Y, Chen Q, Fan LM: A genetic variant of apolipoprotein M increases susceptibility to coronary artery disease in a Chinese population. Clin Exp Pharmacol Physiol, 2008; 35: 546-551 [DOI] [PubMed] [Google Scholar]


