Abstract
Aim: Smoking cessation is particularly important for maintaining health; however, the subsequent risk of an increased body weight is of major concern. The present study investigated the influence of smoking cessation on the incidence of metabolic syndrome and its components in the Japanese general population.
Methods: This study enrolled individuals without metabolic syndrome or a history of smoking via our annual health checkup program (n = 5,702, 55.2 ± 11.5 years). Participants were divided into three groups mentioned below and followed up with the endpoint being the development of metabolic syndrome: (1) subjects who had never smoked and did not smoke during the observation period (non-smoker); (2) those who continued smoking during the observation period (continuous smoker); and (3) those who ceased smoking during the observation period (smoking cessation).
Results: During the observation period (median 1,089 days), 520 subjects developed metabolic syndrome, and Kaplan–Meier analysis showed a higher incidence of metabolic syndrome in the smoking cessation group than in the other groups. Smoking cessation was confirmed as an independent predictor of the new onset of metabolic syndrome by multivariate Cox proportional hazard analysis after adjustment. Subjects only from the smoking cessation group showed a significant deterioration in metabolic factors during the study in correlation with an increased waist circumference after smoking cessation.
Conclusions: Smoking cessation without instruction could be followed by the development of metabolic syndrome, and the incidence of metabolic syndrome might reduce the benefit obtained from smoking cessation. Therefore, further educational outreach is needed to prevent the progression of metabolic syndrome during the course of smoking cessation.
Keywords: Smoking, Cessation, Metabolic syndrome, Body weight, Waist circumference
Introduction
Smoking induces systemic vascular damage and is a major risk factor for cardiovascular disease1, 2). However, while smoking cessation can substantially reduce the risk of cardiovascular disease3, 4), an increased body weight following smoking cessation is a frequent phenomenon that cannot be ignored5, 6). Smoking cessation is considered to promote food and calorie intake, and reduce basal metabolism due to the reduced energy needed for nicotine metabolism7), thereby resulting in an increase in body weight. According to a recent study, smokers who ceased smoking had a significant association with an increase in body weight8). Besides obesity also being a risk factor for cardiovascular disease9, 10), an increased body weight due to hyperphagia often causes accumulation of visceral fat, which is frequently accompanied by other risk factors, such as impaired glucose tolerance, elevated blood pressure, and lipid metabolism disorder, leading to the development of metabolic syndrome11). Thus, smoking cessation may increase body weight and the risk of metabolic syndrome, and thus counteract the beneficial effects of not smoking.
Despite several studies on body weight gain after smoking cessation, there are limited data on the subsequent development of metabolic syndrome5, 6, 12, 13), particularly on how ethnicity might affect the impact of an increasing body weight as a risk factor for cardiovascular disease. For instance, obesity is not a significant risk factor for myocardial infarction in the Japanese population14), while the incidence of diabetes is higher in Japan than in Western countries among individuals with similar body weight gain15, 16). This study thus investigated the effects of smoking cessation on the incidence of metabolic syndrome and its components in the Japanese general population.
Methods
Study Design
This was a retrospective observational study of participants in our annual health checkup program at the Department of Health Checkup, Enshu Hospital. The study protocol was approved by the Ethics Committee of Enshu Hospital and written informed consent was obtained from all subjects prior to the start of the study.
Study Subjects and Procedures
Individuals who visited the Department of Health Checkup, Enshu Hospital two times or more from July 2008 to June 2013 (n = 9,018) were screened for eligibility for this study. Subjects with metabolic syndrome at the first visit (n = 1,273) and those with incomplete data (n = 39) were first excluded. Then, those participants who did not smoke at the first visit, but had a history of smoking (n = 1,918), and those who started smoking during the observation period (n = 86) were excluded. The remaining 5,702 participants (males: 49.7%, average age: 55.2 ± 11.5 years) were finally enrolled in this study and followed up with the endpoint being the onset of metabolic syndrome.
We divided subjects into the following three groups: (1) subjects who had never smoked and did not smoke during the observation period (non-smoker); (2) those who continued smoking during the observation period (continuous smoker); and (3) those who ceased smoking during the observation period (smoking cessation). Information about the smoking status was obtained by questionnaire. Data obtained at the first health checkup during the study period were considered as baseline data in the non-smoker and continuous smoker groups and data obtained at the last health checkup as a smoker (data collected immediately prior to smoking cessation) were considered as baseline data in the smoking cessation group. Group differences in the incidence of metabolic syndrome after the collection of baseline data during the observation period were analyzed using the Kaplan–Meier method. Changes in each component of the metabolic syndrome during the observation period were compared among the groups.
Metabolic syndrome was defined based on the Japanese diagnostic criteria (waist circumference ≥ 85 cm for males and ≥ 90 cm for females and 2 or more of the following three criteria: (1) triglyceride ≥ 150 mg/dL and/or high-density lipoprotein (HDL) cholesterol < 40 mg/dL; (2) systolic pressure ≥ 130 mmHg and/or diastolic pressure ≥ 85 mmHg; and (3) fasting plasma glucose ≥ 110 mg/dL)17). Dyslipidemia was defined as low-density lipoprotein (LDL) cholesterol ≥ 140 mg/dL, triglyceride ≥ 150 mg/dL, HDL-cholesterol < 40 mg/dL, and/or use of anti-lipid drugs18). Hypertension was defined as systolic pressure ≥ 140 mmHg, diastolic pressure ≥ 90 mmHg, and/or use of anti-hypertensive drug. Diabetes mellitus was defined as fasting plasma glucose ≥ 126 mg/dL and/or use of anti-diabetic medication19, 20).
Statistical Analysis
All the data are expressed as the mean ± SD and were analyzed using IBM SPSS statistics 24 software (Chicago, IL). The paired Student's t test was used for the comparison of data obtained at baseline and at the end of the observation period. Differences in continuous variables among the three groups were tested using analysis of variance (ANOVA) followed by Scheffe's post-hoc analysis. The chi-squared test was used for comparison of categorized data among three groups, which was followed by 2-way contingency table analysis. Cumulative incidence rates of metabolic syndrome were calculated using the Kaplan–Meier method. Differences in the cumulative incidence rates were evaluated by the log-rank test and adjusted by multivariate Cox proportional hazard regression models. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. Statistical significance was set as < 5%.
Results
The number of visits per participant after baseline assessment during the observation period was as follows: 2 visits (n = 1,722), 3 visits (n = 1,167), 4 visits (n = 1,032), 5 visits (n = 1,671), and 6 visits (n = 110). A total of 398 subjects ceased smoking during the observation period. Table 1 lists the subject demographics across the three groups at baseline. The smoking cessation group was older and had lower triglyceride levels than the continuous smoker group, while BMI, fasting plasma glucose, and HDL cholesterol were similar in both these groups.
Table 1. Demographics of study participants at baseline; comparison by smoking status.
| Non-smoker group | Continuous smoker group | Smoking cessation group | |
|---|---|---|---|
| (n = 3,968) | (n = 1,336) | (n = 398) | |
| Age (years) | 55.6 ± 11.3 | 50.9 ± 11.1* | 55.9 ± 11.3§ |
| Male, n (%) | 1,262 (31.8%) | 1,205 (90.2%) | 368 (92.5%) |
| Body mass index (kg/m2) | 22.0 ± 2.9 | 22.3 ± 2.9* | 22.0 ± 2.9 |
| Waist circumference (cm) | 81.8 ± 8.0 | 82.7 ± 8.2 | 82.2 ± 7.9 |
| Systolic blood pressure (mmHg) | 123.8 ± 16.1 | 118.8 ± 14.5 | 119.9 ± 15.4* |
| Diastolic blood pressure (mmHg) | 75.4 ± 9.6 | 74.2 ± 9.6* | 74.4 ± 9.8 |
| Pulse rate (bpm) | 63.8 ± 9.5 | 61.1 ± 8.7* | 60.0 ± 8.0* |
| Fasting plasma glucose (mg/dL) | 94.2 ± 14.1 | 94.4 ± 15.8 | 93.8 ± 13.7 |
| LDL-cholesterol (mg/dL) | 121.1 ± 27.0 | 116.0 ± 28.9* | 115.9 ± 27.1 * |
| HDL-cholesterol (mg/dL) | 62.6 ± 13.8 | 56.1 ± 13.2 | 57.3 ± 12.5 |
| Triglyceride (mg/dL) | 92.5 ± 51.0 | 120.0 ± 85.6* | 105.7 ± 69.7*§ |
| Serum creatinine (mg/dL) | 0.70 ± 0.21 | 0.80 ± 0.27* | 0.80 ± 0.14* |
| Uric acid (mg/dL) | 4.9 ± 1.2 | 5.8 ± 1.3* | 5.7 ± 1.2* |
| Comorbidities: | |||
| Hypertension, n (%) | 979 (24.7%) | 196 (14.7%) | 80 (20.1%)*§ |
| Diabetes mellitus, n (%) | 170 (4.3%) | 57 (4.3%) | 15 (3.8%) |
| Dyslipidemia, n (%) | 1,428 (36.0%) | 502 (37.6%) | 122 (30.%)*§ |
P < 0.05 vs. non-smoker group,
P < 0.05 vs. continuing smoker group by ANOVA-Scheffe or chi-square test
LDL-cholesterol, low-density lipoprotein-cholesterol; HDL-cholesterol, high-density lipoprotein-cholesterol
During the median follow up of 1,089 days, 520 subjects were newly diagnosed as having metabolic syndrome (33.3/1,000 person-years; Fig. 1). The incidence was higher in males (48.0/1,000 person-years) than in females (18.7/1,000 person-years), and Kaplan–Meier analysis indicated a higher incidence of metabolic syndrome in the smoking cessation group than in the non-smoker and continuing smoker groups (Fig. 2). The results obtained by Kaplan–Meier analysis were confirmed by multivariate analysis. The new onset of metabolic syndrome was significantly associated with smoking cessation in a multivariate Cox proportional hazard model adjusted for age, gender, waist circumference, triglyceride, HDL-cholesterol, systolic blood pressure, and fasting plasma glucose at baseline (Table 2). Similar results were obtained after further adjustment for diastolic blood pressure (data not shown). In line with these observations, waist circumference, and systolic and diastolic pressures were significantly increased during the follow-up (median, 2.0 years) in the smoking cessation group, but not in the non-smoker or continuous smoker groups (Table 3), although the followup period was significantly shorter in the former than in the latter groups (both 3.0 years, p < 0.001). The mean increase in body weight and waist circumference was 1.8 kg and 1.4 cm, respectively, in the smoking cessation group, while these were 0.2 kg and −0.2 cm, respectively, in the continuous smoker group. Changes in body weight and waist circumference after smoking cessation were also correlated with each other (r = 0.775) and changes in waist circumference were significantly correlated with those in systolic blood pressure (r = 0.119), diastolic blood pressure (r = 0.171), triglyceride (r = 0.200), and HDL-cholesterol (r = −0.109).
Fig. 1.
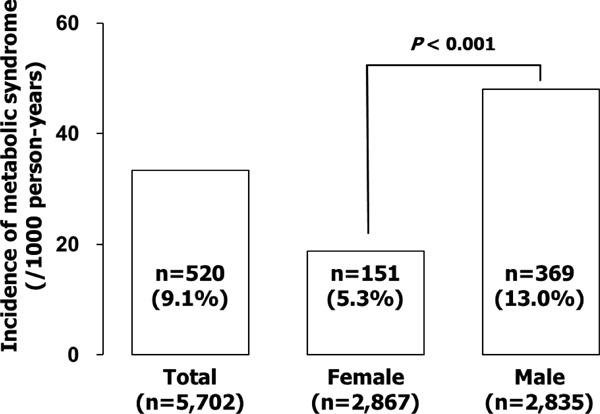
Development of metabolic syndrome during the observation period. Bar graphs show the incidence of metabolic syndrome in total, female, and male subjects. Note that the incidence was higher in males than in females.
Fig. 2.
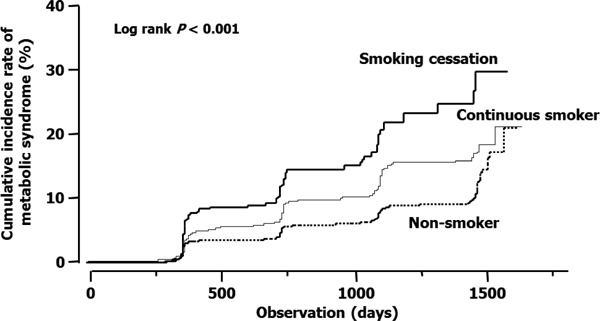
Plots of incidence rates of metabolic syndrome in the non-smoker (subjects who had never smoked and did not smoke during the observation period), continuous smoker (subjects who continued smoking during the observation period), and smoking cessation (subjects who ceased smoking during the observation period) groups. Data obtained at first visit were considered as baseline data in the non-smoker and continuous smoker groups and data obtained at the last health checkup as a smoker (data collected immediately prior to smoking cessation) were considered as baseline data in the smoking cessation group.
P < 0.001 by log-rank test.
Table 2. Cox-Hazard analysis for the incidence of metabolic syndrome.
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Smoking status: | ||||
| Non-smoker group | 1 (reference) | – | 1 (reference) | – |
| Continuous smoker group | 1.609 (1.328–1.950) | <0.001 | 1.194 (0.946–1.508) | 0.135 |
| Smoking cessation group | 2.584 (1.956–3.412) | <0.001 | 1.727 (1.276–2.338) | <0.001 |
| Age (years) | 1.026 (1.018–1.034) | <0.001 | 1.030 (1.021–1.039) | <0.001 |
| Sex (male) | 2.589 (2.142–3.128) | <0.001 | 1.976 (1.581–2.468) | <0.001 |
| Waist circumference (cm) | 1.122 (1.112–1.132) | <0.001 | 1.113 (1.102–1.124) | <0.001 |
| Triglyceride (mg/dL) | 1.003 (1.003–1.004) | <0.001 | 1.002 (1.001–1.003) | <0.001 |
| HDL-cholesterol (mg/dL) | 0.957 (0.950–0.964) | <0.001 | 0.982 (0.973–0.990) | <0.001 |
| Systolic blood pressure (mmHg) | 1.030 (1.025–1.035) | <0.001 | 1.023 (1.018–1.023) | <0.001 |
| Fasting plasma glucose (mg/dL) | 1.014 (1.012–1.017) | <0.001 | 1.012 (1.009–1.016) | <0.001 |
HDL-cholesterol, high-density lipoprotein cholesterol
Table 3. Changes in metabolic factors during the observation period.
| Non-smoker group | Continuing smoker group | Smoking cessation group | ||||
|---|---|---|---|---|---|---|
| (n = 3968) |
(n = 1336) |
(n = 398) |
||||
| Metabolic syndrome factors | Baseline | Last visit | Baseline | Last visit | Baseline | Last visit |
| Body weight (kg) | 55.3 ± 9.6 | 55.3 ± 10.0 | 62.8 ± 10.3 | 63.0 ± 10.6* | 61.0 ± 9.9 | 62.8 ± 10.2* |
| Waist circumference (cm) | 81.8 ± 8.0 | 81.5 ± 8.4* | 82.7 ± 8.2 | 82.5 ± 8.5* | 82.2 ± 7.9 | 83.6 ± 8.2* |
| Systolic blood pressure (mmHg) | 123.8 ± 16.1 | 121.9 ± 14.5* | 118.8 ± 14.5 | 119.0 ± 14.5 | 119.9 ± 15.4 | 123.4 ± 13.9* |
| Diastolic blood pressure (mmHg) | 75.4 ± 9.6 | 74.0 ± 9.0* | 74.2 ± 9.6 | 73.9 ± 9.2 | 74.4 ± 9.8 | 75.5 ± 8.9* |
| Fasting plasma glucose (mg/dL) | 94.2 ± 14.1 | 94.9 ± 13.2* | 94.4 ± 15.8 | 95.5 ± 15.3* | 93.8 ± 13.7 | 97.9 ± 14.9* |
| Triglyceride (mg/dL) | 92.5 ± 51.0 | 93.7 ± 51.6 | 120.0 ± 85.6 | 123.4 ± 95.0 | 105.7 ± 69.7 | 110.4 ± 58.1 |
| HDL-cholesterol (mg/dL) | 62.6 ± 13.8 | 62.1 ± 13.7* | 56.1 ± 13.2 | 55.8 ± 13.2 | 57.3 ± 12.5 | 59.0 ± 13.1* |
P < 0.05 vs. baseline by paired Student's t test. Data at baseline were imported from Table 1 for comparison with data obtained at the last visit. HDL-cholesterol, high-density lipoprotein cholesterol.
Discussion
This study demonstrated that smoking cessation may be followed by the new onset of metabolic syndrome in the general population without metabolic syndrome at baseline. Since deterioration in metabolic factors was closely associated with the increase in waist circumference after smoking cessation in our study cohort, health professionals and educational programs need to provide appropriate instruction against these unfavorable changes for individuals who are going to stop smoking.
The increase in body weight after smoking cessation observed in this study has already been reported in Western countries and Japan5, 6, 21–24). However, body weight gain itself does not always mean the development of metabolic syndrome and, to the best of our knowledge, this is the first study demonstrating a higher risk of metabolic syndrome in subjects who ceased smoking as compared to those who continued smoking. A previous study demonstrating an increased risk of metabolic syndrome in male subjects who stopped smoking was an observational study where non-smokers, but not continuous smokers, were considered as a reference25). Thus, this study further expands the concept that smoking cessation might increase the risk of developing metabolic syndrome in the general population.
The results of Kaplan–Meier analysis suggested that smoking cessation increases the risk of metabolic syndrome and multivariate analysis confirmed smoking cessation as an independent predictor of developing metabolic syndrome. The incidence of metabolic syndrome in non-smokers seemed to increase after 1,500 days of observation. However, Kaplan–Meier analysis of data obtained during an extended follow-up period (until March 2014) showed a clear separation of the cumulative incidence curves in continuous smokers and non-smokers (data not shown). Thus, the incidence of metabolic syndrome remained higher in continuous smokers than in non-smokers, confirming previous findings of a higher risk of metabolic syndrome in active smokers than in non-smokers26). This study does not clarify the mechanisms underlying the close relationship between smoking cessation and the development of metabolic syndrome; however, an increased waist circumference is likely to play a major role as a risk factor for metabolic syndrome. Notably, changes in body weight and waist circumference were correlated with each other in the smoking cessation group, although the continuous smoking group showed an increased body weight and a reduced waist circumference. Thus, an increase in body weight is not necessarily followed by an increase in waist circumference, and an increase in body weight may not be the only crucial determinant of developing metabolic syndrome. An increase in waist circumference indicates the accumulation of visceral fat, which is the fundamental cause of metabolic syndrome, leading to insulin resistance, elevation of blood pressure, and activation of the sympathetic nerve system. Indeed, changes in blood pressure, triglyceride, fasting plasma glucose levels were correlated with changes in waist circumference in the smoking cessation group in this study. It should be noted that waist circumference was not different at baseline in the continuous smoker and smoking cessation groups. Smoking cessation could therefore play a major role in increasing waist circumference. The observation that subjects who stopped smoking experienced an increase in both body weight and HDL-cholesterol in this study appears somewhat contrary to the overall results; however, similar findings have been previously reported23, 27). HDL-cholesterol might have increased due to an increase in physical activity, such as exercise28). Alternatively, an increased HDL-cholesterol associated with smoking cessation might be attributable to an improvement from an individual's deconditioning due to smoking29).
A cross-sectional analysis, including non-smokers, continuous smokers, and past smokers, at the first visit indicated that the prevalence of metabolic syndrome was 9.8%, 14.2%, and 19.9%, respectively. Moreover, the incidence of metabolic syndrome in past smokers at baseline was higher than in non-smokers or continuous smokers (data not shown). These results are compatible with the present study results. A meta-analysis of prospective studies demonstrated that active smoking is associated with the development of metabolic syndrome and that smoking cessation seems to reduce the risk of metabolic syndrome26). It is noteworthy this meta-analysis included studies with varying lengths of follow-up, from 1 to 28 years, suggesting that the impact of smoking cessation on the incidence of metabolic syndrome could differ markedly among studies with different observational periods. Indeed, the risk of incident metabolic syndrome within 3 years of smoking cessation was higher than that for sustained smoking in one study30) and smoking cessation was associated with metabolic syndrome incidence in a 7-year follow-up study owing to a subsequent body weight gain31); however, in studies with 20 to 28 years of follow-up32, 33), the risk of metabolic syndrome was increased in current smokers, but not in ex-smokers, as compared to subjects who had never smoked. Thus, the risk of metabolic syndrome might continue to increase after smoking cessation, at least for several years, but reduce thereafter to the level of non-smokers with increasing periods after smoking cessation. This concept supports our present study results that smoking cessation contributed to the development of metabolic syndrome during the median of 3 years of observation. Sub-analysis of incident metabolic syndrome in male and female subjects was performed, because the criteria of metabolic syndrome were different in male and female subjects. Multivariate Cox hazard analysis indicated that the incidence of metabolic syndrome was higher with smoking cessation than in continuous smokers only for male subjects (data not shown). The results may be attributable to the small number of female subjects in the smoking cessation and continuous smoker groups and to the small number of female subjects who developed metabolic syndrome.
There are several limitations in our study. Subjects in this study were participants in our physical checkup program and a selection bias cannot be ruled out. Information about the smoking status was obtained by questionnaire without laboratory examination. Furthermore, information on lifestyle, such as physical activity and diet, was not available, and such changes were possible especially among the smoking cessation group who obviously had concerns for their health. The present study results therefore should be interpreted with caution considering the above-mentioned conditions. Long, prospective, observational studies are necessary to further define the relationship between the progression of metabolic syndrome and cardiovascular events after smoking cessation.
Conclusions
Smoking cessation without educational instruction could be followed by the development of metabolic syndrome. Although smoking is one of the major risk factors for cardiovascular disease and smoking cessation is essential for health maintenance, the incidence of metabolic syndrome may reduce or overcome the benefit obtained from smoking cessation. Sufficient instruction should be provided to the public about the risk of smoking and metabolic syndrome, and further educational outreach is needed to prevent the progression of metabolic syndrome during the course of smoking cessation.
Disclosure
N. Ohte has received honoraria from Daiichi-Sankyo, Tanabe-Mitsubishi Pharma, Bayer Yakuhin, Astra-Zeneka, and Boehringer Ingelheim.
Department of Cardio-Renal Medicine and Hypertension, Nagoya City University has received clinical research founding from Daiichi-Sankyo, Novartis Pharma, Otsuka Pharmaceutical, Astellas Pharma, Bayer Yakuhin, Actelion Pharma, MSD, and Takeda Pharmaceutical.
References
- 1). LaCroix AZ, Lang J, Scherr P, Wallace RB, Cornoni-Huntley J, Berkman L, Curb JD, Evans D, Hennekens CH: Smoking and mortality among older men and women in three communities. N Engl J Med, 1991; 324: 1619-1625 [DOI] [PubMed] [Google Scholar]
- 2). Sugiura T, Dohi Y, Hirowatari Y, Yamashita S, Ohte N, Kimura G, Fujii S: Cigarette smoking induces vascular damage and persistent elevation of plasma serotonin unresponsive to 8 weeks of smoking cessation. Int J Cardiol, 2013; 166: 748-749 [DOI] [PubMed] [Google Scholar]
- 3). Wolf PA, D'Agostino RB, Kannel WB, Bonita R, Belanger AJ: Cigarette smoking as a risk factor for stroke. The Framingham Study. JAMA, 1988; 259: 1025-1029 [PubMed] [Google Scholar]
- 4). Iso H, Date C, Yamamoto A, Toyoshima H, Watanabe Y, Kikuchi S, Koizumi A, Wada Y, Kondo T, Inaba Y, Tamakoshi A, JACC Study Group : Smoking cessation and mortality from cardiovascular disease among Japanese men and women: the JACC Study. Am J Epidemiol, 2005; 161: 170-179 [DOI] [PubMed] [Google Scholar]
- 5). Filozof C, Fernandez Pinilla MC, Fernandez-Cruz A: Smoking cessation and weight gain. Obes Rev, 2004; 5: 95-103 [DOI] [PubMed] [Google Scholar]
- 6). Williamson DF, Madans J, Anda RF, Kleinman JC, Giovino GA, Byers T: Smoking cessation and severity of weight gain in a national cohort. N Engl J Med, 1991; 324: 739-745 [DOI] [PubMed] [Google Scholar]
- 7). Moffatt RJ, Owens SG: Cessation from cigarette smoking: changes in body weight, body composition, resting metabolism, and energy consumption. Metabolism, 1991; 40: 465-470 [DOI] [PubMed] [Google Scholar]
- 8). Harris KK, Zopey M, Friedman TC: Metabolic effects of smoking cessation. Nat Rev Endocrinol, 2016; 12: 299-308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Cui R, Iso H, Toyoshima H, Date C, Yamamoto A, Kikuchi S, Kondo T, Watanabe Y, Koizumi A, Wada Y, Inaba Y, Tamakoshi A, JACC Study Group : Body mass index and mortality from cardiovascular disease among Japanese men and women: the JACC study. Stroke, 2005; 36: 1377-1382 [DOI] [PubMed] [Google Scholar]
- 10). Chen Y, Copeland WK, Vedanthan R, Grant E, Lee JE, Gu D, Gupta PC, Ramadas K, Inoue M, Tsugane S, Tamakoshi A, Gao YT, Yuan JM, Shu XO, Ozasa K, Tsuji I, Kakizaki M, Tanaka H, Nishino Y, Chen CJ, Wang R, Yoo KY, Ahn YO, Ahsan H, Pan WH, Chen CS, Pednekar MS, Sauvaget C, Sasazuki S, Yang G, Koh WP, Xiang YB, Ohishi W, Watanabe T, Sugawara Y, Matsuo K, You SL, Park SK, Kim DH, Parvez F, Chuang SY, Ge W, Rolland B, McLerran D, Sinha R, Thornquist M, Kang D, Feng Z, Boffetta P, Zheng W, He J, Potter JD: Association between body mass index and cardiovascular disease mortality in east Asians and south Asians: pooled analysis of prospective data from the Asia Cohort Consortium. BMJ, 2013; 347: f5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Eckel RH, Grundy SM, Zimmet PZ: The metabolic syndrome. Lancet, 2005; 365: 1415-1428 [DOI] [PubMed] [Google Scholar]
- 12). Ponciano-Rodriguez G, Paez-Martinez N, Villa-Romero A, Gutierrez-Grobe Y, Mendez-Sanchez N: Early changes in the components of the metabolic syndrome in a group of smokers after tobacco cessation. Metab Syndr Relat Disord, 2014; 12: 242-250 [DOI] [PubMed] [Google Scholar]
- 13). Song YM, Chang WD, Hsu HY, Chen MD: A short-term smoking cessation may increase the risk of developing metabolic syndrome. Diabetes Metab Syndr, 2015; 9: 135-137 [DOI] [PubMed] [Google Scholar]
- 14). Kawano H, Soejima H, Kojima S, Kitagawa A, Ogawa H, Japanese Acute Coronary Syndrome Study (JACSS) Investigators : Sex differences of risk factors for acute myocardial infarction in Japanese patients. Circ J, 2006; 70: 513-517 [DOI] [PubMed] [Google Scholar]
- 15). Sone H, Yoshimura Y, Tanaka S, Iimuro S, Ohashi Y, Ito H, Seino H, Ishibashi S, Akanuma Y, Yamada N, Japan Diabetes Complications Study (JDCS) Group : Diabetes Res Clin Pract, 2007; 77: S23-29 [DOI] [PubMed] [Google Scholar]
- 16). Fukushima M, Suzuki H, Seino Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes: Diabetes Res Clin Pract, 2004; 66: S37-43 [DOI] [PubMed] [Google Scholar]
- 17). An examination committee for criterion of metabolic syndrome. Definition and criteria of metabolic syndrome. Nippon Naika Kakkai Zasshi (in Japanese), 2005; 94: 794-809 [PubMed] [Google Scholar]
- 18). Teramoto T, Sasaki J, Ueshima H, Egusa G, Kinoshita M, Shimamoto K, Daida H, Biro S, Hirobe K, Funahashi T, Yokote K, Yokode M, Japan Atherosclerosis Society (JAS) Committee for Epidemiology and Clinical Management of Atherosclerosis : Diagnostic criteria for dyslipidemia. Executive summary of Japan Atherosclerosis Society (JAS) guideline for diagnosis and prevention of atherosclerotic cardiovascular diseases for Japanese. J Atheroscler Thromb, 2007; 14: 155-158 [DOI] [PubMed] [Google Scholar]
- 19). Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Kim-Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S, Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension : The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res, 2014; 37: 253-392 [DOI] [PubMed] [Google Scholar]
- 20). Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, Araki E, Ito C, Inagaki N, Iwamoto Y, Kasuga M, Hanafusa T, Haneda M, Ueki K, Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus : Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig, 2010; 1: 212-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Kikuchi E, Oda K, Yoshida K, Sen T, Kawakubo K: The effect of changes in smoking habits on HDL cholesterol, waist circumference and body weight over four years. Health Evaluation and Promotion, 2015; 44: 243-252 [Google Scholar]
- 22). CDC. Reducing the health consequences of smoking: 25 years of progress- a report of the Surgeon General, 1989. Rockville, Maryland: U.S. Department of Health and Human Services,Public Health Service; DHHS publication no.(CDC) 89-8411: Washington DC, 1989 [Google Scholar]
- 23). Tamura U, Tanaka T, Okamura T, Kadowaki T, Yamato H, Tanaka H, Nakamura M, Okayama A, Ueshima H, Yamagata Z, HIPOP-OHP research group : Changes in weight, cardiovascular risk factors and estimated risk of coronary heart disease following smoking cessation in Japanese male workers: the high-risk and population strategy for occupational health promotion (HIPOP-OHP) study. J Atheroscler Thromb, 2010; 17: 12-20 [DOI] [PubMed] [Google Scholar]
- 24). Komiyama M, Shimada S, Wada H, Yamakage H, Satoh-Asahara N, Shimatsu A, Akao M, Morimoto T, Takahashi Y, Hasegawa K. Time-dependent Changes of Atherosclerotic LDL Complexes after Smoking Cessation. J Atheroscler Thromb, 2016; 23: 1270-1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Matsushita Y, Nakagawa T, Yamamoto S, Takahashi Y, Noda M, Mizoue T: Associations of smoking cessation with visceral fat area and prevalence of metabolic syndrome in men: the Hitachi health study. Obesity (Silver Spring), 2011; 19: 647-651 [DOI] [PubMed] [Google Scholar]
- 26). Sun K, Liu J, Ning G. Active smoking and risk of metabolic syndrome: a meta-analysis of prospective studies. PLoS One, 2012; 7: e47791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Gepner AD, Piper ME, Johnson HM, Fiore MC, Baker TB, Stein JH: Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. Am Heart J, 2011; 161: 145-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Ministry of Health, Labour and Welfare Department of Health Cancer Control and Health Promotion Division: Physical standards for health promotion 2013. Ministry of Health, Labour and Welfare, 2013. (online) URL (http://www.mhlw.go.jp/stf/houdou/2r9852000002xple-att/2r9852000002xpqt.pdf), (access on 2014-4-17) [Google Scholar]
- 29). Takata K, Imaizumi S, Kawachi E, Suematsu Y, Shimizu T, Abe S, Matsuo Y, Tsukahara H, Noda K, Yahiro E, Zhang B, Uehara Y, Miura S, Saku K: Impact of cigarette smoking cessation on high-density lipoprotein functionality. Circ J, 2014; 78: 2955-2962 [DOI] [PubMed] [Google Scholar]
- 30). Kim BJ, Kim BS, Sung KC, Kang JH, Lee MH, Park JR. Association of smoking status, weight change, and incident metabolic syndrome in men: a 3-year follow-up study. Diabetes Care, 2009; 32: 1314-1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Nakanishi N, Takatorige T, Suzuki K. Cigarette smoking and the risk of the metabolic syndrome in middle-aged Japanese male office workers. Ind Health, 2005; 43: 295-301 [DOI] [PubMed] [Google Scholar]
- 32). Wannamethee SG, Shaper AG, Whincup PH. Modifiable lifestyle factors and the metabolic syndrome in older men: Effects of lifestyle changes. J Am Geriatr Soc, 2006; 54: 1909-1914 [DOI] [PubMed] [Google Scholar]
- 33). Holme I, Tonstad S, Sogaard AJ, Larsen PG, Haheim LL. Leisure time physical activity in middle age predicts the metabolic syndrome in old age: results of a 28-year follow-up of men in the Oslo study. BMC Public Health, 2007; 7: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]


