Abstract
We describe a novel model for investigation of genetically normal human osteoblasts in culture. SK11 is a clonal progenitor cell line derived from human embryonic stem cells. Initially selected based on the expression of chondrogenic markers when differentiated in micromass culture, SK11 cells display typical mRNA expression patterns of bone phenotypic genes under osteogenic conditions. These include osterix, α1(I) collagen, alkaline phosphatase, osteonectin, osteopontin, and osteocalcin. Similar to well-characterized murine osteoblast cultures, the osteoblast master regulator RUNX2 was present during the first few days after plating, but the protein disappeared during the first week of culture. Loss of RUNX2 expression is considered an important regulatory feature for osteoblast maturation. Indeed, following ~2 weeks of differentiation, SK11 cultures exhibited robust calcium deposition, evidenced by alizarin red staining. We also introduced a lentiviral vector encoding doxycycline (dox)-inducible FLAG-tagged RUNX2 into SK11 cells. Dox-mediated enhancement of RUNX2 expression resulted in accelerated mineralization, which was further increased by co-treatment with BMP-2. Like the endogenous RUNX2, expression of the virally coded FLAG-RUNX2 was lost during the first week of culture despite persistent dox treatment. By following RUNX2 decay after dox withdrawal from day-5 versus day-3 cultures, we demonstrated a developmentally regulated decrease in RUNX2 stability. Availability of culture models for molecular investigation of genetically normal human osteoblasts is important because differences between murine and human osteoblasts, demonstrated here by the regulation of matrix Gla Protein, may have significant biomedical implications.
Many cell culture models for the investigation of osteoblast growth and differentiation have contributed to the understanding of cellular and molecular processes associated with bone formation and its regulation by intrinsic and extrinsic factors. Primary osteoblast cultures derived from embryonic and newborn mouse and rat calvariae faithfully recapitulate in vivo osteoblast differentiation and bone formation (Peck et al., 1964; Luben et al., 1976). Primary mesenchymal cultures from rat and mouse bone marrow are less homogenous but quite useful for the investigation of pluripotent cells but nevertheless widely studied to investigate cellular responses to growth and differentiation factors (Friedenstein et al., 1974; Beresford, 1989). Immortalized cell culture models have become popular for studies of osteoblast biology because they eliminate animal use and are readily amenable for molecular manipulation. Cancer-derived cell culture models such as the rat osteosarcoma cell lines ROS17/2.8 (Majeska and Rodan, 1982) and UMR-106 (Partridge et al., 1983) and human osteosarcoma cell lines such as Saos-2 (Rodan et al., 1987), U2-OS (Key et al., 1988) and MG-63 (Dedhar et al., 1987) have provided robust and reproducible models to study a variety of molecular mechanisms that regulate osteoblast differentiation. However, their abnormal genotype casts a shadow on whether their behavior reflects normal osteogenesis in vivo.
Immortalized osteoblast cell lines derived from non-cancerous tissue generally recapitulate specific regulatory processes with a fidelity close to that offered by primary cultures. For example, the mesenchymal pluripotent ST2 cell line, derived from the mouse bone marrow stroma, has been extensively employed to investigate how cells select the osteoblastic versus the alternative adipocytic cell fate (Udagawa et al., 1989; Otsuka et al., 1999). Likewise, the MBA-15 cell line has been employed, for example, to study the effect of glucocorticoids on MAPK/ERK activity in osteoblasts (Benayahu et al., 1989; Engelbrecht et al., 2003). The mouse C2C12 cell line, otherwise a model for muscle cell differentiation (Yaffe and Saxel, 1977), offers a useful model for the investigation of BMP-induced trans-differentiation into osteoblasts (Katagiri et al., 1994). Finally, the mouse calvaria-derived MC3T3-E1 cell line consistently displays many aspects of osteoblast growth and differentiation that have been initially established using primary cultures (Sudo et al., 1983).
In contrast to the plethora of non-cancerous murine cell culture models for the investigation of the osteoblast phenotype, the selection of such human cell lines is relatively limited. Osteoblasts cultures initiated from bone chips obtained during surgery suffer the obvious disadvantages of limited tissue availability and scalability, as well as variability among patients, which hinders data reproducibility. Scarcity of young patient donors in particular limits proliferative capacity and lifespan (Evans et al., 1990). Scalability is partially overcome when osteoblasts are derived from human bone marrow stroma (Colter et al., 2000). In addition to the human osteosarcoma cell lines mentioned above, a handful of immortalized osteoblast cell lines have been established from human bone, including the hFOB 1.19 (Harris et al., 1995), the HOB 04-T8, the 03-CE6 and the 03-CE10 cell lines (Prince et al., 2001). These provide excellent models for the investigation of specific aspects of osteoblast differentiation, but do not necessarily recapitulate the process as a whole (Harris et al., 1995; Prince et al., 2001).
Recent progress with isolation and propagation of embryonic stem cells provides an opportunity for the development of normal diploid human cell culture models from all somatic cell lineages. Embryonic stem cell-derived cell lines that maintain the potential to develop terminal phenotypes may represent unique models to follow in detail complex differentiation processes in vitro. During screening of dozens of cell lines derived from human embryonic progenitors (hEP), we identified several that preferentially express osteo/chondrogenic marker genes (West et al., 2008). Here we show that one of them, SK11, possesses the potential for development into fully differentiated osteoblasts, including expression of characteristic genes and elaboration of a robust extracellular mineralized matrix. We further employed the SK11 cell line to investigate the molecular basis for the established decay of RUNX2 expression during osteoblast differentiation.
Materials and Methods
Cells
Newborn mouse calvarial osteoblasts (NeMCO) were isolated from the partial bones of 1–2 day-old pups as previously described (Gabet et al., 2011). NeMCO and MC3T3-E1 cells were maintained in αMEM supplemented with 10% fetal bovine serum (FBS) (Gemini Bio-Products, West Sacramento, CA). ST2 cells were maintained in RPMI1640 (Mediatech Inc. Manassas, VA) supplemented with 10% FBS. The human embryonic stem cell-derived cell line SK11 (PureStem™, BioTime Inc., Alameda, CA) was maintained in αMEM supplemented with 5% FBS, 50 μg/ml Fetuin (Calbiochem, Billerica, MA), 10 ng/ml EGF, 1 ng/ml bFGF, 10 μg/ml insulin and 10−8 M dexamethasone (all from Sigma-Aldrich, St. Louis, MO). For experimentation, cells were plated on 0.1% gelatin (Sigma-Aldrich) and differentiated in αMEM supplemented with 10% FBS, 10 mM sodium β-glycerophosphate, 50 μg/ml ascorbic acid, and 10−8 M dexamethasone. SK11/Rx2dox and MC3T3-E1/Rx2dox cells were engineered with a dox-inducible RUNX2 lentiviral vector (Rx2dox) as previously described (Baniwal et al., 2010; Baniwal et al., 2012).
Alizarin red staining
Cells were washed once in PBS and fixed in 70% ethanol for 1 h at 4°C. After extensive washes with ddH2O, cultures were stained with 1.3 mg/ml alizarin (Sigma-Aldrich) for 5 min, thoroughly rinsed with ddH2O and air dried.
Chromatin immunoprecipitation
FLAG-RUNX2 ChIP was performed essentially as previous described (Little et al., 2012). Precipitated DNA fragments were quantified by qPCR using the primers listed in Table 1.
TABLE 1.
Primers used for RT-qPCT and ChIP-qPCR
| Primer sequence (5′ to 3′) | ||
|---|---|---|
| ALPL mRNA | F | GAGATACAAGCACTCCCACTTC |
| R | TGGCTCGAAGAGACCCAATA | |
| BGLAP mRNA | F | ACACTCCTCGCCCTATTGGC |
| R | TGCTTGGACACAAAGGCTGC | |
| COL1A1 mRNA | F | GCCAAGACGAAGACATCCCA |
| R | GGCAGTTCTTGGTCTCGTCA | |
| GAPDH mRNA | F | GTCATGGGTGTGAACCATGAGA |
| R | GGTCATGAGTCCTTCCACGATAC | |
| MGP mRNA | F | GGCCGCCTTAGCGGTAGTAA |
| R | AGCGTTCTCGGATCCTCTCT | |
| Osterix mRNA | F | TGCTTGAGGAGGAAGTTCACTAT |
| R | GGCTTCTTTGTGCCTGCTTT | |
| RUNX2 mRNA | F | ATCATCGCCGACCACCCGGC |
| R | GGCTACCACCTTGAAGGCCACG | |
| SPARC mRNA | F | TGGCAGAGGTGACTGAGGTAT |
| R | CGTGTTTGCAGTGGTGGTTC | |
| SPP1 mRNA | F | GCCGAGGTGATAGTGTGGTT |
| R | TCAGGGTACTGGATGTCAGGT | |
| BGLAP ChIP | F | AAATAGCCCTGGCAGATTCC |
| R | CAGCCTCCAGCACTGTTTAT | |
| Control ChIP | F | ATGGTTGCCACTGGGGATCT |
| R | TGCCAAAGCCTAGGGGAAGA |
Quantitative RT-PCR (qPCR)
RNA extraction, cDNA synthesis and quantification of mRNA by qPCR was performed as previously described (Baniwal et al., 2013) using the primers listed in Table 1. Expression levels of mRNAs of interest were normalized for the respective GAPDH mRNA levels, which themselves were relatively stable.
Western blot analysis
Cells were lysed in a 50 mM Tris-HCl buffer (pH 7.4) containing 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, and fresh protease inhibitor cocktail (1X; Sigma-Aldrich). Protein concentrations were determined using Bio-Rad Protein Assay Kit (Bio-Rad Laboratories, Inc., Hercules, CA) and aliquots of 20 μg were mixed with an equal volume of Laemmli buffer. Proteins were resolved by 8% SDS–PAGE, transferred to AmershamHybond™-P PVDF membranes (GE Healthcare, Piscataway, NJ), and detected with mouse monoclonal antibodies against FLAG epitope (M2, Sigma-Aldrich), or rabbit polyclonal antibodies against RUNX2 (M-70 Santa Cruz Biotechnology, Santa Cruz, CA), or Actin (I-19 Santa Cruz Biotechnology).
Results
Toward investigation of molecular mechanisms that regulate osteoblast growth and differentiation in normal dipoloid human cells in vitro, we initially engineered the human embryonic progenitor cell line SK11 (Sternberg et al., 2013) with the FLAG-RUNX2dox lentiviral system (Baniwal et al., 2010). FLAG-RUNX2 was undetectable in SK11/Rx2dox cells in the absence of doxycycline (dox) (Fig. 1A). Upon treatment with 100 ng/ml dox, RUNX2 was expressed at levels two- to threefold higher than those observed in control cultures (Fig. 1A). ChIP assay demonstrated binding of the dox-induced FLAG-RUNX2 to its classical binding site at the osteocalcin (BGLAP) promoter (Ducy and Karsenty, 1995; Banerjee et al., 1997) (Fig. 1B) and RT-qPCR showed robust induction of osteocalcin gene expression in response to RUNX2 (Fig. 1C). We next assessed mineral deposition by alizarin red staining of day-6 cultures. Robust mineralization was apparent in the dox-treated SK11/Rx2dox cultures (Fig. 1D). This was attributable to RUNX2 induction, because no significant calcium deposition was detectable in naïve SK11 cultures on day 6 (Fig. 1D). Mineralization of the dox-treated SK11/Rx2dox cultures was further stimulated by 10 ng/ml recombinant human BMP2 (rhBMP2), which did not induce mineralization on its own (Fig. 1D). This is attributed to enhancement of RUNX2 activity by BMP-SMADs (Lee et al., 2000). Higher rhBMP2 concentrations, up to 100 ng/ml, did not further enhance mineral deposition (data not shown).
Fig. 1.

RUNX2 expression and mineralization in SK11 and SK11/Rx2dox human embryonic progenitor-derived osteoblasts. (A) Day-3 SK11/Rx2dox cell cultures were treated with 100 ng/ml dox for 24 h and subjected to Western blot analysis using either anti-FLAG (left panel) or anti-RUNX2 (right panel) antibodies. Arrow depicts the dox-inducible FLAG-RUNX2. Arrowhead in this and the following figures depicts an endogenous RUNX2 isoform of approximately 55 kDa. Additional RUNX2 isoforms detected by the anti-RUNX2 antibody are not labeled. Bottom panel shows β-Actin loading control. (B) SK11/Rx2dox cells were treated with 100 ng/ml dox and subjected to ChIP assay with FLAG antibodies. Immunoprecipitated DNA was analyzed with primers (Table 1) designed to amplify RUNX2 binding site at the osteocalcin promoter or an unrelated region as a negative control. (C) SK11/Rx2dox cells were treated with 100 ng/ml dox and subjected to RT-qPCR analysis of BGLAP mRNA. (D) SK11/Rx2dox or naïve SK11 cells were treated with 100 ng/ml dox and/or 10 ng/ml recombinant human BMP-2 from plating to the indicated day. Calcium deposition in representative wells is demonstrated by alizarin red staining.
Because endogenous RUNX2 was expressed, albeit at lower levels, in day-3 SK11/Rx2dox cultures that had not been treated with dox (Fig. 1A), we asked whether mineralization would be detectable in naïve SK11 cultures after prolonged periods of time. In the presence of differentiation medium, these cells exhibited robust calcium deposition by day 12–16, demonstrated by alizarin red staining (Fig. 1D). Interestingly, mineral deposition by the naive cells was not increased by rhBMP-2 (Fig. 1D).
We next performed RT-qPCR time course analysis to characterize the expression of osteoblast marker genes in naive SK11 cultures. Counting from confluence (defined as day 0), α1 (I) collagen (COL1A1) mRNA increased by 69% from day 2 to 4 with no significant change until day 9 (Fig. 2A). Osteonectin (SPARC) expression was upregulated by 68% between days 2 and 3, with a second increase on days 5–7 (Fig. 2A). The second phase of SPARC upregulation on days 5–7 was accompanied with increase in the expression of, alkaline phosphatase (ALPL; Fig. 2A) osterix (OSX), osteocalcin (BGLAP; Fig. 2B) and osteopontin (SPP1; Fig. 2C). Both osteopontin and osteocalcin mRNA levels continued to increase after day 5, and this was surprisingly paralleled by a threefold increase in MGP expression (Fig. 2C). Because this parallelism contrasts with anti-parallel expression of BGLAP and MGP in other osteoblast culture models (Baniwal et al., 2012; Wu et al., 2014), we wondered if RUNX2 stimulated MGP expression in SK11 cells, as it did BGLAP (Fig. 1C). Indeed, RT-qPCR analysis of SK11/Rx2dox cells shows that induction of RUNX2 by dox dramatically increases MGP expression (Fig. 2D), the opposite of the effect of RUNX2 on MGP in ST2/Rx2dox cells (Baniwal et al., 2012).
Fig. 2.
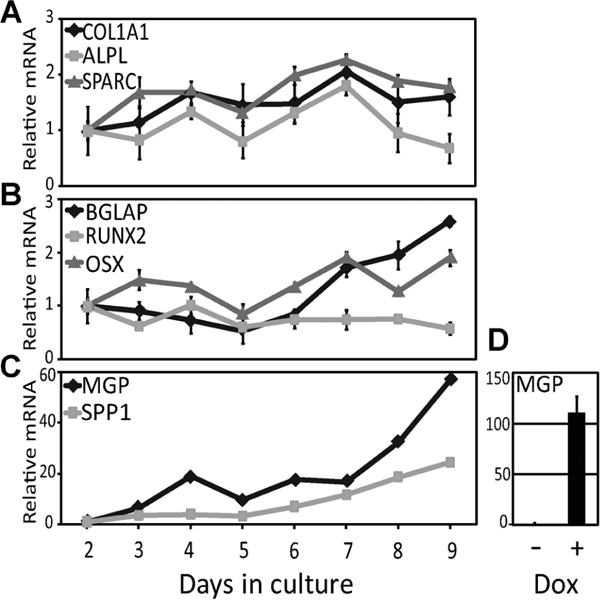
Osteoblast marker gene expression in SK11 cell cultures. (A–C) SK11 cells were subjected to differentiation conditions as described in Materials and Methods and the mRNA levels of indicated genes were measured between day 2 and day 9 by RT-qPCR (mean ± SD, n = 3). (D) SK11/Rx2dox cells were treated with dox and subjected to RT-qPCR analysis of MGP mRNA.
The upregulation of the osteogenic marker genes OSX, ALPL, SPP1 and BGLAP in SK11 cultures between days 5 and 7 was not attributable to an increase in RUNX2 mRNA expression, which was essentially unchanged throughout the time-course experiment (Fig. 2B). We therefore performed a time-course analysis of RUNX2 expression in the SK11 culture model at the protein level. As shown in Figure 3A, RUNX2 did not increase, but in fact decreased on day 5. This is consistent with the idea that persistent RUNX2 expression after cellular commitment to the osteoblast lineage interferes with proper osteoblast maturation (Komori, 2010). Indeed, similar to SK11 cells, we confirmed the downregulation of RUNX2 expression during the progression of other osteoblast culture models, including ST2 (Fig. 3B), MC3T3-E1 (Fig. 3C), as well as primary osteoblast cultures derived from newborn mouse calvariae (Fig. 3D).
Fig. 3.

Loss of RUNX2 protein during osteoblast differentiation. (A–D) SK11 (A), ST2 (B), MC3T3-E1 (C), and NeMCO (D) cultures were subjected to Western analysis of RUNX2 on the indicated days. (E–G) SK11/Rx2dox (E), ST2/Rx2dox (F), and MC3T3-E1/Rx2dox (G) cultures were treated with dox and subjected to Western analysis with anti-FLAG antibodies on the indicated days. Western analysis of Actin (SK11) and Coomassie Blue staining (other cell lines) were used as loading control. The arrow and the arrowhead indicate FLAG-RUNX2 and an endogenous RUNX2 isoform of approximately 55 kDa, respectively.
RUNX2 expression is subjected to alternative mechanisms of transcriptional initiation, transcriptional termination, as well as RNA splicing (Geoffroy et al., 1998; Xiao et al., 1998; Ogawa et al., 2000; Xiao et al., 2001). To address the contribution of these alternative expression mechanisms to the abrupt decrease in RUNX2 levels in SK11 cultures (Fig. 3A), we asked whether such downregulation is recapitulated by RUNX2 that is expressed from the dox-inducible lentiviral cassette in SK11/Rx2dox cells (Fig. 1A). As shown by Western analysis of SK11/Rx2dox cells, the exogenous, dox-induced FLAG-RUNX2 was subjected to a similar expression pattern, with abrupt disappearance of the protein between days 6 and 7 (Fig. 3E). Western blot analysis of FLAG-RUNX2 in ST2/Rx2dox and MC3T3-E1/Rx2dox cells also demonstrated virtual disappearance of the virally expressed protein within a short period of 3–4 days (Figs. 3F–G). Thus, loss of RUNX2 expression during progression of the osteoblast phenotype is a feature shared by culture models of diverse species and tissue of origin.
To test whether the loss of RUNX2 during development of the osteoblast phenotype was attributable to protein destabilization, we induced FLAG-RUNX2 in day-3 and in day-5 SK11/Rx2dox cultures by a 24 h dox treatment, and examined the protein decay rate after dox withdrawal. As demonstrated by the Western analysis time-course, RUNX2 was significantly less stable in the day-5 compared to the day-3 cultures (Fig. 4). These results indicate that the decline in RUNX2 expression during osteoblast differentiation is attributable to developmentally regulated loss of RUNX2 stability.
Fig. 4.

Decreased RUNX2 stability as a function of osteoblast differentiation. Day-3 and Day-5 SK11/Rx2dox cultures under osteogenic condition were treated with dox for 24 h and cell lysates were subjected to Western blot analyses with anti-FLAG antibodies between 0 and 12 h after dox withdrawal. Arrow indicates FLAG-RUNX2 in the top part. Bottom part shows Western blot analysis of Actin used as a loading control.
Discussion
While RUNX2 plays a critical role in osteoblast differentiation in vivo and in vitro (Banerjee et al., 1997; Ducy et al., 1997; Komori et al., 1997; Otto et al., 1997), persistent expression of the protein in mature osteoblasts has deleterious consequences (Liu et al., 2001; Geoffroy et al., 2002; Komori, 2010). RUNX2 expression is tightly controlled at the transcriptional level (Komori, 2011). The present work suggests that loss of RUNX2 during osteoblast differentiation is attributable to post-translational mechanism(s) because its mRNA level did not decrease and because the protein lost its stability during the first week of culture when expressed from an exogenous lentiviral vector. The mechanism underlying the loss of RUNX2 stability remains to be elucidated, and may involve covalent modifications such as phosphorylation, acetylation, sumoylation, and ubiquitination (Bae and Lee, 2006) and/or protein–protein interactions (Li et al., 2010).
Osteocalcin (BGLAP) is the best-established RUNX2 target gene in osteoblasts (Banerjee et al., 1997; Ducy et al., 1997). Interestingly, BGLAP mRNA levels continue to increase after RUNX2 expression is lost. Presumably, RUNX2 initiates osteocalcin transcription, which later persists and even increases under the regulation of other transcription factors. Increased osteocalcin expression despite reduced RUNX2 levels in the SK11 human osteoblasts, which is similar to the regulation of the orthologous murine gene (Hassan et al., 2004; Pregizer et al., 2008), is not paralleled by some of the other osteoblastic marker genes. For example, ALPL expression in SK11 cells decreases after the disappearance of RUNX2. An interesting feature of the SK11 osteoblast model is the remarkable upregulation of MGP expression during culture progression, which exceeds that of BGLAP, and is not shared by the orthologous gene in murine osteoblast cultures (Meyer et al., 2014; Wu et al., 2014). Furthermore, MGP in SK11 cells is strongly stimulated by RUNX2, which is in sharp contrast to the RUNX2-mediated repression observed in ST2 cells undergoing osteoblast differentiation (Baniwal et al., 2012). It remains to be seen whether the robust upregulation of MGP in SK11 cells, and its stimulation by RUNX2, are shared with other human osteoblast culture models. This may have important biomedical implications given the powerful regulation of biological calcification by MGP (Luo et al., 1997).
SK11 is one of several human embryonic progenitor cell lines selected in our previous study based on the expression of chondrogenic markers such as COL2A1, COMP, OGN and MGP (Sternberg et al., 2013). Although these cells express type II collagen when differentiated as cell pellets, micromasses, or Hystem-4D array under the combined influence of TGFß3 and BMP2, 4, 6 or 7, they do not express type II collagen in the undifferentiated state in which they are normally propagated (Sternberg et al., 2013). This, and a robust proliferative capacity, were the considerations based on which we selected SK11 for the present study. Indeed, we show here that SK11 spontaneously develop the ultimate bone phenotype of mineral deposition when cultured in osteogenic medium. This cell line and the derived SK11/Rx2dox cells provide useful research tools for in vitro investigation of molecular mechanisms underlying human osteoblast differentiation.
Acknowledgments
BF holds the J. Harold and Edna L. LaBriola Chair in Genetic Orthopaedic Research.
Contract grant sponsor: California Institute for Regenerative Medicine;
Contract grant number: TG2-01161.
Contract grant sponsor: National Institute of Diabetes and Digestive and Kidney Diseases;
Contract grant number: RO1DK71122.
Footnotes
Conflict of Interest: J Jiang, H Sternberg, and MD West are employees of BioTime Inc. and may have equity interest in BioTime or its subsidiaries and have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Literature Cited
- Bae SC, Lee YH. Phosphorylation, acetylation and ubiquitination: The molecular basis of RUNX regulation. Gene. 2006;366:58–66. doi: 10.1016/j.gene.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Banerjee C, McCabe LR, Choi JY, Hiebert SW, Stein JL, Stein GS, Lian JB. Runt homology domain proteins in osteoblast differentiation: AML3/CBFA1 is a major component of a bone-specific complex. J Cell Biochem. 1997;66:1–8. doi: 10.1002/(sici)1097-4644(19970701)66:1<1::aid-jcb1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Baniwal SK, Chimge NO, Jordan VC, Tripathy D, Frenkel B. Prolactin-induced protein (PIP) regulates proliferation of luminal A type breast cancer cells in an estrogen-independent manner. PloS One. 2013;8:e62361. doi: 10.1371/journal.pone.0062361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniwal SK, Khalid O, Gabet Y, Shah RR, Purcell DJ, Mav D, Kohn-Gabet AE, Shi Y, Coetzee GA, Frenkel B. Runx2 transcriptome of prostate cancer cells: Insights into invasiveness and bone metastasis. Mol Cancer. 2010;9:258. doi: 10.1186/1476-4598-9-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniwal SK, Shah PK, Shi Y, Haduong JH, Declerck YA, Gabet Y, Frenkel B. Runx2 promotes both osteoblastogenesis and novel osteoclastogenic signals in ST2 mesenchymal progenitor cells. Osteoporos Int. 2012;23:1399–1413. doi: 10.1007/s00198-011-1728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benayahu D, Kletter Y, Zipori D, Wientroub S. Bone marrow-derived stromal cell line expressing osteoblastic phenotype in vitro and osteogenic capacity in vivo. J Cell Physiol. 1989;140:1–7. doi: 10.1002/jcp.1041400102. [DOI] [PubMed] [Google Scholar]
- Beresford JN. Osteogenic stem cells and the stromal system of bone and marrow. Clin Orthop Relat Res. 1989;240:270–280. [PubMed] [Google Scholar]
- Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci USA. 2000;97:3213–3218. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedhar S, Argraves WS, Suzuki S, Ruoslahti E, Pierschbacher MD. Human osteosarcoma cells resistant to detachment by an Arg-Gly-Asp-containing peptide overproduce the fibronectin receptor. J Cell Biol. 1987;105:1175–1182. doi: 10.1083/jcb.105.3.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Karsenty G. Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol Cell Biol. 1995;15:1858–1869. doi: 10.1128/mcb.15.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Engelbrecht Y, de Wet H, Horsch K, Langeveldt CR, Hough FS, Hulley PA. Glucocorticoids induce rapid up-regulation of mitogen-activated protein kinase phosphatase-1 and dephosphorylation of extracellular signal-regulated kinase and impair proliferation in human and mouse osteoblast cell lines. Endocrinology. 2003;144:412–422. doi: 10.1210/en.2002-220769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CE, Galasko CS, Ward C. Effect of donor age on the growth in vitro of cells obtained from human trabecular bone. J Orthop Res. 1990;8:234–237. doi: 10.1002/jor.1100080212. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- Gabet Y, Noh T, Lee C, Frenkel B. Developmentally regulated inhibition of cell cycle progression by glucocorticoids through repression of cyclin A transcription in primary osteoblast cultures. J Cell Physiol. 2011;226:991–998. doi: 10.1002/jcp.22412. [DOI] [PubMed] [Google Scholar]
- Geoffroy V, Corral DA, Zhou L, Lee B, Karsenty G. Genomic organization, expression of the human CBFA1 gene, and evidence for an alternative splicing event affecting protein function. Mamm Genome. 1998;9:54–57. doi: 10.1007/s003359900679. [DOI] [PubMed] [Google Scholar]
- Geoffroy V, Kneissel M, Fournier B, Boyde A, Matthias P. High bone resorption in adult aging transgenic mice overexpressing cbfa1/runx2 in cells of the osteoblastic lineage. Mol Cell Biol. 2002;22:6222–6233. doi: 10.1128/MCB.22.17.6222-6233.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SA, Enger RJ, Riggs BL, Spelsberg TC. Development and characterization of a conditionally immortalized human fetal osteoblastic cell line. J Bone Miner Res. 1995;10:178–186. doi: 10.1002/jbmr.5650100203. [DOI] [PubMed] [Google Scholar]
- Hassan MQ, Javed A, Morasso MI, Karlin J, Montecino M, van Wijnen AJ, Stein GS, Stein JL, Lian JB. Dlx3 transcriptional regulation of osteoblast differentiation: temporal recruitment of Msx2, Dlx3, and Dlx5 homeodomain proteins to chromatin of the osteocalcin gene. Mol Cell Biol. 2004;24:9248–9261. doi: 10.1128/MCB.24.20.9248-9261.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key LL, Jr, Weichselbaum RR, Carnes DL., Jr A link between calcitriol and bone resorption. Bone Miner. 1988;3:201–209. [PubMed] [Google Scholar]
- Komori T. Regulation of osteoblast differentiation by Runx2. Adv Exp Med Biol. 2010;658:43–49. doi: 10.1007/978-1-4419-1050-9_5. [DOI] [PubMed] [Google Scholar]
- Komori T. Signaling networks in RUNX2-dependent bone development. J Cell Biochem. 2011;112:750–755. doi: 10.1002/jcb.22994. [DOI] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Lee KS, Kim HJ, Li QL, Chi XZ, Ueta C, Komori T, Wozney JM, Kim EG, Choi JY, Ryoo HM, Bae SC. Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol Cell Biol. 2000;20:8783–8792. doi: 10.1128/mcb.20.23.8783-8792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Decker M, Westendorf JJ. TEThered to Runx: Novel binding partners for runx factors. Blood Cells Mol Dis. 2010;45:82–85. doi: 10.1016/j.bcmd.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little GH, Noushmehr H, Baniwal SK, Berman BP, Coetzee GA, Frenkel B. Genomewide Runx2 occupancy in prostate cancer cells suggests a role in regulating secretion. Nucleic Acids Res. 2012;40:3538–3547. doi: 10.1093/nar/gkr1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Toyosawa S, Furuichi T, Kanatani N, Yoshida C, Liu Y, Himeno M, Narai S, Yamaguchi A, Komori T. Overexpression of Cbfa1 in osteoblasts inhibits osteoblast maturation and causes osteopenia with multiple fractures. J Cell Biol. 2001;155:157–166. doi: 10.1083/jcb.200105052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luben RA, Wong GL, Cohn DV. Biochemical characterization with parathormone and calcitonin of isolated bone cells: Provisional identification of osteoclasts and osteoblasts. Endocrinology. 1976;99:526–534. doi: 10.1210/endo-99-2-526. [DOI] [PubMed] [Google Scholar]
- Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- Majeska RJ, Rodan GA. Alkaline phosphatase inhibition by parathyroid hormone and isoproterenol in a clonal rat osteosarcoma cell line. Possible mediation by cyclic AMP. Calcif Tissue Int. 1982;34:59–66. doi: 10.1007/BF02411210. [DOI] [PubMed] [Google Scholar]
- Meyer MB, Benkusky NA, Pike JW. The RUNX2 Cistrome in Osteoblasts: Characterization, down-regulation following differentiation, and relationship to gene expression. J Biol Chem. 2014;289:16016–16031. doi: 10.1074/jbc.M114.552216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Harada H, Fujiwara M, Tagashira S, Katsumata T, Takada H. Cbfa1, an essential transcription factor for bone formation, is expressed in testis from the same promoter used in bone. DNA Res. 2000;7:181–185. doi: 10.1093/dnares/7.3.181. [DOI] [PubMed] [Google Scholar]
- Otsuka E, Yamaguchi A, Hirose S, Hagiwara H. Characterization of osteoblastic differentiation of stromal cell line ST2 that is induced by ascorbic acid. Am J Physiol. 1999;277:C132–C138. doi: 10.1152/ajpcell.1999.277.1.C132. [DOI] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- Partridge NC, Alcorn D, Michelangeli VP, Ryan G, Martin TJ. Morphological and biochemical characterization of four clonal osteogenic sarcoma cell lines of rat origin. Cancer Res. 1983;43:4308–4314. [PubMed] [Google Scholar]
- Peck WA, Birge SJ, Jr, Fedak SA. Bone cells: Biochemical and biological studies after enzymatic isolation. Science. 1964;146:1476–1477. doi: 10.1126/science.146.3650.1476. [DOI] [PubMed] [Google Scholar]
- Pregizer S, Baniwal SK, Yan X, Borok Z, Frenkel B. Progressive recruitment of Runx2 to genomic targets despite decreasing expression during osteoblast differentiation. J Cell Biochem. 2008;105:965–970. doi: 10.1002/jcb.21900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M, Banerjee C, Javed A, Green J, Lian JB, Stein GS, Bodine PV, Komm BS. Expression and regulation of Runx2/Cbfa1 and osteoblast phenotypic markers during the growth and differentiation of human osteoblasts. J Cell Biochem. 2001;80:424–440. doi: 10.1002/1097-4644(20010301)80:3<424::aid-jcb160>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Rodan SB, Imai Y, Thiede MA, Wesolowski G, Thompson D, Bar-Shavit Z, Shull S, Mann K, Rodan GA. Characterization of a human osteosarcoma cell line (Saos-2) with osteoblastic properties. Cancer Res. 1987;47:4961–4966. [PubMed] [Google Scholar]
- Sternberg H, Kidd J, Murai JT, Jiang J, Rinon A, Erickson IE, Funk WD, Wang Q, Chapman KB, Vangsness CT, Jr, West MD. Seven diverse human embryonic stem cell-derived chondrogenic clonal embryonic progenitor cell lines display site-specific cell fates. Reg Med. 2013;8:125–144. doi: 10.2217/rme.12.117. [DOI] [PubMed] [Google Scholar]
- Sudo H, Kodama HA, Amagai Y, Yamamoto S, Kasai S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol. 1983;96:191–198. doi: 10.1083/jcb.96.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udagawa N, Takahashi N, Akatsu T, Sasaki T, Yamaguchi A, Kodama H, Martin TJ, Suda T. The bone marrow-derived stromal cell lines MC3T3-G2/PA6 and ST2 support osteoclast-like cell differentiation in cocultures with mouse spleen cells. Endocrinology. 1989;125:1805–1813. doi: 10.1210/endo-125-4-1805. [DOI] [PubMed] [Google Scholar]
- West MD, Sargent RG, Long J, Brown C, Chu JS, Kessler S, Derugin N, Sampathkumar J, Burrows C, Vaziri H, Williams R, Chapman KB, Larocca D, Loring JF, Murai J. The ACTCellerate initiative: Large-scale combinatorial cloning of novel human embryonic stem cell derivatives. Reg Med. 2008;3:287–308. doi: 10.2217/17460751.3.3.287. [DOI] [PubMed] [Google Scholar]
- Wu H, Whitfield TW, Gordon JA, Dobson JR, Tai PW, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Genomic occupancy of Runx2 with global expression profiling identifies a novel dimension to control of osteoblastogenesis. Genome Biol. 2014;15:R52. doi: 10.1186/gb-2014-15-3-r52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao ZS, Liu SG, Hinson TK, Quarles LD. Characterization of the upstream mouse Cbfa1/Runx2 promoter. J Cell Biochem. 2001;82:647–659. doi: 10.1002/jcb.1192. [DOI] [PubMed] [Google Scholar]
- Xiao ZS, Thomas R, Hinson TK, Quarles LD. Genomic structure and isoform expression of the mouse, rat and human Cbfa1/Osf2 transcription factor. Gene. 1998;214:187–197. doi: 10.1016/s0378-1119(98)00227-3. [DOI] [PubMed] [Google Scholar]
- Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]


