Abstract
BACKGROUND
Obesity (BMI≥30) may be an etiologic and prognostic factor in inflammatory breast cancer (IBC). We examined the relationship between BMI, pathologic complete response (pCR), and circulating-tumor-cell (CTC) levels in IBC.
METHODS
Cohort included IBC patients diagnosed 2005–2015 who had neoadjuvant chemotherapy during a prospective trial on CTCs and pathologic review describing pCR. Chi-square, logistic regression, and Cox proportional hazards models were used to identify clinicopathologic associations with event-free survival (EFS).
RESULTS
Of 73 patients, 61 (84%) had CTC values, 22 (30%) achieved a pCR, and 39 (53%) were obese. There was no difference between obese and non-obese patients for pCR rates (31% vs. 29%, p=0.90) or presence of CTCs (23% vs. 26%, p=0.80). Among non-obese patients, CTCs were associated with worse EFS (HR 11.69, p<0.01), but among obese patients, there was no difference in EFS between those with and without CTCs.
CONCLUSIONS
BMI mediates CTCs’ prognostic significance in IBC.
Keywords: body mass index, circulating tumor cells, inflammatory breast cancer, obesity, pathologic complete response, prognosis
Table of Contents Summary
Inflammatory breast cancer (IBC) is an aggressive malignancy more commonly seen among obese patients. The impact of obesity (body mass index [BMI] ≥ 30) on the etiology and prognosis of IBC is unclear. We examine the relationship between BMI and two established prognosticators for breast cancer – pathologic complete response (pCR) and circulating tumor cells (CTCs) – in patients with IBC.
INTRODUCTION
Breast cancer is increasingly recognized as a heterogeneous disease, the prognosis for which is shaped by numerous biochemical, molecular, and clinical characteristics. Breast cancer biomarkers – specifically, tumor expression of the estrogen receptor (ER) and the progesterone receptor (PR) as well as amplification of HER2/neu – not only serve as indicators of tumor differentiation and aggressiveness but have also been utilized as targets for numerous effective therapies. Furthermore, there is an established and expanding body of evidence that tumor biomarker status can have strong, reproducible associations with the clinical characteristics of patients in whom breast cancer develops. For example, breast cancers diagnosed in obese, postmenopausal women are more likely to be ER-positive (ER+), in keeping with the observed phenomenon of greater estrogen production in obese postmenopausal women when compared with their normal-weight counterparts.1 Indeed, patient obesity – often defined using body mass index (BMI) – may play an important role in both the pathogenesis of breast cancer and in patient response to breast-cancer treatments, with proposed mechanisms of interaction including not only increased estrogen production but also insulin resistance and chronic, low-grade inflammation.2
As rates of obesity continue to increase both in the United States (US) and around the world, the impact of obesity on breast cancer prognosis is a topic of increasing relevance to both clinical practice and translational investigation. Elevated BMI is associated with elevated risk both for the development of breast cancer and for relapse following treatment.3 A number of studies examining the impact of BMI on outcome following neoadjuvant chemotherapy (NACT) have demonstrated lower rates of pathologic complete response (pCR, i.e., absence of residual invasive disease in the breast and lymph nodes after NACT) in overweight (BMI ≥ 25) and obese (BMI ≥ 30) patients.2, 4 Both pCR and the presence of residual systemic microscopic disease in the form of circulating tumor cells (CTCs) after receipt of NACT are important positive and negative prognosticators, respectively, of breast cancer outcomes, but their relationship to each other remains unclear. Also unknown is whether BMI affects the likelihood of CTCs’ being present after NACT and what relationship, if any, exists between BMI, pCR, and CTC levels. Here, we describe an analysis of the relationship between patient BMI at diagnosis, pCR, and CTC levels after NACT in a cohort of patients with inflammatory breast cancer (IBC), a rare but aggressive variant of breast cancer that is more commonly seen in obese women5 and is often triple negative.6 We hypothesized that higher BMI would be associated with lower likelihood of attaining pCR and an increased likelihood of having CTCs after NACT. We also hypothesized that higher BMI would diminish the prognostic significance of pCR and CTCs, making them less reliable prognosticators in obese patients with breast cancer.
MATERIALS AND METHODS
We used the TNM staging system of the American Joint Commission on Cancer (AJCC) for pathologic stage and Black’s nuclear grading system for histologic grade. We defined clinical stage as the TNM stage established at the time of the first diagnostic procedure confirming invasive breast cancer, which, at our institution, also involves sonographic and pathologic staging of all adjacent nodal basins including the axilla. BMI (weight in kilograms [kg] divided by height in square meters [m2], i.e., kg/m2) is calculated for all patients at our institution at their initial visit and is automatically updated at each subsequent visit by incorporating the most current patient weight. For this study, we utilized the BMI values from each participant’s first visit at The University of Texas MD Anderson Cancer Center with a breast surgical oncologist (A.L, H.M.K., and S.M.D.) and considered this to be a patient’s BMI at diagnosis.
This study included T4d breast cancer patients diagnosed between March 2005 and March 2015 who received NACT as part of a prospective trial at MD Anderson Cancer Center examining the relationship between CTC levels and patient outcomes. All participants either received NACT at MD Anderson or consulted an MD Anderson medical oncologist even if they chose to receive NACT closer to home. Following completion of NACT and prior to surgical resection, a peripherally drawn, venous blood sample (7.5 mL) was obtained from each participant. Within 72 hours of collection, CTCs were identified via the CellSearch® method using previously described techniques.7
Patients with bilateral breast cancer or another malignancy diagnosed within 5 years of the primary breast cancer were excluded from the trial. Furthermore, inclusion in this statistical analysis was limited to patients whose postoperative pathologic review definitively described whether pCR had or had not occurred in the breasts and axillary lymph nodes. The institutional review board at MD Anderson approved this prospective study (04-0698; PI: A.L.), and written informed consent was obtained from all patients prior to blood collection.
Chi-square tests and logistic regression were used to examine the relationships between pCR, CTCs, and clinical variables including BMI (categorically divided into non-obese [BMI<30] and obese [BMI≥30] for regression analyses); age; race/ethnicity; tumor biomarkers stratified as (1) ER+ (includes all ER+ tumors regardless of PR and HER/neu status), (2) HER2/neu-amplified (HER2+) only, (3) ER-negative (ER−) and HER2/neu-non-amplified (HER2−), and (4) triple-negative (TNBC) (see Appendix Table 1 for number of participants per receptor status); tumor grade (1 = well-differentiated/low grade, 2 = moderately differentiated/intermediate grade, and 3 = poorly differentiated/high grade); tumor histology (i.e., ductal, lobular, mixed, or other); menopausal status; type of neoadjuvant chemotherapy received (anthracycline [i.e., doxorubicin or Epirubicin] only, taxane [i.e., docetaxel or paclitaxel] only, anthracycline and taxane, none; axillary nodal status (N0–N3); and the presence of lymphovascular invasion (LVI). These variables were also included in Cox proportional hazards models to calculate predicted event-free survival (EFS, i.e., no recurrence or death between date of diagnosis and date of last follow-up). We report proportions, adjusted odds ratios (OR), and adjusted hazards ratios (HR) with 95% confidence intervals (CI) significant at 2-tailed p<0.05. For the EFS analysis, we also report Harrell’s C indices in order to indicate the ordinal predictive power of the survival models, with values closer to 1 demonstrating greater predictive power than values closer to 0.5.8 Statistical analysis was performed using STATA 13 (StataCorp, College Station, TX).
RESULTS
From a cohort of 113 trial-enrolled patients, 73 (65%) patients with IBC were identified (Table 1). Median follow-up time was 40.4 months. Of the 73 IBC patients, 61 (84%) had CTC values and 22 (30%) achieved a pCR (Table 1). Only 16 (22%) patients had a normal BMI (25>BMI≥18.5) or were underweight (BMI<18.5), while 18 (25%) were overweight (30>BMI≥25) and 39 (53%) were obese (BMI≥30). Twenty-six patients (37%) had TNBC, which was the most common biomarker subtype in our cohort and has been more frequently observed among IBC patients as compared to non-IBC patients.6, 9, 10
Table 1.
Patient Demographics
| Clinical characteristic | Overall cohort | BMI | |||
|---|---|---|---|---|---|
| N | % | Obese | Non-obese | p-value | |
| Total patients | 73 | 39 | 34 | ||
| Age | |||||
| Mean (standard deviation) | 51 (11.2) | 49.85 (11.3) | 53 (11.02) | 0.233 | |
| Pathologic complete response | |||||
| (+) | 22/73 | 30% | 12/22 (55%) | 10/22 (45%) | 0.900 |
| (−) | 51/73 | 70% | 27/51 (53%) | 24/51 (47%) | |
| Circulating tumor cells | |||||
| (+) | 18/61 | 30% | 9/18 (50%) | 9/18 (50%) | 0.804 |
| (−) | 43/61 | 70% | 23/43 (53%) | 20/43 (47%) | |
| Pathologic nodal status (N) | |||||
| Node negative (N0) | 7/73 | 10% | 3/7 (42.86%) | 4/7 (57.14%) | 0.693 |
| 1–3 Lymph nodes (N1) | 25/73 | 34% | 14/25 (56%) | 11/25 (4%) | |
| 4–9 lymph nodes (N2) | 7/73 | 10% | 5/7 (71.43%) | 2/7 (28.57%) | |
| N3 | 34/73 | 46% | 17/34 (50%) | 17/34 (50%) | |
| Receptors/Biomarkers | |||||
| ER+ | 23/71 | 32% | 13/23 (56.52%) | 10/23 (43.48%) | 0.229 |
| ER− | 2/71 | 3% | 2/2 (100%) | 0/2 (0%) | |
| HER2+ only | 20/71 | 28% | 12/20 (60%) | 8/20 (40%) | |
| Triple-negative (TNBC) | 26/71 | 37% | 10/26 (38.46%) | 16/26 (61.54%) | |
| Histology | |||||
| Ductal | 65/73 | 89% | 36/65 (55.38%) | 29/65 (44.62%) | 0.666 |
| Lobular | 2/73 | 3% | 1/2 (50%) | 1/2 (50%) | |
| Mixed | 4/73 | 5% | 2/4 (50%) | 2/4 (50%) | |
| Other | 2/73 | 3% | 0/2 (0%) | 2/2 (100%) | |
| Menopausal status | |||||
| Premenopausal | 26/73 | 36% | 15/26 (57.69%) | 11/26 (42.31%) | 0.587 |
| Postmenopausal | 47/73 | 64% | 24/47 (51.06%) | 23/47 (48.94%) | |
| Race/Ethnicity | |||||
| White | 59/73 | 81% | 29/59 (49.15%) | 30/59 (50.85%) | 0.140 |
| Black | 4/73 | 5% | 4/4 (100%) | 0/4 (0%) | |
| Hispanic/Latino | 8/73 | 11% | 4/8 (50%) | 4/8 (50%) | |
| Asian/Other | 2/73 | 3% | 2/2 (100%) | 0/2 (0%) | |
| Chemotherapy regimen | |||||
| Anthracycline only | 6/73 | 8% | 4/6 (66.67%) | 2/6 (33.33%) | 0.762 |
| Taxane only | 9/73 | 12% | 4/9 (44.44%) | 5/9 (55.56%) | |
| Anthracycline & Taxane | 58/73 | 80% | 31/58 (53.45%) | 27/58 (46.55%) | |
| Lymphovascular invasion | |||||
| (+) | 32/65 | 49% | 16/32 (50%) | 16/32 (50%) | 0.267 |
| (−) | 33/65 | 51% | 21/33 (63.64%) | 12/33 (36.36%) | |
| Histological grade | |||||
| 1 | 0/73 | 0% | -- | -- | 0.835 |
| 2 | 31/73 | 42% | 17/31 (54.84%) | 14/31 (45.16%) | |
| 3 | 42/73 | 58% | 22/42 (52.38%) | 20/42 (47.62%) | |
There was no difference between obese and non-obese patients with regards to pCR rates (31% [12/39] vs. 29% [10/34], p=0.90) or the presence of CTCs (23% [9/39] vs. 26% [9/34], p=0.80). In univariate regression, the presence of LVI (OR 0.07, CI 0.01–0.35, p<0.01) was associated with lower likelihood of pCR. HER2/neu amplification (OR 4.4, CI 1.17–16.57, p=0.03) and receiving either epirubicin (vs. doxorubicin; OR 4.65, CI 1.39–15.52, p=0.01) or no anthracycline (vs. doxorubicin; OR 7.75, CI 1.53–39.12, p=0.01) were associated with an increased likelihood of achieving pCR. In bivariate regression in which BMI and another covariate served as independent variables, anthracycline regimen (epirubicin: OR 4.73, CI 1.39–16.05, p=0.01; none: OR 7.74, CI 1.53–39.07, p=0.01) and the presence of LVI (OR 0.07, CI 0.01–0.35, p<0.01) continued to be associated with likelihood of achieving pCR, but HER2/neu amplification was not. There were no covariates associated with increased likelihood of CTCs’ being present in either univariate or bivariate analyses. Additionally, there was no association between BMI and pCR, BMI and CTCs, or pCR and CTCs in univariate or bivariate regression analyses. Multivariate modeling was limited by sample size.
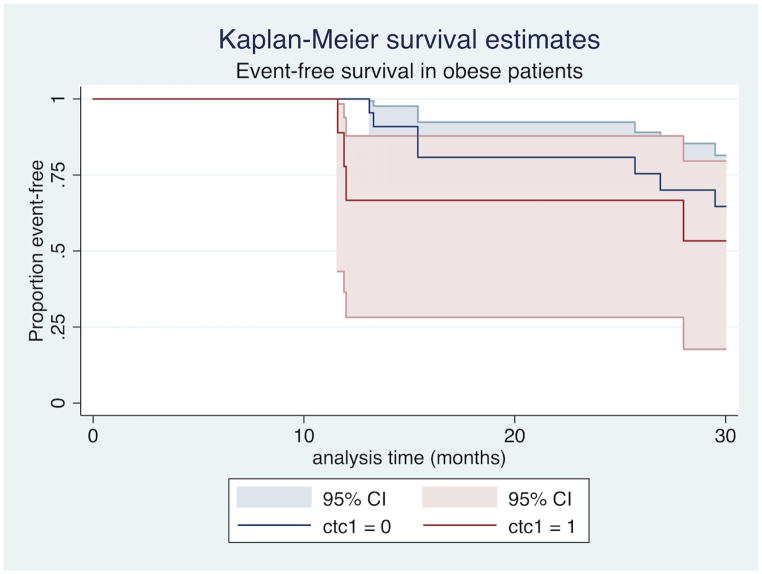
In bivariate modeling that included BMI as an independent covariate, pCR was associated with improved EFS (HR 0.04, CI 0.001–0.86, p=0.04) while the presence of CTCs was associated with worse EFS (HR 27.09, CI 4.54–161.51, p<0.01) at 30-month follow-up. When patients with IBC were stratified by BMI, CTCs continued to be associated with worse EFS in non-obese patients (HR 11.69, CI 2.75–49.66, p<0.01, Figure 1), while among obese patients, there was no statistically significant difference in EFS between those with and without CTCs (HR 1.89, CI 0.55–6.49, p=0.312, Figure 2). The addition of BMI stratified by obesity (BMI<30 vs. BMI≥30) did not substantively change the predictive power of CTCs and pCR (Harrell’s C index of 0.8305 for model without BMI vs. 0.8317 for model with BMI).
Figure 1.
CTC presence is associated with worse event-free survival among non-obese patients with IBC.
Figure 2.
CTC presence is not associated with event-free survival in obese patients with IBC.
DISCUSSION
In our cohort, none of the clinicopathologic variables we examined – including BMI and pCR – were associated with CTCs, though the presence of CTCs was independently associated with EFS following receipt of NACT. Our findings are concordant with previously published work and illustrate the important function CTCs could play as a unique prognosticator for breast cancer patients that might be at elevated risk for relapse despite achieving pCR. Notably, however, the prognostic significance of CTCs was mediated by patient BMI, and CTCs were not associated with worse EFS among obese patients.
A high proportion of the IBC patients in our cohort had TNBC and hormone-receptor-negative (HR−), HER2+ breast cancers (see Appendix), which is consistent with epidemiologic studies demonstrating high rates of HR− and HER2+ subtypes among IBC patients.6 Although obesity is known to be a risk factor for IBC, the epidemiology of TNBC and obesity is more complex and is also mediated by menopausal status.11 IBC and TNBC are both histologically aggressive and associated with worse survival than other subtypes of breast cancer,12–14 but the extent of overlap between these two entities – while significant – is nonlinear and the exact etiological link is unknown. A candidate mediator for this relationship is the fat mass and obesity associated (FTO) gene, which is important for energy balance15–17 and has been found via genome wide association studies (GWAS) to be associated with TNBCs including triple-negative IBC. In addition to its potential etiological significance, the FTO gene could also serve as a therapeutic target for TNBC in certain subsets of patients in a manner akin to how HER2/neu amplification, historically an adverse characteristic of breast cancer, now provides a highly responsive, therapeutic target for HER2+ IBC. However, obesity may also mediate the clinical course of HER2+ patients and/or their response to therapy, as evidenced by the difference between our univariate and bivariate regression analyses examining the effect of HER2/neu amplification on likelihood of achieving pCR.
In other studies, obesity has been demonstrated to adversely affect the likelihood of a patient’s achieving pCR, but the relationship between pCR attainment and obesity is neither linear nor consistent. In the 2008 study by Litton et al. examining the relationship between pCR and obesity in women with nonmetastatic, noninflammatory breast cancer treated at MD Anderson, there was no significant difference in pCR for obese patients compared with normal/underweight patients, but overweight patients compared with normal/underweight patients were less likely to achieve pCR; furthermore, when overweight and obese patients were combined, they were less likely to attain pCR than the combined group of normal/underweight patients.4 It is possible that with a larger data set, we might have had more patients in each weight class to provide a more granular picture of the relationship between BMI and pCR. However, it is also possible that IBC represents a class of disease (notably excluded from Litton’s study and many other studies examining the pCR-obesity relationship) in which the association between pCR and BMI differs significantly what has historically been observed in other types of breast cancer. In aggregate, our findings highlight the importance of appreciating the mechanistic diversity of breast cancer and support the need for greater investigation into how the development of breast cancer in patients with elevated BMI may differ from that in non-obese individuals, particularly in breast cancer phenotypes such as IBC for which obesity is a risk factor. In-depth molecular profiling, as reported by Ross and colleagues, may play an important role in lending granularity to the management and prognostication of IBC.6
As this study examines CTC levels following NACT, one reason CTCs may not be as prognostic in obese patients is that their presence may matter less in the context of other factors that adversely affect both the natural history of breast cancer in obese patients as well as their response to systemic therapy. In addition, CTCs often have low levels of ER expression, even in patients with ER+ primary breast cancers.18 The estrogen-rich milieu in obese patients may do less to promote the dissemination and implantation of CTCs than the relatively estrogen-poor environment within non-obese patients. Furthermore, the presence of ER− CTCs – as one might expect to see in HR−/HER2+ breast cancers and TNBC – may be due to a number of factors including heterogeneity within the population of primary tumor cells and de-differentiation of primary tumor cells that are more likely to be found amongst non-obese patients whose tumors are less likely to be fed by estrogen. Finally, patients with higher BMI may be more likely to have ER+ cells that persist both locally and in a disseminated fashion after NACT, but these relatively well-differentiated cells may have little metastatic potential and, accordingly, little impact on long-term outcome even if they are detected as CTCs.
As previously stated, women who are obese have lower rates of overall and breast-cancer-specific survival than non-obese patients, but the mechanism for this disparity remains unclear. With regards to chemotherapy, obesity is associated with differences in dosing and administration, but there is conflicting evidence as to whether obesity affects the bioavailability and efficacy of these medications.2, 4, 19–25 Most chemotherapy drugs are dosed using body surface area (BSA), and their narrow therapeutic window has historically raised concerns as to how best to administer efficacious doses of chemotherapy to obese patients without inducing the toxicity that might be incurred by basing dosage on their actual, as opposed to their ideal, weight. While there is evidence that the elimination of chemotherapy drugs may be delayed in obese patients, there is little evidence to support reduced dosage in these patients. However, obese women are still more likely to be under-dosed than non-obese women.23 This disparity in practice may not only contribute to worse outcomes when obese patients are compared to non-obese patients but also to greater heterogeneity among obese patients who, at the population level, may have less standardized dosing than their non-obese counterparts. Such heterogeneity in the administration of systemic treatment would, as a result, mitigate the prognostic significance of any variable that might be influenced by receipt of systemic therapy, including the presence of residual microscopic disease in the form of CTCs.
Our study had some limitations. Our sample size was small, and it is possible that the failure to detect an association between CTCs and BMI among obese patients is due to the study’s sample size being underpowered. However, our cohort actually had more obese patients than overweight, normal weight, or underweight patients, so we have reason to believe that the failure to observe an association between CTCs and obesity reflects a yet-to-be elucidated difference in the role CTCs may play in obese and non-obese patients with breast cancer. Second, BMI is only one of many ways in which to assess obesity and differences in adipose deposition; research utilizing other measures of obesity for which we do not have patient data – including bioelectrical impedance,26 skinfold thickness,27 waist-to-hip ratio,28 and calculations of percent body fat using CT or MRI scans29 – may yield different associations with the likelihood of having CTCs following NACT or on the impact of CTCs on breast cancer prognosis. Third, our follow-up time was limited to 30 months; long-term follow up of our cohort may reveal different or stronger associations, as a greater proportion of our patients will have had sufficient time for an event to occur. Finally, our study is limited to patients with IBC, a rare form of breast cancer; thus, we acknowledge that the applicability of our findings to the general population of breast cancer patients in the US – where most patients present with early-stage disease30 – may be relatively limited.
CONCLUSIONS
In our study, BMI was not independently associated with the likelihood of either experiencing pCR or having CTCs after NACT; furthermore, pCR and BMI were not associated with each other. BMI did, however, mediate the prognostic significance of CTCs, serving as a negative prognosticator in non-obese patients but not demonstrating any association with EFS among obese patients. To our knowledge, our study is the first to demonstrate an interaction between BMI and the prognostic strength of CTCs, and our work highlights the need for ongoing investigation into the unique challenges associated with treating breast cancer in obese patients and in forecasting their prognosis.
Our study represents an important contribution to the literature as it further illustrates the need to better incorporate BMI into our understanding and management of a very aggressive form of breast cancer. While not independently associated with EFS in our cohort, BMI may nevertheless be important in predicting prognosis in IBC patients following NACT, and the significance of both CTCs, pCR, and other prognosticators in obese patients warrants further investigation.
Acknowledgments
Presented in part at the 2016 annual meetings of the Society of Surgical Oncology (SSO), March 2–5, 2016, and the American Society of Breast Surgeons (ASBS), April 13–17, 2016. This work was supported by an SSO Clinical Investigator Award (A. Lucci), the Morgan Welch Inflammatory Breast Cancer Program and the Institute for Personalized Therapy at the University of Texas MD Anderson Cancer Center, the State of Texas Rare and Aggressive Breast Cancer Research Program, and philanthropic funds for which we thank our many generous donors. Dr. Lucci has served on the Genomic Health, Inc., Speaker’s Bureau. All other authors declare that they have no competing interests.
Appendix
Table 1.
Participant receptor status
| Receptor Variable | Receptors | n* | ||
|---|---|---|---|---|
| ER | PR | HER2/neu | ||
| ER+ | ER+ | PR+ | HER2+ | 4 |
| ER+ | PR+ | HER2− | 11 | |
| ER+ | PR− | HER2+ | 3 | |
| ER+ | PR− | HER2− | 5 | |
| ER− | ER− | PR+ | HER2+ | 0 |
| ER− | PR+ | HER2− | 2 | |
| HER2+ only | ER− | PR− | HER2+ | 20 |
| TNBC | ER− | PR− | HER2− | 26 |
| Total | 71 | |||
Five patients did not have HER2 receptor status reported.
ER – estrogen receptor; HER2+ – HER2/neu-amplified; PR – progesterone receptor; TNBC – triple negative breast cancer.
References
- Lorincz AM, Sukumar S. Molecular links between obesity and breast cancer. Endocr Relat Cancer. 2006 Jun;13(2):279–92. doi: 10.1677/erc.1.00729. [DOI] [PubMed] [Google Scholar]
- Fontanella C, Lederer B, Gade S, et al. Impact of body mass index on neoadjuvant treatment outcome: a pooled analysis of eight prospective neoadjuvant breast cancer trials. Breast Cancer Res Treat. 2015 Feb;150(1):127–39. doi: 10.1007/s10549-015-3287-5. [DOI] [PubMed] [Google Scholar]
- Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010 Oct;123(3):627–35. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- Litton JK, Gonzalez-Angulo AM, Warneke CL, et al. Relationship between obesity and pathologic response to neoadjuvant chemotherapy among women with operable breast cancer. J Clin Oncol. 2008 Sep 1;26(25):4072–7. doi: 10.1200/JCO.2007.14.4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Buzdar AU, Hursting SD. Inflammatory breast cancer and body mass index. J Clin Oncol. 1998 Dec 1;16(12):3731–5. doi: 10.1200/JCO.1998.16.12.3731. [DOI] [PubMed] [Google Scholar]
- Ross JS, Ali SM, Wang K, et al. Comprehensive genomic profiling of inflammatory breast cancer cases reveals a high frequency of clinically relevant genomic alterations. Breast Cancer Res Treat. 2015;154(1):155–162. doi: 10.1007/s10549-015-3592-z. [DOI] [PubMed] [Google Scholar]
- Hall C, Karhade M, Laubacher B, et al. Circulating Tumor Cells After Neoadjuvant Chemotherapy in Stage I–III Triple-Negative Breast Cancer. Ann Surg Oncol. 2015 Dec;22(Suppl 3):552–8. doi: 10.1245/s10434-015-4600-6. [DOI] [PubMed] [Google Scholar]
- Harrell FE, Jr, Lee KL, Califf RM, et al. Regression modelling strategies for improved prognostic prediction. Stat Med. 1984 Apr-Jun;3(2):143–52. doi: 10.1002/sim.4780030207. [DOI] [PubMed] [Google Scholar]
- Biswas T, Efird JT, Prasad S, et al. Inflammatory TNBC Breast Cancer: Demography and Clinical Outcome in a Large Cohort of Patients With TNBC. Clin Breast Cancer. 2016;16(3):212–216. doi: 10.1016/j.clbc.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Masuda H, Brewer TM, Liu DD, et al. Long-term treatment efficacy in primary inflammatory breast cancer by hormonal receptor- and HER2-defined subtypes. Ann Oncol. 2013;25(2):384–391. doi: 10.1093/annonc/mdt525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P. Triple-negative breast cancer: epidemiological considerations and recommendations. Ann Oncol. 2012 Aug;23(Suppl 6):vi7–12. doi: 10.1093/annonc/mds187. [DOI] [PubMed] [Google Scholar]
- Matro JM, Li T, Cristofanilli M, et al. Inflammatory breast cancer management in the national comprehensive cancer network: the disease, recurrence pattern, and outcome. Clin Breast Cancer. 2015 Feb;15(1):1–7. doi: 10.1016/j.clbc.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SP, Roth A, Goya R, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012 Jun 21;486(7403):395–9. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke C, Mazouni C, Hess KR, et al. Response to Neoadjuvant Therapy and Long-Term Survival in Patients With Triple-Negative Breast Cancer. J Clin Oncol. 2008 Mar 10;26(8):1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- Berulava T, Ziehe M, Klein-Hitpass L, et al. FTO levels affect RNA modification and the transcriptome. Eur J Hum Genet. 2013 Mar;21(3):317–23. doi: 10.1038/ejhg.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson R, Hagglund M, Olszewski PK, et al. The obesity gene, FTO, is of ancient origin, up-regulated during food deprivation and expressed in neurons of feeding-related nuclei of the brain. Endocrinology. 2008 May;149(5):2062–71. doi: 10.1210/en.2007-1457. [DOI] [PubMed] [Google Scholar]
- Gulati P, Cheung MK, Antrobus R, et al. Role for the obesity-related FTO gene in the cellular sensing of amino acids. Proc Natl Acad Sci U S A. 2013 Feb 12;110(7):2557–62. doi: 10.1073/pnas.1222796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babayan A, Hannemann J, Spotter J, et al. Heterogeneity of estrogen receptor expression in circulating tumor cells from metastatic breast cancer patients. PLoS One. 2013;8(9):e75038. doi: 10.1371/journal.pone.0075038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce-Salinas C, Aguilar-Ponce JL, Villarreal-Garza C, et al. Overweight and obesity as poor prognostic factors in locally advanced breast cancer patients. Breast Cancer Res Treat. 2014 Jul;146(1):183–8. doi: 10.1007/s10549-014-2977-8. [DOI] [PubMed] [Google Scholar]
- Berclaz G, Li S, Price KN, et al. Body mass index as a prognostic feature in operable breast cancer: the International Breast Cancer Study Group experience. Ann Oncol. 2004 Jun 1;15(6):875–884. doi: 10.1093/annonc/mdh222. [DOI] [PubMed] [Google Scholar]
- Crozier JA, Moreno-Aspitia A, Ballman KV, et al. Effect of body mass index on tumor characteristics and disease-free survival in patients from the HER2-positive adjuvant trastuzumab trial N9831. Cancer. 2013 Jul 1;119(13):2447–54. doi: 10.1002/cncr.28051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Fabbro E, Parsons H, Warneke CL, et al. The relationship between body composition and response to neoadjuvant chemotherapy in women with operable breast cancer. Oncologist. 2012;17(10):1240–5. doi: 10.1634/theoncologist.2012-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs JJ, Sorbero ME, Lyman GH. Undertreatment of obese women receiving breast cancer chemotherapy. Arch Intern Med. 2005 Jun 13;165(11):1267–73. doi: 10.1001/archinte.165.11.1267. [DOI] [PubMed] [Google Scholar]
- Iwase T, Sangai T, Nagashima T, et al. Impact of body fat distribution on neoadjuvant chemotherapy outcomes in advanced breast cancer patients. Cancer Med. 2016 Jan;5(1):41–8. doi: 10.1002/cam4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogawa T, Fouad TM, Wei C, et al. Association of Body Mass Index Changes during Neoadjuvant Chemotherapy with Pathologic Complete Response and Clinical Outcomes in Patients with Locally Advanced Breast Cancer. J Cancer. 2015;6(4):310–318. doi: 10.7150/jca.10580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu Khaled M, McCutcheon MJ, Reddy S, et al. Electrical impedance in assessing human body composition: the BIA method. Am J Clin Nutr. 1988 May;47(5):789–92. doi: 10.1093/ajcn/47.5.789. [DOI] [PubMed] [Google Scholar]
- Reilly JJ, Wilson J, Durnin JV. Determination of body composition from skinfold thickness: a validation study. Arch Dis Child. 1995 Oct;73(4):305–10. doi: 10.1136/adc.73.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelsteyn CJ, Joffres MR, MacLean DR, Flowerdew G. A comparative evaluation of waist circumference, waist-to-hip ratio and body mass index as indicators of cardiovascular risk factors. The Canadian Heart Health Surveys. Int J Obes Relat Metab Disord. 2001 May;25(5):652–61. doi: 10.1038/sj.ijo.0801582. [DOI] [PubMed] [Google Scholar]
- Silver HJ, Welch EB, Avison MJ, Niswender KD. Imaging body composition in obesity and weight loss: challenges and opportunities. Diabetes Metab Syndr Obes. 2010;3:337–47. doi: 10.2147/DMSOTT.S9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal J, Ginsburg O, Rochon PA, et al. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA. 2015 Jan 13;313(2):165–73. doi: 10.1001/jama.2014.17322. [DOI] [PubMed] [Google Scholar]