Abstract
Objectives
This study examined how different quantifications of pain (average vs. day-to-day inconsistency) are related to sleep in older adults beyond known predictors.
Methods
Baseline measures from the Active Adult Mentoring Project were used for secondary analyses. Participants included 82 adults in mid- to late-life. Depression was assessed with the BDI-II. Pain intensity was assessed over seven days on a 11-point Likert-scale, while sleep efficiency (SE), total sleep time (TST), and total wake time (TWT) were assessed using a self-report diary.
Results
Regression analyses revealed that pain inconsistency was associated with both SE and TWT and accounted for significant variance over age, gender, and depression. In contrast, average pain was not associated with SE, TST, or TWT.
Conclusions
The findings indicate that pain inconsistency may be a more meaningful predictor of sleep disturbance than average pain level, suggesting that one’s ability to regulate pain may be related to one’s ability to engage in optimal sleep in mid- to late-life.
Clinical Implications
Pain inconsistency appears to contribute more to sleep disturbance than average pain. Pain inconsistency in late-life warrants greater attention and may be an area of clinical intervention through activity-pacing or coping skills training.
Keywords: Older adults, pain, pain inconsistency, sleep
Sleep has been identified as a marker of healthy aging (Driscoll et al., 2008). Yet, sleep is known to decline in late-life, with approximately 50% of older adults complaining of sleep disturbance (Ohayon, Carskadon, Guilleminault, & Vitiello, 2004). Sleep disturbance in older adults warrants clinical attention due to its association with a wide range of adverse physical and mental health outcomes (Neikrug & Ancoli-Israel, 2010). Although the etiology of disrupted sleep is multifactorial, depression is one commonly cited risk factor for the development of sleep disturbance in community-dwelling older adults (Buysse, 2004).
Pain is another common health problem in late-life that contributes to sleep disturbance (Ohayon, 2005). Older adults are disproportionally affected by both acute and chronic pain (Hwang & Platts-Mills, 2013; Tsang et al., 2008), with approximately 50% of older adults reporting pain in the past month that interfered with their ability to function normally (Patel, Guralnik, Dansie, & Turk, 2013). Although pain is often assessed in terms of its average intensity during the past week or month, pain is known to be inconsistent from day to day (Jensen & McFarland, 1993). Moreover, changes in pain intensity are a hallmark of several chronic pain conditions (Allen, Coffman, Golightly, Stechuchak, & Keefe, 2009; Johnson, Zautra, & Davis, 2006).
Sleep disturbance and pain frequently co-occur, especially in late-life (Eslami, Zimmerman, Grewal, Katz, & Lipton, 2016). Although the relationship between sleep and pain is complex and not fully understood, evidence suggests a bidirectional relationship between sleep and pain (Finan, Goodin, & Smith, 2013). In addition, the two have been found to covary in older adults (Dzierzewski et al., 2010), suggesting that common physiological systems may underlie both sleep disturbance and pain. Moreover, average pain intensity has been shown to be associated with sleep disturbance for older adults, even after controlling for demographics, comorbidities, and stress (Eslami et al., 2016). Despite the strong link between pain and sleep in older adults, a lack of research exists concerning the association between pain inconsistency and sleep in late-life. This gap in knowledge is relevant given that pain inconsistency can be a debilitating factor for individuals who experience pain (Hutchings et al., 2007) and may clarify the relationship between pain and sleep.
The purpose of the present study was to explore whether different quantifications of pain (average vs. daily inconsistency) are related to sleep above and beyond known predictors, such as depression. Sleep disturbance and pain are common in older adults and associated with adverse consequences; however, relatively little research has examined the association between pain inconsistency and sleep, even though we know that pain demonstrates significant day-to-day inconsistency. Furthermore, pain inconsistency may be an area of clinical intervention for health-care providers who work with older adults. Integrating past findings on both pain and sleep, it was hypothesized that both average pain and pain inconsistency would predict poorer sleep (i.e., lower sleep efficiency, increased total sleep time, and lower total wake time) over and above depression. In addition, we explored whether pain inconsistency or average pain would be a more consistent predictor of sleep.
Methods
This project included secondary analysis of baseline data from the Active Adult Mentoring Project (AAMP). The primary aim of the AAMP study was to examine whether a social-cognitive lifestyle intervention could help promote moderate intensity exercise in older adults. A description of methods pertinent to the present study are provided here, while the complete methods of the AAMP study are presented elsewhere (Buman et al., 2011).
Participants
Participants included 82 community-dwelling older adults recruited between 2006 and 2008 via a university community in the southeastern United States. Announcements were placed in a local newspaper, a university older adult participant registry, and in community gathering places.
Inclusion criteria for the AAMP study included an age of 50 years old or older and a self-reported sedentary lifestyle (defined by the Physical Activity Guidelines Advisory Committee [2008] as less than 150 minutes per week of moderate or vigorous physical activity during the past six months). Exclusion criteria included the presence of medical conditions that would significantly interfere with the ability to complete unsupervised exercise (e.g., major cardiovascular disease, pulmonary disease, recent cancer treatment) or the presence of factors that would significantly interfere with study compliance or assessment (e.g., cognitive impairment, psychosis, hearing or speech impairment).
Procedure
Individuals who were interested in participating in the study first completed a brief screening over the phone in order to ensure that they met eligibility criteria. Next, qualified individuals completed demographic items and a measure of depression. Participants were then asked to complete daily sleep and pain measures each morning upon awakening for seven consecutive days.
Measures
Sleep
Participants completed a daily sleep diary upon awakening for seven consecutive days. The daily sleep diaries assessed a variety of sleep parameters including: total sleep time (TST: the total amount of time spent asleep during the night), and total wake time (TWT: the total amount of time spent awake during the night, including time to initially fall asleep, time awake during the middle of the night, and time spent ‘snoozing’ in the morning). Finally, sleep efficiency (SE: the percentage of time asleep over the total time spent in bed), was also calculated.
Pain
Pain was assessed subjectively using a single item, “What is your current level of pain?”. Participants recorded their response to this item on a 11-point Likert-type scale where 0 = “no pain” and 10 = “worst pain possible.” Although brief, this item captures day-to-day variations in pain and meets criteria recommended in a consensus statement by chronic pain researchers (Dworkin et al., 2008). In addition, even small changes on this scale reflect meaningful and clinically relevant changes in pain (Ferreira-Valente, Pais-Ribeiro, & Jensen, 2011).
Depression
Depressive symptoms were assessed using the Beck-Depression Inventory, Second Edition (BDI-II; Beck, Steer, & Brown, 1996). The BDI-II consists of 21 self-report items of cognitive and somatic depressive symptoms experienced in the past two weeks. In contrast to the Geriatric Depression Scale which is typically used for individuals 65 years old or older, the BDI-II has been validated for use with adults over the age of 50, making it more suitable for the present study (Segal, Coolidge, Cahill, & O’Riley, 2008).
Data Analysis
In line with previous research examining pain inconsistency (Farrar et al., 2014), pain inconsistency was operationalized as the seven-day individual standard deviation, while average pain was defined as the seven-day average. Figure 1 provides a visual representation of both average pain level and day-to-day inconsistency. Six separate three-block hierarchical multiple regressions were conducted with SE, TWT, and TST acting as the criterion variable. For all analyses, age and gender were entered in the first block, while depression was entered in the second block. For half of the analyses, average pain was entered in the third block, while for the other half of the analyses, pain inconsistency was entered in the third block.
Figure 1.
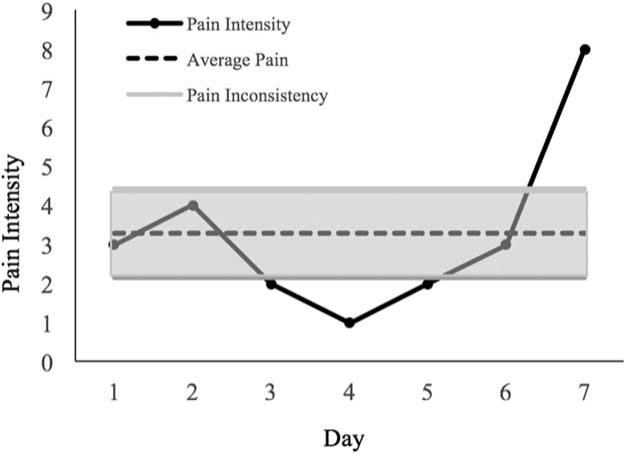
Depiction of different quantifications of pain for a randomly selected participant over the course of seven days. The solid black lines represents reported daily pain intensity levels. The dotted black line represents average pain level during the week, and the shaded area represents average pain inconsistency (seven-day standard deviation).
Results
Participants’ mean age was 63.37 (SD = 8.58). Participants were predominately female (82.9%) and White (91.4%). Complete demographic information and clinical characteristics are presented in Table 1. Model statistics (F-Statistics for the overall model fit and for ΔR2) are presented in Table 2. In block one, age and gender did not predict sleep for any of the models. In contrast, in block two, depression resulted in a significant improvement in the overall models for SE (ΔR2 = .24, p < .001) and TWT (ΔR2 = .28, p < .001) but not TST (ΔR2 = .05, p = .88). Similarly, when pain inconsistency was entered in block three, it accounted for a significant improvement in the overall models for SE (ΔR2 = .04, p < .05) and TWT (ΔR2 = .06, p = .01) but not TST (ΔR2 < .01, p = .68). With pain inconsistency as a predictor, the final model accounted for approximately 28% (adjusted R2) of the total variance for SE and approximately 30% (adjusted R2) of the total variance for TWT. In contrast, when average pain was entered in block three of the model, it failed to account for a significant improvement in the SE (ΔR2 = .01, p = .28), TST (ΔR2 = .01, p = .39), and TWT models (ΔR2 = .01, p = .29).
Table 1.
Participant descriptive statistics (N = 82).
| Variable | Mean (Std. Deviation) |
|---|---|
| Participant Demographics | |
| Agea | 63.37 (8.58) |
| Educationa | 16.15 (2.23) |
| Gender (Number of Males) | 14 |
| Race/Ethnicity (Number of | 7 |
| Racial/Ethnic Minorities) | |
| Depressionb | 6.07 (4.72) |
| Sleep Characteristics | |
| Sleep Efficiencyc | 87.44 (8.21) |
| Total Wake Timed | 64.01 (47.84) |
| Total Sleep Timed | 429.32 (56.02) |
| Pain Characteristics | |
| Average Pain Ratinge | 1.55 (1.50) |
| Pain Inconsistencyf | .84 (.64) |
Notes:
units of measurement in years
depression measured on a scale from 0 to 63
sleep efficiency measured as a percentage
sleep variables measured in minutes
pain rated on a scale from 0 to 10
measured as the within-person standard deviation of pain
Table 2.
Model fit statistics for each block in the model-building process.
| Variable | F | df | R2 | ΔR2 | F for ΔR2 |
|---|---|---|---|---|---|
| Sleep Efficiency | |||||
| Average Pain | |||||
| Block 1 | .35 | 2, 73 | .01 | .01 | .35 |
| Block 2 | 7.60** | 3, 72 | .24 | .23 | 21.89** |
| Block 3 | 6.01** | 4, 71 | .25 | .01 | 1.19 |
| Pain Inconsistency | |||||
| Block 1 | .35 | 2, 73 | .01 | .01 | .35 |
| Block 2 | 7.60** | 3, 72 | .24 | .23 | 21.89** |
| Block 3 | 6.94** | 4, 71 | .28 | .04 | 4.02* |
| Total Sleep Time | |||||
| Average Pain | |||||
| Block 1 | 1.87 | 2, 73 | .05 | .05 | 1.87 |
| Block 2 | 1.24 | 3, 72 | .05 | <.01 | .02 |
| Block 3 | 1.11 | 4, 71 | .06 | .01 | .76 |
| Pain Inconsistency | |||||
| Block 1 | 1.87 | 2, 73 | .05 | .05 | 1.87 |
| Block 2 | 1.24 | 3, 72 | .05 | <.01 | .02 |
| Block 3 | .96 | 4, 71 | .05 | <.01 | .17 |
| Total Wake Time | |||||
| Average Pain | |||||
| Block 1 | .76 | 2, 73 | .02 | .02 | .76 |
| Block 2 | 9.22** | 3, 72 | .28 | .26 | 25.63** |
| Block 3 | 7.22** | 4, 71 | .29 | .01 | 1.15 |
| Pain Inconsistency | |||||
| Block 1 | .76 | 2, 73 | .02 | .02 | .76 |
| Block 2 | 9.22** | 3, 72 | .28 | .26 | 25.63** |
| Block 3 | 9.17** | 4, 71 | .34 | .06 | 6.78* |
Note:
p < .001,
p < .05.
Block 1: Age and gender, Block 2: depression, Block 3: pain.
In the final models for SE, pain inconsistency was negatively associated with SE (β = −.21, p < .05), while average pain showed no association (β = −.12, p = .28). Similarly, in the final models for TWT, pain inconsistency was positively associated with TWT (β = .26, p = .01), however, average pain showed no association with TWT (β = .11, p = .29). Table 3 provides a complete listing of the standardized and unstandardized regression coefficients in the final models.
Table 3.
Summary of hierarchical regression analyses predicting sleep.
| Sleep Efficiency (Average Pain)
|
Sleep Efficiency (Pain Inconsistency)
|
|||||
|---|---|---|---|---|---|---|
| Variables | B | SE(B) | β | B | SE(B) | B |
| Age | −.03 | .09 | −.04 | −.01 | .09 | −.02 |
| Gender | .55 | 2.17 | .03 | .44 | 2.13 | .02 |
| Depression | −.75 | .18 | −.46*** | −.70 | .18 | −.42*** |
| Pain | −.59 | .54 | −.12 | −2.54 | 1.27 | −.21* |
|
| ||||||
| Total Sleep Time (Average Pain)
|
Total Sleep Time (Pain Inconsistency)
|
|||||
| B | SE(B) | β | B | SE(B) | B | |
|
| ||||||
| Age | 1.24 | .72 | .20 | 1.23 | .72 | .20 |
| Gender | −15.52 | 17.46 | −.10 | −15.38 | 17.53 | −.10 |
| Depression | .11 | 1.42 | .01 | −.38 | 1.44 | −.03 |
| Pain | −3.80 | 4.37 | −.10 | 4.28 | 10.40 | .05 |
|
| ||||||
| Total Wake Time (Average Pain)
|
Total Wake Time (Pain Inconsistency)
|
|||||
| B | SE(B) | β | B | SE(B) | B | |
|
| ||||||
| Age | .31 | .50 | .06 | .19 | .49 | .04 |
| Gender | −7.43 | 12.27 | −.06 | −6.65 | 11.82 | −.06 |
| Depression | 4.61 | 1.00 | .48*** | 4.16 | .97 | .44*** |
| Pain | 3.30 | 3.07 | .11 | 18.24 | 7.01 | .26* |
Note:
p < .001,
p < .01,
p < .05.
Discussion
The present study examined whether average pain or pain inconsistency predicted sleep disturbance in mid- to late-life adults, above known predictors such as depression. In addition, the study explored whether pain inconsistency or average pain was a more consistent predictor of sleep. Pain inconsistency, but not average pain, was found to significantly predict sleep for adults in mid- to late-life. Specifically, pain inconsistency accounted for a significant amount of variance in both SE and TWT above and beyond age, gender, and depression. In contrast, average pain was not associated with either SE, TWT, or TST.
Beyond reaffirming the significant contribution of depression on sleep in mid- to late-life, the results reveal the association between pain inconsistency and sleep in late-life. Specifically, the findings indicate that pain inconsistency may be a more meaningful predictor of sleep disturbance than average pain level. Furthermore, the relationship between pain inconsistency and sleep found in the present study suggests that the ability to regulate pain may be related to the ability to engage in optimal nightly sleep. Homeostenosis, the progressive constriction of homeostatic reserve that occurs naturally with age (Karp, Shega, Morone, & Weiner, 2008), may be the mechanism through which both pain inconsistency and sleep disturbance are induced, leading to increased difficulty in regulating both processes.
Beyond reiterating the need to address depressive symptoms in older adults with disrupted sleep, the findings from the present study have several important clinical implications. First, they suggest that pain inconsistency warrants greater clinical attention in the assessment of older adults with sleep disturbance. Secondly, clinical interventions that aim to minimize fluctuations in pain may be warranted, particularly for individuals who experience frequent flare-ups in their day-to-day experience of pain. Interventions that reduce pain inconsistency may also have beneficial secondary effects on sleep in mid- to late-life. Examples of such interventions include activity-pacing, which teaches patients to pace daily activities and exercises in order to counteract the over-activity-underactivity cycle that contributes to pain inconsistency in many pain populations (Andrews, Strong, & Meredith, 2012). Alternatively, given that pain catastrophizing is associated with pain inconsistency (Schneider et al., 2012), identifying and correcting maladaptive thoughts and beliefs might reduce pain inconsistency and promote better sleep in older adults.
Despite its strengths, the present study has several limitations. First, due to the cross-sectional nature of the study, no casual conclusions can be drawn between pain inconsistency and sleep disturbance. Relatedly, previous research has shown evidence for a bidirectional relationship between pain and sleep (Finan et al., 2013), thus, sleep may also serve as a predictor of pain in the current sample. Given the cross-sectional nature of the study, alternative mechanisms through which sleep contribute to pain are also possible (e.g., changes in circadian rhythm, light exposure). Second, both sleep and pain measures were self-reported and thus subject to recall bias and potential noncompliance. Third, general health status is likely related to both sleep and pain, however, the present study lacked the necessary data to include this information in the analyses. Relatedly, the study did not include information about participants’ use of pain medications which may have contributed to pain variations, nor could it categorize participants based on the presence or absence of clinical pain. Fourth, the physical demands required by the parent study may have limited the number of individuals with chronic pain from participating in the study, thus, the findings of the present study may not generalize to older individuals with chronic pain. In addition, given the relatively low levels of average pain and pain inconsistency found in the present study, the nonsignificant association between average pain and sleep may have resulted in a floor effect. Finally, the sample contained few racial and ethnic minorities thereby limiting the generalizability of the present findings. Given that minority populations are disproportionally affected by pain and report experiencing greater pain intensity (Edwards, Fillingim, & Keefe, 2001), pain inconsistency might have particularly important implications on sleep for ethnic and racial minorities.
Future research would benefit from experimentally exploring the role of pain inconsistency and sleep disturbance in older adults. In addition, given the link between pain inconsistency and sleep found in the present study, future research would benefit from exploring the role of pain inconsistency and sleep in older adults with diagnosed chronic pain conditions. Finally, exploration of temporal daily association between sleep and pain also warrants further exploration.
Clinical Implications.
Pain inconsistency is a more consistent predictor of sleep disturbance in mid- to late-life adults than average pain.
Pain inconsistency in mid- to late-life merits greater clinical attention and may be targeted through activity-pacing or pain coping skills training.
Acknowledgments
Funding
This work was supported by the National Institute on Aging (1R36AG029664-01, PI: Aiken-Morgan; T32AG020499, PI: Dzierzewski; F31AG032802, PI: Dzierzewski; 1K23AG049955, PI: Dzierzewski) and University of Florida (Age Network research award, PI: McCrae).
Footnotes
ORCID
Peter R. Giacobb http://orcid.org/0000-0003-1978-7424
Michael Marsiske http://orcid.org/0000-0001-5973-2116
References
- Allen KD, Coffman CJ, Golightly YM, Stechuchak KM, Keefe FJ. Daily pain variations among patients with hand, hip, and knee osteoarthritis. Osteoarthritis and Cartilage. 2009;17(10):1275–1282. doi: 10.1016/j.joca.2009.03.021. [DOI] [PubMed] [Google Scholar]
- Andrews NE, Strong J, Meredith PJ. Activity pacing, avoidance, endurance, and associations with patient functioning in chronic pain: A systematic review and meta-analysis. Archives of Physical Medicine and Rehabilitation. 2012;93(11):2109–2121 e7. doi: 10.1016/j.apmr.2012.05.029. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck depression inventory-II. San Antonio, TX: Psychological Corporation; 1996. pp. 78204–772498. [Google Scholar]
- Buman MP, Giacobbi PR, Jr, Dzierzewski JM, Morgan AA, McCrae CS, Roberts BL, Marsiske M. Peer volunteers improve long-term maintenance of physical activity with older adults: A randomized controlled trial. Journal of Physical Activity and Health. 2011;8(s2):S257–S266. doi: 10.1123/jpah.8.s2.s257. [DOI] [PubMed] [Google Scholar]
- Buysse DJ. Insomnia, depression and aging. Assessing sleep and mood interactions in older adults. Geriatrics. 2004;59(2):47–51. [PubMed] [Google Scholar]
- Driscoll HC, Serody L, Patrick S, Maurer J, Bensasi S, Houck PR, Reynolds CF. Sleeping well, aging well: A descriptive and cross-sectional study of sleep in “successful agers” 75 and older. The American Journal of Geriatric Psychiatry. 2008;16(1):74–82. doi: 10.1097/JGP.0b013e3181557b69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Zavisic S. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. The Journal of Pain. 2008;9(2):105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Dzierzewski JM, Williams JM, Roditi D, Marsiske M, McCoy K, McNamara J, McCrae CS. Daily variations in objective nighttime sleep and subjective morning pain in older adults with insomnia: Evidence of covariation over time. Journal of the American Geriatrics Society. 2010;58(5):925–930. doi: 10.1111/j.1532-5415.2010.02803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CL, Fillingim RB, Keefe F. Race, ethnicity and pain. Pain. 2001;94(2):133–137. doi: 10.1016/S0304-3959(01)00408-0. [DOI] [PubMed] [Google Scholar]
- Eslami V, Zimmerman ME, Grewal T, Katz M, Lipton RB. Pain grade and sleep disturbance in older adults: Evaluation the role of pain, and stress for depressed and non-depressed individuals. International Journal of Geriatric Psychiatry. 2016;31(5):450–457. doi: 10.1002/gps.4349. [DOI] [PubMed] [Google Scholar]
- Farrar JT, Troxel AB, Haynes K, Gilron I, Kerns RD, Katz NP, Dworkin RH. Effect of variability in the 7-day baseline pain diary on the assay sensitivity of neuropathic pain randomized clinical trials: An ACTTION study. Pain®. 2014;155(8):1622–1631. doi: 10.1016/j.pain.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain®. 2011;152(10):2399–2404. doi: 10.1016/j.pain.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Finan PH, Goodin BR, Smith MT. The association of sleep and pain: An update and a path forward. The Journal of Pain. 2013;14(12):1539–1552. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings A, Calloway M, Choy E, Hooper M, Hunter DJ, Jordan JM, Palmer L. The Longitudinal Examination of Arthritis Pain (LEAP) study: Relationships between weekly fluctuations in patient-rated joint pain and other health outcomes. The Journal of Rheumatology. 2007;34(11):2291–2300. [PubMed] [Google Scholar]
- Hwang U, Platts-Mills TF. Acute pain management in older adults in the emergency department. Clinics in Geriatric Medicine. 2013;29(1):151–164. doi: 10.1016/j.cger.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Jensen MP, McFarland CA. Increasing the reliability and validity of pain intensity measurement in chronic pain patients. Pain. 1993;55(2):195–203. doi: 10.1016/0304-3959(93)90148-I. [DOI] [PubMed] [Google Scholar]
- Johnson LM, Zautra AJ, Davis MC. The role of illness uncertainty on coping with fibromyalgia symptoms. Health Psychology. 2006;25(6):696–703. doi: 10.1037/0278-6133.25.6.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp JF, Shega JW, Morone NE, Weiner DK. Advances in understanding the mechanisms and management of persistent pain in older adults†. British Journal of Anaesthesia. 2008;101(1):111–120. doi: 10.1093/bja/aen090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neikrug AB, Ancoli-Israel S. Sleep disorders in the older adult – A mini-review. Gerontology. 2010;56(2):181–189. doi: 10.1159/000236900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM. Relationship between chronic painful physical condition and insomnia. Journal of Psychiatric Research. 2005;39(2):151–159. doi: 10.1016/j.jpsychires.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1274. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- Patel KV, Guralnik JM, Dansie EJ, Turk DC. Prevalence and impact of pain among older adults in the United States: Findings from the 2011 national health and aging trends study. Pain®. 2013;154(12):2649–2657. doi: 10.1016/j.pain.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Junghaenel DU, Keefe FJ, Schwartz JE, Stone AA, Broderick JE. Individual differences in the day-to-day variability of pain, fatigue, and well-being in patients with rheumatic disease: Associations with psychological variables. Pain. 2012;153(4):813–822. doi: 10.1016/j.pain.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal DL, Coolidge FL, Cahill BS, O’Riley AA. Psychometric properties of the Beck Depression Inventory II (BDI-II) among community-dwelling older adults. Behavior Modification. 2008;32(1):3–20. doi: 10.1177/0145445507303833. [DOI] [PubMed] [Google Scholar]
- Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Angermeyer MC, Watanabe M. Common chronic pain conditions in developed and developing countries: Gender and age differences and comorbidity with depression-anxiety disorders. The Journal of Pain. 2008;9(10):883–891. doi: 10.1016/j.jpain.2008.05.005. [DOI] [PubMed] [Google Scholar]


