Abstract
A G3P2 patient who conceived while using an intrauterine contraceptive device presented at 20 weeks of gestation with mild irregular uterine contractions and vaginal bleeding. Sonographic examination at admission showed the presence of dense amniotic fluid “sludge” and a long sonographic uterine cervix (42 mm). To assess the microbiologic significance of amniotic fluid “sludge,” we performed a transabdominal amniocentesis. The procedure was performed under real-time ultrasound, and fluid resembling pus at gross examination was noted. Rapid amniotic fluid analysis showed the presence of a high white blood cell count and structures resembling hyphae. Amniotic fluid cultures were positive for Candida albicans. Treatment was begun with broad-spectrum antibiotics, including Fluconazole, upon the visualization of pus in the “sludge” material because of the presence of hyphae in the Gram stain. Despite treatment, the patient went into spontaneous preterm labor and delivered five days after admission. Placental examination revealed acute fungal histologic chorioamnionitis and funisitis. This represents the first report of transabdominal collection and analysis of amniotic fluid “sludge” and the microbiologic detection of Candida albicans in this material. This report provides evidence that transabdominal retrieval of “sludge” is possible and may be of significant value for patient management and selection of antimicrobial agents.
Keywords: amniocentesis, biofilms, chorioamnionitis, funisitis, intra-amniotic infection/inflammation, intrauterine contraceptive device, microbial invasion of the amniotic cavity, preterm prelabor rupture of the membranes, spontaneous preterm labor, transvaginal ultrasound
Introduction
Conception while using an intrauterine contraceptive device (IUD) is a risk factor for adverse pregnancy outcome, including intrauterine infection often due to fungi [1–4], spontaneous preterm labor/birth (with or without ruptured membranes) [1, 5–14], maternal sepsis [6, 7, 15], and even death [16]. Amniotic fluid “sludge” is frequently detected in patients who present with an episode of suspected preterm labor [17–19], cervical insufficiency [20, 21], and an asymptomatic short cervix [20, 22–26]. The presence of “sludge” is a risk factor for preterm delivery, histologic chorioamnionitis, and intra-amniotic infection [19, 21–24, 27–29]. Although originally thought by some to represent a blood clot in the amniotic fluid [30], “sludge” is now known to represent intra-amniotic inflammation/infection due to microbial biofilms in patients with preterm parturition [31].
Previous cases of “sludge,” which have been the subject of studies using microbiologic techniques, have been retrieved transvaginally from patients who have inevitable preterm delivery [31, 32]. However, it is technically feasible to retrieve “sludge” by transabdominal aspiration. This would allow the analysis of the material in the context of an ongoing pregnancy.
We report herein the first transabdominal collection of “sludge” and identification of Candida albicans in a patient who conceived with an IUD.
Case Report
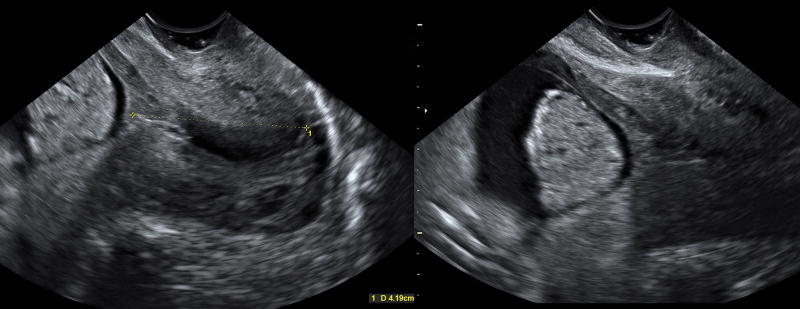
A 34 year-old Hispanic woman, gravida 3 para 2, who had two previous term deliveries (one vaginal, one Cesarean), was admitted at 20 weeks of gestation due to vaginal bleeding and irregular uterine contractility that did not meet the criteria for preterm labor because there were no changes in her cervix. The patient had conceived despite having a T380A copper IUD. A vaginal speculum examination revealed a few blood clots with no active bleeding. Transvaginal ultrasound showed a cervical length of 42 mm and floating, dense hyperechogenic particulate matter, amniotic fluid "sludge," in close proximity to the internal cervical os (Figure 1 and Videoclip 1). After obtaining written informed consent from the patient, a transabdominal amniocentesis was performed using a 20-gauge needle to exclude intra-amniotic infection [1–15, 33–7] based on the combination of amniotic fluid "sludge" [17, 18, 21, 22, 28, 31, 32], idiopathic vaginal bleeding [48, 49], and pregnancy co-existing with an IUD [1]. The tip of the needle was directed toward the “sludge” and allowed aspiration of this material (Videoclip 2). The gross characteristics of the amniotic fluid and "sludge" are depicted in Figure 2 and Videoclip 3.
Figure 1.
Two-dimensional transvaginal ultrasound showing amniotic fluid "sludge" in a patient with an intrauterine device, vaginal bleeding, and a long cervix at 20 weeks of gestation.
Figure 2.
Amniotic fluid samples collected during the transabdominal amniocentesis: a) from the lower uterine compartment and b) directly from the "sludge." The amniotic fluid looks turbid, while the "sludge" was thick and dense.
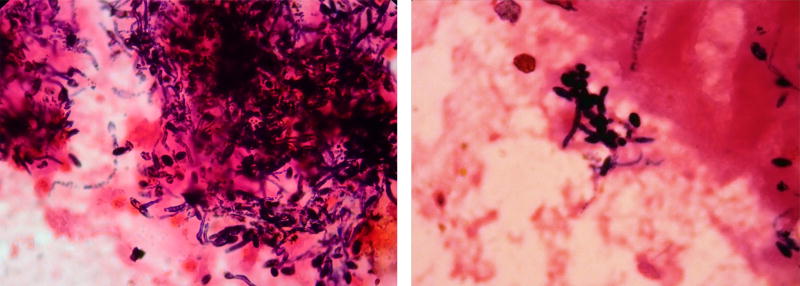
Amniotic fluid analysis revealed a white blood cell count of 1627 cells/mm3 (under normal circumstances, there are no white blood cells in the amniotic fluid, and a nucleated cell count above 50 is considered indicative of intra-amniotic inflammation). Amniotic fluid glucose was not detectable (under normal circumstances, a concentration below 14 mg/dl is suggestive of intra-amniotic infection/inflammation), and a Gram stain of the amniotic fluid revealed fusiform structures consistent with hyphae (Figure 3).
Figure 3.
Gram stain of amniotic fluid "sludge" showing the presence of fungal hyphae.
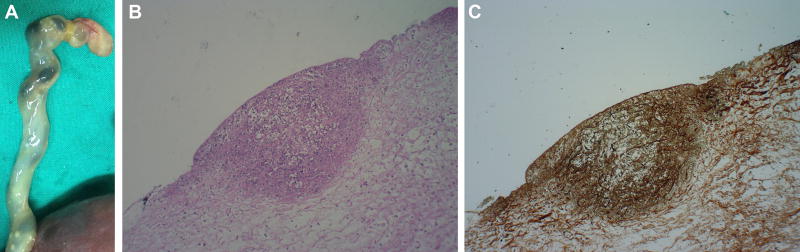
Broad-spectrum antibiotic treatment was initiated, including Clindamycin 900 mg IV every 8 hours, Ceftriaxone 1 g IV every 12 hours, Azithromycin 1 g PO once a day, and Fluconazole 400 mg IV once a day. Two days after the amniocentesis, cultures from the amniotic fluid and “sludge” were positive for Candida albicans. Five days after admission, the intensity and frequency of uterine contractions increased, and the patient progressed into well-established preterm labor, delivering a non-viable male fetus weighing 421 grams. Histological examination of the placenta revealed acute chorioamnionitis and severe funisitis. The umbilical cord presented several small, well-circumscribed white nodules—fungal infection microabscesses—on the surface [50] (Figure 4).
Figure 4.
a) Macroscopic examination of the umbilical cord revealed several small, well-circumscribed, white nodules on the surface. b and c) Histological section of the umbilical cord showing a microabscess in its surface with inflammatory cells (H&E) and fungal microorganisms (Groccott).
Discussion
Amniotic fluid “sludge” as a sonographic sign of intra-amniotic infection/inflammation
Amniotic fluid “sludge” is particulate matter attributed to bacterial biofilms [32] floating in close proximity to the cervix. “Sludge” was reported in asymptomatic patients at risk for preterm delivery [20–22, 24, 28] and those with preterm labor [17, 27] and/or acute cervical insufficiency [20, 21, 29]. Indeed, this particulate matter was reported as a risk factor for spontaneous preterm delivery [17, 19, 22, 25–29], preterm prelabor rupture of the membranes (PROM) [17, 22], acute histologic chorioamnionitis and funisitis as well as intra-amniotic infection/inflammation [17–26, 28, 32, 51] in asymptomatic women [28], patients with spontaneous preterm labor with intact membranes [17–19], asymptomatic women at high risk for spontaneous preterm delivery [22, 26], singleton and twin pregnancies with a sonographic short cervix [20, 22–26], acute cervical insufficiency [21], and in cases with a subchorionic hematoma [51].
Microorganisms isolated from the amniotic fluid in patients with "sludge" include the following: Ureaplasma spp., Mycoplasma hominis, Fusobacterium nucleatum, Candida albicans, Peptostreptococcus spp., Group B Streptococcus, Gardnerella vaginalis, Acinetobacter spp., Streptococcus mutans, Aspergillus flavus, and Staphylococcus warneri [17, 18, 21, 22, 28, 32].
Retrieval of “sludge”
Thus far, the retrieval of “sludge” from the intra-amniotic cavity has only been performed vaginally [31, 32]. Therefore, the available information regarding the microbiology and the nature of “sludge” is confined to these patients. This case report is the first to demonstrate a transabdominal collection of amniotic fluid “sludge” (Videoclip 2). This is important because women with amniotic fluid “sludge” may have negative amniotic fluid cultures, despite the presence of microorganisms within the particulate matter, as is typical in biofilms [31, 32]. This information has the potential to impact the clinical management of patients; for example, those with cervical insufficiency and “sludge” who have an amniocentesis with negative amniotic fluid cultures may undergo placement of a cerclage, a procedure that would not have occurred in the presence of known microbial invasion of the amniotic cavity.
A previous report demonstrated that women with a cerclage who have “sludge” are at a substantially greater risk for preterm birth [52], intra-amniotic infection/inflammation, and clinical chorioamnionitis compared to those without this particulate matter [22]. The feasibility of collecting amniotic fluid “sludge” transabdominally will provide physicians with more accurate information regarding the microbiological status of the amniotic cavity, enabling a tailored patient management, e.g., the placement of a cerclage in women with cervical insufficiency or the administration of antibiotic treatment that may eradicate microbial invasion in the amniotic cavity [53–58].
In the case presented herein, the identification of Candida albicans in the amniotic fluid and the demonstration of hyphae in the sample of “sludge” changed patient management by adding antifungal agents.
Pregnancy conceived with an intrauterine contraceptive device: a risk factor for intra-amniotic infection
Conceiving in the presence of an IUD is associated with increased risk for maternal and fetal morbidity. In cases where the IUD cannot be removed in the beginning of pregnancy, there are increased rates of complications, including: intrauterine infection often due to fungi [1–4], late spontaneous abortion [6, 8], spontaneous preterm labor/birth (with or without ruptured membranes) [1, 5–14], histological chorioamnionitis/funisitis [50], abruptio placentae [1], adverse neonatal outcome [1–15, 33–45], maternal sepsis [6, 7, 15], and even death [16]. Indeed, in a large cohort study including 12,297 patients of whom 196 conceived with an IUD, the rate of preterm birth in the latter group was 56.1%, and approximately two-thirds of these births were due to preterm PROM [1]. Women who conceive with an IUD have a 5-fold higher risk of having an intra-amniotic infection than those who conceived without an IUD [1]. The prevalence of a positive amniotic fluid culture in patients who had an IUD was 45.9%, and 31% of them had Candida spp., a rate 5 times higher than in patients without an IUD [1].
Conclusion
We report, for the first time, the transabdominal collection of “sludge” material, which made possible the diagnosis of intra-amniotic infection due to Candida albicans. This observation is important because it indicates that, in selected cases, amniotic fluid “sludge” can be retrieved without rupturing the membranes. This would allow assessment of the inflammatory and microbiologic status of the amniotic cavity and create opportunities for tailored treatment and patient management.
Supplementary Material
Acknowledgments
This research was supported, in part, by the Perinatology Research Branch, Program for Perinatal Research and Obstetrics, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C.
The authors would also like to thank the invaluable work and support of Mr. Pat Schoff and Mr. Rolando Pérez.
Footnotes
Disclosure Statement: The authors report no conflicts of interest.
References
- 1.Spaun E, Klunder K. Candida chorioamnionitis and intra-uterine contraceptive device. Acta Obstet Gynecol Scand. 1986;65:183–184. doi: 10.3109/00016348609158377. [DOI] [PubMed] [Google Scholar]
- 2.Smith CV, Horenstein J, Platt LD. Intraamniotic infection with Candida albicans associated with a retained intrauterine contraceptive device: a case report. Am J Obstet Gynecol. 1988;159:123–124. doi: 10.1016/0002-9378(88)90505-4. [DOI] [PubMed] [Google Scholar]
- 3.Michaud P, Lemaire B, Tescher M. Spontaneous abortion with an IUD and Candida chorioamnionitis. Article in French. Rev Fr Gynecol Obstet. 1989;84:45–46. [PubMed] [Google Scholar]
- 4.Kim SK, Romero R, Kusanovic JP, Erez O, Vaisbuch E, Mazaki-Tovi S, et al. The prognosis of pregnancy conceived despite the presence of an intrauterine device (IUD) J Perinat Med. 2010;38:45–53. doi: 10.1515/JPM.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dreishpoon IH. Complications of pregnancy with an intrauterine contraceptive device in situ. Am J Obstet Gynecol. 1975;121:412–413. doi: 10.1016/0002-9378(75)90022-8. [DOI] [PubMed] [Google Scholar]
- 6.Tatum HJ, Schmidt FH, Jain AK. Management and outcome of pregnancies associated with the Copper T intrauterine contraceptive device. Am J Obstet Gynecol. 1976;126:869–879. doi: 10.1016/0002-9378(76)90675-x. [DOI] [PubMed] [Google Scholar]
- 7.Waites KB, Bobo RA, Davis RO, Brookings ES, Cassell GH. Clinically silent polymicrobial amnionitis and intrauterine fetal death associated with a Cu-7 intrauterine contraceptive device. Am J Obstet Gynecol. 1984;150:998–999. doi: 10.1016/0002-9378(84)90398-3. [DOI] [PubMed] [Google Scholar]
- 8.Ríos R, Aguilar R, Zaror L. Chorioamnionitis caused by candida associated with intrauterine devices. Article in Spanish. Rev Chil Obstet Ginecol. 1988;53:297–298. [PubMed] [Google Scholar]
- 9.von Theobald P, Duchemin JM, Levy G. The outcome of continuing pregnancies in patients with intrauterine devices. A retrospective study from the Maternity Unit of the University Hospital Center at Caen during the period 1985–1988. Article in French. J Gynecol Obstet Biol Reprod (Paris) 1990;19:863–868. [PubMed] [Google Scholar]
- 10.Chaim W, Mazor M. Pregnancy with an intrauterine device in situ and preterm delivery. Arch Gynecol Obstet. 1992;252:21–24. doi: 10.1007/BF02389602. [DOI] [PubMed] [Google Scholar]
- 11.Chaim W, Mazor M, Meril T, Peleg R, Maor E. Late miscarriage and intraamniotic candidiasis in a woman with a retained intrauterine contraceptive device. Arch Gynecol Obstet. 1993;253:157–160. doi: 10.1007/BF02767335. [DOI] [PubMed] [Google Scholar]
- 12.Ganer H, Levy A, Ohel I, Sheiner E. Pregnancy outcome in women with an intrauterine contraceptive device. Am J Obstet Gynecol. 2009;201:381.e1–e5. doi: 10.1016/j.ajog.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 13.Brahmi D, Steenland MW, Renner RM, Gaffield ME, Curtis KM. Pregnancy outcomes with an IUD in situ: a systematic review. Contraception. 2012;85:131–139. doi: 10.1016/j.contraception.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Ozgu-Erdinc AS, Tasdemir UG, Uygur D, Aktulay A, Tasdemir N, Gulerman HC. Outcome of intrauterine pregnancies with intrauterine device in place and effects of device location on prognosis. Contraception. 2014;89:426–430. doi: 10.1016/j.contraception.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Mermet J, Bolcato C, Rudigoz RC, Dargent D. Outcome of pregnancies with an intrauterine devices and their management. Article in French. Rev Fr Gynecol Obstet. 1986;81:233–235. [PubMed] [Google Scholar]
- 16.Potasman I, Leibovitz Z, Sharf M. Candida sepsis in pregnancy and the postpartum period. Rev Infect Dis. 1991;13:146–149. doi: 10.1093/clinids/13.1.146. [DOI] [PubMed] [Google Scholar]
- 17.Espinoza J, Goncalves LF, Romero R, Nien JK, Stites S, Kim YM, et al. The prevalence and clinical significance of amniotic fluid 'sludge' in patients with preterm labor and intact membranes. Ultrasound Obstet Gynecol. 2005;25:346–352. doi: 10.1002/uog.1871. [DOI] [PubMed] [Google Scholar]
- 18.Gauthier S, Tetu A, Himaya E, Morand M, Chandad F, Rallu F, et al. The origin of Fusobacterium nucleatum involved in intra-amniotic infection and preterm birth. J Matern Fetal Neonatal Med. 2011;24:1329–1332. doi: 10.3109/14767058.2010.550977. [DOI] [PubMed] [Google Scholar]
- 19.Ventura W, Nazario C, Ingar J, Huertas E, Limay O, Castillo W. Risk of impending preterm delivery associated with the presence of amniotic fluid sludge in women in preterm labor with intact membranes. Fetal Diagn Ther. 2011;30:116–121. doi: 10.1159/000325461. [DOI] [PubMed] [Google Scholar]
- 20.Vaisbuch E, Romero R, Mazaki-Tovi S, Erez O, Kusanovic JP, Mittal P, et al. The risk of impending preterm delivery in asymptomatic patients with a nonmeasurable cervical length in the second trimester. Am J Obstet Gynecol. 2010;203:446.e1–e9. doi: 10.1016/j.ajog.2010.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhalmi N, Himaya E, Girard M, Bujold E. Intra-amniotic sludge in a woman with asymptomatic cervical dilatation. J Obstet Gynaecol Can. 2011;33:1201. doi: 10.1016/S1701-2163(16)35099-X. [DOI] [PubMed] [Google Scholar]
- 22.Paules C, Moreno E, Gonzales A, Fabre E, Gonzalez de Aguero R, Oros D. Amniotic fluid sludge as a marker of intra-amniotic infection and histological chorioamnionitis in cervical insufficiency: a report of four cases and literature review. J Matern Fetal Neonatal Med. 2016;29:2681–2684. doi: 10.3109/14767058.2015.1101445. [DOI] [PubMed] [Google Scholar]
- 23.Boyer A, Cameron L, Munoz-Maldonado Y, Bronsteen R, Comstock CH, Lee W, et al. Clinical significance of amniotic fluid sludge in twin pregnancies with a short cervical length. Am J Obstet Gynecol. 2014;211:506.e1–e9. doi: 10.1016/j.ajog.2014.05.044. [DOI] [PubMed] [Google Scholar]
- 24.Bujold E, Pasquier JC, Simoneau J, Arpin MH, Duperron L, Morency AM, et al. Intra-amniotic sludge, short cervix, and risk of preterm delivery. J Obstet Gynaecol Can. 2006;28:198–202. doi: 10.1016/S1701-2163(16)32108-9. [DOI] [PubMed] [Google Scholar]
- 25.Kusanovic JP, Espinoza J, Romero R, Goncalves LF, Nien JK, Soto E, et al. Clinical significance of the presence of amniotic fluid 'sludge' in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2007;30:706–714. doi: 10.1002/uog.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaisbuch E, Romero R, Erez O, Kusanovic JP, Mazaki-Tovi S, Gotsch F, et al. Clinical significance of early (< 20 weeks) vs. late (20–24 weeks) detection of sonographic short cervix in asymptomatic women in the mid-trimester. Ultrasound Obstet Gynecol. 2010;36:471–481. doi: 10.1002/uog.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatanaka AR, Mattar R, Kawanami TE, Franca MS, Rolo LC, Nomura RM, et al. Amniotic fluid "sludge" is an independent risk factor for preterm delivery. J Matern Fetal Neonatal Med. 2016;29:120–125. doi: 10.3109/14767058.2014.989202. [DOI] [PubMed] [Google Scholar]
- 28.Himaya E, Rhalmi N, Girard M, Tetu A, Desgagne J, Abdous B, et al. Midtrimester intra-amniotic sludge and the risk of spontaneous preterm birth. Am J Perinatol. 2011;28:815–820. doi: 10.1055/s-0031-1295638. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs F, Boucoiran I, Picard A, Dube J, Wavrant S, Bujold E, et al. Impact of amniotic fluid "sludge" on the risk of preterm delivery. J Matern Fetal Neonatal Med. 2015;28:1176–1180. doi: 10.3109/14767058.2014.947575. [DOI] [PubMed] [Google Scholar]
- 30.Rust O, Atlas R, Rawlinson K, Van Gaalen J, Balducci J. 0097 Sonographic description of the cervix at risk for preterm birth. Am J Obstet Gynecol. 2001;184:S41. [Google Scholar]
- 31.Romero R, Schaudinn C, Kusanovic JP, Gorur A, Gotsch F, Webster P, et al. Detection of a microbial biofilm in intraamniotic infection. Am J Obstet Gynecol. 2008;198:135.e1–e5. doi: 10.1016/j.ajog.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romero R, Kusanovic JP, Espinoza J, Gotsch F, Nhan-Chang CL, Erez O, et al. What is amniotic fluid 'sludge'? Ultrasound Obstet Gynecol. 2007;30:793–798. doi: 10.1002/uog.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brandsma MA, Braaksma JT, van der Harten JJ. Immature delivery after intrauterine Candida albicans infection. Eur J Obstet Gynecol Reprod Biol. 1975;5:331–335. doi: 10.1016/0028-2243(75)90062-3. [DOI] [PubMed] [Google Scholar]
- 34.Delprado WJ, Baird PJ, Russell P. Placental candidiasis: report of three cases with a review of the literature. Pathology. 1982;14:191–195. doi: 10.3109/00313028209061293. [DOI] [PubMed] [Google Scholar]
- 35.Honore LH. Placental candidiasis: report of two cases, one associated with an IUCD in situ. Contraception. 1984;30:555–560. doi: 10.1016/0010-7824(84)90005-2. [DOI] [PubMed] [Google Scholar]
- 36.Bruner JP, Elliott JP, Kilbride HW, Garite TJ, Knox GE. Candida chorioamnionitis diagnosed by amniocentesis with subsequent fetal infection. Am J Perinatol. 1986;3:213–218. doi: 10.1055/s-2007-999870. [DOI] [PubMed] [Google Scholar]
- 37.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, Hobbins JC. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–824. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 38.Bider D, Ben-Rafael Z, Barkai G, Mashiach S. Intrauterine fetal death apparently due to Candida chorioamnionitis. Arch Gynecol Obstet. 1989;244:175–177. doi: 10.1007/BF00931296. [DOI] [PubMed] [Google Scholar]
- 39.Donders GG, Moerman P, Caudron J, Van Assche FA. Intra-uterine Candida infection: a report of four infected fetuses from two mothers. Eur J Obstet Gynecol Reprod Biol. 1991;38:233–238. doi: 10.1016/0028-2243(91)90298-y. [DOI] [PubMed] [Google Scholar]
- 40.Mazor M, Chaim W, Pak I, Goldstein D. Intraamniotic infection with Candida albicans associated with a retained intrauterine device. A case report. J Reprod Med. 1992;37:950–952. [PubMed] [Google Scholar]
- 41.Nichols A, Khong TY, Crowther CA. Candida tropicalis chorioamnionitis. Am J Obstet Gynecol. 1995;172:1045–1047. doi: 10.1016/0002-9378(95)90044-6. [DOI] [PubMed] [Google Scholar]
- 42.Marelli G, Mariani A, Frigerio L, Leone E, Ferrari A. Fetal Candida infection associated with an intrauterine contraceptive device. Eur J Obstet Gynecol Reprod Biol. 1996;68:209–212. doi: 10.1016/0301-2115(96)02471-2. [DOI] [PubMed] [Google Scholar]
- 43.Roque H, Abdelhak Y, Young BK. Intra amniotic candidiasis. Case report and meta-analysis of 54 cases. J Perinat Med. 1999;27:253–262. doi: 10.1515/JPM.1999.036. [DOI] [PubMed] [Google Scholar]
- 44.Horn LC, Nenoff P, Ziegert M, Hockel M. Missed abortion complicated by Candida infection in a woman with rested IUD. Arch Gynecol Obstet. 2001;264:215–217. doi: 10.1007/s004040000117. [DOI] [PubMed] [Google Scholar]
- 45.Barth T, Broscheit J, Bussen S, Dietl J. Maternal sepsis and intrauterine fetal death resulting from Candida tropicalis chorioamnionitis in a woman with a retained intrauterine contraceptive device. Acta Obstet Gynecol Scand. 2002;81:981–982. doi: 10.1034/j.1600-0412.2002.811014.x. [DOI] [PubMed] [Google Scholar]
- 46.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Sem Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deveer R, Engin-Ustun Y, Sarikaya E, Aydogan P, Doganay M, Mollamahmutoglu L. Comparison of C-reactive protein levels in pregnancies with retained and removed intrauterine device. J Matern Fetal Neonatal Med. 2011;24:1152–1154. doi: 10.3109/14767058.2010.545925. [DOI] [PubMed] [Google Scholar]
- 48.De Felice C, Toti P, Picciolini E, Massafra C, Pecciarini L, Palmeri ML, et al. High incidence of histologic chorioamnionitis in women with gestational vaginal bleeding. Acta Obstet Gynecol Scand. 1997;76:85–86. doi: 10.3109/00016349709047792. [DOI] [PubMed] [Google Scholar]
- 49.Gomez R, Romero R, Nien JK, Medina L, Carstens M, Kim YM, et al. Idiopathic vaginal bleeding during pregnancy as the only clinical manifestation of intrauterine infection. J Matern Fetal Neonatal Med. 2005;18:31–37. doi: 10.1080/14767050500217863. [DOI] [PubMed] [Google Scholar]
- 50.Qureshi F, Jacques SM, Bendon RW, Faye-Peterson OM, Heifetz SA, Redline R, et al. Candida funisitis: A clinicopathologic study of 32 cases. Pediatr Dev Pathol. 1998;1:118–124. doi: 10.1007/s100249900014. [DOI] [PubMed] [Google Scholar]
- 51.Tskitishvili E, Tomimatsu T, Kanagawa T, Sawada K, Kinugasa Y, Mimura K, et al. Amniotic fluid 'sludge' detected in patients with subchorionic hematoma: a report of two cases. Ultrasound Obstet Gynecol. 2009;33:484–486. doi: 10.1002/uog.6348. [DOI] [PubMed] [Google Scholar]
- 52.Kusanovic J, Romero R, Espinoza J, Goncalves L, Gotsch F, Camacho N, et al. OC144: Clinical significance of the presence of amniotic fluid ‘sludge’ in patients with cervical cerclage. Ultrasound Obstet Gynecol. 2007;30:411. doi: 10.1002/uog.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Romero R, Scioscia AL, Edberg SC, Hobbins JC. Use of parenteral antibiotic therapy to eradicate bacterial colonization of amniotic fluid in premature rupture of membranes. Obstet Gynecol. 1986;67(3 Suppl):15S–17S. doi: 10.1097/00006250-198603001-00005. [DOI] [PubMed] [Google Scholar]
- 54.Mazor M, Horowitz S, Meril T, Bar-Am I. Eradication of Ureaplasma urealyticum from amniotic fluid. Isr J Med Sci. 1992;28:296–298. [PubMed] [Google Scholar]
- 55.Romero R, Hagay Z, Nores J, Sepulveda W, Mazor M. Eradication of Ureaplasma urealyticum from the amniotic fluid with transplacental antibiotic treatment. Am J Obstet Gynecol. 1992;166:618–620. doi: 10.1016/0002-9378(92)91686-5. [DOI] [PubMed] [Google Scholar]
- 56.Mazor M, Chaim W, Meirovitz M, Yohay D, Leiberman JR, Glezerman M. Eradication of viridans streptococci from the amniotic cavity by parenteral antibiotic administration. A case report. J Reprod Med. 1995;40:820–822. [PubMed] [Google Scholar]
- 57.Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med. 2006;34:13–19. doi: 10.1515/JPM.2006.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morency AM, Rallu F, Laferriere C, Bujoldg E. Eradication of intra-amniotic Streptococcus mutans in a woman with a short cervix. J Obstet Gynaecol Can. 2006;28:898–902. doi: 10.1016/S1701-2163(16)32269-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.