Abstract
Background and study aims
Endoscopic ultrasound fine-needle aspiration (EUS-FNA) is a keystone in diagnosing and staging of pancreatic masses. Recently, a microfiber that can pass through a 19-gauge needle has been introduced for confocal laser endomicroscopy (nCLE). The aims of this study were to evaluate the diagnostic value and the reproducibility of nCLE criteria for solid malignant lesions.
Patients and methods
This prospective dual-center study included patients with pancreatic masses suspicious of malignancy referred for EUS-FNA. Endomicroscopic imaging was performed under EUS-guidance until organ-specific structures were obtained. Afterwards, standard cytology was obtained and patients were followed for up to 12 months. All nCLE parameters included in former studies were correlated with the final diagnosis (dark lobular structures/normal acinar cells, dark cell aggregates > 40 µm, dilated irregular vessels with fluorescein leakage, fine white fibrous bands, small black cell movements, pseudoglandular structures). Finally, three CLE novices and three CLE experts assessed the unedited movies from all patients.
Results
Twenty-eight patients were enrolled in the study. A final diagnosis was obtained in 24 patients (86 %). One patient (3 %) died before a diagnosis was obtained, while 3 were lost to follow-up (11 %). In 18/24 patients (74 %) the diagnosis was malignant. The mean sensitivity, specificity, and accuracy for the nCLE parameters ranged from 19 – 93 %, 0 – 56 %, 26 – 69 %, respectively. The inter-observer values ranged from κ = 0.20 – 0.41 for novices and κ = –0.02 – 0.38 for experts.
Conclusions
The diagnostic value of nCLE in solid pancreatic masses is questionable and the inter-observer agreement for both novices and CLE experts appears limited.
Introduction
Pancreatic cancer is one of the most aggressive gastrointestinal malignancies with mortality rates closely following the incidence rates 1 . The incidence is increasing and the prognosis is grim especially because of late diagnosis and metastatic potential. While surgical treatment is currently the only potential curative intervention, 80 – 85 % of the pancreatic cancer cases are unfortunately detected in advanced unresectable stages of the disease 2 . Furthermore, in spite of advances in the diagnosis and management of pancreatic cancer, less than 5 % of patients are alive at five years 3 .
Endoscopic ultrasound (EUS) represents a highly valuable tool in the management of pancreatic cancer patients. As a minimal invasive technique that enables high-resolution imaging of the pancreatic parenchyma and surrounding structures, it is considered the most sensitive method for the detection of clinically suspected pancreatic tumors, with a negative predictive value close to 100 % 4 . Its diagnostic sensitivity was shown by previous studies to be superior compared to other imaging methods, especially in the case of small tumors 5 6 . Additionally, EUS enables guided fine needle aspiration (EUS-FNA), which is currently recommended as the first-line procedure whenever pathological diagnosis is required 7 . However, EUS-FNA as a sampling technique has its drawbacks, mainly represented by the relatively low negative predictive value in diagnosing pancreatic cancer. It thus cannot reliably rule out a diagnosis of malignancy in a patient with a focal mass and a negative EUS-FNA and therefore patients with a high clinical suspicion of malignancy usually need repeated FNA 8 .
Confocal laser endomicroscopy (CLE) has emerged as a novel technique that enables in vivo microscopic imaging during ongoing endoscopy. Endomicroscopy can be performed either with dedicated endoscopes (eCLE) or with probe-based systems (pCLE) 9 . The principle of the method is based on a laser beam of defined wavelength being focused towards the targeted tissue, with the recaptured signal displayed as ‘optical biopsies’ in the horizontal plane. CLE is a contrast-based method; the most widely used agent being intravenously administered fluorescein, although other agents are in preclinical stages 10 . The potential role of CLE has been explored in both the upper and lower gastrointestinal tract, showing good accuracy for predicting the final histopathological diagnosis based on immediate evaluation of tissue, vascular patterns, and functional defects of the intestinal barrier function 11 12 . Recently, CLE has gone beyond the luminal indications with the introduction of a novel microprobe that can be passed through a 19-gauge EUS-FNA needle 13 . Thus, under EUS guidance solid and cystic lesions can be accessed for real-time endomicroscopic information with a needle-based CLE approach (nCLE) 14 . The feasibility of the method has been tested and gained substantial clinical use in pancreatic cystic neoplasms 15 16 17 18 19 . However, a limited number of cases of solid pancreatic masses have been described with nCLE and evidence of the suggested imaging criteria are warranted 20 21 22 .
The aim of this study was to estimate the feasibility and safety of EUS-guided nCLE for evaluation of solid pancreatic masses and validate the diagnostic value of nCLE criteria for malignant lesions. Furthermore, the reproducibility of the nCLE parameters and the movie quality were analyzed for both nCLE novices and international experts.
Patients and methods
The present study was a prospective, dual-center, cohort study in selected patients referred to our departments between November 2012 and July 2015 for EUS and EUS-FNA of a suspected pancreatic mass. The study was approved by the regional ethics committee and the Danish Data collection authorities, and was registered at clinicaltrials.gov (NCT01734967). All patients signed informed consent for EUS with FNA and nCLE examination. This paper includes only patients where nCLE of pancreatic masses were performed. The probe-based endomicroscopy system consists of a flexible catheter probe representing a bundle of optical fibers linked to a micro-objective, a laser scanning unit, and the control and acquisition software (Mauna Kea Technology, Paris, France). Throughout our study, we used a small diameter probe developed for usage through a 19G needle (nCLE) for EUS-guided procedures ( AQ-Flex 19™, Mauna Kea, Paris, France ). This miniprobe has a diameter of 0.85 mm, a field of view of 325 µm and a 3.5 µm lateral resolution for the resulting images.
Patients
The indication for this investigation was based on the patient’s clinical history and previous imaging studies (abdominal US, CT, or MRI). Furthermore, the EUS examination had to identify a solid pancreatic mass with an indication for EUS-FNA. Patients with known allergy to fluorescein, being pregnant or breast feeding, or presenting with a contraindication for EUS-FNA were excluded. For each patient personal data, EUS variables, histological, and cytological findings were registered.
nCLE procedures
All patients with a suspicion of pancreatic masses were evaluated by EUS, nCLE, and EUS-FNA for cytopathological diagnosis. For the EUS examination linear instruments were used (Pentax EG 3870 UTK, Hamburg, Europe or Olympus GF-UCT180, Tokyo, Japan) to perform complete examination of the pancreas. P. V., H. H., or A. S carried out the procedures, while T. C. or J. G. K. assisted in order to optimize the nCLE image acquisition. Tumor characteristics (echogenicity, echostructure, size, and vascular invasion) were described. The presence of regional lymph nodes was reported with their maximal size, echogenicity, shape, and margins. Identification of liver metastases was also looked upon by careful examinations of the liver. nCLE was performed after EUS identification of the pancreatic mass. Initially, the confocal microprobe was preloaded into a 19-gauge EUS-FNA needle as previously described and advanced into the lesion under real-time EUS guidance 13 . The nCLE examination followed after intravenous administration of five mL of fluorescein 10 % (Skanderborg Pharmacy, Skanderborg, Denmark). Acquisition time was registered and image data were stored digitally for offline analysis. Confocal imaging was initiated when the needle was positioned in the lesion and continued until organ specific images representing the examined lesion were obtained. If the needle was repositioned, movie sequencing was paused. In order to enable a final pathological diagnosis, EUS-FNA was performed after the nCLE procedure. Following nCLE imaging, the fiber was removed and EUS-FNA performed using the same needle. All adverse events were registered systematically for the first 30 days after the procedure.
Assessment of the nCLE movies
All confocal images were registered in regard to dark lobular structures (normal acinar cells), dark aggregates > 40 µm, dilated irregular vessels with fluorescein leakage, fine white fibrous bands, small black cell movements, and pseudoglandular structures 20 21 22 . Furthermore, in order to validate the composite scores proposed by Giovannini and Kongkam, these were assessed 20 21 . These analyses were post hoc as no criteria had been described at the initiation of the study. Finally, the quality of the nCLE images was evaluated using a five-point scale ranging from 1 to 5 13 . Three experts with extensive experience in CLE (H. B., M. B., and P. V.) and three CLE novices (B. K., C. C., and S. D.) carried out the assessments in a blinded manner (post hoc). While the novices were completely naive to CLE, the experts had all conducted and analyzed nCLE cases from pancreatic cysts and at least 100 luminal CLE cases. However, none were familiar with interpretation in solid masses. Prior to the assessment, all assessors received and reviewed a training chart including three examples of each parameter (supplementary material). In order to analyze the intra-observer variability, the movies were re-assessed after 48 hours by C. C. and P. V. The nCLE were unedited during all analyses.
Final diagnosis
The final diagnosis was based on EUS-FNA cytopathology and/or histological specimens in patients referred for surgery. Furthermore, these patients were followed for up to one year. Pathology samples obtained from surgical resections with curative intent as well as microhistological fragments obtained by EUS-FNA were processed by paraffin embedding with usual staining (hematoxylin and eosin) and subsequent immunohistochemistry if necessary. For patients without positive cytology or histology, the diagnosis was based on follow-up for up to one year. Both pancreatic adenocarcinoma and neuroendocrine tumors were defined as malignant
Statistical analysis
Mean (standard deviation [SD]) or when appropriate median (interquartile range [IQR]) are presented. Normal distribution was tested using visual inspection and the Shapiro – Wilk test. Confidence intervals (CI) for proportions were calculated using an exact binomial method. For the inter- and intra-observer study, kappa statistics and intra-class correlations were interpreted according to Landis and Koch (poor = 0, slight = 0 – 0.2, fair = 0.21 – 0.4, moderate = 0.41 – 0.6, substantial = 0.61 – 0.8, almost perfect = 0.81 – 1) 22 . Fleiss’ kappa was used for the single parameters in the inter-observer study, while intra-class correlations were applied for movie quality. In the intra-observer study Cohen’s kappa was used. Confidence intervals for kappa statistics were calculated using a nonparametric bootstrap method. For all statistics, SPSS Statistics v. 23 (IBM, Armonk, New York, USA) or R version 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria) was used.
Results
Patients
A total of 28 eligible patients were enrolled in the study, 12 female and 16 male patients with a mean age 65 years (SD ± 12). The majority of pancreatic lesions was located in the head ( Table 1 ). Liver metastasis or suspicious peripancreatic lymph nodes were found in 7 (25.0 %) and 10 patients (36.0 %), respectively. EUS-FNA was inconclusive in 2 patients (7.1 %), benign in 11 cases (39.3 %), and malignant in 15 (53.6 %) cases. The final diagnosis was not obtained in 3 cases (10.7 %) due to loss of follow-up and was further missing in 1 case (3.6 %) as the patients died before a final diagnosis was obtained. In the remaining 24 patients the diagnosis was malignant in 18 cases (64.2 %) and benign in 6 cases (21.4 %). Two of the patients with pancreatic adenocarcinomas had benign cytology in the FNA and were finally diagnosed by analyzing ascites and on a Tru-cut liver biopsy, respectively.
Table 1. Patient characteristics.
| Patients | |
| No | 28 |
| Age/years (SD) | 64.7 (11.8) |
| Sex/females (%) | 12 (42.8) |
| Location of tumor | |
|
1 (3.6) |
|
19 (67.9) |
|
5 (17.9) |
|
3 (10.7) |
| Size of tumor (maximal diameter) | |
|
2 (7.1) |
|
57.1) |
|
10 (35.7) |
| FNA diagnosis | |
|
2 (7.1) |
|
14 (50.0) |
|
1 (3.6) |
|
11 (39.3) |
| Final diagnosis | |
|
4 (14.3) |
|
17 (60.7) |
|
1 (3.6) |
|
6 (21.4) |
| Metastasis | |
|
7 (25.0) |
|
10 (35.7) |
SD, standard deviation; FNA, fine-needle aspiration; PDAC, pancreatic ductal adenocarcinoma; NET, neuroendocrine tumor
Procedures
No adverse advents were registered during a 30-day follow-up. Moreover, in all patients it was clearly feasible to do nCLE inside pathological lesions and organ specific tissue was visualized in all procedures ( Fig. 1a , Fig. 1b , Fig. 1c , Fig. 1d , Video 1 , Video 2 ). The mean quality of movies was 3.3 (SD ± 1.0) when assessed by experts and 3.5 (SD ± 1.2) for CLE novices. The mean image acquisition time was 214 seconds (SD ± 76) and the median number of needle repositioning in order to optimize nCLE imaging was 3 (IQR 3.0).
Fig. 1 a.
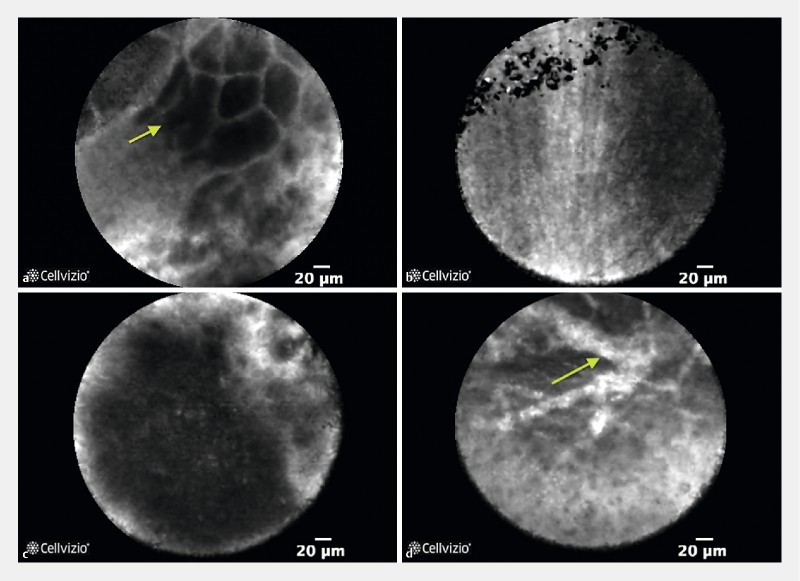
nCLE of benign pancreatic lesion (chronic pancreatitis) displaying normal acinar cells (arrow). b Fine white fibrous bands from a benign pancreatic lesion probably representing fibrotic tissue. c Dark aggregates > 40 µm in a pancreatic ductal adenocarcinoma. d Dilated irregular vessels with fluorescein leakage in a patient with pancreatic ductal adenocarcinoma.
Diagnostic value
For single nCLE parameters, the mean sensitivity, specificity, accuracy, negative predictive value, and positive predictive value are presented in Table 2 . While the most sensitive parameter for malignancy was small black cell movements for the experts (93 %, 95 %CI: 79 – 98 %), dark aggregates > 40 µm was the sensitive parameter for the CLE novices (70 %, 95 %CI: 56 – 82 %). When we evaluated the composite score of dark aggregates > 40 µm or dilated irregular vessels with fluorescein leakage (Giovannini et al.), we found a sensitivity of 80 % (95 %CI: 62 – 91 %) for experts and 78 % (95 %CI: 67 – 88 %) for CLE novices, while the specificity was 5 % (95 %CI: 0 – 22 %) and 24 % (95 %CI: 7 – 44 %) for experts and CLE novices, respectively ( Table 3 ) 21 . With the presence of dark lobular structures/normal acinar cells alone, the sensitivity and specificity of a benign diagnosis was 50 % (95 %CI: 20 – 75 %) and 82 % (95 %CI: 74 – 89 %), respectively for experts and 50 % (95 %CI: 20 – 75 %) and 89 % (95 %CI: 80 – 95 %), respectively for CLE novices. However, when dark lobular structures/normal acinar cells was combined with fine white fibrous bands, the sensitivity rose to 83 % (95 %CI: 67 – 93 %) and 89 % (95 %CI = 67 %-100 %) for experts and CLE novices, respectively. Of note, the negative predictive value of this combination was 88 % (95 %CI: 60 – 97 %) for experts and 94 % (95 %CI: 72 – 100 %) for CLE novices.
Table 2. Assessment of single nCLE parameters.
| Experts | Novices | |||||||||
| Sensitivity (%) | Specificity (%) | Accuracy (%) | Positive predictive value (%) | Negative predictive value (%) | Sensitivity (%) | Specificity (%) | Accuracy (%) | Positive predictive value (%) | Negative predictive value (%) | |
| Dark lobular structures/normal acinar cells (95 %CI) | 19 (11 – 26) | 50 (21 – 73) | 26 (17 – 36) | 53 (24 – 80) | 17 (6 – 36) | 11 (4 – 19) | 39 (11 – 73) | 18 (8 – 31) | 35 (10 – 67) | 13 (3 – 33) |
| Dark aggregates > 40 µm (95 %CI) | 72 (53 – 84) | 17 (0 – 42) | 58 (39 – 71) | 72 (50 – 89) | 17 (0 – 53) | 70 (56 – 82) | 44 (11 – 75) | 64 (50 – 74) | 79 (56 – 93) | 33 (6 – 61) |
| Dilated irregular vessels with fluorescein leakage (95 %CI) | 61 (44 – 77) | 39 (17 – 56) | 56 (40 – 68) | 75 (51 – 91) | 25 (8 – 51) | 33 (16 – 49) | 72 (40 – 93) | 43 (26 – 57 | 78 (47 – 95) | 27 (11 – 50) |
| Fine white fibrous bands (95 %CI) | 57 (38 – 74) | 39 (11 – 75) | 53 (36 – 67) | 74 (46 – 90) | 23 – 6-52) | 41 (23 – 61) | 50 (20 – 71) | 43 (28 – 57) | 71 (44 – 91) | 22 (7 – 46) |
| Small black cell movements (95 %CI) | 93 (79 – 98) | 0 | 69 (46 – 83) | 74 (51 – 87) | 0 | 65 (45 – 80) | 33 (0 – 53) | 57 (39 – 69) | 75 (47 – 89) | 24 (8 – 63) |
| Pseudoglandular structures (95 %CI) | 52 (37 – 67) | 56 (33 – 67 | 53 (40 – 63) | 78 (53 – 91) | 28 (10 – 49) | 35 (21 – 52) | 56 (0 – 87) | 40 (25 – 56) | 70 (37 – 92) | 22 (6 – 47) |
CI, confidence interval
Table 3. Assessment of proposed nCLE criteria.
| Experts | Novices | |||||||||
| Sensitivity (%) | Specificity (%) | Accuracy (%) |
Positive predictive value
(%) |
Negative predictive value (%) | Sensitivity (%) | Specificity (%) | Accuracy (%) | Positive predictive value (%) | Negative predictive value (%) | |
| Dark aggregates > 40 µm or dilated irregular vessels with fluorescein leakage (95 %CI) (for malignancy) | 80 (62 – 91) | 5 (0 – 22) | 58 (38 – 71) | 67 (45 – 84) | 9 (0 – 50) | 78 (67 – 88) | 24 (7 – 44) | 63 (44 – 74) | 71 (48 – 87) | 31 (8 – 65) |
| Dark lobular structures/normal acinar cells (95 %CI) (for benign diagnosis) | 50 (20 – 75) | 82 (74 – 89) | 74 (58 – 82) | 47 (21 – 77) | 83 (62 – 94) | 50 (20 – 75) | 89 (80 – 95) | 82 (67 – 90) | 65 (30 – 88) | 87 (67 – 97) |
| Dark lobular structures/normal acinar cells or fine white fibrous bands (95 %CI) (for benign diagnosis | 83 (67 – 93) | 41 (26 – 59) | 51 (36 – 64) | 32 (13 – 55) | 88 (60 – 97) | 89 (67 – 100) | 56 (33 – 73) | 64 (43 – 76) | 40 (16 – 68) | 94 (72 – 100) |
CI, confidence interval
Inter and intra-observer analysis
For experts, the inter-observer analysis for the nCLE parameters showed poor or slight agreement for dark lobular structures/normal acinar cells, small black cell movements, and pseudoglandular structures, while the agreement was fair for dark aggregates > 40 µm, dilated irregular vessels with fluorescein leakage, fine white fibrous bands, and nCLE movie quality ( Table 4 ). For CLE novices, the inter-observer analysis for the nCLE parameters showed fair agreement for dark lobular structures / normal acinar cells, dark aggregates > 40 µm, dilated irregular vessels with fluorescein leakage, small black cell movements, pseudoglandular structures, and nCLE movie quality, while it was moderate for fine white fibrous bands ( Table 4 ). Both for experts and CLE novices, the intra-observer variability ranged from slight to almost perfect ( Table 4 ). The highest value was found for pseudoglandular structures with κ = 0.93 (95 %CI: 0.63 – 1.00) and 0.93 (95 %CI: 0.52 – 1.00) for experts and CLE novices, respectively.
Table 4. Inter- and intra-observer analysis of nCLE parameters.
| Experts | CLE novices | |||
| Inter-observer variability | Intra-observer variability | Inter-observer variability | Intra-observer variability | |
| Dark lobular structures/normal acinar cells (95 %CI) (κ) | –0.02 (–0.20 – 0.39) | 0.52 (–0.05 – 0.89) | 0.28 (0.01 – 0.59) | 0.78 (0.00 – 1.00) |
| Dark aggregates > 40 µm (95 %CI) (κ) | 0.30 (0.02 – 0.62) | 0.62 (0.19 – 0.91) | 0.20 (–0.05 – 0.53) | 0.00 (–0.24 – 0.51) |
| Dilated irregular vessels with fluorescein leakage (95 %CI) (κ) | 0.31 (0.08 – 0.60) | 0.14 (–0.26 – 0.45) | 0.35 (0.13 – 0.64) | 0.69 (0.30 – 0.90) |
| Fine white fibrous bands (95 %CI) (κ) | 0.38 (0.14 – 0.65) | 0.71 (0.40 – 0.93) | 0.41 (0.13 – 0.64) | 0.86 (0.57 – 1.00) |
| Small black cell movements (95 %CI) (κ) | 0.10 (–0.08 – 0.48) | –0.05 (–0.16 – 0.00) | 0.28 (0.04 – 0.56) | 0.26 (0.52 – 1.00) |
| Pseudoglandular structures (95 %CI) (κ) | 0.00 (–0.16 – 0.29) | 0.93 (0.63 – 1.00) | 0.23 (–0.03 – 0.51) | 0.93 (0.52 – 1.00) |
| nCLE movie quality (95 %CI) (κ) | 0.26 (0.07 – 0.45) | 0.71 (0.54 – 0.87) | 0.33 (0.18 – 0.53) | 0.71 (0.54 – 0.87) |
nCLE, needle-based confocal laser endomicroscopy
Discussion
In this prospective validation study we investigated the feasibility and safety of EUS-guided nCLE examination of pancreatic masses and the diagnostic value of the single nCLE parameters as well as the proposed nCLE criteria. We found the method feasible and safe, while the diagnostic value and the reproducibility were limited.
When introducing new methods and equipment, safety is crucial. nCLE is a non-traumatic method guided by EUS though a standard 19-gauge needle, so apart from the known adverse events related to FNA itself, one would not expect problems related to the insertion of the miniprobe and the acquisition of images. Furthermore, fluorescein as an intravenously administered contrast agent has been used for decades with adverse events being mild and transient 10 . In this study we did not register any adverse events, which corresponds well with other nCLE studies in solid pancreatic masses, which reported rates of adverse events ranging from 0 % – 4.5 % 20 21 . nCLE has been used more widespread in pancreatic cysts, but also for that indication, the rate of adverse events are low, thus the method can be considered safe 23 .
With the use of nCLE, Giovaninni et al. and Kongkam et al. presented an accuracy of pancreatic ductal adenocarcinoma of 85.0 % and 90.9 %, respectively. When we assessed the proposed criteria, we were unable to reproduce these excellent results. For experts the accuracy of dark aggregates > 40 µm or dilated irregular vessels with fluorescein leakage was limited to 58 % (95 %CI: 38 – 71), while it was 63 % (95 %CI: 44 – 74) for CLE novices. Furthermore, the presumed predictors of a benign diagnosis (dark lobular structures/normal acinar cells fine white fibrous bands) also failed to reach diagnostic values that in combination with EUS-FNA would benefit in a clinical setting. This might reflect a lack of experience and that initial nCLE sequences included in the study were collected in the early learning phase. However, the experts in our study were highly experienced endomicroscopists with both extra- and intraluminal experience. Furthermore, the diagnostic values were comparable for experts and CLE novices. A second explanation could be that the mean length of nCLE movies in our study was limited to 3.5 minutes, compared to 6 (range 2 – 10) and 8.2 (range 1 – 32) minutes for Giovaninni and Kongkam, respectively. The criteria analysed in our study were clearly described, but further precision of the criteria, might increase the diagnostic value. Due to the high specificity for IPMN’s and serous cystic lesions of the pancreas, nCLE has gained widespread use for this indication 16 24 25 . However, with the technology available and the current criteria, we find nCLE unable to distinguish benign from malignant solid lesions in the pancreas.
A limitation in our study was the number of patients included and the fact that the patients were not consecutively enrolled. The limited number of patients calls for cautious interpretation of the results, while the latter might have increased the risk of selection bias. The study period was prolonged mainly due to limited research resources, as the nCLE fibers are not reimbursed. Furthermore, larger prospective trials may have the power to estimate if location and size of the lesion affect the imaging quality and subsequently the diagnostic value.
The reproducibility of luminal CLE parameters has been extensively studied for Barrett’s esophagus, IBD and colorectal polyps. In general, the results have been suitable for a clinical setting with acceptable inter- and intra-observer values 26 27 28 29 30 31 . Recently, the nCLE criteria for pancreatic cystic neoplasms have been evaluated. Napoleon et al found inter-observer values ranging from fair for mucinous cystic neoplasms to perfect for pancreatic pseudocysts. Overall inter-observer agreement was substantial (κ = 0.72) 17 . In contrast, Karia et al found relatively low inter-observer agreement ranging from poor to fair 24 . In solid masses, the reproducility of the nCLE parameters has been less extensively investigated. Both in the inter- and the intra-observer study, we found values ranging from slight to moderate. Intensified training might increase the inter- and intra-observer variability; nonetheless, with the current criteria the reproducibility of the nCLE parameters is unacceptable for implementation in a clinical or even an investigational setting. Furthermore, it is important to keep in mind that EUS-FNA alone has a sensitivity around 90 % for malignancy thus it is questionable whether the adjunct of nCLE can improve the diagnostic value further.
Conclusion
EUS-guided nCLE procedures in focal pancreatic masses are feasible and safe. Nevertheless, the diagnostic value as an adjunct to EUS-FNA seems limited and further development of the technology and precision of the diagnostic criteria are needed before further studies can be undertaken.
Acknowledgements
Establishment of nCLE in Copenhagen was possible due to generous contributions of A. P. Møller and Chastine McKinney Møllers Foundation, Foundation Jochum, The Toyota Foundation and the Foundation of Aase and Ejnar Danielsen. The activity of J. G. K. and A. S. was supported by The Foundation of Arvid Nilsson and The Lundbeck Foundation. Also, the activity of T. C. and A. S. was supported by the research grant “Minimal invasive assessment of angiogenesis in pancreatic cancer based on imaging methods and molecular techniques (Angio-PAC)”, Ideas programme, 164/2011, National Research Council – UEFISCDI, project number PN-II-ID-PCE-2011-3-0589.
Footnotes
Competing interests None
References
- 1.Quaresma M, Coleman M P, Rachet B. 40-year trends in an index of survival for all cancers combined and survival adjusted for age and sex for each cancer in England and Wales, 1971 – 2011: a population-based study. Lancet. 2015;385:1206–1218. doi: 10.1016/S0140-6736(14)61396-9. [DOI] [PubMed] [Google Scholar]
- 2.Vincent A, Herman J, Schulick R et al. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma C, Eltawil K M, Renfrew P D et al. Advances in diagnosis, treatment and palliation of pancreatic carcinoma: 1990 – 2010. World J Gastroenterol. 2011;17:867–897. doi: 10.3748/wjg.v17.i7.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saftoiu A, Vilmann P. Role of endoscopic ultrasound in the diagnosis and staging of pancreatic cancer. J Clin Ultrasound. 2009;37:1–17. doi: 10.1002/jcu.20534. [DOI] [PubMed] [Google Scholar]
- 5.DeWitt J, Devereaux B, Chriswell M et al. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med. 2004;141:753–763. doi: 10.7326/0003-4819-141-10-200411160-00006. [DOI] [PubMed] [Google Scholar]
- 6.Iglesias Garcia J, Larino Noia J, Dominguez Munoz JE. Endoscopic ultrasound in the diagnosis and staging of pancreatic cancer. Rev Esp Enferm Dig. 2009;101:631–638. doi: 10.4321/s1130-01082009000900006. [DOI] [PubMed] [Google Scholar]
- 7.Dumonceau J M, Polkowski M, Larghi A et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2011;43:897–912. doi: 10.1055/s-0030-1256754. [DOI] [PubMed] [Google Scholar]
- 8.Hartwig W, Schneider L, Diener M K et al. Preoperative tissue diagnosis for tumours of the pancreas. Br J Surg. 2009;96:5–20. doi: 10.1002/bjs.6407. [DOI] [PubMed] [Google Scholar]
- 9.Goetz M, Malek N P, Kiesslich R. Microscopic imaging in endoscopy: endomicroscopy and endocytoscopy. Nat Rev Gastroenterol Hepatol. 2014;11:11–18. doi: 10.1038/nrgastro.2013.134. [DOI] [PubMed] [Google Scholar]
- 10.Wallace M B, Meining A, Canto M I et al. The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment Pharmacol Ther. 2010;31:548–552. doi: 10.1111/j.1365-2036.2009.04207.x. [DOI] [PubMed] [Google Scholar]
- 11.Kiesslich R, Duckworth C A, Moussata D et al. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut. 2012;61:1146–1153. doi: 10.1136/gutjnl-2011-300695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasmussen D N, Karstensen J G, Riis L B et al. Confocal Laser Endomicroscopy in Inflammatory Bowel Disease -- A Systematic Review. J Crohns Colitis. 2015;9:1152–1159. doi: 10.1093/ecco-jcc/jjv131. [DOI] [PubMed] [Google Scholar]
- 13.Konda V J, Aslanian H R, Wallace M B et al. First assessment of needle-based confocal laser endomicroscopy during EUS-FNA procedures of the pancreas (with videos) Gastrointest Endosc. 2011;74:1049–1060. doi: 10.1016/j.gie.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 14.Costache M I, Iordache S, Karstensen J G et al. Endoscopic ultrasound-guided fine needle aspiration: from the past to the future. Endosc Ultrasound. 2013;2:77–85. doi: 10.4103/2303-9027.117691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konda V J, Meining A, Jamil L H et al. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy. 2013;45:1006–1013. doi: 10.1055/s-0033-1344714. [DOI] [PubMed] [Google Scholar]
- 16.Napoleon B, Lemaistre A I, Pujol B et al. A novel approach to the diagnosis of pancreatic serous cystadenoma: needle-based confocal laser endomicroscopy. Endoscopy. 2015;47:26–32. doi: 10.1055/s-0034-1390693. [DOI] [PubMed] [Google Scholar]
- 17.Napoleon B, Lemaistre A I, Pujol B et al. In vivo characterization of pancreatic cystic lesions by needle-based confocal laser endomicroscopy (nCLE): proposition of a comprehensive nCLE classification confirmed by an external retrospective evaluation. Surg Endosc. 2016;30:2603–2612. doi: 10.1007/s00464-015-4510-5. [DOI] [PubMed] [Google Scholar]
- 18.Bhutani M S, Koduru P, Joshi V et al. EUS-Guided Needle-Based Confocal Laser Endomicroscopy: A Novel Technique With Emerging Applications. Gastroenterol Hepatol. (N Y) 2015;11:235–240. [PMC free article] [PubMed] [Google Scholar]
- 19.Nakai Y, Iwashita T, Park D H et al. Diagnosis of pancreatic cysts: EUS-guided, through-the-needle confocal laser-induced endomicroscopy and cystoscopy trial: DETECT study. Gastrointest Endosc. 2015;81:1204–1214. doi: 10.1016/j.gie.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 20.Kongkam P, Pittayanon R, Sampatanukul P et al. Endoscopic ultrasound-guided needle-based confocal laser endomicroscopy for diagnosis of solid pancreatic lesions (ENES): a pilot study. Endosc Int Open. 2016;4:E17–23. doi: 10.1055/s-0034-1393183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giovannini M, Caillol F, Monges G et al. Endoscopic ultrasound-guided needle-based confocal laser endomicroscopy in solid pancreatic masses. Endoscopy. 2016;48:892–898. doi: 10.1055/s-0042-112573. [DOI] [PubMed] [Google Scholar]
- 22.Karstensen J G, Cartana T, Klausen P H et al. Endoscopic ultrasound-guided needle-based confocal laser endomicroscopy: a pilot study for use in focal pancreatic masses. Pancreas. 2015;44:833–835. doi: 10.1097/MPA.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 23.Krishna S G, Lee J H. Appraisal of needle-based confocal laser endomicroscopy in the diagnosis of pancreatic cysts. World J Gastroenterol. 2016;22:1701–1710. doi: 10.3748/wjg.v22.i4.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karia K, Waxman I, Konda V J et al. Needle-based confocal endomicroscopy for pancreatic cysts: the current agreement in interpretation. Gastrointest Endosc. 2016;83:924–927. doi: 10.1016/j.gie.2015.08.080. [DOI] [PubMed] [Google Scholar]
- 25.Krishna S G, Brugge W R, Dewitt J M et al. Needle-based confocal laser endomicroscopy for the diagnosis of pancreatic cystic lesions: an international external interobserver and intraobserver study (with videos) Gastrointest Endosc. 2017;86:644–654. doi: 10.1016/j.gie.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Rzouq F, Vennalaganti P, Pakseresht K et al. In-class didactic versus self-directed teaching of the probe-based confocal laser endomicroscopy (pCLE) criteria for Barrettʼs esophagus. Endoscopy. 2016;48:123–127. doi: 10.1055/s-0034-1393118. [DOI] [PubMed] [Google Scholar]
- 27.Gaddam S, Mathur S C, Singh M et al. Novel probe-based confocal laser endomicroscopy criteria and interobserver agreement for the detection of dysplasia in Barrettʼs esophagus. Am J Gastroenterol. 2011;106:1961–1969. doi: 10.1038/ajg.2011.294. [DOI] [PubMed] [Google Scholar]
- 28.Kuiper T, Kiesslich R, Ponsioen C et al. The learning curve, accuracy, and interobserver agreement of endoscope-based confocal laser endomicroscopy for the differentiation of colorectal lesions. Gastrointest Endosc. 2012;75:1211–1217. doi: 10.1016/j.gie.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 29.Karstensen J G, Saftoiu A, Brynskov J et al. Confocal laser endomicroscopy: a novel method for prediction of relapse in Crohnʼs disease. Endoscopy. 2016;48:364–372. doi: 10.1055/s-0034-1393314. [DOI] [PubMed] [Google Scholar]
- 30.Neumann H, Vieth M, Atreya R et al. Assessment of Crohnʼs disease activity by confocal laser endomicroscopy. Inflamm Bowel Dis. 2012;18:2261–2269. doi: 10.1002/ibd.22907. [DOI] [PubMed] [Google Scholar]
- 31.Karstensen J G, Saftoiu A, Brynskov J et al. Confocal laser endomicroscopy in ulcerative colitis: a longitudinal study of endomicroscopic changes and response to medical therapy (with videos) Gastrointest Endosc. 2016;84:279–286 e271. doi: 10.1016/j.gie.2016.01.069. [DOI] [PubMed] [Google Scholar]


