Abstract
Background and study aims
The full-thickness resection device (FTRD) represents a novel endoscopic treatment method for lesions unresectable with conventional endoscopic techniques. The overall aim of this study was to evaluate technical success and in toto resection rates, recurrence rates, as well as immediate or late complications in patients who underwent polyp removal with the FTRD.
Patients and methods
Data from a prospectively collected database of 12 patients who underwent 13 over-the-scope clip-based full-thickness resections between June 2015 and June 2017 were analyzed. Follow-up endoscopy was performed in 11 out of 12 patients.
Results
13 full-thickness resections were performed in 7 males and 5 females (mean age 64.3 ± 6.3 years). Mean size of the lesions removed with FTRD was 17 ± 4 mm. Location was rectum (n = 6), cecum (n = 2), ascending colon (n = 2), left flexure (n = 1) and right flexure (n = 2). Mean procedure time was 68 ± 35 minutes and mean hospital stay was 2.5 ± 1.2 days. 2 patients developed post-polypectomy syndrome, which resolved after conservative treatment. No perforations and no immediate surgical revision were needed. Histology of the 13 lesions removed with FTRD showed 5 adenomas with low grade intraepithelial neoplasia (IEN), 4 high grade IEN, 1 fibrosis, 1 fibrosis without dysplasia and 2 adenocarcinomas. Technical success was achieved in all procedures (13/13, 100 %). R0 resection was achieved in 10/12 patients (83.3 %). 2 patients underwent surgery because of recurrence or not evaluable margins. In 1 patient no residual malignancy was proven in histological examination, in the other patient residual low grade IEN adenoma.
Conclusion
FTRD is a minimally invasive approach with good success rate of complete resection and minimal side effects.
Introduction
Transluminal minimally invasive treatment of early neoplasms of the gastrointestinal tract include endoscopic mucosal resection (EMR) 1 and endoscopic submucosal dissection (ESD) 2 for lesions preserving the muscularis propria 3 . However, non-pedunculated non-lifting lesions of the lower gastrointestinal tract are often unsuitable for resection with the conventional resection techniques. For such cases, endoscopic full-thickness resection (FTR) approaches have been developed and early animal studies have proven this technique to allow for endoscopic resection of the entire wall in the colon and stomach 4 5 6 7 . Recently, a commercially available full-thickness resection device has been introduced to the European market (FTRD, Ovesco, Germany) 8 9 and is now CE marked for colonic resections. Apart from non-lifting polyps as the classical indication for performing FTR, this technique may be used also for removing fibrotic lesions that have been previously sampled, incomplete polypectomy, for small colonic subepithelial tumors, lesions located in difficult anatomic places where the perforation risk during resection is significant (e. g. para-diverticular or para-appendicular lesions) or even for diagnostic purposes (e. g. for diagnosing Hirschsprung’s disease) 10 11 12 . As main advantage, FTR provides valid histologic evaluation of en-bloc specimen according to residual tumor (R) classification with minimal thermal artifact, because it removes all layers of suitable lesions including the serosa. Immediately before resection, the intestinal wall defect is closed with an over-the-scope clip (OTSC) without allowing any contact between lumen and peritoneal cavity. Perforation closure of the intestinal wall with OTSC has been proved effective in many studies, also in the setting of postsurgical situations 13 14 . The single-step defect closure of the FTRD is time effective and allows a minimal peritoneal irritation during resection.
The limitation of such a technique is the size of the lesion that can be removed, which corresponds to the amount of tissue that can be grasped in the cap, which is variable according to the location and the scirrous component of the lesion 15 . Another limitation of such technique is the difficulty of advancing the endoscope through the colon with the cap mounted, thereby limiting flexibility and visibility, particularly in the presence of adhesions or an elongated tortuous colon.
In this study we present our experience with the FTRD system emphasizing technical success, in toto resection and recurrence rates, as well as complications.
Patients and methods
Data from a prospectively collected database of all patients who were candidates for full-thickness resection were analyzed. From June 2015 to June 2017, 12 patients underwent colonoscopy or sigmoidoscopy for FTR of colonic or rectal polyps. Indications for FTR were non-pedunculated colonic lesions with non-lifting sign, relapse of adenomatous lesions resected with conventional endoscopic techniques such as EMR or snare polypectomy, lesion presenting with subepithelial invasion, and lesions located in difficult anatomic places where the perforation risk during EMR resection is significant. Technical success was defined as appropriate grasping of the lesion, deployment of the OTSC and en bloc resection. In 1 patient (excluded from the analysis) the lesion in the right transverse colon could not be reached with the device due to severely twisted colon and the presence of diverticula. All procedures were performed under deep sedation with propofol and pethidine. Intravenous butylscopolamine was given at discretion of the operator to reduce bowel peristaltic. Blood pressure, heart rate and oxygen saturation were constantly monitored during the procedure. All patients received prophylactic antibiotic therapy starting immediately before the procedure. All patients provided informed consent to undergo endoscopic resection. After the procedure, patients were monitored for at least 1 night ( Table 1 ). Immediate technical success was reported in all 12 patients. Follow-up endoscopy was performed in 11 out of 12 patients (8.7 ± 7.2 months). Postpolypectomy syndrome was defined as the development of abdominal pain, fever and leukocytosis due to the peritoneal inflammation in the absence of perforation after resection.
Table 1. Patient characteristics and outcomes following FTRD.
| Patient no. | Sex | Age (yrs) | Indication | Histology | Location | Size mm | Time(min) | R0 | Follow up (mo) | Hospital stay | Complication | Macroscopic recurrence | Microscopic recurrence | Surgery |
| 1 | M | 51 | Paradiverticular adenoma | LG IEN | Caecum | 23 | 120 | y | 3 | 3 | n | y | n | n |
| 2 | F | 76 | Relapse after EMR | HG IEN | Rectum | 15 | 80 | y | 6 | 4 | n | y | n | n |
| 3 | F | 65 | Non lifting LST | LG IEN, | Ascending colon | 25 | 105 | y | 3 | 5 | y | y | y | n |
| 4 | M | 55 | NET with submucosal infiltration | Fibrosis | Rectum | 15 | 25 | y | 1 | 1 | n | n | n | n |
| 5 | M | 59 | Non lifting LST with central depression | LG IEN | Rectum | 19 | 25 | y | 12 | 2 | n | y | y | n |
| 5 bis | M | 60 | Adenoma relapse | pT1 | Rectum | 13 | 20 | x | 12 | 2 | n | n | n | y |
| 6 | M | 74 | non lifting adenoma | HG IEN | Right Flexure | 14 | 125 | x | 8 | 2 | n | y | n | n |
| 7 | M | 71 | non lifting adenoma after polypectomy | LG IEN | Ascending colon | 12 | 50 | y | 5 | 3 | n | n | n | n |
| 8 | F | 68 | non lifting adenoma | Fibrosis without dysplasia | Right Flexure | 16 | 65 | n/a | 15 | 1 | n | y | y | y |
| 9 | F | 60 | non lifting adenoma | LG IEN | Caecum | 12 | 65 | y | 3 | 4 | y | y | n | n |
| 10 | M | 68 | non lifting adenoma | HG IEN | Rectum | 21 | 35 | y | 27 | 2 | n | n | n | n |
| 11 | M | 64 | partial adenoma resection | pT1 sm1 | Left flexure | 18 | 105 | y | 10 | 3 | n | y | n | n |
| 12 | F | 65 | Residual adenoma after EMR | SSA | Rectum | 20 | 65 | y | n/a | 1 | n | n/a | n/a | n |
No, number; n/a, not assessed/not applicable; X, not evaluable; yrs, years; min, minutes; mo, months; EMR, endoscopic mucosal resection; LST, lateral spreading tumor; NET, neuroendocrine tumor; R0, complete resection; FTR, full-thickness resection; SSA sessile serrate adenoma.
Description of the technique
Polyp removal with the full-thickness resection device (FTRD, Ovesco Endoscopy, Tübingen, Germany) was performed following a standardized method. First resection area limits were marked using argon plasma coagulation ( Fig. 1a ). Next the FTRD System, which consists of an applicator cap that delivers the over the scope clip (OTSC), was mounted on the colonoscope. The lesion was then grasped into the cap ( Fig. 1b and Fig. 1c ), the 14-mm OTSC clip was deployed, and the lesion was resected en bloc above the clip using the pre-mounted electrosurgical snare included in the device ( Fig.1d ).
Fig. 1 .
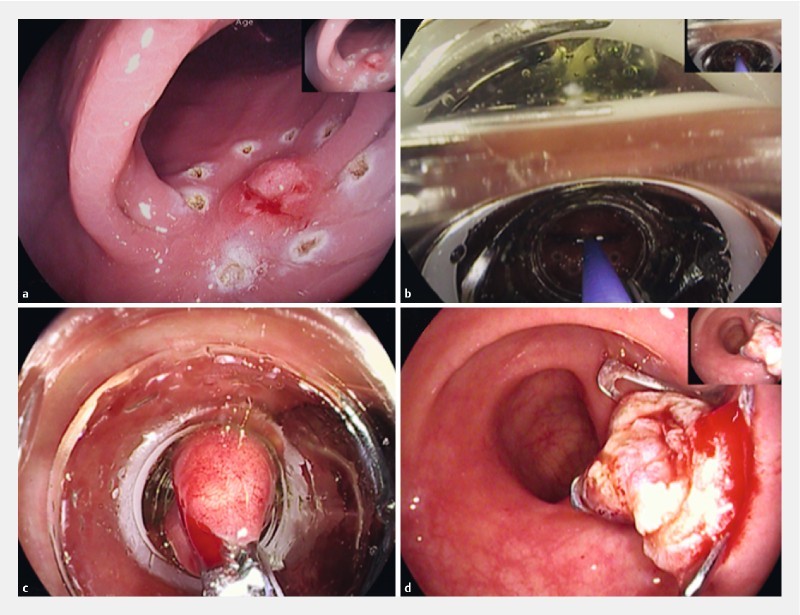
Technical overview of the FTR procedure. a Marking the lesion with argon plasma coagulation. b Grasping the lesion with the forceps into the cap. c Endoscopic view with the complete lesion inside the cap. d Resection site with the OTSC Clip in situ.
Results
Thirteen full-thickness resections were performed in 12 patients (7 males and 5 females). Mean age was 64.3 ± 6.3 years. Mean size of the resection specimen was 17 ± 4 mm. As shown in Table 1 , lesions were located in the rectum (n = 6), the cecum (n = 2), the ascending colon (n = 2), and within the left (n = 1) and right flexure (n = 2).
Mean procedure time (time from insertion to withdrawal of the endoscope) was 68 ± 38 minutes. R0 resection margins were achieved in 10/12 patients (83.3 %). Histology showed 5 adenomas with low grade intraepithelial neoplasia (IEN), 4 adenomas with high grade IEN, 1 fibrosis and 2 adenocarcinomas. Importantly, the 2 cases of adenocarcinoma (T1, sm1) were completely removed (R0 resection) with FTR.
2 patients were referred to surgery: In 1 patient with LGIEN adenoma (as diagnosed in private practice), only fibrosis but no dysplasia was histologically detected after FTR of the polyp (Patient 8). During follow-up endoscopy, a macroscopic recurrence was detected by a gastroenterologist in private practice who subsequently referred this patient to surgery. Another patient (Patient 5) underwent FTRD twice because of recurrent adenoma of the rectum: initially a 19-mm low-grade IEN adenoma was completely removed with FTR (R0 resection). However, after 12 months a 13-mm recurrent adenoma was detected on control endoscopy and was successfully removed via repeat full-thickness resection in the same location. Histology showed a T1 adenocarcinoma with Rx margins, which was then surgically removed (patient no. 5 bis).
Macroscopic recurrence was suspected at follow-up endoscopy in 8 of 11 patients (73 %). However, histological examination confirmed the presence of recurrence in only 3 patients (Patient 3, 5, and 8), while histopathology showed only inflammatory granulomatous tissue or scar tissue in the remaining patients. During follow-up endoscopy the clip was not in situ anymore in all patients.
Technical resection success was achieved in all procedures (13/13, 100 %), all the systems deployed the clip correctly and the grasped tissue could be safely removed. However, in 1 patient with a 20-mm non-lifting SSA in the very distal rectum 4 cm from the anal verge, the snare within the cap did not sufficiently close despite appropriately delivered clip. This was most likely due to the missing possibility to fully angulate the tip of the scope at this locale. In this patient, the endoscope with the FTR system was removed and immediately thereafter, the lesion was completely removed directly above the deployed clip with a standard polypectomy snare and histology confirmed R0 resection.
There were no complications associated with sedation or the endoscopic procedure. No perforations or major bleedings were observed. 2 patients developed a post-polypectomy syndrome with abdominal pain, fever and laboratory chemical signs of inflammation. Intestinal perforation was ruled out radiologically in these patients. In 1 of these patients, the post-polypectomy syndrome developed with a delayed onset of 6 days after the procedure, leading to re-hospitalization. Both patients with post-polypectomy syndrome were managed conservatively with intravenous hydration, antibiotics and analgesics. In patients presenting post polypectomy syndrome the hospital stay was 4 and 6 days, respectively, while the mean hospital stay in the other patients undergoing FTR was 2.5 ± 1.2 days.
Discussion
FTR is an emerging method for minimally invasive resection that obviates the need for surgical therapy in selected patients. It is approved for lesions of the lower intestinal tract and is suitable for non-lifting lesions ranging from 0.5 up to 2.5 cm (according to literature polyps within a range of 12 to 40 mm have been successfully removed with FTR) or small submucosal lesions 10 .
Compared to other similar techniques of FTR, where the defect is closed successfully with normal clips, stapler or suturing, the utilization of an OTSC allows a single-step defect closure. The Padlock clip (Aponos Medical Corp., Kingston, NH, USA) represents an alternative to the FTRD, with some differences since it contains 6 inner needles which “lifts” the lesion allowing suctioning into the cap without the help of a grasping forceps used instead for the OTSC assisted full thickness resection. However, angulation of the lesion may be a risk factor for failing in deploying the clip, which usually is successful in the OTSC procedures 12 16 . Also in our study, clip deployment was successful in all cases. Future prospective comparison studies may investigate on this issue and directly compare different devices for FTR.
A quite common side effect of the procedure is post-polypectomy syndrome, due to peritoneal reaction after resection. That was seen in 2 patients in our series, in 2 out to 25 patients as reported from Schmidt and co-workers 10 and in 1 of 20 patients in the series of Andrisani and colleagues 17 In case series published up to now, perforation requiring subsequent surgical revision was never reported 8 9 10 12 16 17 . 1 minor bleeding requiring endoscopic hemostasis, was reported form Schmidt and coll. 10 while 1 surgical resection of the duplicated intestinal wall was required in the series from Richter-Schrag and co-workers 18 . Technical success rates range from 75 % up to 100 % 9 10 17 18 .
Hence, the procedure is safe and can be performed under conscious sedation without the need of tracheal intubation, thereby rendering it feasible especially in elderly patients whose clinical condition may preclude extraluminal surgery.
Follow-up endoscopy after FTR is recommended since dysplasia can be missed and recurrence of adenoma can occur at the original resection site, even when the histology confirms a R0 resection. Both of these scenarios occurred in our series (Patients 8 and 3, respectively), thereby reiterating the need for endoscopic follow-up after FTR.
In our study, the rate of suspected macroscopic recurrence was quite high (73 %). This was based on granulation tissue induced by the OTSC, endoscopically appearing as large (in our case up to 6 mm) nodular pseudopolyps mimicking adenomatous tissue. Such aberrant polypoid nodule scar has also been described after endoscopic submucosal dissections 19 20 , particularly in the antrum of the stomach. Although no contact occurs between peritoneal cavity and intestinal lumen during FTR, the mucosal epithelial barrier is disrupted during intervention. This may cause a redundant inflammatory reaction, which may play a role in the formation of this pseudopolypous granulation tissue. This hypothesis is consistent with the chronic inflammation reaction with formation of granulomatous tissue and fibrosis as observed on histopathology in these lesions. Further, it is not unlikely that the clips used for FTR promote a foreign body reaction, appearing macroscopically as granulomatous tissue. While histological examination confirmed true adenomatous tissue and thereby adenoma recurrence in only 3 cases, this illustrates at the same time that a certain learning curve is required when evaluating the mucosa in which a previous FTR has been performed and future studies should assess on how to best discriminate recurrent adenoma from granulomatous pseudopolyps endoscopically.
Surprisingly, despite R0 resection, local recurrence was histologically proven in 3 patients during follow-up. It may be speculated that minute fragments of neoplastic tissue might have become entrapped during FTR procedure by the clip arms, leading to local recurrence. This scenario might have been favored in situations where the ideal position for resection is lost with the cap mounted during FTR, thereby potentially leading to entrapment of microscopic fragments in the clip arms.
In the current study, the majority of lesions were removed due to their non-lifting character as the typical indication for performing FTR. However, in 1 patient, FTR was performed due to a neuroendocrine tumor with submucosal infiltration and in 1 patient with a location of an adenoma in direct proximity to a diverticulum. As shown in these cases and also confirmed in other case series 10 11 12 18 21 22 , utilization of FTR techniques might well go beyond non-lifting lesion and extend to submucosal lesions or those in complicated anatomy with involvement of diverticula or the appendix. Further, our report is the first to describe repeat FTR at the same anatomical site due to recurrent adenoma. In this patient, an adenoma recurrence was successfully and completely removed with a second FTR 12 months after the initial FTR, thereby indicating that FTR can also be safely and effectively performed twice at the same anatomical locale. However, further dedicated studies analyzing these indications are clearly necessary.
Conclusion
In summary, FTRD is a minimal invasive approach for resection of selected non-lifting lesions also in challenging locations with good success rate of complete resection. Minimal side effects have been reported but follow-up endoscopy is recommended in order to rule out recurrence. Prospective randomized studies are needed to further evaluate this device and compare it to other available resection techniques.
Footnotes
Competing interests None
References
- 1.Pellise M, Burgess N G, Tutticci N et al. Endoscopic mucosal resection for large serrated lesions in comparison with adenomas: a prospective multicentre study of 2000 lesions. Gut. 2017;66:644–653. doi: 10.1136/gutjnl-2015-310249. [DOI] [PubMed] [Google Scholar]
- 2.Kuroki Y, Hoteya S, Mitani T et al. Endoscopic submucosal dissection for residual/locally recurrent lesions after endoscopic therapy for colorectal tumors. J Gastroenterol Hepatol. 2010;25:1747–1753. doi: 10.1111/j.1440-1746.2010.06331.x. [DOI] [PubMed] [Google Scholar]
- 3.Fujiya M, Tanaka K, Dokoshi T et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015;81:583–595. doi: 10.1016/j.gie.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 4.Schurr M O, Buess G, Raestrup H et al. Full thickness resection device (FTRD) for endoluminal removal of large bowel tumours: development of the instrument and related experimental studies. Minim Invasive Ther Allied Technol. 2001;10:301–309. doi: 10.1080/136457001753337357. [DOI] [PubMed] [Google Scholar]
- 5.Schurr M O, Baur F E, Krautwald M et al. Endoscopic full-thickness resection and clip defect closure in the colon with the new FTRD system: experimental study. Surg Endosc. 2015;29:2434–2441. doi: 10.1007/s00464-014-3923-x. [DOI] [PubMed] [Google Scholar]
- 6.Kaehler G F, Langner C, Suchan K L et al. Endoscopic full-thickness resection of the stomach: an experimental approach. Surg Endosc. 2006;20:519–521. doi: 10.1007/s00464-005-0147-0. [DOI] [PubMed] [Google Scholar]
- 7.Elmunzer B J, Trunzo J A, Marks J M et al. Endoscopic full-thickness resection of gastric tumors using a novel grasp-and-snare technique: feasibility in ex vivo and in vivo porcine models. Endoscopy. 2008;40:931–935. doi: 10.1055/s-2008-1077587. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt A, Damm M, Caca K.Endoscopic full-thickness resection using a novel over-the-scope device Gastroenterology 2014147740–742.e742 [DOI] [PubMed] [Google Scholar]
- 9.Fahndrich M, Sandmann M. Endoscopic full-thickness resection for gastrointestinal lesions using the over-the-scope clip system: a case series. Endoscopy. 2015;47:76–79. doi: 10.1055/s-0034-1377975. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt A, Bauerfeind P, Gubler C et al. Endoscopic full-thickness resection in the colorectum with a novel over-the-scope device: first experience. Endoscopy. 2015;47:719–725. doi: 10.1055/s-0034-1391781. [DOI] [PubMed] [Google Scholar]
- 11.Valli P V, Kaufmann M, Vrugt B et al. Endoscopic resection of a diverticulum-arisen colonic adenoma using a full-thickness resection device. Gastroenterology. 2014;147:969–971. doi: 10.1053/j.gastro.2014.07.053. [DOI] [PubMed] [Google Scholar]
- 12.Al-Bawardy B, Rajan E, Wong Kee Song L M. Over-the-scope clip-assisted endoscopic full-thickness resection of epithelial and subepithelial GI lesions. Gastrointest Endosc. 2017;85:1087–1092. doi: 10.1016/j.gie.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Raithel M, Albrecht H, Scheppach W et al. Outcome, comorbidity, hospitalization and 30-day mortality after closure of acute perforations and postoperative anastomotic leaks by the over-the-scope clip (OTSC) in an unselected cohort of patients. Surg Endosc. 2017;31:2411–2425. doi: 10.1007/s00464-016-5242-x. [DOI] [PubMed] [Google Scholar]
- 14.Haito-Chavez Y, Law J K, Kratt T et al. International multicenter experience with an over-the-scope clipping device for endoscopic management of GI defects (with video) Gastrointest Endosc. 2014;80:610–622. doi: 10.1016/j.gie.2014.03.049. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt A, Meier B, Caca K. Endoscopic full-thickness resection: Current status. World J Gastroenterol. 2015;21:9273–9285. doi: 10.3748/wjg.v21.i31.9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinelli M, Omazzi B, Andreozzi P et al. First clinical experiences with a novel endoscopic over-the-scope clip system. Endosc Int Open. 2017;5:E151–E156. doi: 10.1055/s-0043-101692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrisani G, Pizzicannella M, Martino M et al. Endoscopic full-thickness resection of superficial colorectal neoplasms using a new over-the-scope clip system: A single-centre study. Dig Liver Dis. 2017;49:1009–1013. doi: 10.1016/j.dld.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Richter-Schrag H J, Walker C, Thimme R et al. [Full thickness resection device (FTRD). Experience and outcome for benign neoplasms of the rectum and colon] Chirurg. 2016;87:316–325. doi: 10.1007/s00104-015-0091-z. [DOI] [PubMed] [Google Scholar]
- 19.Arantes V, Uedo N, Pedrosa M S et al. Clinical relevance of aberrant polypoid nodule scar after endoscopic submucosal dissection. World J Gastrointest Endosc. 2016;8:628–634. doi: 10.4253/wjge.v8.i17.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito S KS, Ishikawa K, Uragami K et al. A case of gastric ulcer scar with type IIa-like elevation in center of the lesion. Gastroenterol Endosc. 1974;16:194–197. [Google Scholar]
- 21.Salerno R, Gherardi G, Paterno E et al. Endoscopic full-thickness resection of a submucosal right colon lesion. Endoscopy. 2016;48:E376–E377. doi: 10.1055/s-0042-120338. [DOI] [PubMed] [Google Scholar]
- 22.Guo J, Liu Z, Sun S et al. Endoscopic full-thickness resection with defect closure using an over-the-scope clip for gastric subepithelial tumors originating from the muscularis propria. Surg Endosc. 2015;29:3356–3362. doi: 10.1007/s00464-015-4076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


