Abstract
There is no known root-knot nematode (Meloidogyne spp.) resistance in caladium (Caladium × hortulanum), an ornamental foliage crop grown from tubers, but cultivars have been reported to differ in their level of susceptibility. Research was conducted to assess the relative susceptibility of seven widely grown caladium cultivars to the species of Meloidogyne which occur in the southeastern United States, where caladium cultivars are commonly planted in commercial and residential landscapes. Root-knot nematode species tested were Meloidogyne arenaria, Meloidogyne enterolobii (=M. mayaguensis), Meloidogyne floridensis, Meloidogyne incognita, and Meloidogyne javanica. All of the caladium cultivars tested were susceptible to galling by all species of Meloidogyne tested; however M. javanica caused the least severe galling. Meloidogyne enterolobii produced high numbers of eggs per gram of fresh root on all cultivars tested, with cv. Freida Hemple having the highest number (14,799 eggs/g fresh root). Meloidogyne javanica also reproduced at a high level on most cultivars tested. Overall, the number of eggs of M. arenaria, M. floridensis, and M. incognita was low on all caladium cultivars tested. Meloidogyne javanica was isolated from caladium roots in high numbers regardless of the cultivar. Meloidogyne incognita had low numbers of second stage root-knot nematode juveniles (J2) isolated from soil of all cultivars. The high level of reproduction of M. enterolobii and the high rate of isolation of M. javanica from roots, as well as the low rate of isolation of M. incognita from soil, are not reflected in gall ratings where M. javanica ratings were low but high numbers of eggs and J2 were present in roots. An increased understanding of cultivar susceptibility levels and the reproductive capacity of common root-knot nematode on caladium under various environmental conditions is needed to better manage nematode-infested planting sites and improve caladium growth.
Keywords: caladium, Meloidogyne arenaria, Meloidogyne enterolobii, Meloidogyne floridensis, Meloidogyne incognita, Meloidogyne javanica, root-knot nematodes, susceptibility
Caladium (Caladium × hortulanum Birdsey) is an ornamental foliage crop grown from tubers and planted extensively in landscapes in the southeastern United States. Most of the caladium species originated in the Amazon basin in Brazil but are also found in equatorial tropical rain forests of Latin America, South America, southern Mexico, Peru, Puerto Rico, and the Lesser Antilles (Hartman, personal communication). During the past 150 yr, more than 2,000 named cultivars of caladium have been developed (Hartman, personal communication).
Commercial production of caladium tubers in the United States is concentrated in Highlands County, Florida, where approximately 85% of the world’s supply of tubers is field grown. Historically, caladium growers have relied heavily on soil fumigants for effective parasitic nematode control in this long-season crop, which can be in the field for approximately 9 mon from planting to harvest of tubers. Caladium is susceptible to root-knot nematodes (Meloidogyne spp.) and symptoms of root-knot nematode infestation include leaf dieback, stunted plants, galling on roots, and low tuber yield. Tubers also become infested with root-knot nematodes but may remain either symptomless or produce nondescript corky lesions. Esser (1973) isolated Meloidogyne spp. from 52.8% of soil samples and 27.9% of caladium tuber samples. In that study, 56 of the 75 varieties tested were found to be susceptible to Meloidogyne spp. There is no known root-knot nematode resistance in caladium; however, cultivars differ in their level of susceptibility (McSorley et al., 2004; Dover et al., 2005). Currently efforts in breeding for resistance are limited to work done at the University of Florida and private grower breeding programs.
Limited studies have been conducted to identify nematode resistance in existing caladium cultivars, as well as alternative soil chemical treatments for nematode, weed, and pathogen control (Gilreath and West, 1996; Gilreath et al., 1999; Kokalis-Burelle et al., 2010). In order to successfully manage the production of numerous caladium cultivars, it is necessary to understand the relative susceptibility of cultivars to different species of Meloidogyne. This will also enable growers to better manage nematode-infested fields.
The objectives of this research were to assess the relative susceptibility of seven caladium cultivars to five species of Meloidogyne under controlled greenhouse conditions. All species of Meloidogyne tested occur naturally in Florida and were M. arenaria, M. enterolobii, M. floridensis, M. incognita, and M. javanica.
Materials and Methods
Experimental design:
Tubers of seven field-grown caladium cultivars including ‘Pink Beauty’, ‘White Christmas’, ‘Candidum’, ‘Freida Hemple’, ‘Red Flash’, ‘Carolyn Whorton’, and ‘Postman Joyner’ were harvested using standard practices and provided to USDA-ARS by a commercial grower for greenhouse nematode host-range studies. Tubers were planted into 15-cm-diam. plastic pots containing a mixture of builder’s grade sand and peat-based growing mix (commercial builder’s sand and 4P Fafard’s peat moss in the ratio 4:1). This mixture will be referred to as soil. One tuber seed piece was planted per pot, and each seed piece contained at least one auxiliary bud. After 10 d, each plant was inoculated by pipetting 1 ml of water containing a total of 2,000 eggs of either M. arenaria, M. enterolobii, M. floridensis, M. incognita, or M. javanica into two depressions in the soil near the emerged stems. Gravid females were extracted from roots of Rutgers tomato plants used to culture each species of Meloidogyne and identified based on enzyme phenotypes using the PhastSystem (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) (Esbenshade and Triantaphyllou, 1985, 1990). Five plants of root-knot nematode–susceptible tomato cultivar Tiny Tim were inoculated simultaneously with the caladium tubers to confirm viability and infectivity of all nematode inoculum. All plants were maintained in the greenhouse, watered daily, and fertilized once a week with Peters 20–20–20 (J. R. Peters, Inc., Allentown, PA). Ambient lighting conditions were used in the greenhouse and temperatures were maintained between 10°C and 32°C. After 12 wk, caladium tubers and roots were removed from soil and assessed for disease and nematode reproduction. Caladium tubers of each cultivar used in this study were also grown without nematode inoculum to determine if significant levels of root-knot nematodes were present in the field-grown tubers used in the experiments.
Nematode extraction and disease evaluation:
Caladium roots were assessed for root fresh weight, root condition, root galling, number of eggs isolated per gram of root tissue, and number of second stage root-knot nematode juveniles (J2) present in the roots and soil at harvest. At the end of experiments after 12 wk, 100 cm3 of soil was collected from each pot and processed to determine the number of J2 present in the soil at harvest using a modified Baermann funnel technique (McSorley et al., 1999). Also, 10 g of root tissue was used for extraction of J2 using the modified Baermann funnel technique. Root condition and gall ratings were assessed by eye using the entire root system. Root condition ratings were based on visible root disease/necrosis, which is sometimes more extensive in severely galled root systems but is not a measure of nematode susceptibility. Root condition was assessed using a subjective scale of 0 to 5 with 0 to 1 = 0% to 20% diseased/necrotic, 1 to 2 = 21% to 40%, 2 to 3 = 41% to 60%, 3 to 4 = 61% to 80%, and 4 to 5 = 81% to 100%. Root gall ratings were assessed using a scale of 0 to 5, such that 0 = 0 galls or egg masses; 1 = 1 to 2 galls or egg masses; 2 = 3 to 10 galls or egg masses, 3 = 11 to 30 galls or egg masses; 4 = 31 to 100 galls or egg masses; and 5 ≥ 100 galls or egg masses per root system (Taylor and Sasser, 1978). Nematode eggs were extracted from 10 g of caladium roots and tubers using 0.525% NaOCl (Hussey and Barker, 1973). Nematode J2 and eggs were counted on inverted microscopes at 40× using petri dishes and nematode counting slides, respectively.
Statistical analysis:
Nematode species were tested separately on caladium cv. Control tubers did not receive nematode inoculum and did not have nematodes present in soil or roots at the end of experiments. Cultivars in all experiments were replicated five times and pots were arranged in a completely randomized design in greenhouses. All experiments were conducted two times for each nematode species at the USDA-ARS lab in Ft. Pierce. In repeated experiments, data from both experiments were subjected to a t-test and subsequently combined if no statistically significant differences were found between tests (P ≤ 0.05). Data were analyzed using analysis of variance to determine significance of main effects (P ≤ 0.05). Calculations were performed with the general linear model procedure of SAS (SAS 9.2, Cary, NC). Where analysis of variance detected significance, means were separated and planned comparisons made using least significance difference (LSD).
Results
Root disease and gall ratings on plants that did not receive nematode inoculum were not significant for any cultivar, with no statistically significant differences among cultivars. Low numbers of nematode eggs were isolated from epidermal tissue of tubers of ‘Pink Beauty’, ‘Freida Hemple’, and ‘Carolyn Whorton’ but were not significant (data not shown). Tomatoes inoculated simultaneously with the caladium tubers were heavily galled indicating good viability and infectivity of all nematode inoculum (data not shown). Results from repeated experiments for all Meloidogyne species were not significantly different (P < 0.05) and data were subsequently combined.
Susceptibility to M. arenaria:
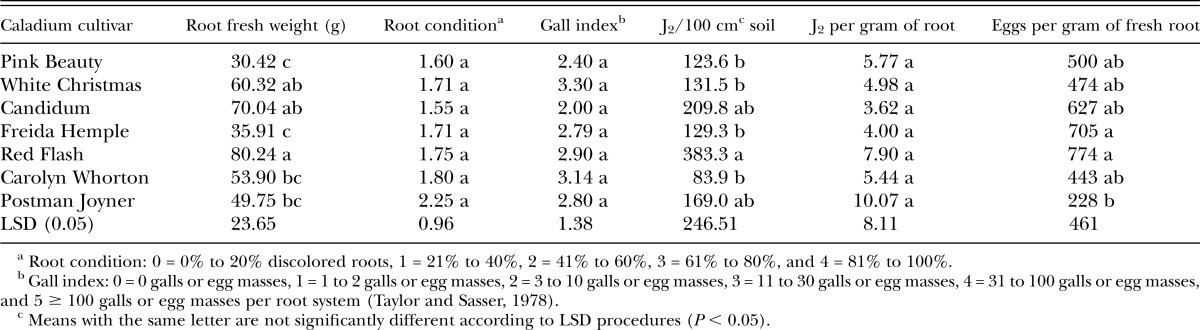
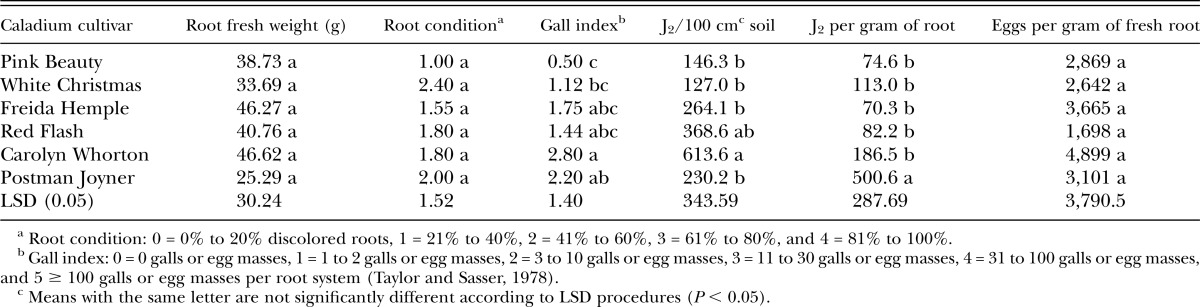
Greenhouse experiments using M. arenaria were harvested on 9 October and 19 October 2012. ‘Red Flash’ had the highest root weight, whereas the lowest weights were recorded from ‘Pink Beauty’ and ‘Freida Hemple’. There were no statistically significant differences among the cultivars tested in root condition, galling, or J2 per gram of root, in response to M. arenaria inoculation (Table 1). Gall index values were moderate for all cultivars, indicating all cultivars were moderately susceptible to M. arenaria (Table 1). The number of J2 isolated from soil was higher in ‘Red Flash’ than several other cultivars tested, and ‘Red Flash’ had very high numbers of eggs per gram of fresh root (Table 1).
Table 1.
Response of caladium cultivars to inoculation with Meloidogyne arenaria eggs in greenhouse experiments performed at USDA-ARS, Ft. Pierce, FL.
Susceptibility to M. enterolobii:
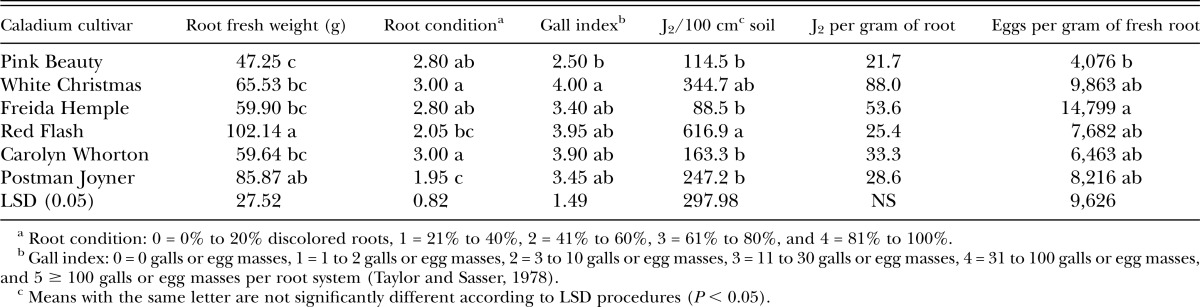
Greenhouse experiments using M. enterolobii were harvested on 3 October 2016 and 2 February 2017. The cultivar Candidum was not tested for susceptibility to M. enterolobii because of unavailability of tubers. Fresh root weight varied among cultivars tested with ‘Red Flash’ having the highest root weight and ‘Pink Beauty’ having the lowest root weight (Table 2). Root condition ratings were best for ‘Postman Joyner’, which had significantly healthier roots than several other cultivars. ‘White Christmas’ and ‘Carolyn Whorton’ had the least healthy root condition ratings (Table 2). Galling induced by M. enterolobii was significantly higher on ‘White Christmas’ than on ‘Pink Beauty’, which had the lowest root gall index value. ‘Red Flash’ had the highest number of J2 isolated from soil, which was higher than most other cultivars. However, there were no statistically significant differences in the number of M. enterolobii J2 recovered from roots. All cultivars had high numbers of M. enterolobii eggs per gram of root with ‘Freida Hemple’ having significantly more eggs per gram of root than ‘Pink Beauty’ (Table 2).
Table 2.
Response of caladium cultivar to inoculation with Meloidogyne enterolobii eggs in greenhouse experiments at USDA-ARS, Ft. Pierce, FL.
Susceptibility to M. floridensis:
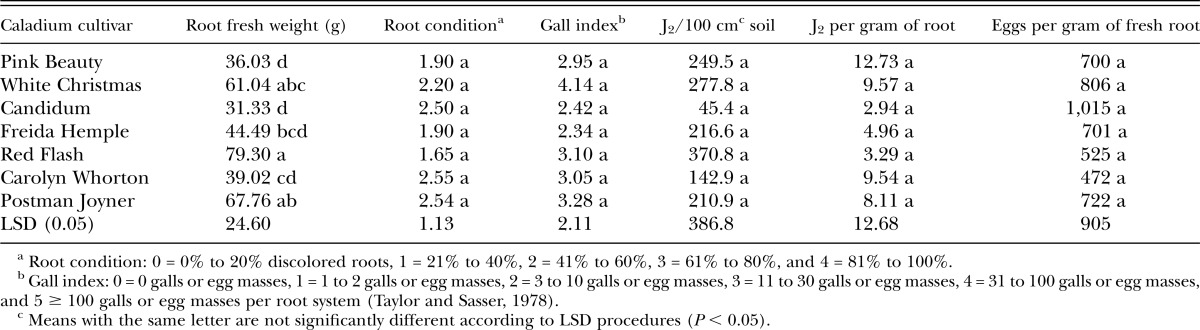
Greenhouse experiments using M. floridensis were harvested on 16 October and 26 October 2012. There were no statistically significant differences among caladium cultivars in root condition, gall index, nematode J2 isolated from soil or roots, and eggs per gram of fresh root in response to inoculation with M. floridensis (Table 3). All cultivars were susceptible to this nematode species. As in other experiments, in this study, root fresh weights were different among caladium cultivars (Table 3). High gall index values and numbers of eggs isolated per gram of root indicate high levels of M. floridensis reproduction on all cultivars.
Table 3.
Response of caladium cultivars to inoculation with Meloidogyne floridensis eggs in greenhouse experiments performed at USDA-ARS, Ft. Pierce, FL.
Susceptibility to M. incognita:
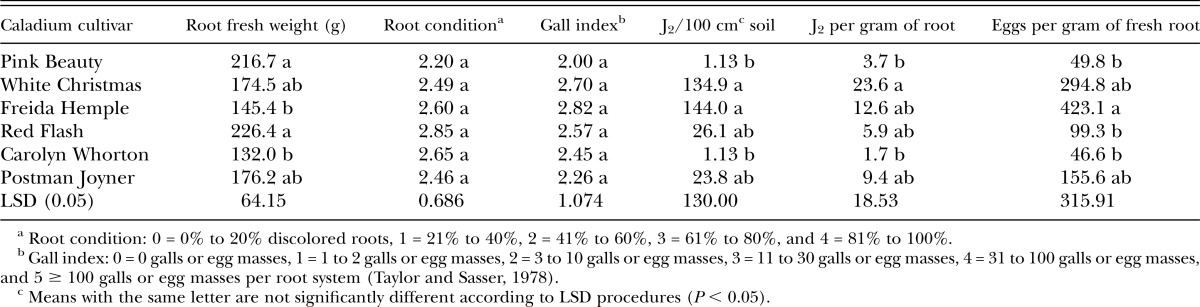
Greenhouse experiments using M. incognita were harvested on 1 November and 15 November 2016. The cultivar Candidum was not tested for susceptibility to M. incognita because of unavailability of tubers. Root fresh weights differed among caladium cultivars being tested for susceptibility to M. incognita with ‘Pink Beauty’ and ‘Red Flash’ having the largest root systems (Table 4). ‘Red Flash’ consistently had the heaviest root systems in tests with all nematode species except M. javanica. Root condition and gall index values did not differ among caladium cultivars in response to M. incognita infection (Table 4). However, more M. incognita J2 were isolated from ‘White Christmas’ roots than ‘Pink Beauty’ and ‘Carolyn Whorton’. Also, ‘Freida Hemple’ had the most M. incognita eggs isolated per gram of root than several other cultivars (Table 4).
Table 4.
Response of caladium cultivars to inoculation with Meloidogyne incognita eggs in greenhouse experiments performed at USDA-ARS, Ft. Pierce, FL.
Susceptibility to M. javanica:
Greenhouse experiments using M. javanica were harvested on 22 August and 29 August 2016. The cultivar Candidum was not tested for susceptibility to M. javanica because of unavailability of tubers. Root fresh weight, root condition, and eggs per gram of fresh root did not differ among caladium cultivars inoculated with M. javanica (Table 5). However, galling was low in ‘Pink Beauty’, which also had low numbers of J2 isolated from soil and roots (Table 5). ‘Carolyn Whorton’ had the highest gall ratings and number of M. javanica J2 isolated from soil whereas ‘Postman Joyner’ had higher numbers of J2 isolated from roots than all other cultivars (Table 5).
Table 5.
Response of caladium cultivars to inoculation with Meloidogyne javanica eggs in greenhouse experiments at USDA-ARS, Ft. Pierce, FL.
Discussion
There is limited literature available on susceptibility of caladium cultivars to plant-parasitic nematodes, including important species of root-knot nematodes. The work presented here is comprised of data on up to seven commonly propagated caladium cultivars and their susceptibility to five important root-knot nematode species. In addition, this is the first study demonstrating the potential ability of two emerging root-knot nematodes in Florida, M. floridensis and M. enterolobii, to cause disease on selected caladium cultivars currently available on the market. Results of the repeated experiments for all Meloidogyne spp. tested were consistent, enabling the data for the replicated experiments to be combined, and indicating a uniform range of susceptibility among the caladium cultivars for each nematode species. These studies demonstrate that the caladium cultivars tested are all susceptible to galling by all Meloidogyne species tested. Also, there was not a great deal of difference among the caladium cultivars in their response to infestation by each nematode species, as evidenced by root gall development. Interestingly, although M. javanica produced the lowest overall gall ratings, it also resulted in the highest number of J2 isolated from roots. Also, with respect to nematode reproduction, the highest egg production was observed on cultivars inoculated with M. enterolobii and M. javanica. These results are somewhat unexpected as the predominant species of Meloidogyne isolated from previous fumigation trials in caladium production fields in Florida has been M. arenaria (Kokalis-Burelle et al., 2010). Dover et al., (2005) found that ‘Pink Beauty’, ‘White Christmas’, ‘Freida Hemple’, and ‘Postman Joyner’ were relatively resistant to M. incognita compared with several other cultivars. Results of our research are similar with regard to ‘Pink Beauty’, which had low numbers of J2 isolated from roots and soil, and eggs isolated from roots.
To successfully manage commercial production of numerous caladium cultivars, an understanding of the relative susceptibility of each cultivar to common species of Meloidogyne will be beneficial, particularly for areas aiming to produce planting material free of these pathogens. This information will also be useful to consumers when planting caladium cultivars in the landscape.
Literature Cited
- Dover KD, McSorley R, Wang KH. Resistance and tolerance of caladium cultivars to Meloidogyne incognita. Soil and Crop Science Society of Florida Proceedings. 2005;64:98–102. [Google Scholar]
- Esbenshade PR, Triantaphyllou AC. Use of enzyme phenotypes for identification of Meloidogyne species. Journal of Nematology. 1985;17(1):6–20. [PMC free article] [PubMed] [Google Scholar]
- Esbenshade PR, Triantaphyllou AC. Isozyme phenotypes for the identification of Meloidogyne species. Journal of Nematology. 1990;22(1):10–15. [PMC free article] [PubMed] [Google Scholar]
- Esser RP. Nematodes associated with caladium in Florida. Plant Disease Reptorer. 1973;57:558–560. [Google Scholar]
- Gilreath JP, West DW. Preliminary investigations with fumigant alternatives to methyl bromide in floricultural crops. Proceedings of the Florida State Horticultural Society. 1996;109:25–28. [Google Scholar]
- Gilreath JP, McSorley R, McGovern RJ. Soil fumigant and herbicide combinations for soilborne pest control in caladium. Proceedings of the Florida State Horticultural Society. 1999;112:285–290. [Google Scholar]
- Hussey RS, Barker KR. A comparison of methods of collecting inocula for Meloidogyne spp., including a new technique. Plant Disease Reporter. 1973;57:1025–1028. [Google Scholar]
- Kokalis-Burelle N, Rosskopf EN, Hartman RD. Evaluation of soil treatments for control of Meloidogyne arenaria in caladium tubers (Caladium × hortulanum) and nematode susceptibility of selected cultivars. Nematropica. 2010;40(2):177–189. [Google Scholar]
- McSorley R, Frederick JJ, McGovern RJ. Extraction of Meloidogyne incognita from caladium corms. Nematropica. 1999;29:245–248. [Google Scholar]
- McSorley R, Wang K-H, Frederick JJ. Host suitability of caladium varieties to Meloidogyne incognita. Nematropica. 2004;34:97–101. [Google Scholar]
- Taylor AL, Sasser JN. Biology, identification and control of root-knot nematodes (Meloidogyne species) Raleigh, NC: Department of Plant Pathology, North Carolina State University and the United States Agency for International Development, North Carolina State University Graphics; 1978. [Google Scholar]