Abstract
Plant parasitic nematodes (PPN) are important crop pests within the global agri-sector. Critical to their success is a complex and highly sensitive chemosensory system used to locate plants by detecting host cues. In addition to this, the nematode neuronal system has evolved mechanisms to allow adaptation to a changing environment. Clearly, there is a need to better understand the host–parasite relationship and the mechanisms by which PPN successfully locate and infect host plants. Here, we demonstrate the chemotactic response of two economically important PPN species, Meloidogyne incognita and Globodera pallida to selected phytochemicals. We further reveal an adapted chemotactic response in M. incognita second-stage juveniles preexposed to ethephon (Eth), potato root diffusate (PRD), and salicylic acid (SA), and present pharmacological evidence supporting the existence of long-term habituation traits acting via serotonergic-dependent neurotransmission.
Keywords: bioassay, biological control, behavior, chemosensory, chemotaxis, Globodera pallida, habituation, Meloidogyne incognita, phytochemicals, serotonin
Plant parasitic nematodes (PPN) are major economic pests within the global agri-sector with annual estimates of the damage they cause exceeding $100 billion (Abad et al., 2008). PPN are among the most difficult crop pests to control, given that some of the most damaging genera (Globodera, Heterodera, and Meloidogyne) remain within the host plant for most of their lifecycle (Jones et al., 2013). Traditionally, control methods relied on good cropping practice as well as biological and chemical nematicides (Barker and Koenning, 1998), with chemical fumigants being the primary means of control for many PPN pest populations. However, over the last several decades, many nematicides have been de-registered or restricted, leaving the global agri-sector at a great disadvantage with regard to nematode control (Fleming, 2015).
PPN host–finding ability is reliant on a complex and sensitive chemosensory system (Curtis, 2007). Locating the host plant involves the infective J2 chemo-orientating toward diverse phytochemicals released in root exudates, many of which are released by plants to attract beneficial micro-organisms to the rhizosphere, or to deter pathogens (Curtis, 2008).
Studies have illustrated the plasticity and sensitivity of the PPN chemoperception toward root exudates (Devine and Jones, 2003; Spence et al., 2008; Reynolds et al., 2011) and phytochemicals including phenylpropanoids (Wuyts et al., 2006), pH gradients (Wang et al., 2009), and monosaccharide sugars (Warnock et al., 2016). Accordingly, research into natural phytochemicals as a viable nematode control strategy has been an area of interest (Chitwood, 2002; Dong et al., 2013). Plants release copious compounds into the rhizosphere (Badri and Vivanco, 2009), many of which are used in defense of the plant to deter or inhibit pathogens (Badri et al., 2010). PPN behavior has been a topic of increasing interest amongst researchers, for example, J2 have been shown to efficiently follow concentration gradients or exhibit clumping behaviors (Wang et al., 2010). Although the use of biostimulants in agriculture is rapidly increasing and they are seen as potential replacements to the traditional plant protection products, little is known about their mechanisms of action (Calvo et al., 2014). Effects include direct modification of plant physiology, changes in soil chemical composition, and interactions with the soil microflora and microfauna. A better understanding of PPN behavioral responses to chemical stimuli will help shed light on biostimulant function and will improve understanding of host–parasite relationships and nematode responses in soil.
Learning and memory behavior in nematodes have been established in the model free-living nematode Caenorhabditis elegans (Rankin et al., 1990), which possesses a highly developed chemosensory system (Bargmann, 2006). Notable is the ability of this simple animal to respond to changes in its environment and adjust its behavior accordingly (McDiarmid et al., 2015). Several examples of associative learning in C. elegans have demonstrated the ability to predict nutrient-deficient environments (Nuttley et al., 2002), aversion to pathogenic bacteria (Zhang et al., 2005) and thermotaxis (Li et al., 2013), behaviors that appear to rely on serotonin-dependent signaling and other neuro-regulatory mechanisms. The actions of serotonin in PPN are well known, where applications induce pharyngeal pumping (Rosso et al., 2005) and are routinely used for studies involving stylet activity (Warnock et al., 2016). Habituation is a form of nonassociative learning by an organism to a single stimulus, in contrast to associative learning between two stimuli. The relevance of habituation, in a biological context, allows an organism to ignore irrelevant stimuli and focus on stimuli attributed to survival (Rose and Rankin, 2001). Here, we investigate the chemoperception ability and chemotactic responses in two economically important pest nematodes and test for the presence of long-term habituation behavior in M. incognita J2, with a role for serotonin.
Materials and Methods
Nematode cultures:
Meloidogyne incognita used in the experiments were collected from cultures raised on tomato cv. Moneymaker. Roots were cut into 10-cm segments and homogenized in 10% sodium hypochlorite solution for 1 min and roots were washed thoroughly in water over three stacked sieves in the order of decreasing mesh size (180, 150, and 38 μM, respectively). Egg-containing sediment was collected from the 38-μM sieve and added to 15-ml Eppendorf tubes and centrifuged at 1,811 relative centrifugal force (rcf) for 3 min. The water layer was removed and replaced with 100% sucrose solution to a total volume of 14 ml. Tubes were shaken to disturb sediment into suspension and then 1 ml of water was added to the tube before centrifuging at 1,881 rcf for 3 min. Eggs were collected from the top layer and incubated 1× antibiotic antimycotic (Sigma Aldrich, Irvine, UK) for 1 hr before being transferred to a 90-mm diameter petri dish (Fisher Scientific, UK) containing sterile water (pH 7.2). Eggs were incubated at 24°C in darkness before hatching. Globodera pallida (pathotype Pa2/3) used in this study were cultured on potato cv. Cara maintained at the Agri-Food and Biosciences Institute, Belfast, Northern Ireland. Cysts were hatched in PRD at 16°C in darkness. To facilitate PRD collection, a plastic collection container was placed under a potted potato plant. De-ionized water was gradually added until runoff was observed in the collection container. At this point, an additional 50 ml of water was added to the pot and allowed to drain thoroughly. The collected diffusate was then carefully transferred back to the pot and allowed to drain through for the second time (second wash). The diffusate transfer was repeated again for the final time (third wash). The collected diffusate from 4-, 5-, and 6-wk-old potted potato tubers was combined for a single stock and stored at 4°C in the dark before use.
Nematistatic bioassays:
The effects of the phenolic compounds on nematode survival were performed by incubating duplicate assays of 100 M. incognita J2 in 10 ml of the respective pH adjusted (pH 7.2, achieved using 1 M NaOH) solutions of SA, p-coumaric acid, and t-cinnamic acid in a 60-mm diameter petri dish. A separate assay was performed with pH unaltered SA solutions, which maintained the acidic nature of the compounds in the solution. Nematodes were soaked for 24 hr in each of the phenolic solutions before being transferred to a petri dish containing sterilized water (pH 7.2) for 24 hr. After the recovery period, nematodes were counted under a stereoscopic microscope and the percentage of J2 exhibiting no motility was recorded; nematodes were deemed immobile if they exhibited nematistatic phenotypes, including the “poker straight” (Dalzell et al., 2009) or “walking stick” (Soumi et al., 2012) phenotypes. All sample groups presented were derived from two sets of independently performed experiments, where nematode J2 were collected from separate tomato root stocks in each case.
Chemotaxis bioassays:
Our chemotaxis bioassay design was selected based on the need to allow J2 to easily chemo-orientate toward a phytochemical. Previous in vitro designs made use of preset water agar allowing J2 chemotaxis motion on a single plane, whereas others used Pluronic F-127 gel allowing three-dimensional motion; however, J2 are exposed to cold shock while preparing the bioassay. Simply, our bioassay offered a two-choice preference, where J2 were placed centrally on a petri dish and offered equidistant positive or negative agar plugs (50 mm diameter, 50 mm height [Alpha Laboratories, Eastleigh, UK]) on either side of the center position. Plugs were added immediately before the addition of J2. Bioassays were prepared using a 60-mm diameter petri dish (Fisher Scientific, UK) filled with 15 ml of 0.25% w/v agar. The substrate for the upper layer was made from 0.25% w/v agar (pH 7.2); the agar was allowed to set at room temperature and then agitated on a magnetic stirrer for several hr until the emulsified agar gained a smooth consistency. Three milliliters of the homogenized agar was evenly spread over the preset agar base layer. The phytochemicals under investigation were mixed into 0.25% agar. Once set, agar plugs were produced using a pastette (with the tapered tip cut off) to cut a cylindrical plug from the agar. A template was used to identify the position to pipette approximately 100 J2 in 10 µl H2O to the center of the dish and mark the placements of the positive and negative chemical agar plugs equidistant (25 mm) from the dish center. Lines transecting the dish 0.5 mm either side of the center were used to help monitor nematode movement. Bioassays were incubated at 16 and 24°C for G. pallida and M. incognita, respectively, and the bioassay stopped for analysis 16 hr after the addition of J2. All sample groups presented were derived from a minimum of six assays from two sets of independently performed experiments (a minimum of three assays per experiment), where nematode J2 were collected from separate tomato root stocks in each case.
Determination of J2 chemotaxis:
Measuring the attraction or repulsion of J2 in response to the chemical plugs was based on recording total numbers of J2 which had moved beyond the 0.5-mm marked lines. Assay replicates were only included where a minimum of 10 J2 had moved out of the neutral zone. A chemotaxis index was produced following the formula:
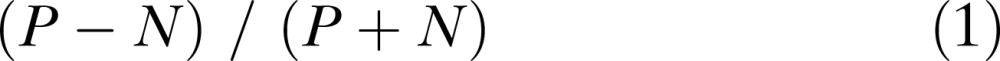 |
where P is the number of J2 in the positive zone and N is the number of J2 in the negative zone. Statistical and graphical displays were generated using GraphPad Prism version 6.0d. Data were initially checked for normality using Kolmogorov–Smirnov normality test and comparisons tested using one-way analysis of variance with Fisher’s LSD tests. Statistical comparisons were made between all treatment groups or compared individually to negative controls, as stated in the figure legends.
Preparation of phytochemicals:
Phytochemicals were either obtained from Sigma-Aldrich (UK) or kindly supplied by Dr. Chris Selby (Agri-Food and Biosciences Institute). Our rationale for selecting concentrations was based on previous studies (Wuyts et al., 2006; Nahar et al., 2011; Fleming, 2015); chemicals were dissolved in sterile ethanol (0.025% v/v, final conc. in plugs), the final concentrations in the 0.25% agar plugs were as follows: SA, 100 µM; Eth, 50 µM; methyl jasmonate (MeJA), 100 µM; vanillic acid, 240 µM; trans-cinnamic acid, 270 µM; p-coumaric acid, 240 µM; 6-benzylaminopurine hydrochloride, 150 µM; gentisic acid, 230 µM; gibberellic acid (GA3), 115 µM; indole-3-acetic acid (IAA), 230 µM; kinetin, 185 µM; indole-3-butyric acid, 200 µM; naphthaleneacetic acid, 215 µM; quinic acid, 200 µM; 6-dimethylallylamino purine (2iP), 200 µM; caffeic acid, 220 µM; mannitol, 5 mM; arginine, 5 mM; glutamine, 5 mM; glutamic acid, 5 mM; alanine, 5 mM; and lysine, 5 mM. The pH readings for the test chemicals used to soak the agar plugs remained close to neutral, with the lowest recorded pH 6.22 for SA and the highest reading for mannitol of pH 8.05. We, therefore, left pH of all chemicals unadjusted. PRD was produced as previously described, and plugs were prepared by adding 1 part PRD to 1 part 0.5% agar. Additional bioassays for Eth plugs at concentrations of 100, 50, 10, 1, 0.1, and 0.01 µM were prepared in the same manner described earlier.
Preexposure and recovery soaks for M. incognita J2:
The initial assay procedure for M. incognita Eth preexposure bioassays involved adding approximately 1,500 J2 to 200 µl Eth solutions of 50, 10, and 1 µM in 1.5-ml round-bottom Eppendorf tubes (Fisher Scientific, UK) for 24 hr before transferring the J2 to the bioassays. Subsequent preexposure soaks involved approximately 100 M. incognita J2 which were incubated for a set time period (1, 6 or 24 hr) in 1.5 ml Eppendorf tubes containing 200 µl of the phytochemical solutions, Eth (50 µl), SA (100 µl), and PRD (50% of stock). Recovery soaks were prepared by transferring the preexposed J2 into Eppendorf tubes containing 200 µl of sterile water (pH 7.2). Exogenous serotonin was applied to corresponding preexposure soaks at a 100 mM concentration. All treatment groups presented were derived from a minimum of six assays from two sets of independently performed experiments (a minimum of three assays per experiment), where nematode J2 were collected from separate tomato root stocks in each case.
Serotonin (5-HT) preexposure and recovery soaks for M. incognita J2:
Eth bioassays involved adding approximately 1,500 J2 to 200 µl separate solutions of Eth (50 µM), 5-HT (100 mM) or a mixture of Eth (50 µM), and 5-HT (100 mM) in 1.5 ml round-bottom Eppendorf tubes (Fisher Scientific, UK) for 24 hr before transferring 100 J2 to the bioassays. J2 were tested for their chemotactic response to Eth (50 µM) immediately after preexposure in Eth (50 µM) and/or 5-HT (100 mM) for 24 hr. Recovery soaks tested J2 chemotactic response to Eth (50 µM) after a preexposure in Eth (50 µM) for 24 hr and a recovery in water for 1, 6, or 24 hr. SA bioassays involved adding approximately 1,500 J2 to 200 µl separate solutions of SA (100 µM), 5-HT (100 mM), or a mixture of SA (100 µM) and 5-HT (100 mM) in 1.5 ml round-bottom Eppendorf tubes (Fisher Scientific, UK) for 24 hr before transferring 100 J2 to the bioassays and immediately testing the J2 chemotactic response to SA (100 µM). All treatment groups presented were derived from a minimum of four assays from two sets of independently performed experiments (a minimum of two assays per experiment), where nematode J2 were collected from separate tomato root stocks in each case.
Gene ortholog determination:
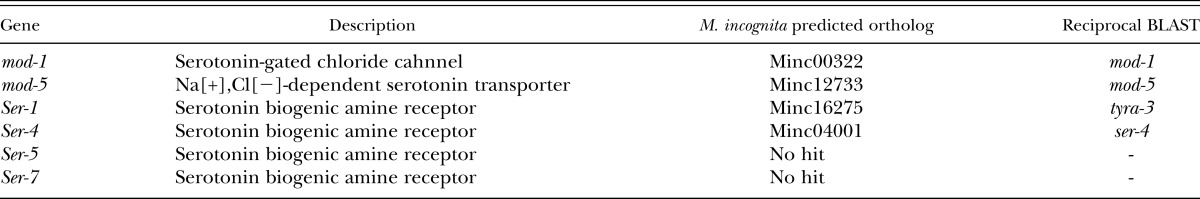
Meloidogyne incognita predicted orthologs were identified by comparing to Caenorhabditis elegans serotonin genes via BLAST analysis (BLASTP and tBLASTn). The WormBase ParaSite BLAST tool criteria followed default settings. Gene orthologs were determined using the highest scoring BLAST hit, except values ≤1,000 (or lowest single hit) and highest bit score rating. A reciprocal BLAST of the top hits confirmed C. elegans homology.
Results
Chemotactic response of M. incognita J2 and G. pallida J2 to phytochemicals:
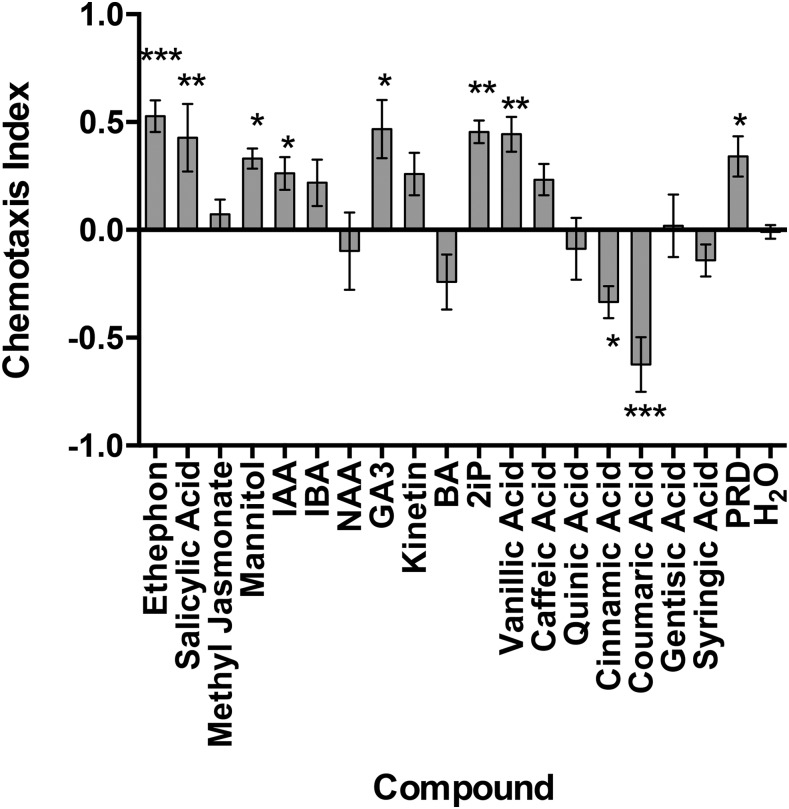
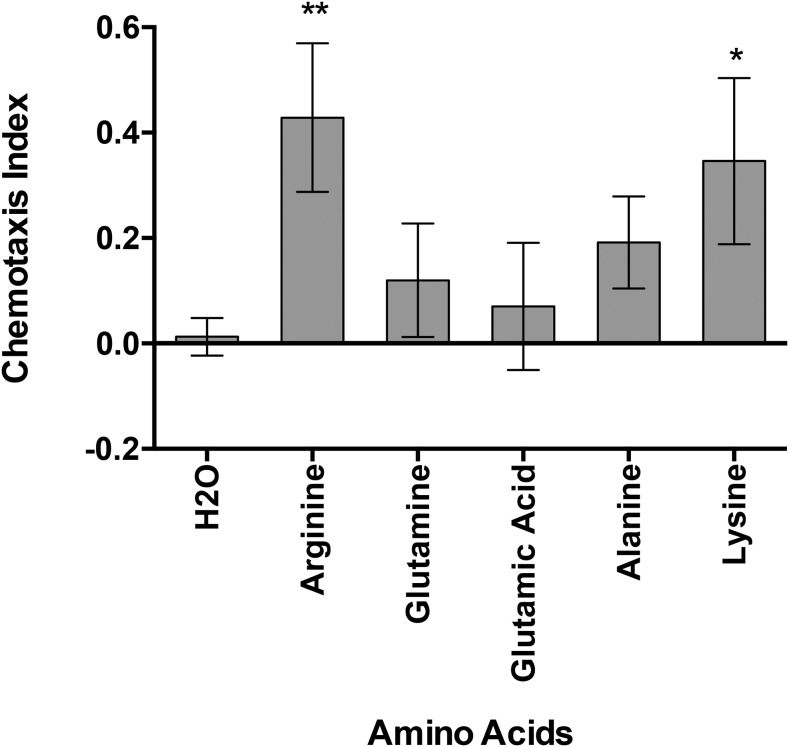
The chemotactic response of M. incognita and G. pallida J2 exposed to a selection of phytochemicals was gauged by either an attraction or repellent phenotype relative to negative controls. Notably Eth, SA, mannitol, IAA, GA3, 2iP, vanillic acid, and PRD were all significant attractants for M. incognita J2 (Fig. 1). In addition, several amino acids were screened, with arginine and lysine identified as attractants for M. incognita J2 (Fig. 2). By contrast, cinnamic and coumaric acid were repellents for M. incognita J2.
Fig. 1.
The chemotactic response of Meloidogyne incognita J2 to phytochemicals. Chemotaxis bioassays show attraction (positive index) or repulsion (negative index) values. Statistical analysis performed using one-way analysis of variance with Fisher’s LSD (P values: *P < 0.05; **P < 0.01; ***P < 0.001) tests comparing with a H2O negative control; error bars represent SE. 2iP = 6-dimethylallylamino purine, BA = 6-benzylaminopurine hydrochloride, GA3 = gibberellic acid, IAA = indole-3-acetic acid, IBA = indole-3-butyric acid, NAA = naphthaleneacetic acid, PRD = potato root diffusate.
Fig. 2.
The chemotactic response of Meloidogyne incognita J2 to amino acids. Chemotaxis bioassays show attraction (positive index) or repulsion (negative index) values. Statistical analysis performed using one-way analysis of variance with Fisher’s LSD (P values: *P < 0.05; **P < 0.01) tests comparing with a H2O negative control; error bars represent SE.
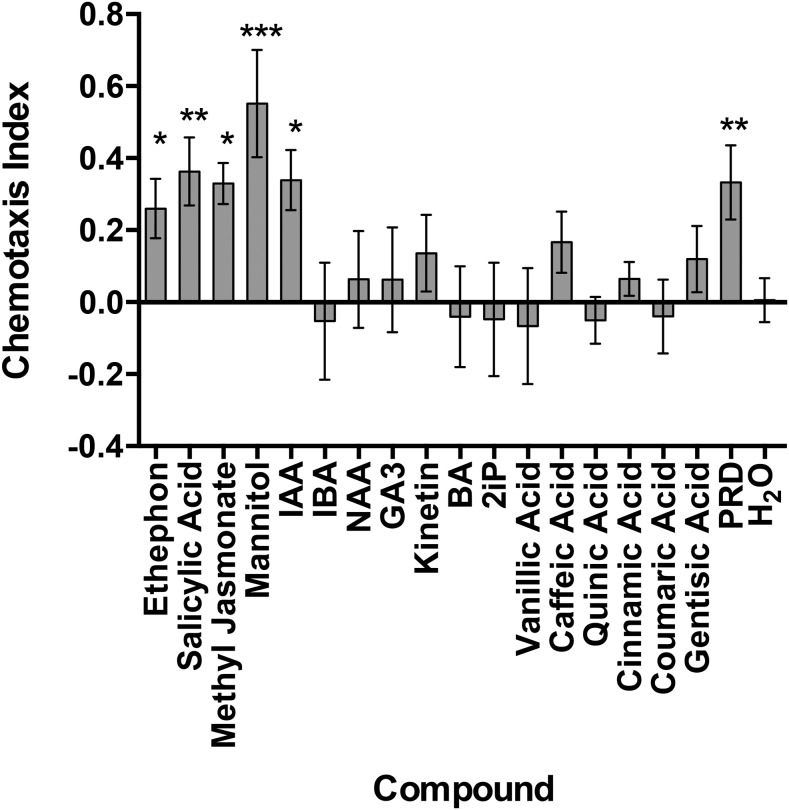
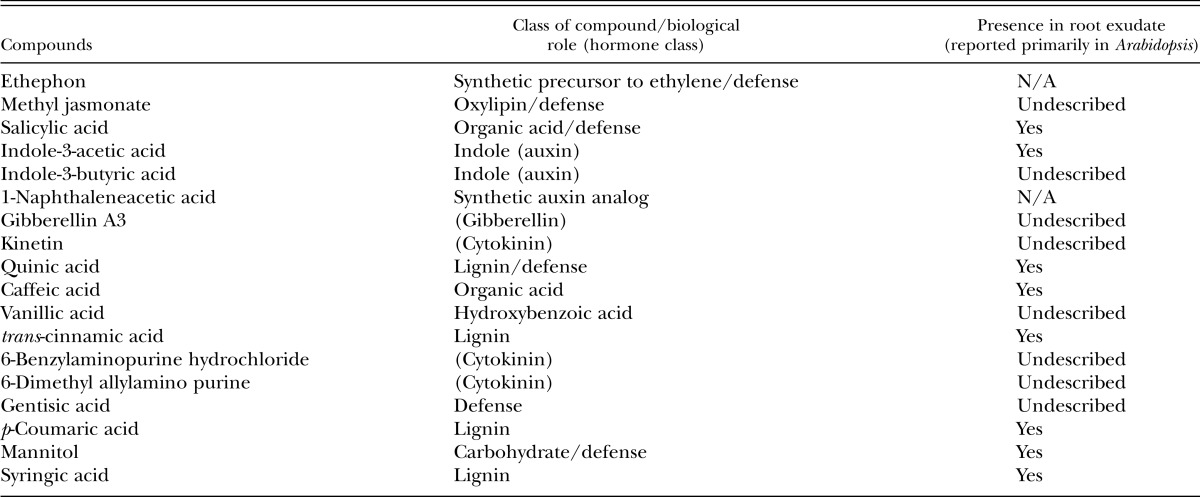
Globodera pallida chemotaxis differed from M. incognita, with fewer compounds eliciting a chemotactic response. No chemicals produced a repellent phenotype; however, Eth, SA, mannitol, IAA, and PRD were identified as attractants. Notably, MeJA was also identified as an attractant for G. pallida J2, but induced no response from M. incognita J2 (Fig. 3). The study by Badri and Vivanco (2009) was reviewed to confirm which chemicals used in the bioassays were present in Arabidopsis thaliana root exudate (Table 1).
Fig. 3.
The chemotactic response of Globodera pallida J2 to phytochemicals. Chemotaxis bioassays show attraction (positive index) or repulsion (negative index) values. Statistical analysis performed using one-way analysis of variance with Fisher’s LSD (P values: *P < 0.05; **P < 0.01; ***P < 0.001) tests comparing with a H2O negative control; error bars represent SE. 2iP = 6-dimethylallylamino purine, BA = 6-benzylaminopurine hydrochloride, GA3 = gibberellic acid, IAA = indole-3-acetic acid, IBA = indole-3-butyric acid, NAA = naphthaleneacetic acid, PRD = potato root diffusate.
Table 1.
Phytochemicals tested for their impact on nematode chemotactic response and known presence in Arabidopsis root exudate (Badri and Vivanco, 2009).
Nematistatic properties of the phenolic compounds salicylic acid, p-coumaric acid, and t-cinnamic acid:
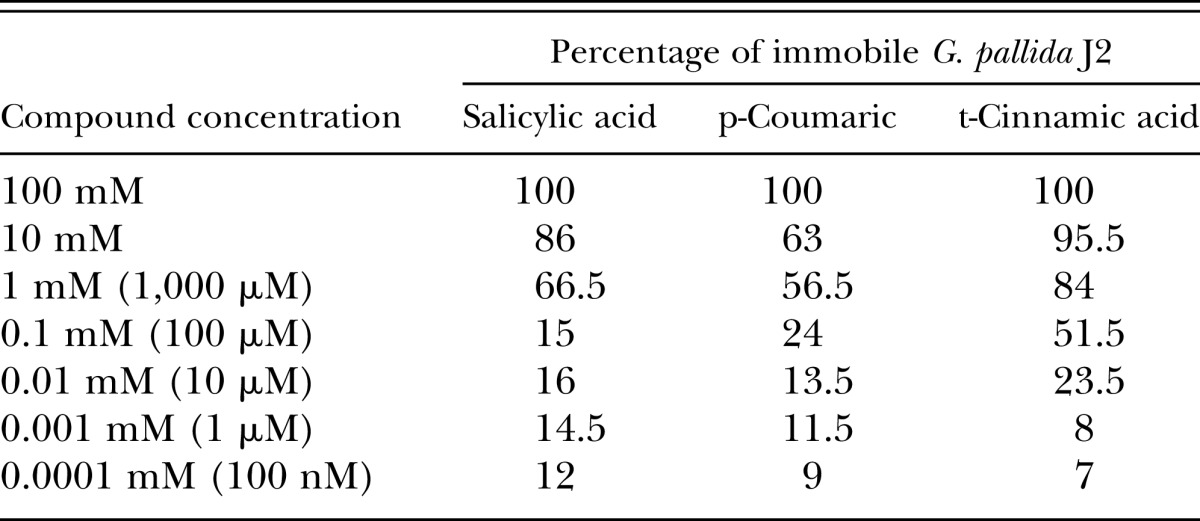
After identifying the phenolics coumaric and cinnamic acids as repellents of M. incognita J2, we wanted to ascertain the concentrations required to kill the J2. Solutions of coumaric acid, cinnamic acid, and SA (the other phenolic compound screened) were assessed for motility/mortality effects on M. incognita J2 (Table 2) and G. pallida J2 (Table 3). The assay conditions involved a 24-hr incubation of M. incognita and G. pallida J2 in chemical solutions (pH 7.2), followed by a 24-hr recovery incubation in water. After incubating nematodes in a dilution series of each chemical and a subsequent recovery incubation in water, a motility inhibition of >50% was achieved at micromolar concentrations in all chemicals. Nematodes were deemed motile if any degree of muscular motion was observed. No quantitative measurement of nematode motility relative to control-treated nematodes was performed; however, based on visual observations, there were substantial reductions in J2 activity across all treatment concentrations. The high acidity of SA solutions was a factor considered; therefore, an unadjusted 1-mM solution of SA was tested, which resulted in 96.7% of immobile M. incognita J2, a marked increase from the 73.2% in the neutralized solution.
Table 2.
Percentages of nematistatic Meloidogyne incognita J2 after incubation in phenolic compounds. Nematode J2 were incubated for 24 hr in serial dilutions of salicylic acid, coumaric acid, and cinnamic acid (adjusted pH 7.2), and then allowed a recovery in water for 24 hr.
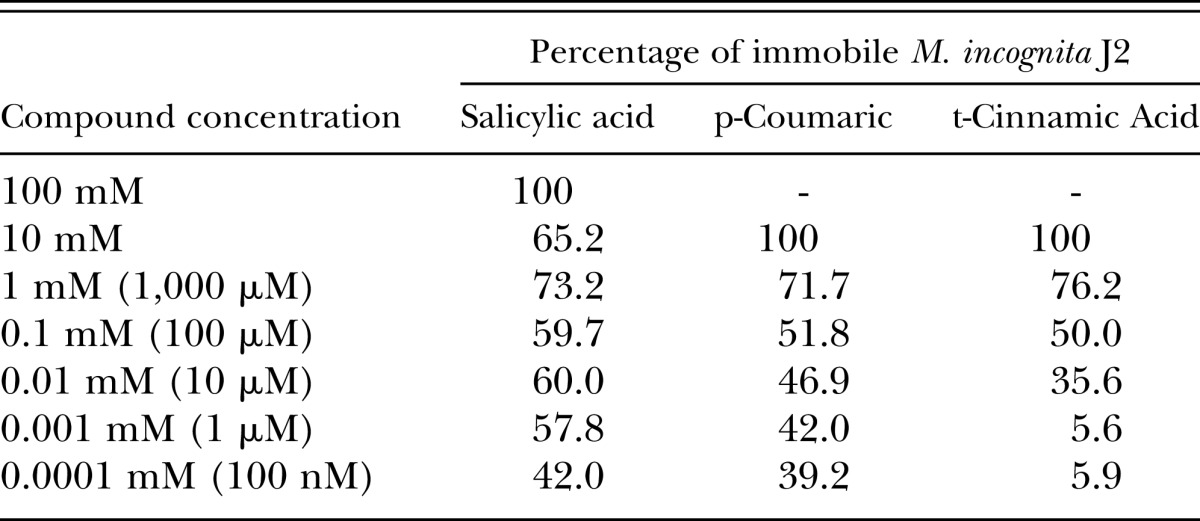
Table 3.
Percentages of nematistatic Globodera pallida J2 after incubation in phenolic compounds. Nematode J2 were incubated for 24 hr in serial dilutions of salicylic acid, coumaric acid, and cinnamic acid (adjusted pH 7.2), and then allowed a recovery in water for 24 hr.
Differential behavioral response of Meloidogyne incognita J2 to chemical preexposure:
Previous studies have illustrated the high degree of chemo-sensitivity that exists in PPN. We, therefore, tested whether naive M. incognita J2 undergo an adaptation state when preexposed to phytochemicals.
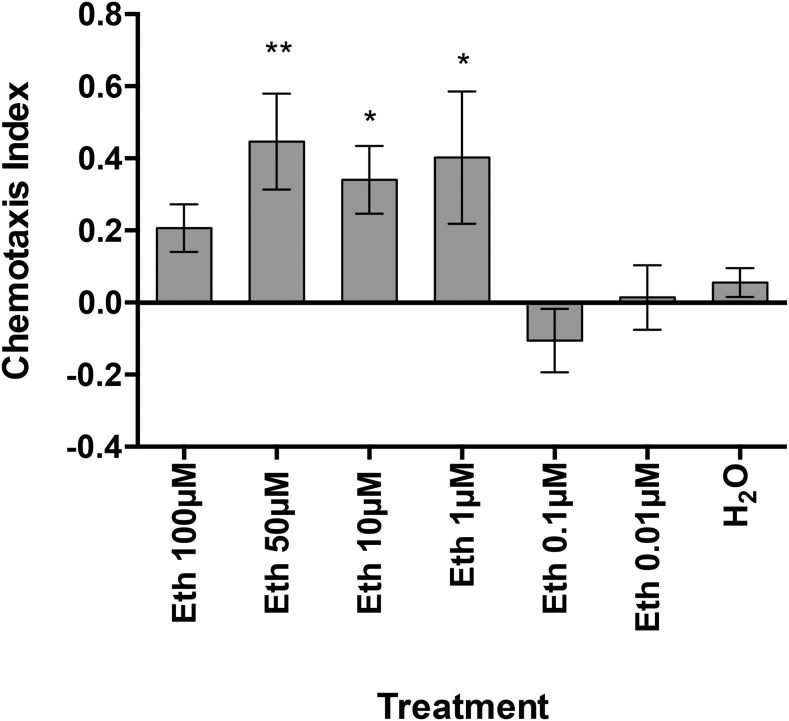
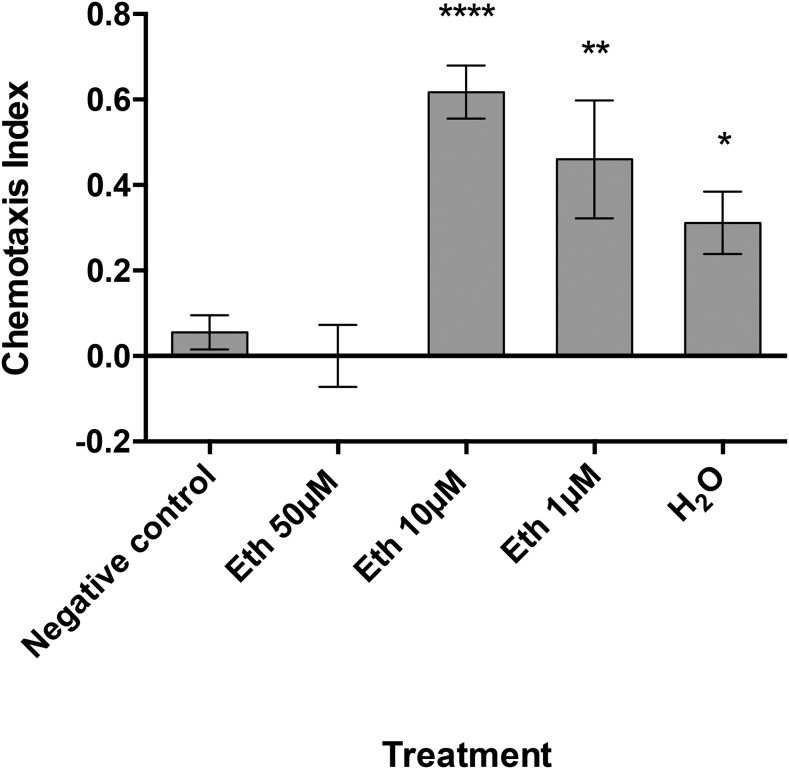
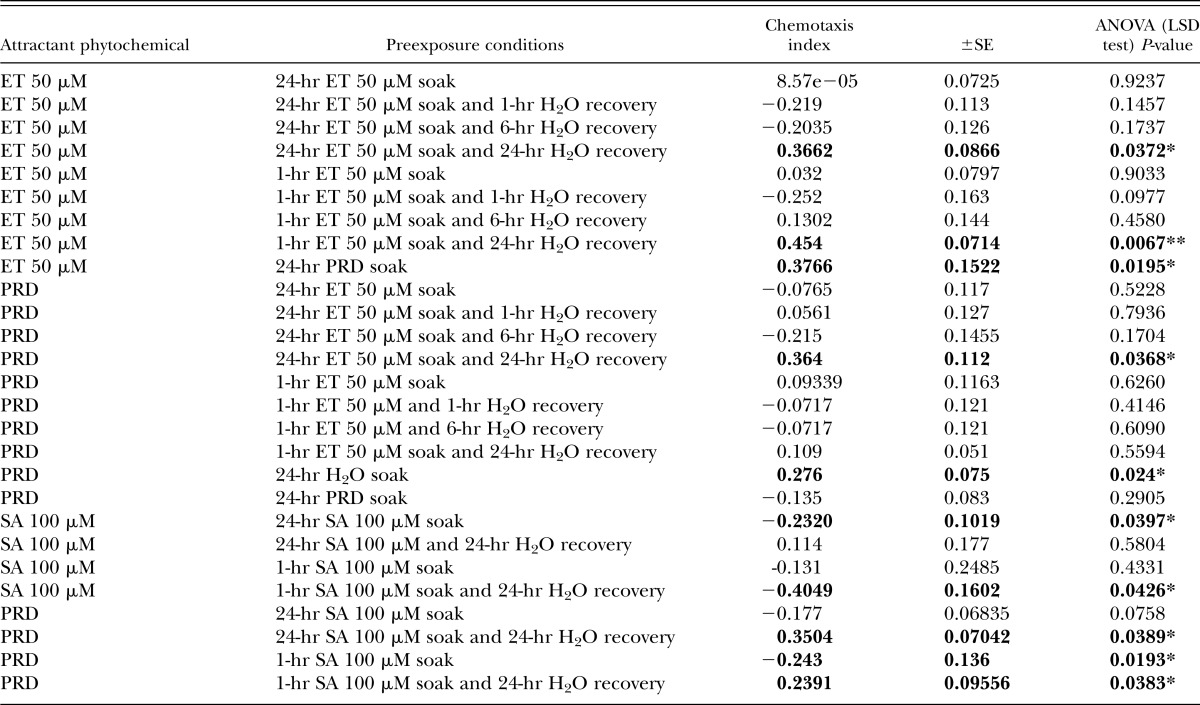
Eth was demonstrated as an M. incognita J2 attractant in preliminary chemotaxis assays (Figs. 1,2) because of the fact Eth readily dissociates in water to form ethylene, a key plant hormone. A serial dilution of Eth was tested in chemotaxis bioassays, with 50 µM identified as the optimal concentration for attraction (Fig. 4). Moreover, preexposing M. incognita J2 to 50 µM Eth for 24 hr before testing in the chemotaxis bioassay resulted in the loss of the original attraction phenotype (Fig. 5). However, preexposing M. incognita to lower Eth concentrations (1 and 10 µM) did not impair the attraction phenotype, and the 10 µM concentration appears to have enhanced the phenotype. Further combinations of chemical and recovery soaks for Eth, SA, and PRD were assessed (Table 4).
Fig. 4.
The chemotactic response of Meloidogyne incognita J2 to different ethephon (Eth) concentrations. Chemotaxis bioassays show attraction (positive index) or repulsion (negative index) values. Statistical analysis performed using one-way analysis of variance with Fisher’s LSD (P values: *P < 0.05; **P < 0.01) tests comparing with a H2O negative control; error bars represent SE.
Fig. 5.
The chemotactic response of Meloidogyne incognita J2 preexposed to different ethephon (Eth) concentrations. J2 were tested for their chemotactic response to Eth (50 µM) immediately after preexposure in Eth (50, 10, 1 µM or H2O) for 24 hr. Chemotaxis bioassays show attraction (positive index) or repulsion (negative index) values. Statistical analysis performed using one-way analysis of variance with Fisher’s LSD (P values: *P < 0.05; **P < 0.01; ***P < 0.001) tests comparing with a H2O negative control; error bars represent SE.
Table 4.
The chemotactic response of Meloidogyne incognita J2 preexposed to different combinations of phytochemicals ethephon (Eth), salicylic acid (SA), and PRD. J2 were tested for their chemotactic response to Eth (50 µM), SA (100 µM), or PRD immediately after preexposure in respective phytochemicals. Recovery soaks tested J2 chemotactic response to respective phytochemicals after a preexposure for 24 hr and a recovery in water for 1, 6, or 24 hr. The chemotaxis indices reported show attraction (positive index) or repulsion (negative index) responses of J2. Statistical analysis performed using one-way analysis of variance (ANOVA) with Fisher’s LSD. Individual P values are stated with significant values in bold (*P < 0.05; **P < 0.01) and SE.
The preexposure of M. incognita J2 to Eth for only 1 hr resulted in the loss of attraction to Eth. Subsequently, the attraction phenotype was only restored after a 24-hr recovery soak in water. Notably, preexposure of M. incognita to PRD resulted in a loss of attraction to PRD, similar to the Eth phenotype; however, the PRD preexposure had no effect on M. incognita attraction in the Eth bioassay.
The effects of preexposing M. incognita to SA were much more pronounced than either Eth or PRD. Not only was the SA attraction phenotype lost, but the preexposed M. incognita exhibited a repellent phenotype. Remarkably, the repellent phenotype was maintained in M. incognita J2 exposed to SA for 1 hr and a 24-hr recovery soak. In the PRD bioassay, the M. incognita J2 preexposed to SA for 1 hr exhibited the repellent phenotype (the 24-hr SA soaked J2 were also repelled by subsequent exposure, but the response was not statistically different to the 1-hr soaked J2); however, the attraction phenotype was restored following a 24-hr recovery soak.
Effect of exogenous serotonin on the behavioral response of M. incognita to chemical preexposure:
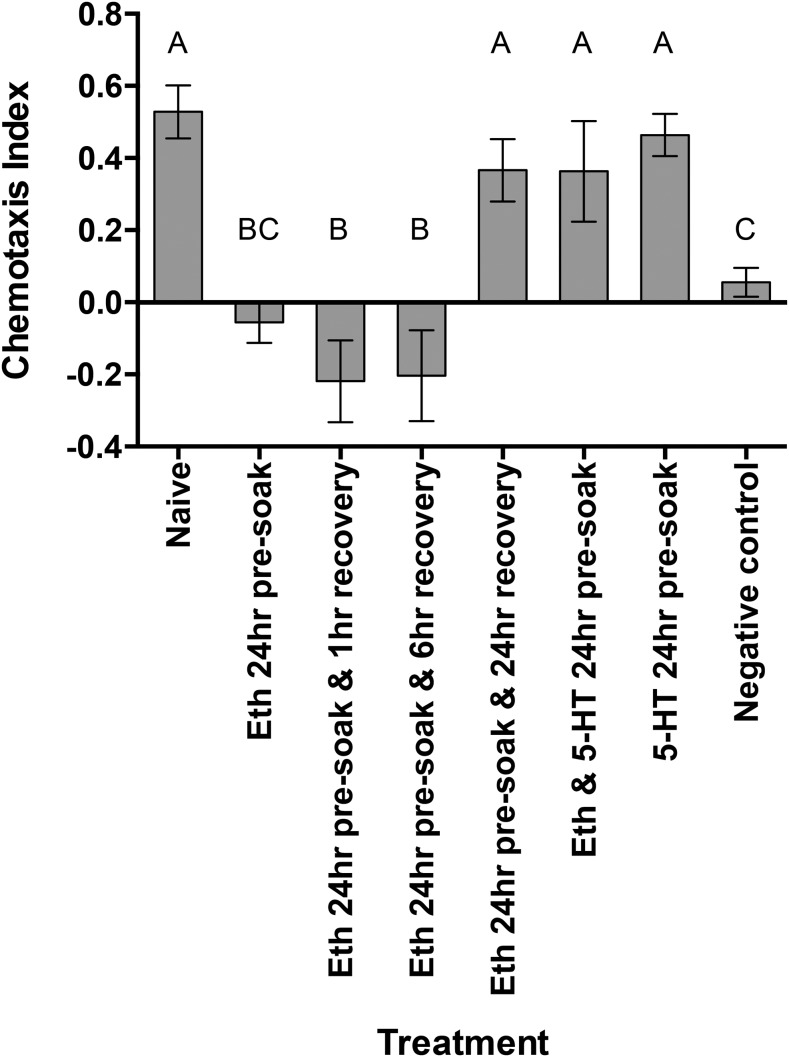
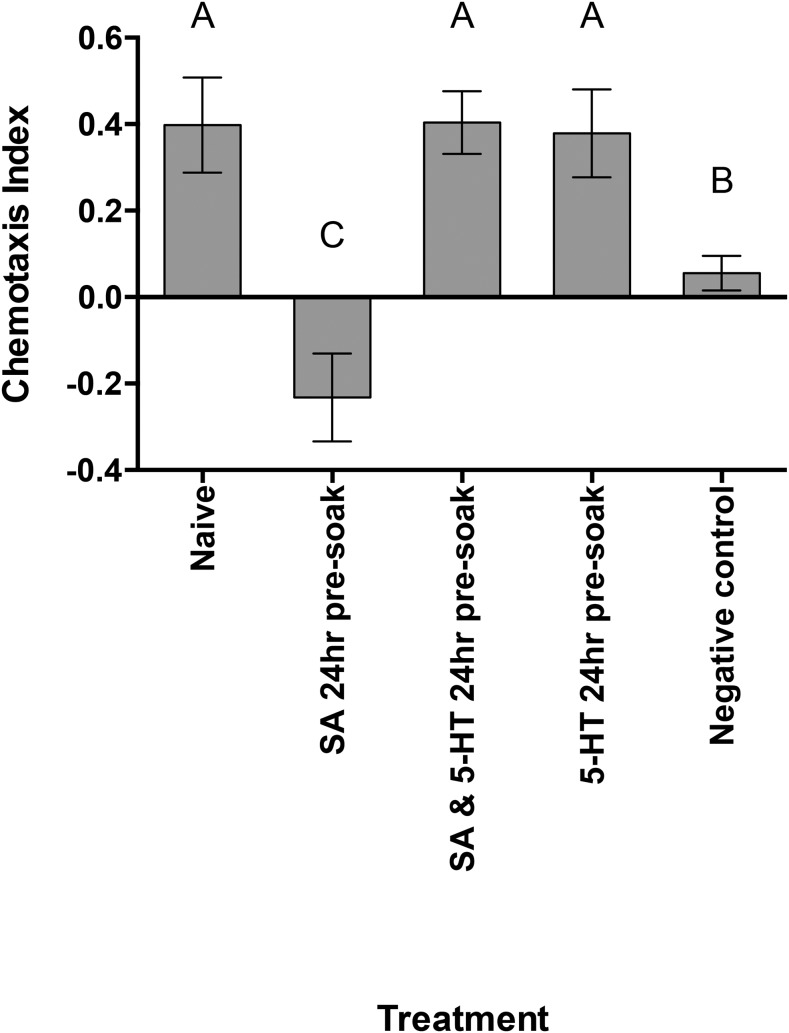
Genetic studies in the model nematode C. elegans have demonstrated the involvement of serotonergic signaling in olfactory learning and memory behavior. Thus, to further decipher the adapted behavioral responses observed in M. incognita J2 to phytochemicals, exogenous 5-HT that is used to inhibit associative learning in C. elegans (Nuttley et al., 2002) was added to the phytochemical preexposure soaks. The Eth 24-hr preexposure bioassay was repeated with the addition of a 5-HT (100 mM) and Eth (50 µM) 24-hr preexposure treatment. This treatment had the effect of restoring the original attraction phenotype observed in naive or Eth recovered M. incognita J2 (Fig. 6). Similarly, exogenous 5-HT (100 mM) successfully restored the attraction phenotype in M. incognita J2 preexposed to SA (Fig. 7).
Fig. 6.
The chemotactic response of Meloidogyne incognita J2 preexposed to ethephon (Eth), serotonin (5-HT) and recovery soaks. J2 were tested for their chemotactic response to Eth (50 µM) immediately after preexposure in Eth (50 µM) and/or 5-HT (100 mM) for 24 hr. Recovery soaks tested J2 chemotactic response to Eth (50 µM) after a preexposure in Eth (50 µM) for 24 hr and a recovery in water for 1, 6, or 24. Chemotaxis bioassays show attraction (positive index) or repulsion (negative index) values. Statistical analysis performed using one-way analysis of variance with Fisher’s LSD. Different letters indicate significance (P < 0.05); error bars represent SE.
Fig. 7.
The chemotactic response of Meloidogyne incognita J2 preexposed to salicylic acid (SA), serotonin (5-HT), and recovery soaks. J2 were tested for their chemotactic response to SA (100 µM) immediately after preexposure in SA (100 µM) and/or 5-HT (100 mM) for 24 hr. Recovery soaks tested J2 chemotactic response to SA (100 µM) after a preexposure in SA (100 µM) for 24 hr. Chemotaxis bioassays show attraction (positive index) or repulsion (negative index) values. Statistical analysis performed using one-way analysis of variance with Fisher’s LSD. Different letters indicate significance (P < 0.05); error bars represent SE.
Discussion
In the present study, a range of phytochemicals were identified as chemotactic elicitors in M. incognita and G. pallida J2. Second, M. incognita J2 exhibited adapted behavioral responses after preexposure to phytochemicals, which were analogous to the long-term habituation behavior observed previously in C. elegans (Rose and Rankin, 2001). Last, the involvement of serotonergic signaling in the apparent learning phenotype was implicated after observing the modified response after the addition of exogenous serotonin in preexposure soaks.
The overall trend from the chemotaxis bioassays was a greater responsiveness from M. incognita J2 to a broader selection of the phytochemicals in comparison with G. pallida J2. An obvious explanation for this difference is the direct influence in the host specificities of the two species, where M. incognita is a promiscuous generalist parasite of flowering plants and G. pallida is a specialized parasite of Solanaceae plants. Notably, many of the phytochemicals (e.g. ethylene, IAA, mannitol, and SA) and amino acids are increasingly used in commercial biostimulants, naturally occurring seaweed extracts (Calvo et al., 2014; Sharma et al., 2014), and plant systemic resistance research (e.g. Eth and MeJA) (Nahar et al., 2011; Wasternack, 2014). Hence, there is a need to investigate how these chemicals will impact the host–parasite relationship.
Eth elicited the strongest attraction response in M. incognita J2, while also acting as an attractant for G. pallida J2. Importantly, Eth is a plant-growth regulator widely used within the agri-sector for fruit ripening and prevention of cereal lodging. Eth dissociates readily above pH 4 or in solution to form ethylene, a key phytohormone involved in plant development and defense (Yang, 1969; Goudey et al., 1987). In this case, ethylene is most likely the compound detected by the PPN, previously Pseudomonas spp. demonstrated chemotactic affinity to ethylene (Kim et al., 2007), whereas the beet cyst nematode Heterodera schachtii had an increased attraction toward ethylene overexpressing plants (Wubben et al., 2001). Conversely, Meloidogyne hapla had decreased attraction to ethylene overexpressing plants and increased attraction to underexpressing plants, providing further evidence for many differences in chemotaxis between nematode species.
SA is probably the most studied phytohormone for inducing plant defenses. SA is also used in several commercial plant-protection products (biostimulants) and is present in root exudates (Badri and Vivanco, 2009); hence, the chemotactic response from both M. incognita and G. pallida was not unexpected. SA was previously shown to attract M. incognita J2 (Wuyts et al., 2008); however, in our bioassay design, nematode perception to the 100 µM concentration in an agar plug was considerably lower, further demonstrating the high sensitivity of nematode chemoperception. Moreover, SA has nematicidal properties. Thus, we performed incubation soaks on M. incognita J2 using pH adjusted SA solutions. These soaks showed substantial numbers of J2 were immobilized by relatively low concentrations of SA. Previous work has demonstrated the benefits of SA application for reducing nematode infection, although the focus has been on the induction of a systemic acquired resistance response in the plant and not on the direct impact of the SA on nematodes (Nandi et al., 2003; Wubben et al., 2008; Nahar et al., 2011).
Other attractants including IAA, mannitol, and vanillic acid are confirmed constituents of root exudates (Badri and Vivanco, 2009), whereas GA3 and 2iP are known phytohormones. A role for IAA has been previously implicated in M. incognita and G. pallida behavior whereby IAA binds the nematode’s neuronal organs and induces stylet thrusting (Curtis et al., 2007). Notably, the only phytochemical that was an attractant for G. pallida J2 and not M. incognita J2 was MeJA, a volatile ester involved in mediating phytohormone signaling Wasternack and Hause, 2013). Phytochemicals that elicited strong repellent responses from M. incognita J2 were cinnamic and coumaric acids, both of which have been previously reported as nematicidal (Wuyts et al., 2006). Likewise, we corroborated the nematistatic properties of these compounds in the 24-hr incubation bioassays.
Nematodes display complex behaviors in response to diverse environmental cues, in particular toward food sources. Here, we reported adapted chemotactic phenotypes in M. incognita J2 in response to Eth, SA, and PRD. Firstly, adaptation of nematodes preexposed to Eth resulted in a loss of the attraction phenotype seen in naive nematodes. Although the adaptation to Eth was observed after a 1-hr exposure, the adaptation in nematodes could be reversed following a 24-hr recovery in water. Interestingly, this adaptation state displays similarities to the serotonergic-dependent learning behaviors in C. elegans. Here, the naive nematodes displayed attraction to the odorant benzaldehyde, but following preexposure to the compound attraction was reduced (Nuttley et al., 2002). To confirm that the adaptation observed in M. incognita J2 was not specific to Eth exposure, we subsequently assessed PRD and SA. Eth adapted nematodes again lost their attraction phenotype on PRD bioassays and nematodes adapted in PRD lost their attraction on PRD bioassays. Similarly, PRD preexposure resulted in the adaptive phenotype; however, normal attraction was observed for PRD preexposed J2 tested in Eth bioassays. This indicated the adaptation was more likely a selective response to the PRD and not general inhibition of J2 chemosensory organs.
The adaptation of M. incognita J2 with SA displayed another interesting phenotype. We recorded a repellent chemotactic response for adapted nematodes, not only a loss of the naive response, but a complete reversal. Of interest was the observation that the 1-hr preexposure also induced a repellent phenotype and a 24-hr recovery failed to restore the attraction to SA displayed by unexposed/naive J2. Although this strong aversion could be due to the nematicidal properties of SA, the behavior shows parallels to the pathogen avoidance learning in C. elegans, again functioning through serotonergic signaling (Zhang et al., 2005).
The collective evidence from the adaptation experiments were strongly suggestive that M. incognita J2 adaptation, particularly the extended period of time that nematodes retained memory of the conditioning, was acting like a long-term habituation response, analogous to that reported in C. elegans (Rose and Rankin, 2001). Moreover, the distinctive loss of attraction, in the case for Eth, showed similarities to context conditioning, where the nematodes were conditioned to associate the chemical stimulus to predict biologically significant events, in this case an absence of a host plant. These mechanisms have been shown to rely on functioning serotonergic signaling, demonstrated by Nuttley et al. (2002) by the aid of serotonin deficient animals or exogenous serotonin application during conditioning. We, therefore, wanted to confirm a role for serotonergic signaling in the plant parasite, M. incognita. Unfortunately, there are no PPN mutants to perform genetic functional testing as those available in the model nematode, C. elegans. However, we could provide pharmacological evidence for the potential mechanism. The addition of serotonin to the preexposure soaks successfully inhibited the adaptation phenotype in both Eth and SA bioassays; thus, substantiating a potential role for serotonergic signaling in the M. incognita learning adaptation. In a biological context, the presence of long-term habituation and/or context conditioning behaviors in M. incognita J2 could be similar to its requirement in C. elegans as a mechanism to optimize successful food detection. Second-stage juveniles are the main free-living stage in the lifecycle of most sedentary PPN. Moreover, these J2 possess limited lipid reserves, which are needed to last until a host plant can be located and successfully infected. Accordingly, these nematodes need to efficiently manage the energy used for locating a host plant, hence efficient learning and memory offers a mechanism whereby the free-living stage parasites can respond to the plasticity of soil environmental conditions.
In the present study, PPN chemotaxis was concisely demonstrated under an in vitro bioassay condition. A range of phytochemicals were identified as attractants or repellents and distinct differences in compound attractiveness were observed for two prominent pest species, M. incognita and G. pallida, which is a reflection of their host specificities. With the recent availability of both these PPN genomes, future studies may be able to investigate genetic differences in chemosensory genes. For instance, we performed basic protein BLAST analyses of the main serotonin receptors, ser-1, ser-4, ser-7, and mod-1 identified previously in C. elegans (Li et al., 2013). The resulting hits indicated there are also multiple serotonin receptors present in M. incognita (Table 5). However, functional studies will be required to validate each gene and its potential role in habituation responses. Moreover, improved knowledge of these pests will be significant, given the expected increased pressure on the global agri-sector (Nicol et al., 2011) and the fact many of the new emerging plant protection and biostimulant products contain naturally occurring phytochemicals which have the potential to modify PPN behaviors (Khan et al., 2009; Sharma et al., 2014). Understanding how these phytochemicals influence PPN in the rhizosphere will enable growers to select the most effective treatments for specific host/parasite combinations. This knowledge also opens up the opportunity to breed crop varieties with modified levels of bioactive root chemicals. Active research into engineering crops that have altered root exudate profiles and in turn make plants more resistant to PPN infection is currently being performed by researchers at the Queen’s University Belfast, funded by the Bill & Melinda Gates Foundation (Anonymous, 2012; Warnock et al., 2016).
Table 5.
Caenorhabditis elegans serotonin genes and predicted Meloidogyne incognita gene orthologs. Meloidogyne incognita–predicted orthologs were identified via BLAST analysis (BLASTP and tBLASTn). The WormBase ParaSite BLAST tool criteria followed default settings. Gene orthologs were determined using the highest scoring BLAST hit, except values ≤1,000 (or lowest single hit) and highest bit score rating. A reciprocal BLAST of the top hits confirmed C. elegans homology.
We provide evidence for long-term habituation in the plant parasite M. incognita and demonstrate that nematodes can be adapted by preexposing to the chemicals Eth and SA, which in turn, alters their chemotactic response to the chemicals. We propose a role for serotonergic signaling in this learning behavior, after serotonin restored the naive chemotactic state in adapted nematodes. Finally, this newly reported behavioral response in a PPN will contribute to the primary aims of understanding the host–parasite interaction and aid in the identification of novel control and management strategies for these important global agri-pests.
Literature Cited
- Abad P, Gouzy J, Aury JM, Castagnone-Sereno P, Danchin EG, Deleury E, Perfus-Barbeoch L, Anthouard V, Artiguenave F, Blok VC, Caillaud MC, Coutinho PM, Dasilva C, De Luca F, Deau F, Esquibet M, Flutre T, Goldstone JV, Hamamouch N, Hewezi T, Jaillon O, Jubin C, Leonetti P, Magliano M, Maier TR, Markov GV, McVeigh P, Pesole G, Poulain J, Robinson-Rechavi M, Sallet E, Segurens B, Steinbach D, Tytgat T, Ugarte E, van Ghelder C, Veronico P, Baum TJ, Blaxter M, Bleve-Zacheo T, Davis EL, Ewbank JJ, Favery B, Grenier E, Henrissat B, Jones JT, Laudet V, Maule AG, Quesneville H, Rosso MN, Schiex T, Smant G, Weissenbach J, Wincker P. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nature Biotechnology. 2008;26:909–915. doi: 10.1038/nbt.1482. [DOI] [PubMed] [Google Scholar]
- Anonymous 2012. Neuropeptides as transgenic nematicides, protecting crop plants, global grand challenges. Available from: http://gcgh.grandchallenges.org/grant/neuropeptides-transgenic-nematicides.
- Badri DV, Vivanco JM. Regulation and function of root exudates. Plant Cell Environment. 2009;32:666–681. doi: 10.1111/j.1365-3040.2008.01926.x. [DOI] [PubMed] [Google Scholar]
- Badri DV, Loyola-Vargas VM, Broeckling CD, Vivanco JM. Root secretion of phytochemicals in Arabidopsis is predominantly not influenced by diurnal rhythms. Molecular Plant. 2010;3:491–498. doi: 10.1093/mp/ssq004. [DOI] [PubMed] [Google Scholar]
- Bargmann CI. 2006. Chemosensation in C. elegans, in WormBook: The online review of C. elegans biology. Available at: http://www.ncbi.nlm.nih.gov/books/NBK19746/.
- Barker KR, Koenning SR. Developing sustainable systems for nematode management. Annual Review of Phytopathology. 1998;36:165–205. doi: 10.1146/annurev.phyto.36.1.165. [DOI] [PubMed] [Google Scholar]
- Calvo P, Nelson L, Kloepper JW. Agricultural uses of plant biostimulants. Plant & Soil. 2014;383:3–41. [Google Scholar]
- Chitwood DJ. Phytochemical based strategies for nematode control. Annual Review of Phytopathology. 2002;40:221–249. doi: 10.1146/annurev.phyto.40.032602.130045. [DOI] [PubMed] [Google Scholar]
- Curtis RH. Plant parasitic nematode proteins and the host-parasite interaction. Genomics and Proteomics. 2007;6(1):50–58. doi: 10.1093/bfgp/elm006. [DOI] [PubMed] [Google Scholar]
- Curtis RHC. Plant-nematode interactions: Environmental signals detected by the nematode’s chemosensory organs control changes in the surface cuticle and behaviour. Parasite. 2008;15:310–316. doi: 10.1051/parasite/2008153310. [DOI] [PubMed] [Google Scholar]
- Dalzell JJ, McMaster S, Johnston MJ, Kerr R, Fleming CC, Maule AG. Non-nematode-derived double-stranded RNAs induce profound phenotypic changes in Meloidogyne incognita and Globodera pallida infective juveniles. International Journal for Parasitology. 2009;39:1503–1516. doi: 10.1016/j.ijpara.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Devine KJ, Jones PW. Investigations into the chemoattraction of the potato cyst nematodes Globodera rostochiensis and G. pallida towards fractionated potato root leachate. Nematology. 2003;5:65–75. [Google Scholar]
- Dong L, Xiaolin L, Huang L, Gao Y, Zhong L, Zheng Y, Zuo Y. Lauric acid in crown daisy root exudate potently regulates root-knot nematode chemotaxis and disrupts Mi-flp-18 expression to block infection. Journal of Experimental Botany. 2013;65:131–141. doi: 10.1093/jxb/ert356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming TR. 2015. Assessment and management of emerging nematode pests of northern Ireland grassland and cereals. Ph.D. thesis, Queen’s University Belfast, Belfast, UK.
- Goudey JS, Saini HS, Spencer MS. Uptake and fate of ethephon ([2-chloroethyl]phosphonic acid) in dormant weed seeds. Plant Physiology. 1987;85:155–157. doi: 10.1104/pp.85.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JT, Haegeman A, Danchin EGJ, Gaur HS, Helder J, Jones MGK, Kikuchi T, Manzanilla-Lopez R, Palomares-Rius JE, Wesemael WML, Perry RN. Top 10 plant-parasitic nematodes in molecular plant pathology. Molecular Plant Pathology. 2013;14:946–961. doi: 10.1111/mpp.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan W, Rayirath UP, Subramanian S, Jithesh MN, Rayorath PD, Hodges M, Critchley AT, Craigie JS, Norrie S, Prithiviraj B. Seaweed extracts as biostimulants of plant growth and development. Journal of Plant Growth Regulation. 2009;28:386–399. [Google Scholar]
- Kim H-E, Shitashiro M, Kuroda A, Takiguchi N, Kato J. Ethylene chemotaxis in Pseudomonas aeruginosa and other Pseudomonas species. Microbes and Environment. 2007;22:186–189. [Google Scholar]
- Li Y, Zhao Y, Huang X, Lin X, Guo Y, Wang D, Li C, Wang D. Serotonin control of thermotaxis memory behavior in nematode Caenorhabditis elegans. Plos One. 2013;8:e77779. doi: 10.1371/journal.pone.0077779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDiarmid TA, Ardiel EL, Rankin CH. The role of neuropeptides in learning and memory in Caenorhabditis elegans. Current Opinion in Behavioral Science. 2015;2:15–20. [Google Scholar]
- Nahar K, Kyndt T, De Vleesschauwer D, Höfte M, Gheysen G. The jasmonate pathway is a key player in systemically induced defense against root knot nematodes in rice. Plant Physiology. 2011;157:305–316. doi: 10.1104/pp.111.177576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi B, Sukul NC, Banewee N, Sengupta S, Das P, Babu SP. Induction of pathogenesis-related protein by salicylic acid and resistance to root-knot nematode in tomato. Indian Journal of Nematology. 2003;33:111–115. [Google Scholar]
- Nicol JM, Turner SJ, Coyne DL, Den Nijs L, Hockland S, Tahna Maafi Z. 2011. Current nematode threats to world agriculture, Ch. 2. Pp. 21–43 in J. Jones, G. Gheysen, and C. Fenoll, eds. Genomics and molecular genetics of plant-nematode interactions. Heidelberg, Germany: Springer.
- Nuttley WM, Atkinson-Leadbeater KP, van der Kooy D. Serotonin mediates food-odor associative learning in the nematode Caenorhabditis elegans. Proceedings of the National Academy of Sciences. 2002;99:12449–12454. doi: 10.1073/pnas.192101699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin CH, Beck CD, Chiba CM. Caenorhabditis elegans: A new model system for the study of learning and memory. Behavioral Brain Research. 1990;37:89–92. doi: 10.1016/0166-4328(90)90074-o. [DOI] [PubMed] [Google Scholar]
- Reynolds AM, Dutta TK, Curtis RHC, Powers SJ, Gaur HS, Kerry BR. Chemotaxis can take plant-parasitic nematodes to the source of a chemo-attractant via the shortest possible routes. Journal of the Royal Society Interface. 2011;8:568–577. doi: 10.1098/rsif.2010.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JK, Rankin CH. Analysis of habituation in Caenorhabditis elegans. Learning & Memory. 2001;8:63–69. doi: 10.1101/lm.37801. [DOI] [PubMed] [Google Scholar]
- Rosso MN, Dubrana MP, Cimbolini N, Jaubert S, Abad P. Application of RNA interference to root-knot nematode genes encoding esophageal gland proteins. Molecular Plant-Microbe Interactions. 2005;18:615–620. doi: 10.1094/MPMI-18-0615. [DOI] [PubMed] [Google Scholar]
- Sharma HSS, Fleming C, Selby C, Rao JR, Martin T. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. Journal of Applied Phycology. 2014;26:465–490. [Google Scholar]
- Soumi J, Gheysen G, Subramaniam K. RNA interference in Pratylenchus coffeae: Knock down of Pc-pat-10 and Pc-unc-87 impedes migration. Molecular and Biochemical Parasitology. 2012;186:51–59. doi: 10.1016/j.molbiopara.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Spence KO, Lewis EE, Perry RN. Host-finding and invasion by entomopathogenic and plant-parasitic nematodes: Evaluating the ability of laboratory bioassays to predict field results. Journal of Nematology. 2008;40:93–98. [PMC free article] [PubMed] [Google Scholar]
- Wang C, Bruening G, Williamson VA. Determination of preferred pH for root-knot nematode aggregation using pluronic F-127 gel. Journal of Chemical Ecology. 2009;35:1242–1251. doi: 10.1007/s10886-009-9703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Lower S, Thomas VP, Williamson VM. Root-knot nematodes exhibit strain-specific clumping behavior that is inherited as a simple genetic trait. Plos One. 2010;5:e15148. doi: 10.1371/journal.pone.0015148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnock ND, Wilson L, Canet-Perez JV, Fleming T, Fleming CC, Maule AG, Dalzell JJ. Exogenous RNA interference exposes contrasting roles for sugar exudation in host-finding by plant pathogens. International Journal for Parasitology. 2016;46:473–477. doi: 10.1016/j.ijpara.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Wasternack C. Action of jasmonates in plant stress responses and development—Applied aspects. Biotechnology Advances. 2014;32:31–39. doi: 10.1016/j.biotechadv.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Wasternack C, Hause B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Annals of Botany. 2013;111:1021–1058. doi: 10.1093/aob/mct067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wubben MJE, Jin J, Baum TJ. Cyst nematode parasitism of Arabidopsis thaliana is inhibited by salicylic acid (SA) and elicits uncoupled SA-independent pathogenesis-related gene expression in roots. Molecular Plant-Microbe Interactions. 2008;21:424–432. doi: 10.1094/MPMI-21-4-0424. [DOI] [PubMed] [Google Scholar]
- Wubben MJE, Su H, Rodermel SR, Baum TJ. Susceptibility to the sugar beet cyst nematode is modulated by ethylene signal transduction in Arabidopsis thaliana. Molecular Plant-Microbe Interactions. 2001;14:1206–1212. doi: 10.1094/MPMI.2001.14.10.1206. [DOI] [PubMed] [Google Scholar]
- Wuyts N, Swennen R, De Waele D. Effects of plant phenylpropanoid pathway products and selected terpenoids and alkaloids on the behaviour of the plant-parasitic nematodes Radopholus similis, Pratylenchus penetrans and Meloidogyne incognita. Nematology. 2006;8:89–101. [Google Scholar]
- Yang SF. Ethylene evolution from 2-chloroethylphosphonic acid. Plant Physiology. 1969;44:1203–1204. doi: 10.1104/pp.44.8.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lu H, Bargmann CI. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature. 2005;438:179–184. doi: 10.1038/nature04216. [DOI] [PubMed] [Google Scholar]