Abstract
Dietary specialization is an important driver of the morphology and performance of the feeding system in many organisms, yet the evolution of phenotypic specialization has only rarely been examined within a species complex. Horned lizards are considered primarily myrmecophagous (ant eating), but variation in diet among the 17 species of horned lizards (Phrynosoma) makes them an ideal group to examine the relationship between dietary specialization and the resultant morphological and functional changes of the feeding system. In this study, we perform a detailed analysis of the jaw adductor musculature and use a biomechanical model validated with in vivo bite force data to examine the evolution of bite force in Phrynosoma. Our model simulations demonstrate that bite force varies predictably with respect to the gape angle and bite position along the tooth row, with maximal bite forces being attained at lower gape angles and at the posterior tooth positions. Maximal bite forces vary considerably among horned lizards, with highly myrmecophagous species exhibiting very low bite forces. In contrast, members of the short‐horned lizard clade are able to bite considerably harder than even closely related dietary generalists. This group appears to be built for performing crushing bites and may represent a divergent morphology adapted for eating hard prey items. The evolutionary loss of processing morphology (teeth, jaw and muscle reduction) and bite force in ant specialists may be a response to the lack of prey processing rather than a functional adaptation per se.
Keywords: biomechanical model, bite force, diet, jaw muscle, lizard
Introduction
The role of dietary specialization in structuring the form and function of the feeding system has been well documented in numerous animals (Grant, 1986; Reiss, 2000; Aguirre et al. 2003; Wittorski et al. 2016). Traditionally, studies have sought to link presumed morphological adaptations with dietary specialization, but only rarely has the functional importance of specialized morphologies been investigated. More recently, studies have begun to address the functional link between morphology and ecology by measuring the performance of ecologically relevant traits (Wainwright & Reilly, 1994; Irschick & Garland, 2001). One such performance trait that has received increasing attention in a wide variety of species is bite force (Kiltie, 1982; Carraway et al. 1996; Hernandez & Motta, 1997; Aguirre et al. 2002; Herrel et al. 2002; Van der Meij & Bout, 2004; Anderson et al. 2008; Sagonas et al. 2014; Dutel et al. 2015; Lopez‐Darias et al. 2015; Santana, 2015; Thomas et al. 2015; Donihue et al. 2016; Wittorski et al. 2016; Dollion et al. 2017). Numerous studies have demonstrated that bite force can be an important determinant of dietary niche by constraining poor biters to a smaller proportion of the available resources (Hernandez & Motta, 1997; Verwaijen et al. 2002; Aguirre et al. 2003; Van der Meij et al. 2004; Kaliontzopoulou et al. 2012; Des Roches et al. 2015).
It has been suggested that dietary specialization in lizard species results in few morphological and functional adaptations (Greene 1982; Schwenk, 2000). However, detailed analyses of omnivorous (Herrel et al. 2004, 2008), durophagous (Dalrymple, 1979; Herrel & Holanova, 2008; Schaerlaeken et al. 2012) and myrmecophagous species (Montanucci, 1989; Meyers & Herrel, 2005; Meyers et al. 2006) have revealed both morphological and functional changes associated with dietary specialization. For instance, Herrel et al. (2004) identified changes in dentition and bite force in lacertid lizards in association with omnivory, and Dalrymple (1979) noted the extreme development of dentition and jaw muscles in Dracaena in response to eating hard prey such as snails. Additionally, studies of the myrmecophagous horned lizards indicate a relationship between ant eating and variation in the morphology of the jaw system (Montanucci, 1989; Meyers et al. 2006). These findings suggest that the influence of diet on morphology and performance in lizards may be more striking than previously thought (Sagonas et al. 2014; Lopez‐Darias et al. 2015; Donihue et al. 2016; Dollion et al. 2017).
Because North American horned lizards (Phrynosoma) are considered to be dietary specialists, they are an ideal group in which to examine how ecological specialization shapes the morphology and performance of the feeding system. Despite a similarity in general shape, species of Phrynosoma differ considerably in the percentage of ants in the diet, ranging from as low as 11% to as high as 89% (Pianka & Parker, 1975). The variation in diet would suggest that some species of horned lizards are in fact not dietary specialists and differ little from lizards considered dietary generalists. In both generalist and specialist species, diet influences prey‐processing behavior, those behaviors resulting in the alteration of the food item before swallowing (Schwenk, 2000; Meyers & Herrel, 2005). Because processing behavior is in part determined by bite force, it is likely that the degree of dietary specialization will be reflected not only in jaw morphology, dentition and associated jaw musculature, but also in bite force capacity. Although it is difficult to predict a priori potential adaptations of the feeding system in ant‐eating lizards, studies on mammalian ant eaters may provide insight into this issue.
Ant‐eating mammals typically exhibit long, slender mandibles with reduced dentition and poorly developed jaw adducting musculature (Reiss, 1997, 2000; Naples, 1999; Sacco & Van Valkenburgh, 2004). It has been suggested that the reduction in these morphological structures is a response to disuse as it is believed that unlike most mammals, many myrmecophagous mammals do not process prey (for a review, see Reiss, 2000). While lizards do not necessarily process prey in the typical mammalian sense (e.g. chewing), the jaws and teeth are still important in the apprehension, restraint and reduction of the food items eaten. If, as in myrmecophagous mammals, ant eating results in relaxation of selective pressures on processing morphology and performance in Phrynosoma, then the evolutionary loss of these traits may be more broadly indicative of a myrmecophagous diet.
In this study, we investigate the evolutionary consequences of dietary specialization in myrmecophagous lizards through a detailed examination of the form and function of their feeding system. We compare the variation in jaw morphology and musculature among 12 species of Phrynosoma and three closely related dietary generalists. To address the functional link between morphology and performance, muscles were dissected, and their force‐generating capacity, position and orientation were used as input for a biomechanical model to investigate variation in bite force capacity among the different species. The model was then validated by in vivo data for a subset of species, which allowed us to determine estimated maximal bite forces in the majority of the species within the genus. We predict that the specialization towards myrmecophagy will be associated with: (1) a reduction in bite force in the most specialized species; and (2) a reduction in jaw robustness, head dimensions and jaw adductor muscle cross‐sectional area.
Materials and methods
Specimens
A total of 37 preserved specimens was dissected, including 12 of the 17 known species of Phrynosoma and three species used for outgroup comparison (i.e. 15 species in total). The following species (samples sizes in parentheses) were included in the analyses: Phrynosoma asio (2); Phrynosoma braconnieri (1); Phrynosoma cornutum (3); Phrynosoma coronatum (3); Phrynosoma ditmarsi (1); Phrynosoma hernandesi (3); Phrynosoma mcallii (3); Phrynosoma modestum (3); Phrynosoma orbiculare (3); Phrynosoma platyrhinos (4); Phrynosoma solare (3); Phyrnosoma taurus (1); Callisaurus draconoides (3); Sceloporus magister (1); and Uma notata (3). Sample sizes for some of the species included in our data set are low due to the fact that they are very rare in collections and extremely hard to find in the field. Yet, for comparative studies where the between‐species differences are much greater than the within‐species differences, this should not bias our analyses (Bickel & Losos, 2002).
Morphology
Both the jaw depressors and all 12 muscle bundles associated with jaw adduction (see Results; Wittorski et al. 2016) were dissected from each individual and stored in a 70% aqueous ethanol solution. Before removing the muscles, the x‐, y‐ and z‐coordinates of the origin and insertion of each muscle were measured using digital calipers (Mitutoyo CD‐15DC; Mitutoyo, Telford, UK). The x‐ and y‐coordinates were measured relative to the center of rotation at the quadrate‐articular joint, while the z‐coordinates were measured relative to the midline of the skull (Fig. 1; Herrel et al. 1998a,b). To calculate physiological cross‐sectional area of the muscles, we determined muscle mass by weighing each muscle to the nearest 0.001 mg using a Metler MT 5 electronic balance. Muscle fiber length was determined by first submersing each muscle in a 30% aqueous nitric acid solution to dissolve the connective tissue. After removal of the nitric acid, muscles were placed in a 50% aqueous glycerol solution and individual fibers were teased apart using blunt‐tipped glass needles. A total of 20 haphazardly chosen fibers from each muscle were drawn using a Wild M3Z dissecting scope with camera lucida and then digitized so that the average muscle fiber length could be calculated. In a few cases in which muscle fibers were damaged during digestion of the connective tissue, estimates of muscle fiber length were based on the same muscle from other similar‐sized specimens. The physiological cross‐sectional area of each muscle was estimated from the ratio of muscle volume over mean fiber length, assuming a muscle density of 1006 kg m−3 (Mendez & Keys, 1960).
Figure 1.
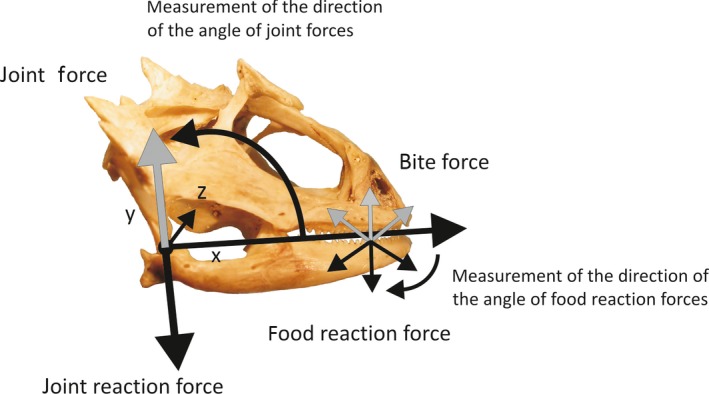
Skull of Phrynosoma hernandesi displaying the measurements made using the static bite force model. The model was set to measure food reaction forces (bite forces are equal magnitude but opposite in direction) at orientations ranging between −30 and −150, which were calculated relative to the long axis of the mandible. The model also calculates the joint force and angle of joint forces about the quadrate joint. The origins and insertions of all muscles input into the model were measured relative to the center of rotation (X = 0, Y = 0) and to the midline of the head (Z = 0).
Bite force modeling
A static bite model was used to examine differences in bite force among the species of Phrynosoma. The model was an adapted version of the model used by Cleuren et al. (1995), which estimates bite forces using the physiological cross‐sectional area of the jaw adducting musculature, a muscle stress of 40 N cm−2 (Gröning et al. 2013), the three‐dimensional position of the muscles, the point of application of the bite forces, and the centers of rotation (see Herrel et al. 1998a,b for a more detailed explanation of the model). The model allows us to calculate several functionally important variables, including bite forces, joint forces and the angle of joint forces (Fig. 1). In the present paper, we will only discuss the variation in bite force calculated at different bite points and gape angles.
To examine the effect of prey position on bite force, we measured two bite points on the mandible. Bite points were defined at the mandibular symphysis (bite point 1) and midway along the tooth row of the mandible (bite point 2). These bite points correspond to positions on the mandible that are used for the initial seizure and subsequent crushing of prey (Meyers & Herrel, 2005). Second, because bite force may also be influenced by the size of a prey item, we examined the effect of gape distance on bite force. To this end, the mandibles were rotated open to both 10 ° and 30 °, mimicking gape angles observed during processing of medium and large prey items, respectively (Meyers & Herrel, 2005). Because the shape, texture and position of a food item may influence the orientation of bite forces, we calculated bite forces corresponding to food reaction force angles ranging between 30 ° and 130 °. As the model calculates forces for only one side of the animal, it is necessary to multiply output forces by 2 to get an estimate of maximal bilateral bite force, as reported in Tables 2 and 3.
Table 2.
Means and standard deviations of estimated bite forces at gape angles of 10 ° and 30 °
| Species | N | Bite force 10 ° (N) | Bite force 30 ° (N) | ||
|---|---|---|---|---|---|
| Bite point 1 | Bite point 2 | Bite point 1 | Bite point 2 | ||
| Callisaurus draconoides | 3 | 2.05 ± 0.03 | 2.90 ± 0.06 | 1.71 ± 0.02 | 2.42 ± 0.04 |
| Phrynosoma asio | 2 | 4.08 ± 0.33 | 5.22 ± 0.36 | 3.50 ± 0.29 | 4.48 ± 0.31 |
| Phrynosoma braconnieri | 1 | 1.37 | 1.79 | 1.02 | 1.33 |
| Phrynosoma cornutum | 3 | 4.06 ± 0.22 | 6.28 ± 0.46 | 3.75 ± 0.13 | 5.78 ± 0.32 |
| Phrynosoma coronatum | 3 | 3.85 ± 0.03 | 5.17 ± 0.13 | 3.24 ± 0.01 | 4.34 ± 0.14 |
| Phrynosoma ditmarsi | 1 | 18.65 | 21.75 | 15.15 | 17.67 |
| Phrynosoma hernandesi | 3 | 4.82 ± 0.83 | 5.89 ± 0.95 | 3.73 ± 0.60 | 4.57 ± 0.67 |
| Phrynosoma mcallii | 3 | 2.11 ± 0.16 | 2.98 ± 0.21 | 1.65 ± 0.11 | 2.34 ± 0.14 |
| Phrynosoma modestum | 3 | 1.42 ± 0.06 | 2.20 ± 0.11 | 1.25 ± 0.02 | 1.94 ± 0.06 |
| Phrynosoma orbiculare | 3 | 4.80 ± 0.49 | 6.24 ± 0.63 | 3.92 ± 0.42 | 5.10 ± 0.54 |
| Phrynosoma platyrhinos | 4 | 2.40 ± 0.13 | 3.11 ± 0.19 | 1.87 ± 0.10 | 2.43 ± 0.14 |
| Phrynosoma solare | 3 | 2.14 ± 0.17 | 3.17 ± 0.15 | 1.61 ± 0.15 | 2.37 ± 0.14 |
| Phrynosoma taurus | 1 | 2.38 | 3.40 | 1.77 | 2.53 |
| Sceloporus magister | 1 | 9.90 | 14.96 | 8.56 | 12.93 |
| Uma notata | 3 | 4.99 ± 0.15 | ± 0.12 | 4.32 ± 0.13 | 6.08 ± 0.05 |
Table 3.
Summary of the results for the linear regressions of the residual contrasts of morphological variables against the residual contrasts of the estimated bite force (bite point 2, gape angle 10 °)
| Variable | Slope | R | P‐value |
|---|---|---|---|
| Head length | −0.65 | 0.28 | 0.30 |
| Head width | 2.42 | 0.66 | 0.007 |
| Head height | 1.20 | 0.34 | 0.21 |
| Mandible length | 3.65 | 0.47 | 0.07 |
| Articular‐coronoid length | 1.99 | 0.64 | 0.01 |
| Anterior dentary height | 1.71 | 0.69 | 0.005 |
| Posterior dentary height | 1.44 | 0.80 | < 0.001 |
| Coronoid height | 1.59 | 0.75 | 0.001 |
| Length tooth row | 2.25 | 0.55 | 0.034 |
| Tooth number | 2.50 | 0.73 | 0.002 |
| Jaw openers PCSA | 0.38 | 0.24 | 0.39 |
| M. adductor mandibulae externus PCSA | 1.20 | 0.97 | < 0.001 |
| M. pseudotemporalis PCSA | 0.52 | 0.62 | 0.01 |
| M. pterygoideus PCSA | 0.80 | 0.74 | 0.002 |
| % Ants | −0.57 | 0.81 | < 0.001 |
Note that muscle data are not independent of the model output. Bold values indicate significant relationships. Note that all regressions are forced through the origin.
PCSA, physiological cross sectional area.
In vivo bite forces
In vivo bite forces were measured in the field using an isometric Kistler force transducer (type 9203, range 7500 N; Kistler, Zurich, Switzerland) mounted on a purpose‐built holder and connected to a Kistler charge amplifier (type 5995A, Kistler; see Herrel et al. 1999 for a more detailed description of the setup). When the bite plates were placed between the jaws of the animal, prolonged and repeated biting resulted. The place of application of bite forces was standardized for all animals by metal stops that were mounted on the bite plates, thus assuring that animals always bit at the same position along the jaw. Gape angle was standardized by setting the plates such that all animals bit at a gape angle of about 10 °. Measurements were repeated five times for each animal, with an inter‐trial interval of at least 30 min. The maximal value obtained during such a recording session was considered to be the maximal bite force for that individual. In Arizona, we collected and measured in vivo bite forces for adults (both males and females) of nine of the 15 species included in our data set (Table 1). Sample sizes for each species were: Callisaurus draconoides, N = 25; P. cornutum, N = 27; P. hernandesi, N = 9; P. mcalli, N = 22; P. modestum, N = 50; P. platyrhinos, N = 65; P. solare, N = 13; S. magister, N = 55; Uma notata, N = 5. All procedures were approved by the Northern Arizona University IACUC protocol number 03‐086. Collecting permits were provided by the Arizona Game and Fish Department numbers SP733156, SP572610 and SP661941.
Table 1.
The percentage of ants in the diet, and the means and standard deviations for all morphological measurements
| C. draconoides | P. asio | P. braconnieri | P. cornutum | P. coronatum | P. ditmarsi | P. hernandesi | P. mcallii | |
|---|---|---|---|---|---|---|---|---|
| % Ants | 5 | 31 | 49 | 61 | 45 | 11 | 41 | 78 |
| Snout‐vent length | 72.46 ± 2.53 | 90.73 ± 17.69 | 53.79 | 91.76 ± 1.18 | 77.17 ± 9.67 | 77.59 | 69.69 ± 16.90 | 75.70 ± 0.51 |
| Head length | 15.27 ± 0.03 | 19.61 ± 3.65 | 14.36 | 19.49 ± 0.69 | 16.96 ± 2.21 | 18.81 | 15.41 ± 1.75 | 16.30 ± 0.65 |
| Head height | 8.69 ± 0.35 | 15.15 ± 2.59 | 12.80 | 17.05 ± 1.18 | 14.18 ± 1.33 | 16.86 | 13.17 ± 1.07 | 11.38 ± 0.49 |
| Head width | 12.21 ± 0.01 | 21.91 ± 3.80 | 17.89 | 20.28 ± 1.02 | 19.04 ± 1.96 | 24.57 | 16.83 ± 5.55 | 16.48 ± 0.42 |
| Mandible length | 15.28 ± 0.90 | 18.27 ± 4.26 | 12.86 | 16.37 ± 1.78 | 14.04 ± 1.08 | 19.13 | 15.17 ± 1.76 | 11.49 ± 0.56 |
| Articular‐coronoid | 4.83 ± 0.16 | 6.75 ± 1.72 | 4.58 | 6.86 ± 0.42 | 4.86 ± 0.30 | 7.34 | 5.24 ± 0.62 | 3.55 ± 0.08 |
| Anterior dentary height | 0.36 ± 0.04 | 0.83 ± 0.30 | 0.41 | 0.75 ± 0.13 | 0.59 ± 0.15 | 0.91 | 0.52 ± 0.07 | 0.64 ± 0.13 |
| Posterior dentary height | 1.94 ± 0.09 | 3.28 ± 0.61 | 2.07 | 3.08 ± 0.17 | 2.17 ± 0.27 | 4.62 | 2.59 ± 0.62 | 1.52 ± 0.14 |
| Coronoid height | 2.71 ± 0.10 | 4.01 ± 0.99 | 1.66 | 3.16 ± 0.10 | 2.67 ± 0.30 | 4.70 | 3.31 ± 0.45 | 1.64 ± 0.14 |
| Tooth row length | 7.47 ± 0.45 | 8.81 ± 1.66 | 5.77 | 8.25 ± 0.47 | 6.92 ± 1.05 | 8.91 | 7.88 ± 1.18 | 5.29 ± 0.19 |
| Tooth number | 21.67 ± 1.53 | 19.50 ± 0.71 | 20.00 | 19.00 ± 1.00 | 20.00 ± 1.73 | 24.00 | 16.33 ± 1.53 | 14.67 ± 2.08 |
| In vivo bite force (N) | 4.02 ± 1.51 | 8.03 ± 1.11 | 8.68 ± 3.79 | 3.15 ± 0.62 |
| P. modestum | P. orbiculare | P. platyrhinos | P. solare | P. taurus | S. magister | U. notata | |
|---|---|---|---|---|---|---|---|
| % Ants | 66 | 41 | 56 | 89 | 56 | 23 | 20 |
| Snout‐vent length | 56.29 ± 2.26 | 76.31 ± 7.93 | 73.19 ± 0.52 | 96.56 ± 3.86 | 74.06 | 100.0 | 95.34 ± 9.97 |
| Head length | 12.57 ± 0.38 | 17.85 ± 1.21 | 16.90 ± 0.77 | 24.86 ± 1.00 | 15.52 | 21.75 | 16.93 ± 1.47 |
| Head height | 10.05 ± 0.22 | 15.45 ± 1.63 | 13.50 ± 0.01 | 15.84 ± 1.16 | 13.46 | 14.60 | 11.61 ± 0.93 |
| Head width | 15.92 ± 0.74 | 20.41 ± 1.96 | 19.24 ± 0.09 | 23.20 ± 1.65 | 19.68 | 20.51 | 15.67 ± 0.15 |
| Mandible length | 10.85 ± 0.72 | 16.36 ± 0.51 | 13.93 ± 0.49 | 16.45 ± 0.63 | 15.94 | 21.86 | 19.18 ± 2.39 |
| Articular‐coronoid | 3.55 ± 0.30 | 5.98 ± 0.49 | 4.47 ± 0.20 | 5.57 ± 0.28 | 6.03 | 7.09 | 6.65 ± 0.64 |
| Anterior dentary height | 0.48 ± 0.05 | 0.54 ± 0.05 | 0.67 ± 0.01 | 0.58 ± 0.05 | 0.58 | 0.85 | 0.54 ± 0.01 |
| Posterior dentary height | 1.19 ± 0.11 | 2.90 ± 0.23 | 1.83 ± 0.16 | 1.85 ± 0.10 | 2.62 | 3.57 | 2.56 ± 0.23 |
| Coronoid height | 1.54 ± 0.10 | 3.23 ± 0.08 | 2.09 ± 0.33 | 2.32 ± 0.09 | 3.08 | 4.72 | 3.70 ± 0.53 |
| Tooth row length | 4.96 ± 0.71 | 8.18 ± 0.59 | 6.33 ± 0.34 | 7.46 ± 0.39 | 7.83 | 11.28 | 9.45 ± 1.01 |
| Tooth number | 18.00 ± 2.65 | 19.00 ± 1.00 | 17.00 ± 1.41 | 16.00 ± 2.00 | 19.00 | 21.00 | 19.33 ± 0.58 |
| In vivo bite force (N) | 2.53 ± 1.15 | 2.94 ± 0.94 | 3.68 ± 1.81 | 18.07 ± 11.69 | 7.16 ± 3.73 |
Values are based on the individuals dissected in this study, except for the in vivo bite forces, which are based on field‐caught adult individuals. All linear measurements are in mm.
Morphometrics
To examine the relationship between head shape and bite force, external morphological measurements were taken on all dissected specimens (0.01 mm) using digital calipers. These included the following external measurements: snout‐vent length (svl), measured from the snout tip to the vent; head length (hl), measured from the snout tip to the posterior edge of the parietal; head height (hh), measured at the highest point posterior to the orbit, excluding horns on the parietal and dentary; head width (hw), measured at the widest part of the skull excluding the horns; and mandible length (ml), measured from the posterior tip of the retro‐articular process to the tip of the dentary. Additionally, we included the following measurements of the dentary taken from Meyers et al. (2006): the length from posterior articular to the midline of the coronoid (ac), height of the dentary at the first (adh) and last (pdh) tooth, coronoid height (ch), length of the tooth row (ltr), and the number of teeth (tn). Means and standard deviations for these variables are presented in Table 1.
Diet
In order to address the relationship between dietary specialization and bite force, we gathered data from the literature describing the percentage of ants in the diet of each species. Much of the dietary data for Phrynosoma was taken from Pianka & Parker's (1975) review of Phrynosoma ecology, but additional dietary information for Phrynosoma hernandesi and Phrynosoma orbiculare (Montanucci, 1981) was also used. The percentages of ants in the diet for Phrynosoma as well as for the outgroup species Uma notata (Turner, 1998), Callisaurus draconoides (Pianka & Parker, 1972) and Sceloporus magister (Parker & Pianka, 1973) are summarized in Table 1.
Statistical analysis
First, we Log10‐transformed all data to meet assumptions of normality and homoscedascity, and calculated species means for all traits. Next, we ran simple regressions on both raw and phylogenetically corrected bite forces to test whether our model output predicted our measured in vivo bite force values for those species for which we had both in vivo data and estimated bite forces based on the biomechanical model (i.e. nine species). These analyses were performed in IBM‐SPSS (V. 23). Thereafter we only used the modeled bite force data in all analyses.
Because closely related species share a part of their evolutionary history, they cannot be considered independent data points (Felsenstein, 1985). Therefore, to take into account species relatedness in our analyses, we analyzed our data in a phylogenetic context. We computed independent contrasts of the morphological, bite force, morphometric, muscle and diet data using the Phenotypic Diversity Analysis Program (Garland et al. 1992) implemented in Mesquite. Independent contrasts require knowledge of the phylogenetic relationships of the species of concern. While there are numerous hypotheses about horned lizard relationships (Reeve, 1952; Presch, 1969; Montanucci, 1987; Reeder & Montanucci, 2001; Hodges & Zamudio, 2004; Leaché & McGuire, 2006), we here use the relationships and branch lengths described in the study of Pyron et al. (2013). The tree used in our analysis (Fig. 2) was obtained by pruning the tree provided in Pyron et al. (2013). This tree is identical in its topological relationships to the one presented by Leaché & McGuire (2006), but includes S. magister.
Figure 2.
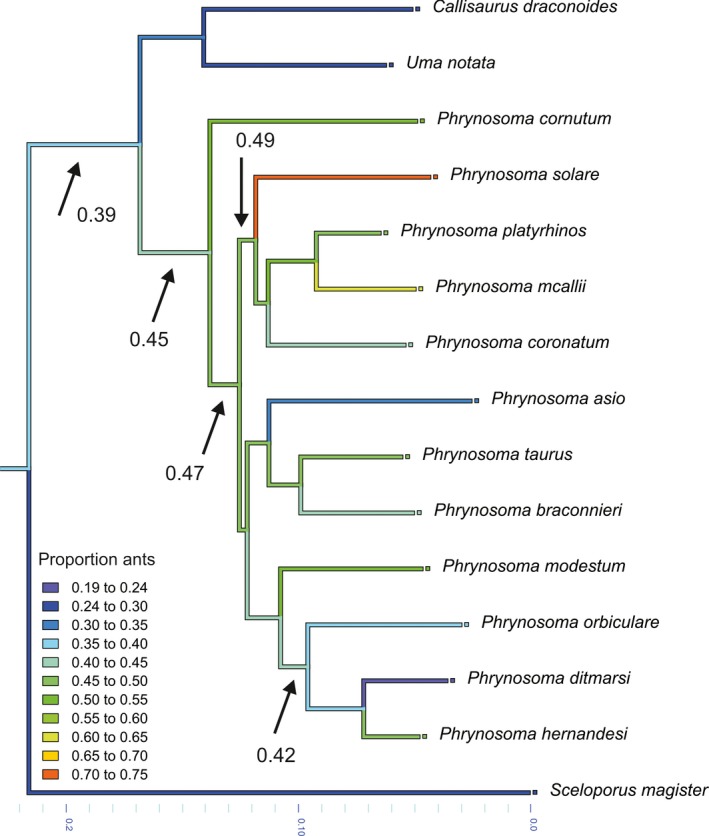
Phylogenetic relationships among Phrynosoma and the closely related species Callisaurus draconoides, Uma notata and Sceloporus magister, as described by Pyron et al. (2013). Illustrated are the results of an ancestral character state reconstruction performed in Mesquite for the proportion of ants in the diet. Note that the ancestor of Phrynosoma had about 45% ants in its diet, a proportion gradually increasing with the evolution of the different species. A notable exception is the clade comprising P. orbiculare, P. ditmarsi and P. hernandesi, showing a secondary reduction in the proportion of ants in the diet.
Because neither percentage (% ants) nor count data (number of teeth) meet the assumptions of normality, the percent ants in the diet was arcsine transformed and the number of teeth was square root transformed before further analyses (Sokal & Rohlf, 1995). After transformation, data were normally distributed. Trait values were input at the tree tips, allowing us to calculate phylogenetic independent contrasts of each variable. Inspection of the diagnostics (Garland et al. 1999) in the PDTREE program implemented in Mesquite allowed us to verify that the branch lengths derived from the molecular phylogeny were adequate for the analyses. The body size of Phrynosoma varies over twofold: because this variation can have a significant effect when making comparisons among species, we calculated the residuals of all variables by regressing the standardized contrasts of each variable against the contrast of snout‐vent length (forced through the origin; Garland et al. 1992) using IBM‐SPSS (V23). These residual‐contrast variables were used in all further analyses.
Because our goal was to examine the variation in bite force among Phrynosoma and to determine which morphological variables influence bite force, we performed simple linear regressions of residual contrasts of all morphological variables, with the residual contrast of bite force as our dependent variable. To determine which variables were the best predictors of bite force, we also performed a stepwise multiple regression analysis using three groupings of variables. As many investigators infer bite force ability based on external head measurements, skeletal measurements or muscle masses, we performed separate stepwise regressions using these three sets of input variables. Lastly, to examine the relationship between dietary specialization and bite force, we performed a linear regression with the contrast of the percentage of ants in the diet and the residual contrast of bite force.
Results
The jaw musculature in lizards is comprised of 12 muscles (terminology follows that of Haas, 1973): M. depressor madibulae (dm), M. levator anguli oris (lao), M. adductor mandibulae externus superficialis anterior (amesa), M. adductor mandibulae externus superficialis posterior (amesp), M. adductor mandibulae externus medialis (amem), M. adductor mandibulae externus profundus (amep), M. adductor mandibulae posterior (amp), P. pseudotemporalis superficialis (psts), M. pseudotemporalis profundus (pstp), M. ptergoideus pars lateralis (ptl), M. pterygoideus pars medialis (ptm), M. levator pterygoidei (lpt), M. protractor pterygoidei (ppt). Because not all 12 jaw muscles are present in every species, Figs 3 and 4 depict those muscles present or visible in the dissections of P. ditmarsi and P. platyrhinos. Because a detailed description of the musculature is beyond the scope of this paper, here we only list the muscles examined and discuss differences in jaw musculature among the species examined in this study.
Figure 3.
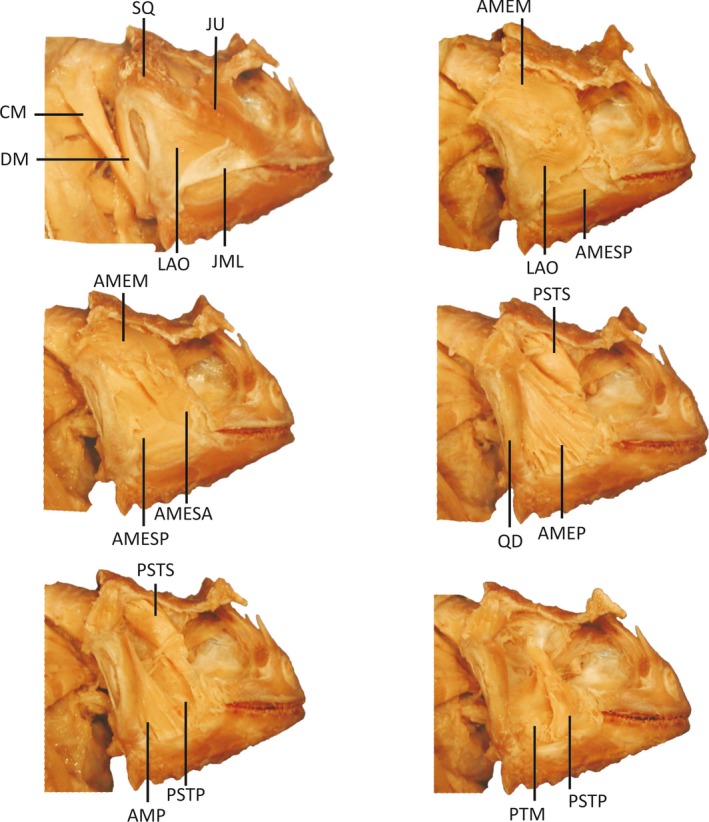
Lateral view of Phyrnosoma ditmarsi displaying successive layers of jaw musculature visible during dissection after removal of the skin. Phrynosoma ditmarsi is representative of species exhibiting enlarged jaw‐closing muscles and reduced jaw‐opening muscles. Muscles and skeletal elements are abbreviated as follows: CM, M. cervicomandibularis; DM, M. depressor mandibulae; LAO, M. levator anguli oris; AMESA, M. adductor mandibulae externus superficialis anterior; AMESP, M. adductor mandiblae externus superficialis posterior; AMEM, M. adductor mandibulae externus medialis; AMEP, M. adductor mandibulae externus profundus; AMP, M. adductor mandibulae posterior; PsTS, M. pseudotemporalis superficialis; PsTP, M. pseudotemporalis profundus; PTM, M. pterygoideus pars medialis; SQ, squamosal; JU, jugal; QD, quadrate; JML, jugal mandibular ligament.
Figure 4.
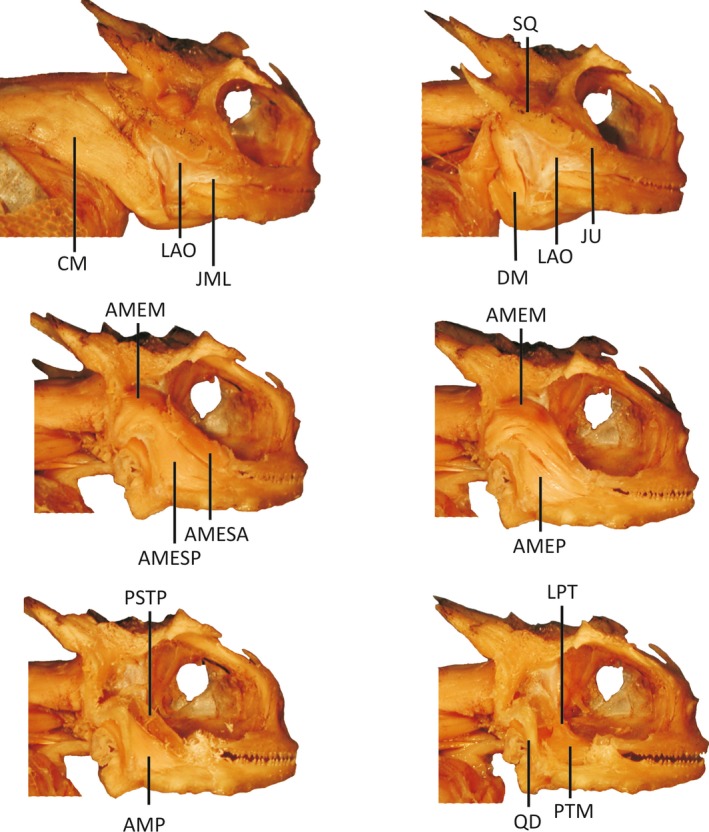
Dissection of Phrynosoma platyrhinos displaying successive layers of jaw musculature visible during dissection after removal of the skin. Phrynosoma platyrhinos is representative of species exhibiting reduced jaw‐closing muscles and enlarged jaw‐opening muscles. Muscles and skeletal elements are abbreviated as follows: CM, M. cervicomandibularis; DM, M. depressor mandibulae; LAO, M. levator anguli oris; AMESA, M. adductor mandibulae externus superficialis anterior; AMESP, M. adductor mandiblae externus superficialis posterior; AMEM, M. adductor mandibulae externus medialis; AMEP, M. adductor mandibulae externus profundus; AMP, M. adductor mandibulae posterior; PsTP, M. pseudotemporalis profundus; PTM, M. pterygoideus pars medialis; LPT, M. levator pterygoidei; SQ, squamosal; JU, jugal; QD, quadrate; JML, jugal mandibular ligament.
The most conspicuous difference in jaw musculature among Phrynosoma is the relative development of the M. adductor manidibulae complex. For example, P. solare, which has the largest snout vent length of any Phrynosoma used in this study (Table 1), has a total jaw adductor mass that is less than all but four of the species of Phrynosoma. At the other extreme is P. ditmarsi, a small‐ to medium‐sized Phrynosoma, which has a jaw adductor mass that is double that of other Phrynosoma and nearly the same as the much larger Sceloporus magister. Because the adductor mandibulae externus group comprises about 60% of the total adductor mass, differences in adductor muscle mass may be attributable primarily to this muscle. Other notable differences include the general reduction and even loss of m. pseudotemporalis pars superficialis. The presence of this muscle was variable in some species and completely absent in others, and its reduction appears to coincide with an overall reduction in total adductor mass. The pterygoideus muscle, whose functional significance in biting is not well understood, is overall poorly developed in Phrynosoma, on average making up only 12% of the adductor mass. The reduced size of this muscle in Phrynosoma is in sharp contrast to its size in the outgroup species C. draconoides, U. notata and S. magister, where it comprises as much as 25% of total adductor mass.
Modeling
Because the model output reveals that species vary in magnitude but respond similarly to parameter changes (angle of the orientation of the food orientation force, bite point, gape angle), we first provide a short description of the model results and then describe how species differ. The orientation of the prey reaction forces had a significant effect on bite force. Bite force was lowest when the food reaction forces were perpendicular (90 °) to the mandible, and increased with increasing deviation from 90 °. Food reaction forces directed posteriorly resulted in higher maximal bite and joint forces then those directed anteriorly (Herrel et al. 1998a,b). As a conservative estimate of bite force, we used bite forces at prey orientation angles of 90 ° for comparisons among species (Table 2). Model outputs indicate distinct differences in bite force for the two bite positions on the tooth row. Bite forces are lowest at the anterior bite point and increase posteriorly (Tables 2 and 3). On average, there is about a 27% increase in bite force when moving from the anterior to posterior bite point. In addition to the position of the prey along the mandible, the size of a prey item will also have a considerable effect on bite force. When simulations were run at a 10 ° gape, bite forces were considerably higher than at a 30 ° gape. The bite forces at the two gapes differ to the same degree among species, so that bite force increases from anterior to posterior at both 10 ° and 30 ° gapes. The increase in gape angle has less of an effect on bite forces than does bite point, with an average decrease in bite force of 18% as gape angle increases from 10 ° to 30 °.
A linear regression of estimated bite force on in vivo bite force for the subset of species for which we had both measures revealed a highly significant relationship between the two data sets (r = 0.98; P < 0.001; slope = 1.045 ± 0.88; Fig. 5). Moreover, the slope of the regression was not significantly different from 1 (P = 0.95), suggesting that our model estimates are a good proxy for in vivo data. Analyses performed on the independent contrasts of both in vivo and modeled bite force showed a similar result (r = 0.96; P < 0.001).
Figure 5.
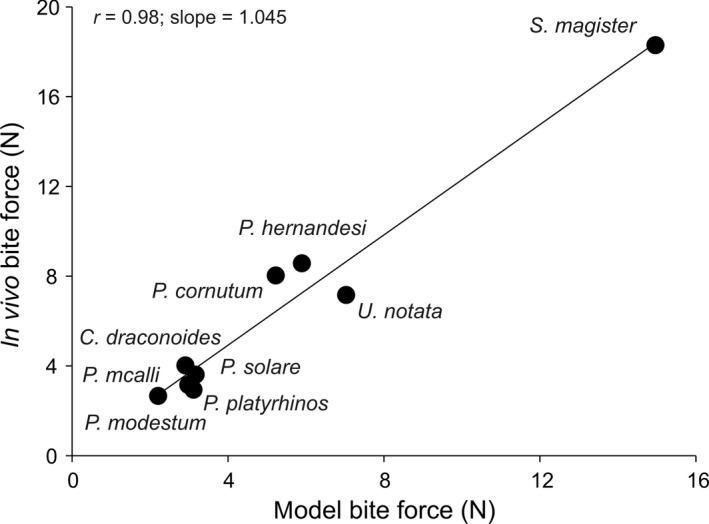
Scatter plot illustrating the relationship between the modeled bite force and the in vivo measured bite force for the nine species for which both in vivo bite forces and model estimates were available. Each point represents a species mean.
Comparisons of bite forces among the 12 species of Phrynosoma reveal striking differences in maximal bite force. As detailed in Table 2, there are about three groupings with regards to bite force. First, we have the species with very low estimated bite forces, including P. braconnieri, P. mcallii, P. modestum, P. platyrhinos, P. solare, P. taurus and C. draconoides. Next are P. asio, P. cornutum, P. coronatum, P. hernandesi, P. orbiculare, S. magister and U. notata whose forces are intermediate. And finally, we have the radically divergent P. ditmarsi, which exhibits estimated bite forces of over 15 N at all bite points and gape angles. While direct comparisons are difficult because of differences in body size, larger species are not necessarily the hardest biters. For example, P. ditmarsi is capable of producing bite forces more than five times greater than the much larger P. solare. After P. ditmarsi, the next highest bite forces in Phrynosoma are found in the closely related short‐horned lizard clade, consisting of P. orbiculare and P. hernandesi. Yet, bite forces in these species are still lower than that of P. ditmarsi (Fig. 6).
Figure 6.
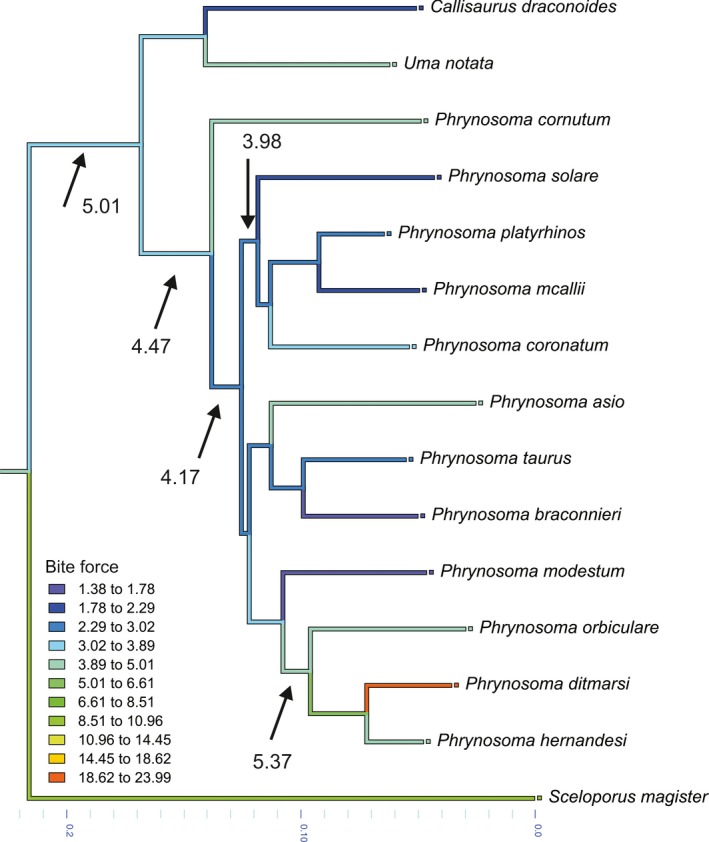
Illustrated are the results of an ancestral character state reconstruction performed in Mesquite for the estimated bite force based on the modeling. Note that the ancestor of Phrynosoma bit with an estimated force of 4.47 N. Bite force then gradually decreases with the evolution of the different species paralleling the increase in the amount of ants consumed. A notable exception is the clade comprising P. orbiculare, P. ditmarsi and P. hernandesi, showing a secondary increase in bite force.
Evolutionary analyses
Linear regression analyses of the residual contrasts of external head measurements vs. bite force revealed that only head width is correlated with bite force (Table 3). However, regressions of the morphological measurements made on the mandibles show that an increase in bite force is associated with a taller mandible, a taller coronoid process, a longer jaw closer in‐lever (articular to coronoid distance), and a mandible with more teeth and a longer tooth row (Table 3). The residual contrasts of the physiological cross‐sectional area of all jaw adductor, but not opener, groups is also significantly related to the residual contrast of bite force (Table 3).
To determine which groups of variables best predict changes in bite force, we performed three multiple stepwise regressions on the following variables: external head measurements; mandible measurements; and jaw muscle physiological cross‐sectional areas. A stepwise regression containing the residual contrasts of the four external head measurements revealed that the residual contrasts of head width (r = 0.66, P = 0.007) is the best predictor of the residual contrast of bite force. Performing these same analyses using the characters measured on the mandible resulted in a single model containing only posterior mandible height (r = 0.80, P < 0.001). The results of a stepwise regression of the residual contrasts of muscle cross‐sectional areas and bite force reveal a model with a single highly significant variable, the residual contrast of the m. adductor mandibulae externus (r = 0.97, P < 0.0001; Fig. 7). Thus, the evolution of high bite force in Phrynosoma has gone hand in hand with the evolution of wider heads, taller mandibles and a larger external adductor muscle cross‐sectional area.
Figure 7.
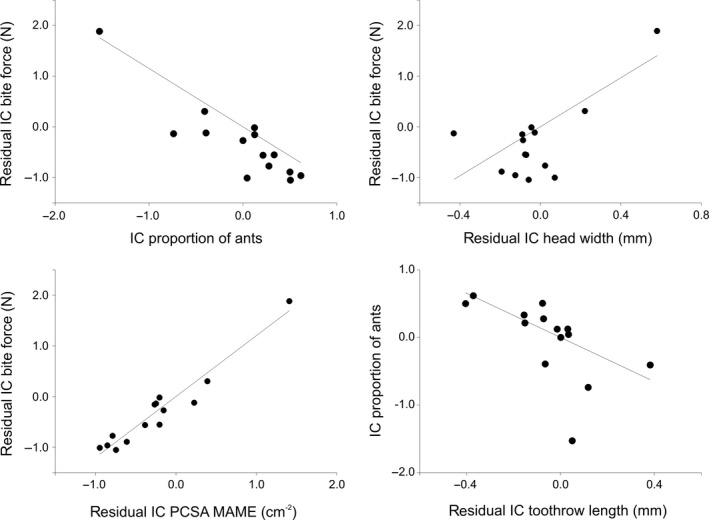
Residual independent contrasts of bite force plotted against the residual independent contrasts of the proportion of ants in the diet, head width and the physiological cross‐sectional area of the external adductor. At the bottom right of the figure is a scatter plot illustrating the relationship between the independent contrasts of the proportion of ants in the diet and the residual independent contrasts of tooth row length. Note that all regressions are forced through the origin. (IC), independent contrasts.
Finally, the residual contrast of the modeled bite force shows a significant negative correlation with the independent contrast of proportion of ants in the diet (r = 0.81, P < 0.001). Thus, the evolution of a higher proportion of ants in the diet is associated with an evolutionary decrease in bite force relative to body size (Fig. 7). The evolutionary increase in the proportion of ants in the diet is also associated with an evolutionary decrease in relative head width (r = −0.54; P = 0.048), relative posterior mandible depth (r = −0.79, P = 0.001), relative coronoid height (r = −0.76, P = 0.002), the relative closing in‐lever (r = −0.67, P = 0.009), relative tooth row length (r = −0.60, P = 0.023; Fig. 7), the relative number of teeth (r = −0.87, P < 0.001), the relative cross‐sectional area of the external adductor (r = −0.86, P < 0.001), the relative cross‐sectional area of the pseudotemporalis group (r = −0.61, P = 0.02) and the relative cross‐sectional area of the m. pterygoideus (r = −0.70, P = 0.006).
Discussion
Our analysis examining the morphology and performance of the feeding system in Phrynosoma reveals several important findings. First, model simulations show a good correspondence with in vivo data. Second, model simulations show that bite force varies predictably with respect to gape angle and bite point, with maximal bite forces being attained at lower gape angles and posterior bite points. Third, bite force ability in this group of lizards is determined primarily by the size of the jaw adductor musculature. Fourth, Phrynosoma exhibit considerable variation in maximum bite force, with some species producing bite forces that are higher than those of closely related dietary generalists. Lastly, the evolution of bite force capacity in Phrynosoma appears to coincide with the degree of myrmecophagy, so that as animals become more myrmecophagous, there is an associated loss of bite force capacity and a reduction of the underlying anatomical structures responsible for bite force generation (muscles, bones, teeth).
Morphology and bite force
Bite force in Phrynosoma is clearly associated with morphological changes in the jaw system. While some species exhibit extremely high bite forces, possibly associated with eating hard prey (Montanucci, 1989), the group as a whole exhibits a reduced bite force compared with other lizards (Fig. 6). The loss of bite force ability is correlated with changes in the size and shape of the mandible, including a reduction in the overall height of the mandible, a decrease in the height of the coronoid, and a decrease in the number of teeth on the tooth row (Table 3). These traits indicate that as well as reduced bite force, most species of horned lizards exhibit a reduced area for muscle insertion (coronoid height), a less stout jaw and fewer teeth with which to perform crushing bites. In conjunction, the loss of this processing morphology suggests that for at least some species of Phrynosoma, the ability to process prey may be unimportant (Meyers & Herrel, 2005).
In many animals, variation in head shape has been used as a predictor of potential bite force. Those species with wider and/or taller heads often exhibit higher bite forces (Herrel et al. 2001; Verwaijen et al. 2002; Vanhooydonck et al. 2010; Lopez‐Darias et al. 2015; Wittorski et al. 2016). Our analyses generally conform to this model, with hard‐biting species tending to have wider heads. The general assumption is that a wider head provides a greater area for increased muscle mass as well as increased area for muscle attachment. Among Phrynosoma, the physiological cross‐sectional area of the external adductor appears to be the most important muscle variable determining bite force, as has been observed in other lizards (Wittorski et al. 2016). While a few species exhibit wider heads and higher bite forces, most Phrynosoma show a reduction in head size and an associated loss of bite force capacity. The relatively smaller head size may then explain the reduction and loss of specific adductor muscles in several species of Phrynosoma.
Modeling parameters
The position of a prey item along the tooth row (bite point) had a significant effect on bite force (Dumont & Herrel, 2003), and the importance of bite point is apparent when considering movement of the prey into the mouth. Like all other iguanians, Phrynosoma use their tongue to capture prey (Schwenk, 2000; Meyers & Herrel, 2005). As the prey is prehended, it is pulled into the mouth, and the jaws close around the prey item. While small prey items are often brought further into the buccal cavity during the capture event, for larger prey items the first point of contact is often at the anterior bite point. The importance of the anterior bite point in performing crushing bites is probably minimal, yet sufficient forces need to be produced anteriorly so that the prey can be restrained until it is transported further into the buccal cavity. Bite forces at the anterior bite point are on average 25% lower than those attained at the posterior bite point. However, joint forces created when biting at the anterior bite point are higher than at the posterior bite point. Low bite forces and high joint forces suggest that the jaw system may not be optimized for performing crushing bites anteriorly, but are likely adequate for restraining most prey items.
An increase in prey size also has a significant effect on bite force because a larger gape is required in order to perform crushing bites. In all the species examined in this study, maximal bite forces occur when biting at lower gape angles, i.e. when feeding on smaller prey. While increasing the gape has less of an effect on bite force than moving from a posterior to an anterior bite point, there is still about an 18% decrease in bite performance. These findings are in accordance with studies on other vertebrates, which found that bite force generally decreases with increasing gape (Cleuren et al. 1995; Herrel et al. 2002; Dumont & Herrel, 2003; Santana, 2015).
Bite force and myrmecophagy
Maximal bite forces vary considerably among horned lizards. Nearly half of the species exhibit bite forces similar in magnitude to those of the outgroup species (Fig. 6), suggesting that although Phrynosoma exhibit a specialized morphology relative to other lizards (Pianka & Parker, 1975), they still possess the morphological structure to process a wide range of prey. However, the remaining species appear to have diverged toward either reduced or well‐developed bite force ability. At one extreme is P. solare, which is the epitome of a highly myrmecophagous animal, exhibiting an extreme loss in performance and reduction in the morphology of prey‐processing structures. At the other end of the spectrum is P. ditmarsi, which displays extreme hypertrophy of the jaw muscles, resulting in bite forces that are greater than those of any other lizard examined in this study. As Montanucci (1989) pointed out, this species appears to be built for producing high bite forces, likely for crushing hard prey items such as beetles. If dietary information supports this contention, then it would seem that within this specialized clade of myrmecophagous lizards, a separate group of durophagous species has evolved. Alternatively, high bite forces could be the result of sexual selection (Herrel et al. 1999; Huyghe et al. 2005; Donihue et al. 2016). This is, however, unlikely given that Phrynosoma are not territorial (Munger, 1984) and that dimorphism in head shape is low in this group in contrast to the marked dimorphism in body size (Zamudio, 1998).
The evolution of bite force capacity in Phrynosoma is highly correlated with the degree of myrmecophagy. Species with high bite forces tend to eat few ants, whereas decreasing bite force is associated with increasing specialization on ants (Figs 2, 6 and 7). While the development of hard biting is beneficial in expanding dietary breadth, an explanation for a loss of bite force is more difficult. Does the loss of dentition, jaw musculature and bite force represent any functional advantage for these animals, or are these characteristics simply a response to disuse? It seems reasonable for lizards like Phrynosoma that swallow ants without crushing them (Meyers & Herrel, 2005) not to maintain energetically expensive tissue like bone or muscle. A similar phenomenon has been well documented in humans after having spent time in reduced gravity or under conditions of prolonged bed‐rest (Brooks et al. 2008; Narici & de Boer, 2011; Brooks & Myburgh, 2014). Evolutionary loss of morphological characters and performance in response to disuse has also been noted in other groups (McPeek, 1997), and may be responsible for the loss of morphology of some horned lizards. Convergent loss of morphology and processing behaviors in myrmecophagous mammals (Reiss, 2000) and horned lizards lends further support to the disuse hypothesis.
In conclusion, our findings indicate that Phrynosoma have undergone significant morphological evolution of the jaw system that is strongly associated with dietary specialization. The loss of bite force is the result of a decrease in the number and size of jaw adductor muscles, and is accompanied by a reduced dentition and size of the mandible. Although the trend is toward a reduction in the form and function of the prey‐processing machinery with an increase in myrmecophagy, the appearance of the hyper‐developed jaw system of P. ditmarsi suggests different evolutionary trajectories within the genus (Fig. 6). The similar response of mammalian myrmecophages and Phrynosoma indicates that the evolution of myrmecophagy may result in predictable changes of the feeding system that span divergent phylogenetic groups.
Author contributions
JM devised the study; JM and AH performed dissections, measured in vivo bite force data, did the biomechanical modeling and statistical analyses; all authors contributed to the writing of the manuscript.
Acknowledgements
The authors would like to thank the following curators and museum staff for allowing them to measure specimens in their care: George Bradley (University of Arizona), Dave Wake and Javier Rodriquez (University of California Berkeley Museum of Vertebrate Zoology), Brad Hollingsworth (San Diego Natural History Museum), Alan Resetar (Chicago Field Museum of Natural History), Kent Beaman (Los Angeles County Natural History Museum), Jonathan Campbell (University of Texas at Arlington), Tad Theimer (Museum of Vertebrates at Northern Arizona University), Robert Drewes and Jens Vindum (California Academy of Sciences). The authors would also like to thank Peter Aerts who developed the static bite force model, Bieke Vanhooydonck who helped with the phylogenetic analysis, and two anonymous reviewers for helpful and constructive comments on an earlier version of the manuscript. J. Meyers was funded by NIH MSD Grant # R25 GM 056931‐07 and a J. Exp. Biology travel grant.
References
- Aguirre LF, Herrel A, Van Damme R, et al. (2002) Ecomorphological analysis of trophic niche partitioning in a tropical savannah bat community. Proc R Soc Lond 269, 1271–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre LF, Herrel A, Van Damme R, et al. (2003) The implications of food hardness for diet in bats. Funct Ecol 17, 201–212. [Google Scholar]
- Anderson R, McBrayer LD, Herrel A (2008) Bite force in vertebrates: opportunities and caveats for use of a nonpareil whole‐animal performance measure. Biol J Linn Soc 93, 709–720. [Google Scholar]
- Bickel R, Losos JB (2002) Patterns of morphological variation and correlates of habitat use in chameleons. Biol J Linn Soc 76, 91–103. [Google Scholar]
- Brooks NE, Myburgh KH (2014) Skeletal muscle wasting with disuse atrophy is multi‐dimensional: the response and interaction of myonuclei, satellite cells and signaling pathways. Front Physiol 5, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks N, Cloutier GJ, Cadena SM, et al. (2008) Resistance training and timed essential amino acids protect against the loss of muscle mass and strength during 28 days of bed rest and energy deficit. J Appl Physiol 105, 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraway LN, Verts BJ, Jones ML, et al. (1996) A search for age‐related changes in bite force and diet in shrews. Am Midl Nat 135, 231–240. [Google Scholar]
- Cleuren J, Aerts P, De Vree F (1995) Bite and joint force analysis in Caiman crocodiles . Belg J Zool 124, 79–94. [Google Scholar]
- Dalrymple GH (1979) On the jaw mechanism of the snail crushing lizards, Dracaena, Daudin, 1802 (Reptilia, Lacertilia, Teiidae). J Herpetol 13, 303–311. [Google Scholar]
- Des Roches S, Brinkmeyer MS, Harmon LJ, et al. (2015) Ecological release and directional change in White Sands lizard trophic ecomorphology. Evol Ecol 29, 1–16. [Google Scholar]
- Dollion A, Measey GJ, Cornette R, et al. (2017) Does diet drive the evolution of head shape and bite force in chameleons of the genus Bradypodion? Funct Ecol 31, 671–684. [Google Scholar]
- Donihue CM, Brock KM, Foufopoulos J, et al. (2016) Feed or fight: testing the impact of food availability and intraspecific aggression on the functional ecology of an island lizard. Funct Ecol 30, 566–575. [Google Scholar]
- Dumont ER, Herrel A (2003) The effects of gape angle and bite point on bite force in bats. J Exp Biol 206, 2117–2123. [DOI] [PubMed] [Google Scholar]
- Dutel H, Herbin M, Clément G, et al. (2015) Bite force in the extant coelacanth Latimeria: the role of the intracranial joint and the basicranial muscle. Curr Biol 25, 1228–1233. [DOI] [PubMed] [Google Scholar]
- Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125, 1–15. [Google Scholar]
- Garland T, Harvey PH, Ives AR (1992) Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst Biol 41, 18–32. [Google Scholar]
- Garland T, Midford PE, Ives AR (1999) An introduction to phylogenetically based statistical methods, with a new method for confidence intervals on ancestral states. Am Zool 39, 374–388. [Google Scholar]
- Grant PR (1986) Ecology and Evolution of Darwin's Finches. Princeton, NJ: Princeton University Press. [Google Scholar]
- Greene HW (1982) Dietary and phenotypic diversity in lizards: why are some organisms specialized? In: Environmental Adaptation and Evolution. (eds Mosskowski D, Roth G.), pp. 107–128. Stuttgart: Gustav Fischer. [Google Scholar]
- Gröning F, Jones MEH, Curtis N, et al. (2013) The importance of accurate muscle modelling for biomechanical analyses: a case study with a lizard skull. J R Soc Interface 10, 20130216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas G (1973) Muscles of the jaws and associated structures in the Rhynchocephalia and Squamata In: Biology of the Reptilia, Vol. 4 (eds Gans C, Parsons T.), pp. 285–490. London: Academic Press. [Google Scholar]
- Hernandez LP, Motta PJ (1997) Trophic consequences of differential performance: ontogeny of oral jaw‐crushing performance in the sheepshead, Archosargus probatocephalus (Teleostei, Sparidae). J Zool Lond 243, 737–756. [Google Scholar]
- Herrel A, Holanova V (2008) Cranial morphology and bite force in Chamaeleolis lizards, adaptations to molluscivory? Zoology 111, 467–475. [DOI] [PubMed] [Google Scholar]
- Herrel A, Aerts P, De Vree F (1998a) Static biting in lizards: functional morphology of the temporal ligaments. J Zool Lond 244, 135–143. [Google Scholar]
- Herrel A, Aerts P, De Vree F (1998b) Ecomorphology of the lizard feeding apparatus: a modeling approach. Neth J Zool 48, 1–25. [Google Scholar]
- Herrel A, Spithoven L, Van Damme R, et al. (1999) Sexual dimorphism of head size in Gallotia galloti; testing the niche divergence hypothesis by functional analyses. Funct Ecol 13, 289–297. [Google Scholar]
- Herrel A, Van Damme R, Vanhooydonck B, et al. (2001) The implications of bite performance for diet in two species of lacertid lizards. Can J Zool 79, 662–670. [Google Scholar]
- Herrel A, Adriaens D, Verraes W, et al. (2002) Bite performance in clariid fishes with hypertrophied jaw adductors as deduced by bite modeling. J Morphol 253, 196–205. [DOI] [PubMed] [Google Scholar]
- Herrel A, Vanhooydonck B, Van Damme R (2004) Omnivory in lacertid lizards: adaptive evolution or constraint. J Evol Biol 17, 974–984. [DOI] [PubMed] [Google Scholar]
- Herrel A, Huyghe K, Vanhooydonck B, et al. (2008) Rapid large scale evolutionary divergence in morphology and performance associated with exploitation of a different dietary resource. Proc Natl Acad Sci USA 105, 4792–4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges WL, Zamudio KR (2004) Horned lizard (Phrynosoma) phylogeny inferred from mitochondrial genes and morphological characters: understanding conflicts using multiple approaches. Mol Phyl Evol 31, 961–971. [DOI] [PubMed] [Google Scholar]
- Huyghe K, Vanhooydonck B, Scheers H, et al. (2005) Morphology, performance and fighting capacity in male lizards, Gallotia galloti . Funct Ecol 19, 800–807. [Google Scholar]
- Irschick DJ, Garland T Jr (2001) Integrating function and ecology in studies of adaptation: investigations of locomotor capacity as a model system. Ann Rev Ecol Syst 32, 367–396. [Google Scholar]
- Kaliontzopoulou A, Adams DC, van der Meijden A, et al. (2012) Relationships between head morphology, bite performance and ecology in two species of Podarcis wall lizards. Evol Ecol 26, 825–845. [Google Scholar]
- Kiltie RA (1982) Bite force as a basis for niche differentiation between rain forest peccaries (Tayassu tajacu and T. pecari). Biotropica 14, 188–195. [Google Scholar]
- Leaché AD, McGuire JA (2006) Phylogenetic relationships of horned lizards (Phrynosoma) based on nuclear and mitochondrial data: evidence for a misleading mitochondrial gene tree. Mol Phyl Evol 39, 628–644. [DOI] [PubMed] [Google Scholar]
- Lopez‐Darias M, Vanhooydonck B, Cornette R, et al. (2015) Sex‐specific differences in ecomorphological relationships in lizards of the genus Gallotia . Funct Ecol 29, 506–514. [Google Scholar]
- McPeek MA (1997) Measuring phenotypic selection on an adaptation: lamellae of damselflies experiencing dragonfly predation. Evolution 51, 459–466. [DOI] [PubMed] [Google Scholar]
- Mendez J, Keys A (1960) Density and composition of mammalian muscle. Metabolism 9, 184–188. [Google Scholar]
- Meyers JJ, Herrel A (2005) Prey capture kinematics of ant‐eating lizards. J Exp Biol 208, 113–127. [DOI] [PubMed] [Google Scholar]
- Meyers JJ, Herrel A, Nishikawa KC (2006) Ecomorphology of the feeding system in a specialized group of lizards (Phrynosoma). Biol J Linn Soc 89, 13–24. [Google Scholar]
- Montanucci RR (1981) Habitat separation between Phrynosoma douglassi and Phrynosoma orbiculare (Lacertilia:Iguanidae) in Mexico. Copeia 1981, 147–153. [Google Scholar]
- Montanucci RR (1987) A phylogenetic study of the horned lizards, genus Phrynosoma, based on skeletal and external morphology. Cont Sci Nat Hist Mus Los Ang Co 390, 1–36. [Google Scholar]
- Montanucci RR (1989) The relationship of morphology to diet in the horned lizard genus Phrynosoma . Herpetologica 45, 208–216. [Google Scholar]
- Munger JC (1984) Home ranges of horned lizards (Phrynosoma): circumscribed and exclusive? Oecologia 61, 351–360. [DOI] [PubMed] [Google Scholar]
- Naples V (1999) Morphology, evolution and function of feeding in the giant anteater (Myrmecophaga tridactyla). J Zool Lond 249, 19–41. [Google Scholar]
- Narici MV, de Boer MD (2011) Disuse of the musculo‐skeletal system in space and on earth. Eur J Appl Physiol 111, 403–420. [DOI] [PubMed] [Google Scholar]
- Parker PS, Pianka ER (1973) Notes on the ecology of the iguanid lizard, Sceloporus magister . Herpetologica 29, 143–152. [Google Scholar]
- Pianka ER, Parker WS (1972) Ecology of the iguanid lizard Callisaurus draconoides . Copeia 1972, 493–508. [Google Scholar]
- Pianka ER, Parker WS (1975) Ecology of horned lizards: a review with special reference to Phrynosoma platyrhinos . Copeia 1975, 141–162. [Google Scholar]
- Presch W (1969) Evolutionary osteology and relationships of the horned lizard genus Phrynosoma (family Iguanidae). Copeia 1969, 250–275. [Google Scholar]
- Pyron RA, Burbrink FT, Wiens JJ (2013) A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol Biol 13, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder TW, Montanucci RR (2001) Phylogenetic analysis of the horned lizards (Phrynosomatidae: Phrynosoma): evidence from mitochondrial DNA and morphology. Copeia 2001, 309–322. [Google Scholar]
- Reeve WL (1952) Taxonomy and distribution of the horned lizard genus Phrynosoma . Univ Kans Sci Bull 34, 817–960. [Google Scholar]
- Reiss K (1997) Myology of the feeding apparatus of myrmecophagid anteaters (Xenarthra: Myrmecophagidae). J Mamm Evol 40, 87–117. [Google Scholar]
- Reiss K (2000) Feeding in myrmecophagous mammals In: Feeding. (ed. Schwenk K.), pp. 459–485. New York: Academic Press. [Google Scholar]
- Sacco T, Van Valkenburgh B (2004) Ecomorphological indicators of feeding behaviour in the bears (Carnivora: Ursidae). J Zool Soc Lond 263, 41–54. [Google Scholar]
- Sagonas K, Pafilis P, Lymberakis P, et al. (2014) Insularity affects head morphology, bite force and diet in a Mediterranean lizard. Biol J Linn Soc 112, 469–484. [Google Scholar]
- Santana SE (2015) Quantifying the effect of gape and morphology on bite force: biomechanical modeling and in vivo measurements in bats. Funct Ecol 30, 557–565. [Google Scholar]
- Schaerlaeken V, Holanova V, Boistel R, et al. (2012) Built to bite: feeding kinematics, bite forces and head shape of a specialized durophagous lizard, Dracaena guianensis (Teiidae). J Exp Zool 317A, 371–381. [DOI] [PubMed] [Google Scholar]
- Schwenk K (2000) Feeding in Lepidosaurs In: Feeding. (ed. Schwenk K.), pp. 175–291. New York: Academic Press. [Google Scholar]
- Sokal R, Rohlf FJ (1995) Biometry. New York: W.H. Freeman. [Google Scholar]
- Thomas P, Pouydebat E, Hardy I, et al. (2015) Sexual dimorphism in bite force in the grey mouse lemur (Microcebus murinus). J Zool 296, 133–138. [Google Scholar]
- Turner DS (1998) Ecology of Cowles fringe‐toed lizard, Uma notata, in Arizona's Mohawk Sand Dunes. Unpublished MS. Thesis, Tucson: University of Arizona. [Google Scholar]
- Van der Meij MAA, Bout RG (2004) Scaling of jaw muscle size and maximal bite force in finches. J Exp Biol 207, 2745–2753. [DOI] [PubMed] [Google Scholar]
- Van der Meij MAA, Griekspoor M, Bout RG (2004) The effect of seed hardness on husking time in finches. Anim Biol 54, 195–205. [Google Scholar]
- Vanhooydonck B, Cruz FB, Abdala CS, et al. (2010) Sex‐specific evolution of bite performance in Liolaemus lizards (Iguania: Iguanidae): the battle of the sexes. Biol J Linn Soc 101, 461–475. [Google Scholar]
- Verwaijen D, Van Damme R, Herrel A (2002) Relationships between head size, bite force, prey handling efficiency and diet in two sympatric lacertid lizards. Funct Ecol 16, 842–850. [Google Scholar]
- Wainwright PC, Reilly SM (1994) Ecological Morphology: Integrative Organismal Biology. Chicago, IL: University of Chicago Press. [Google Scholar]
- Wittorski A, Losos JB, Herrel A (2016) Proximate determinants of bite force in Anolis lizards. J Anat 228, 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamudio KR (1998) The evolution of female‐biased sexual size dimorphism: a population‐level comparative study in horned lizards. Evolution 52, 1821–1833. [DOI] [PubMed] [Google Scholar]


