Abstract
Sexual dimorphism in the human respiratory system has been previously reported at the skeletal (cranial and thoracic) level, but also at the pulmonary level. Regarding lungs, foregoing studies have yielded sex‐related differences in pulmonary size as well as lung shape details, but different methodological approaches have led to discrepant results on differences in respiratory patterns between males and females. The purpose of this study is to analyse sexual dimorphism in human lungs during forced respiration using 3D geometric morphometrics. Eighty computed tomographies (19 males and 21 females) were taken in maximal forced inspiration (FI) and expiration (FE), and 415 (semi)landmarks were digitized on 80 virtual lung models for the 3D quantification of pulmonary size, shape and kinematic differences. We found that males showed larger lungs than females (P < 0.05), and significantly greater size and shape differences between FI and FE. Morphologically, males have pyramidal lung geometry, with greater lower lung width when comparing with the apices, in contrast to the prismatic lung shape and similar widths at upper and lower lungs of females. Multivariate regression analyses confirmed the effect of sex on lung size (36.26%; P < 0.05) and on lung shape (7.23%; P < 0.05), and yielded two kinematic vectors with a small but statistically significant angle between them (13.22°; P < 0.05) that confirms sex‐related differences in the respiratory patterns. Our 3D approach shows sexual dimorphism in human lungs likely due to a greater diaphragmatic action in males and a predominant intercostal muscle action in females during breathing. These size and shape differences would lead to different respiratory patterns between sexes, whose physiological implications need to be studied in future research.
Keywords: expiration, inspiration, landmark, lungs, semilandmark, shape, size
Introduction
The lungs are breathing organs located into the ribcage, and the air enters and leaves them through the main bronchi, which are branches of the trachea. The pulmonary arteries carry deoxygenated blood to the lungs from the right ventricle of the heart, while the oxygenated blood returns to the left atrium through the pulmonary veins. This coordination between respiratory system and cardiovascular system allows for gas exchange between alveoli and blood capillaries (Gray, 2009; West, 2012).
Breathing kinematics of the human respiratory system
The lungs are protected by the ribcage, which has been divided into two functional compartments regarding costal muscle attachments: the pulmonary (upper) thorax (1st–6th ribs, scalenes, parasternal intercostal and sternocleidomastoid muscles); and the diaphragmatic (lower) thorax (7th–12th ribs and diaphragm) (Campbell, 1955; Campbell & Newsom, 1970; Roussos & Macklem, 1982; De Troyer & Estenne, 1984; Ward et al. 1992; West, 2012; Beyer et al. 2013, 2016; Bastir et al. 2017). The mechanics of the chest wall and the ribs allow for an effective movement of the air during breathing (De Troyer & Estenne, 1984; Frappell & MacFarlane, 2005). In quiet inspiration, respiratory muscles increase the mediolateral and anteroposterior dimensions of the chest wall, modifying the volume of the chest cavity and decreasing the intrapulmonary pressure. Besides the intercostal muscles and the diaphragm, throughout forced inspiration (FI), scalene muscles elevate the 1st and 2nd ribs, and the sternocleidomastoid lifts the sternum (Ratnovsky et al. 2008; West, 2012). In quiet expiration, the volume of the chest wall decreases and the recoil force of the lungs drives expiration passively (Stockert, 2003). During forced expiration (FE), abdominal and internal intercostal muscles decrease actively the dimensions of the chest wall (Zach, 2000). Such respiratory patterns are the result of complex and coordinated actions between the ribcage and these muscles, and they are essential elements for respiration (Campbell & Newsom, 1970; De Troyer & Estenne, 1984; Ratnovsky et al. 2008).
Breathing patterns can change according to various factors, including growth and age (Bastir et al. 2013; Shi et al. 2014; Weaver et al. 2014; García‐Martínez et al. 2016a), exercise (Johnson et al. 1992; McClaran et al. 1998; Guenette et al. 2007; Aliverti, 2008; Layton et al. 2011; Howes et al. 2013), disease (Decramer, 1989, 1997; Aliverti & Macklem, 2001; García‐Río, 2005), body position (Wade, 1954; Agostoni et al. 1965; Konno & Mead, 1967; Sharp et al. 1975; Romei et al. 2010) and sex (Bellemare et al. 2003; Parreira et al. 2010; Romei et al. 2010; García‐Martínez et al. 2016b). The aim of this study is to address the influence of sexual dimorphism on 3D pulmonary size, shape and kinematics.
Relevance of the study of sexual dimorphism in human lungs
Sexual dimorphism in the respiratory system has been previously reported at different skeletal levels (Rosas & Bastir, 2002; Bellemare et al. 2003; Bastir et al. 2011; García‐Martínez et al. 2016b). Thus, it has been observed that males have larger cranial airways and taller piriform apertures than females (Enlow & Hans, 1996; Rosas & Bastir, 2002; Bastir et al. 2011). This is consistent with larger ribcages also found in males (Bellemare et al. 2003), which has been related to both body size and pulmonary gas exchange differences between sexes (Mead, 1980; Adams et al. 2004; Hopkins & Harms, 2004; Hall, 2005). Furthermore, smaller ribcages in females are consistent with smaller lung volumes than those of males of the same height and age (Crapo et al. 1981; Thurlbeck, 1982), although Bellemare et al. (2003) reported a disproportionate growth of the ribcage in relation to the lungs. This, along with a greater rib declination and a greater intercostal muscles contribution observed in females during breathing, has been proposed as compensation to spatial problems during pregnancy (Bellemare et al. 2003).
Recently, Romei et al. (2010) studied the effect of sex and body position on respiratory chest wall kinematics of healthy individuals at rest. Their results revealed that the movement of the chest wall was influenced by body position (supine/upright), with a progressive inclination of the trunk leading to a reduction of the displacement of the chest wall and to an increase of abdominal contribution to the tidal volume (Vt). Importantly, this different contribution was more evident in females than in males (Romei et al. 2010), giving rise to the idea that males and females might show different respiratory kinematic patterns. More recently, research on sexual dimorphism of 3D ribcage morphology (Shi et al. 2014; Weaver et al. 2014; García‐Martínez et al. 2016b) reported shape differences between males and females, possibly also reflecting differences in pulmonary shape. Such potential differences in lung morphology could underlie the different breathing patterns detected by Romei et al. (2010), but sexual dimorphism in the 3D morphology and kinematic function of the lungs has not yet been studied.
Sex‐related differences in pulmonary function can be assessed from different approaches. On the one hand, lung function tests such as spirometry have been classically used for assessing lung function. Thus, assessing pulmonary response to exercise and training yielded that females are particularly susceptible to develop expiratory flow limitation due to the smaller diameter of the airways and lung size compared with males of the same age and height (Mead, 1980; Thurlbeck, 1982; Enlow & Hans, 1996; McClaran et al. 1998; Hopkins & Harms, 2004; Guenette et al. 2007). Furthermore, such anatomical differences result in a decrease in respiratory minute volume, and therefore in a reduction of oxygen consumption in females compared with males (Hopkins & Harms, 2004). On the other hand, respiratory plethysmography has been also used as a complementary method for evaluating breathing patterns and pulmonary function (Tobin et al. 1983; Verschakelen & Demedts, 1995; Parreira et al. 2010; Romei et al. 2010; Layton et al. 2011; LoMauro et al. 2012). Thus, this method has allowed for the quantification of sex‐related differences during quiet breathing through respiratory variables such as Vt, respiratory frequency (RF), minute ventilation (VE) and respiratory time (RT) (Tobin et al. 1983; Feltrim, 1994; Parreira et al. 2010). Despite females showing significantly lower Vt, shorter RT and higher RF, these authors concluded that males and females share overall similar breathing pattern. Ragnarsdóttir & Kristinsdóttir (2006) arrived at similar conclusions using respiratory movement measuring instruments.
Aims of this study
Although the studies mentioned above addressed sex‐related differences in the respiratory system from both physiological and biomechanical approaches, a systematic assessment accounting for 3D size, shape and kinematics of lungs has not yet been carried out. In this study, we used 3D geometric morphometrics to shed light on these discrepant results previously reported by investigating sex‐specific differences in lung morphology and function. Therefore, within the previously outlined context, here we addressed the following three hypotheses.
Hypothesis 1
There are sex‐related differences in lung size between males and females. This is expected because previous studies have reported sex‐related differences in lung size, as well as evidence for smaller ribcage dimensions in females from different approaches (Thurlbeck, 1982; Bellemare et al. 2003; Shi et al. 2014; García‐Martínez et al. 2016b).
Hypothesis 2
There are lung shape differences between males and females. This is expected, as sex‐related differences in skeletal thorax configuration have been reported (Bellemare et al. 2003; Shi et al. 2014; Weaver et al. 2014; García‐Martínez et al. 2016b), and close relationships between the form of the ribcage and the form of the lungs are assumed (Gayzik et al. 2008).
Hypothesis 3
There are differences in male and female pulmonary kinematics. This is expected assuming sex‐specific differences in lung shape (Hypothesis 2), and a close functional relationship between internal pulmonary and external chest wall kinematics (Romei et al. 2010).
Materials and methods
Subjects and 3D data
The sample consisted of 80 computed tomography scans (CT scans) of 40 individuals (19 males and 21 females, average age 51.9 ± 1.2 years) in maximal FE and FI. The individuals were part of a healthy control group in a Chronic Obstructive Pulmonary Disease study unrelated to this research (Radiology Service of University Hospital La Paz, Madrid, Spain). Because all the individuals were non‐smokers and none of them suffered from respiratory pathologies, they were considered suitable for this study. According to the Helsinki protocol (Goodyear et al. 2007), all of them signed a written consent that allowed the use of these data for scientific purposes.
All CT scans were performed with a 16‐MDCT scanner (Somatom Sensation 16, Siemens Medical Solutions, Erlangen, Germany). The scanning voltage was 120 kV and the current was 160 mA. CT of the thorax was performed in the supine position, from the lung apex to the level of the diaphragm in maximal FE followed by maximal FI. All imaging was performed with a collimation of 16 × 0.75 mm, table feed of 30 mm per rotation and rotation time of 0.6 s per 360 °. Cross‐sectional images were reconstructed using the standard method, and the resulting 3D CT data were imported in DICOM format using mimics 8.0 software (http://biomedical.materialise.com/mimics). The marching‐cube algorithm was applied to create 3D triangular meshes of the lungs (Lorensen & Cline, 1987) that were ultimately post‐processed (cleaning, smoothing edges and filling gaps) using artec studio software (www.artec.com) to facilitate 3D anatomical measurements.
3D geometric morphometrics and data measurement
3D geometric morphometrics allows for the quantification of size and shape through points called landmarks and semilandmarks, which are defined by 3D Cartesian coordinates (Bookstein, 1991; Zelditch et al. 2012; Gunz & Mitteroecker, 2013). While ‘landmarks’ are homologous and discrete points easily recognizable in all specimens, complex curves and surfaces require points called ‘semilandmarks’ because of uncertainty in terms of their location. We measured a total of 415 landmarks and semilandmarks on 80 virtual lung models using viewbox 4.0 software (www.dhal.com): 12 anatomical landmarks, 103 semilandmarks along 10 curves and 300 semilandmarks on four surfaces. These points were distributed in order to collect as much information as possible on pulmonary morphology (see Table 1 and Fig. 1 for the anatomical position). Because of the lesser homology and the uncertainty in terms of their location on the lungs, semilandmarks were subjected to a ‘sliding’ process along the tangent vectors of their corresponding curves and the tangent planes to the surfaces, in order to minimize shape variation only due to arbitrary position (Mitteroecker & Gunz, 2009; Gunz & Mitteroecker, 2013). The process was repeated twice for each specimen: the first time taking the template of digitization as a reference for the sliding; and the second time taking the mean form of the 80 configurations. This iterative process minimized the bending energy (BE), i.e. the amount of shape deformation of each specimen with respect to the reference configuration (Gunz et al. 2005; Gunz & Mitteroecker, 2013). Finally, this allowed us to mathematically maximize the homology of the curves and surfaces by minimizing the BE of each specimen with respect to the reference (Gunz et al. 2009; Gunz & Mitteroecker, 2013).
Table 1.
Anatomical position of 12 landmarks (lm), 103 curve semilandmarks (Curve sml) and 300 surface semilandmarks (Surface sml)
| Number of (semi)lm | Type | Anatomical position |
|---|---|---|
| 001 | lm | Apex of lung (R) |
| 002 | lm | Apex of lung (L) |
| 003 | lm | Impression costae primae (R) |
| 004 | lm | Impression costae primae (L) |
| 005 | lm | Apex middle lobe (R) |
| 006 | lm | Lingula of left lung (L) |
| 007 | lm | Lower lobe medial point (R) |
| 008 | lm | Lower lobe medial point (L) |
| 009 | lm | Oblique fissure (R) |
| 010 | lm | Oblique fissure (L) |
| 011 | lm | Carina |
| 012 | lm | Bifurcation upper and middle bronchus (R) |
| 013–030 | Curve sml | Anterior margin (R) |
| 031–053 | Curve sml | Inferior margin of lung (R) |
| 054–061 | Curve sml | Apex middle lobe (R)–lower lobe medial point curve (R) |
| 062–079 | Curve sml | Anterior margin (L) |
| 080–097 | Curve sml | Inferior margin of lung (L) |
| 098–105 | Curve sml | Apex middle lobe (L)–lower lobe medial point curve (L) |
| 106–110 | Curve sml | Apex of lung (R)–oblique fissure curve (R) |
| 111–115 | Curve sml | Apex of lung (L)–oblique fissure curve (L) |
| 116–215 | Surface sml | Costal surface (R) |
| 216–265 | Surface sml | Costal surface (L) |
| 266–365 | Surface sml | Diaphragmatic surface (R) |
| 366–415 | Surface sml | Diaphragmatic surface (L) |
L, left; R, right.
Figure 1.
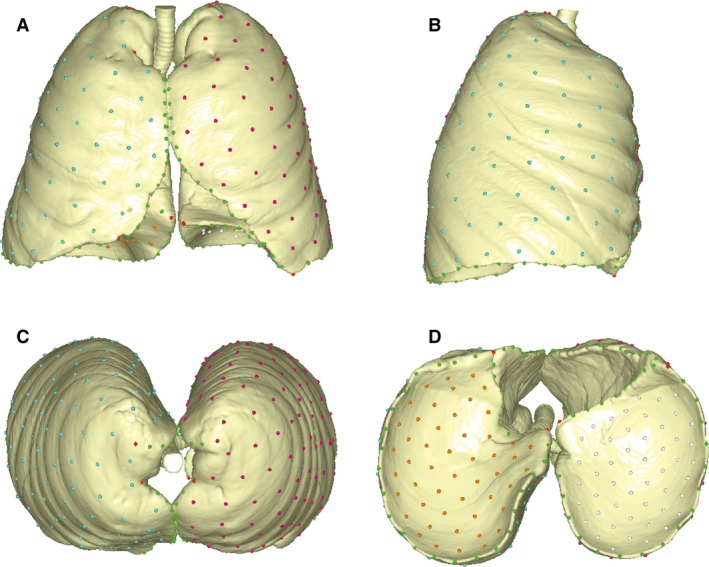
Template of digitization. Landmarks (red), sliding curve semilandmarks (green) and surface semilandmarks (other colors) used to describe lung morphology (see Table 1 for the anatomical position); front view (A), right lateral view (B), axial view (C) and inferior view (D).
The 80 (semi)landmarks configurations were subjected to a generalized Procrustes analysis (GPA), which applied translation, rotation and scaling in order to remove any variation not related to the shape (Rohlf & Slice, 1990). Lung size was measured by centroid size (CS) – the square root of the sum of squared distances of each landmark to the center (Zelditch et al. 2012) – and GPA yielded Procrustes shape coordinates as a measure of 3D lung shape. Lung size was measured by mean CS comparisons, and lung shape was analysed by statistical analysis of the Procrustes shape coordinates and Procrustes distances – the summed, squared inter‐landmark distances between corresponding landmarks once the GPA has been carried out (Bookstein, 1991; Dryden & Mardia, 1998; O'Higgins, 2000; Adams et al. 2004; Mitteroecker & Gunz, 2009).
Lung size, shape and kinematic analyses
To test Hypothesis 1, we calculated lung mean CS at FI and FE for males and females as a proxy for volumetric differences in terms of respiratory capacity. After performing a Kolmogorov–Smirnov normality test and a homoscedasticity test (Levene, 1960) for each group (significance level, SL = 0.05), we compared the CS means at FI and FE between males and females using Student's t‐tests (Sokal & Rohlf, 1998). Additionally, we explored the influence of pulmonary kinematics (FI/FE) and sex (male/female) on lung size (CS) through multivariate regression analyses (SL = 0.05, 10 000 permutations).
To test Hypothesis 2, we quantified lung shape differences due to pulmonary kinematics and sexual dimorphism through mean shape comparisons of Procrustes shape coordinates and Procrustes distances (SL = 0.05; 10 000 permutations). Lung shape variation related to kinematic function and sexual dimorphism was further explored by a principal component analysis (PCA) in form space – which includes size by definition (Mitteroecker & Gunz, 2009). We visualized lung mean shapes and lung shape variation along principal component (PC)1 and PC2 using the thin‐plate spline method on evan toolbox 1.71 software (http://www.evan-society.org/). Finally, we performed multivariate regression analyses (SL = 0.05, 10 000 permutations) to quantify the influence of pulmonary kinematics (FI/FE) and sex (male/female) on lung shape (Procrustes shape coordinates).
To test Hypothesis 3, we quantified kinematic differences through ‘functional size’ (the difference in lung CS between FI and FE for males and females) as a measure of the individual's capacity to change pulmonary size during breathing (Torres‐Tamayo et al. 2015; Bastir et al. 2017) and ‘functional shape’ (Procrustes distances between FE and FI mean shapes for both sexes) as the individual's capacity for lung deformation (Bastir et al. 2017). Multivariate regressions of shape on pulmonary kinematics yielded two vectors of pulmonary shape deformation connecting FI and FE mean shapes for males and females in the same shape space (Bastir et al. 2013, 2016; Klingenberg & Marugán‐Lobón, 2013). The vector directions were compared through an angular comparison (Klingenberg, 2011), i.e. the angle between these vectors provides information about the similarity of breathing pattern between sexes (Bastir et al. 2013, 2017). Finally, we carried out regression analyses (SL=0.05, 10000 permutations) in order to explore the effect, if any, of absolute lung size on functional size, the effect of functional size on functional shape, and the influence of shape on functional size after removing the effect of sex. These analyses were carried out in morphoj software (Klingenberg, 2011).
Results
Size analysis
The four groups analysed were normally distributed and no statistically significant differences between the standard deviations were found (Table S1). Table 2 shows mean comparisons results of the Student's t‐tests. In males, lung size (CS) was larger than in females (P < 0.01) at both FI and FE. The regression results showed that lung size is influenced primarily by breathing kinematics (42.51%; P < 0.05), but also by sex (36.26%; P < 0.05). This evidence supports Hypothesis 1.
Table 2.
Student's t‐test results of CS mean comparisons between males and females in FI and FE
| Males FI | Females FI | Males FE | Females FE | |
|---|---|---|---|---|
| CS | 2652.70 | 2362.58 | 2340.10 | 2123.90 |
| Standard deviation | 109.39 | 64.01 | 119.97 | 90.75 |
| t‐value (P‐value) | −10.35 (P < 0.01) | −6.46 (P < 0.01) | ||
CS, centroid size; FE, forced expiration; FI, forced inspiration.
Shape analysis
Mean shape comparisons revealed significant differences in pulmonary shape between males and females at both FI and FE (Table 3). Specifically, the Procrustes distance between sexes was significantly larger at FE (0.076) than at FI (0.060), indicating greater lung shape differences at FE. The Procrustes distance between FI and FE was greater (P < 0.05) in males (0.123) than in females (0.106), indicating greater lung deformation during breathing in males. Figure 2 illustrates lung mean shapes in frontal, lateral and inferior views, and shows less caudally diverging lateral walls of the lungs in females (Fig. 2A, A′) than in males (Fig. 2D, D′). Regression analyses indicate that breathing kinematics accounts for 20.96% of lung shape (P < 0.05), and sex for 7.23% (P < 0.05). These findings lead us to accept Hypothesis 2.
Table 3.
Procrustes distances between the different groups studied
| Females FI | Females FE | Males FI | |
|---|---|---|---|
| Males FI | 0.060 | – | 0 |
| Males FE | – | 0.076 | 0.123 |
| Females FE | 0.106 | 0 | – |
FE, forced expiration; FI, forced inspiration.
Figure 2.
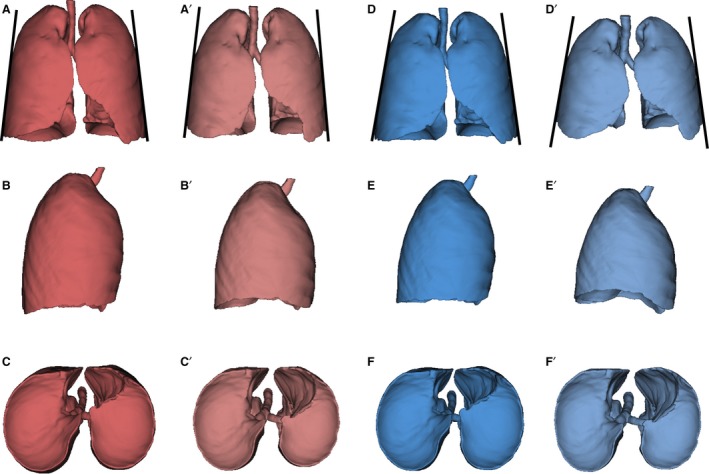
Lung mean forms of: females in forced inspiration (FI; A–C), females in forced expiration (FE; A′–C′), males in forced inspiration (FI; D–F) and males in forced expiration (FE;D′–E′). Frontal view (row 1), right lateral view (row 2) and inferior view (row 3).
In a further exploration, PCA (Fig. 3; Table S2) reveals that PC1–3 explain 74.5% of the total lung shape variation and show a clear differentiation between the different groups in a PC1–PC2 subspace. PC1 reflects variations related to breathing kinematics: FI towards negative scores, FE towards positive scores. Morphologically, at FI (Fig. 4A,C,E), the anterior edges of the lungs (solid arrows) and the medial lower vertices (dashed arrows) approach each other, and the pulmonary cardiac notch acquires a rectilinear morphology. At FE (Fig. 4B,D,F), the anterior edges (solid arrows) and the medial lower vertices (dashed arrows) become more separated from each other, and the pulmonary cardiac notch acquires a curved shape. PC2 shows sexual dimorphism, with males falling towards negative values and females towards positive values (Fig. 3). Negative values (Fig. 5A,C,E) reflect a relative shortening along the cranio‐caudal axis, and an increase in the medio‐lateral axis at the lower lungs, which results in a pyramidal lung shape in males. Positive values (Fig. 5B,D,F) show an increased cranio‐caudal axis and a slightly increased medio‐lateral axis on the upper lungs, leading to a more prismatic shape (see Video [Link], [Link], [Link], [Link], illustrating shape changes along PC1–PC2).
Figure 3.
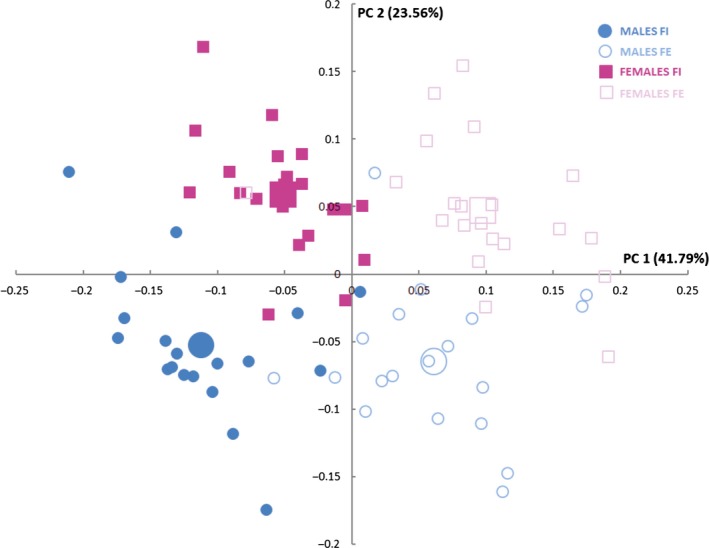
Scatterplot showing the relationship of the different subjects studied at principal component (PC)1–PC2. Females in forced inspiration (FI; pink filled squares), females in forced expiration (FE; pink unfilled squares), males in FI (blue filled circles) and males in FE (light unfilled circles). Larger squares and circles represent the mean of each group.
Figure 4.
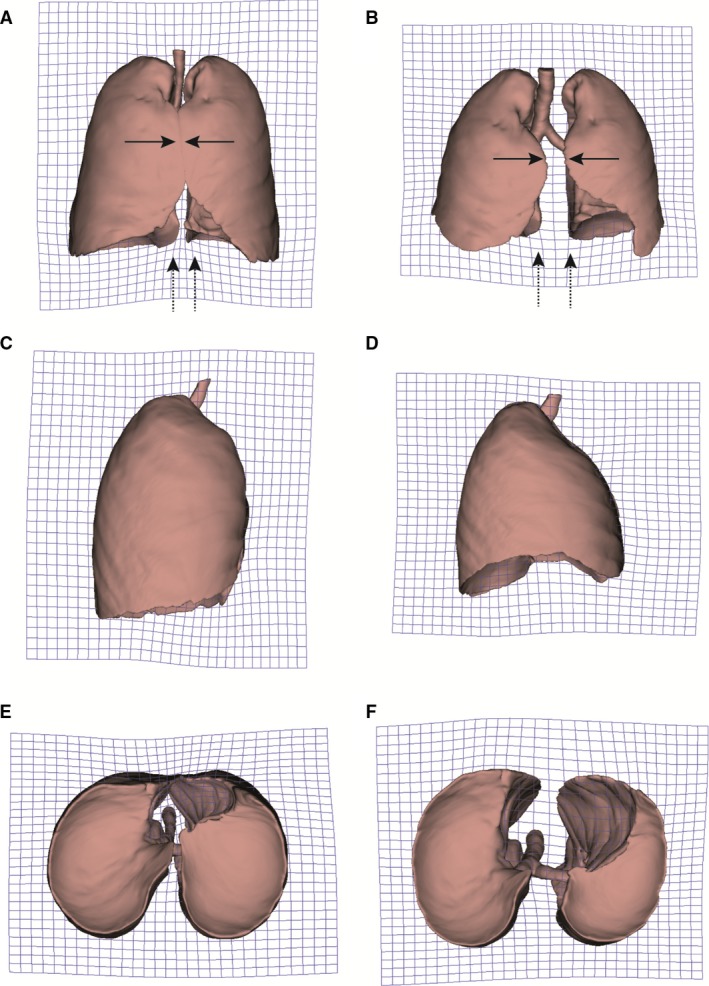
Surface warps associated to principal component (PC)1. Grids were warped from overall lung mean shape to the lung shape at forced inspiration (FI; left: negative values of PC1), and to the lung shape at forced expiration (FE; right: positive values of PC1). Frontal view (row 1), right lateral view (row 2) and inferior view (row 3).
Figure 5.
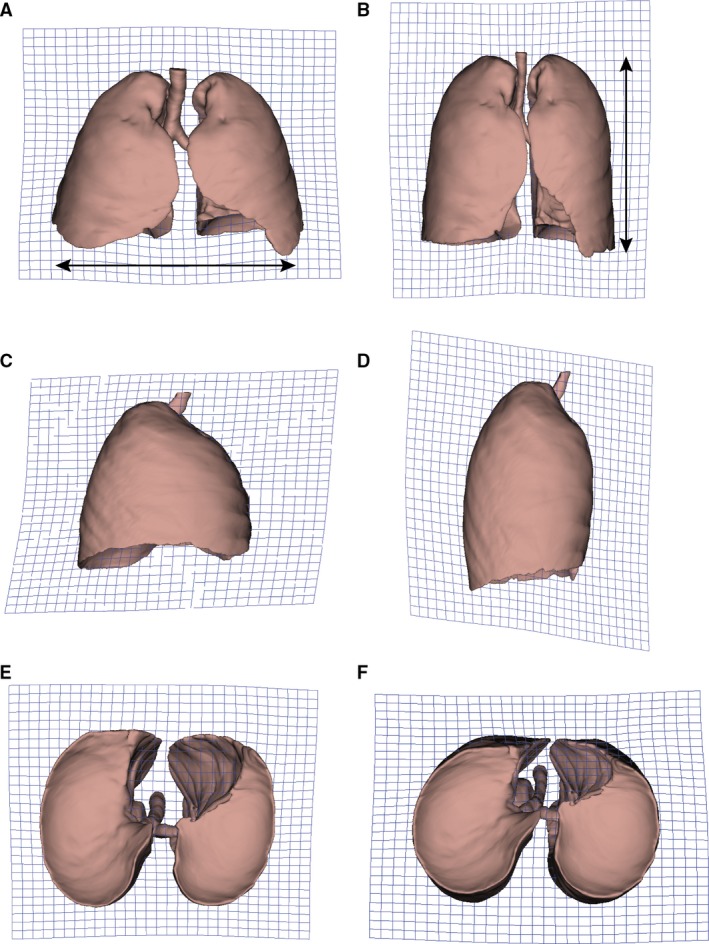
Surface warps associated to principal component (PC)2. Grids were warped from overall lung mean shape to the more pyramidal lung shape of males (left: negative values of PC2), and to the more prismatic lung shape of females (right: positive values of PC2). Frontal view (row 1), right lateral view (row 2) and inferior view (row 3).
Breathing kinematics analysis
Mean functional size is larger in males (312.57) than in females (238.63; t‐value = −3.29; P < 0.01), as well as functional shape (Table 3), with larger Procrustes distances in males between FI and FE (P < 0.05). This indicates that males show a greater capacity to change the pulmonary size and pulmonary shape during breathing, with functional lung size having an important effect on functional lung shape (59.87%; P < 0.01). Importantly, further regression analyses allowed us to discard that the greater functional size in males is due to a greater absolute lung size (0.02%; P = 0.91), and any significant relation between shape and functional size was lost after removal of the effect of sex, both at FE (3.94%; P = 0.18) and FI (1.56%; P = 0.69). The angular comparison between the two kinematic vectors extracted by regression of pulmonary kinematics on shape (α = 13.22 °; P < 0.05) reveals further sex‐specific differences in the patterns of lung deformation during breathing and allows us to accept Hypothesis 3.
Discussion
This is the first study that assesses in vivo 3D size and shape pulmonary sexual dimorphism using 3D geometric morphometrics. Our results indicate clear lung differences between males and females, as well as in their breathing patterns.
Size differences
We found significantly greater lung sizes in males than in females, at both FI and FE (Table 2), confirming previous assertions (Thurlbeck, 1982; Bellemare et al. 2003). The small lung size of females is consistent with the smaller diameter of their airways and alveolar surface compared with males (Thurlbeck, 1982; Enlow & Hans, 1996; Ochs et al. 2004), and could be likely a consequence of both body size differences and sex hormone regulation of lung development (Stahl, 1967; Kimura et al. 2003; Carey et al. 2007; Sheel & Guenette, 2008). However, although it is expected that the development of the ribcage was consistent with that of the lungs (Gayzik et al. 2008; Bastir et al. 2013), Bellemare et al. (2003) proposed a slightly greater, disproportionate growth of the ribcage of females relative to the lungs, speculating about it as a possible adaptation to accommodate the increased abdominal cavity during pregnancy. This reported disproportion between lung size and ribcage size will be assessed in future research. We show that sex explains 36.26% of the variability in lung size, supporting Hypothesis 1 and confirming previous results (McClaran et al. 1998; Bellemare et al. 2003; Romei et al. 2010).
We also found significantly larger functional size in males than in females, probably because of a different contribution of the respiratory muscles during breathing (Verschakelen & Demedts, 1995; Bellemare et al. 2003; LoMauro et al. 2012; Bastir et al. 2017). Respiratory pattern is determined by the action of intercostal muscles , which contribute to FI by elevating the ribs (De Troyer et al. 2005); and the diaphragm, which increases the lung size by expanding the lower part of the lungs mediolaterally (West, 2012). According to LoMauro et al. (2012), the action of the diaphragm on the lower thorax contributes to greater changes in thorax volume during breathing than the intercostal muscles acting on the upper thorax. Therefore, the lower thorax would contribute much more to functional size than the upper thorax, as confirmed by Bastir et al. (2017) on skeletal data. This would explain the greater capacity of volume expansion in males, although we must consider kinematic status as the main factor of variation in lung size (42.51%; P < 0.05).
Shape differences
Differences in pulmonary shape between males and females are shown in the mean shape comparisons (Fig. 2 Table 3) and along PC2 (Fig. 5), revealing the more pyramidal lung shape in males contrasting the more prismatic lung shape observed in females. We propose that these trends are the morphological reflection of a greater contribution of the diaphragm in males during breathing (Romei et al. 2010), which expands the lower lungs mediolaterally (Fig. 5A), as well as a greater action of the intercostal muscles in females on the upper lungs (Fig. 5B) (Bellemare et al. 2003; Binazzi et al. 2006). The greater contribution of intercostal muscles in females has been proposed as an adaptation to gestation period (Bellemare et al. 2003; LoMauro & Aliverti, 2015), as pregnancy leads to hormonal and anatomical effects on the respiratory system (Contreras et al. 1991; García‐Río et al. 1996; LoMauro & Aliverti, 2015). This could explain different patterns of muscle recruitment between males and females, and the resulting different lung shapes.
In the light of the close spatial relationships between the transverse processes and the ribs, Bastir et al. (2014) found more dorsally oriented transverse processes in males at T1 and T5–T9 levels, and more superiorly oriented facets for the articulation of the rib tubercles in females, indicating a different position of the ribs at the costotransverse articulation. In males, the transverse processes orientation leads to a reorientation of the ribs that would increase the medio‐lateral diameter of the ribcage at lower levels (T5–T9). This, along with the more ventrally oriented processes in females at these levels, as well as the greater rib curvature recently reported by Chapman et al. (2017), could lead to the sex‐related shape differences observed in this study. We confirmed these pulmonary shape differences by comparing male and female mean shapes at FI and FE, noting that lung shape differences (Procrustes distances) between males and females are greater in FE than in FI (Fig. 2 Table 3). Because sex explains 7.23% (P < 0.05) of lung shape, these results allow us to accept Hypothesis 2.
Lung deformation (functional shape) was significantly greater in males than in females (Table 3), which is consistent with significantly greater functional sizes, as confirmed by regression analysis (59.87%; P < 0.05). Bellemare et al. (2003) suggested a different pattern of respiratory muscle recruitment between males and females, with females showing a greater contribution of the intercostal muscles due to a greater inclination of the ribs, and males having a greater mediolateral expansion of the lower lungs due to a predominant diaphragmatic action. Functionally, these different contributions of the respiratory muscles lead to three types of rib movements classically described during breathing: ‘pump‐handle’ movement, more important in the pulmonary thorax and involving the anteroposterior expansion of the ribcage; and ‘bucket‐handle’ movement, which along with ‘spreading‐caliper’ movement is predominant in the diaphragmatic thorax and allows for the mediolateral expansion of the ribcage (Aiello & Dean, 1990; Franciscus & Churchill, 2002). Our results on lung shape differences between sexes suggest that females have a predominant ‘pump‐handle’ rib movement, while males show a ‘bucket‐handle’ movement of the ribs. Given that the greater functional size is not related to the pulmonary size in males (0.02%; P = 0.91), we conclude that sex‐specific musculoskeletal features such as a greater diaphragmatic action, a larger muscle length (Bellemare et al. 2003), and consequently a greater capacity of muscle contraction (Roussos & Koutsoukou, 2003; Adams et al. 2004; Gea et al. 2009), allow for a greater kinematic lung deformation in males. Our results are thus consistent with Gea et al. (2009) and Romei et al. (2010), both suggesting greater abdominal contribution in males during breathing.
Finally, the small but statistically significant angle between the male and female kinematic vectors (13.22 °; P < 0.05) confirms slightly different respiratory patterns between sexes. On the basis of 3D geometric morphometrics, our results are in line with previous assertions according to which males and females have different respiratory patterns (Romei et al. 2010; based on chest surface morphology). However, our findings may contrast with other authors who reported similar breathing patterns by measuring respiratory variables (Tobin et al. 1983; Feltrim, 1994; Ragnarsdóttir & Kristinsdóttir, 2006; Parreira et al. 2010). Therefore, although our results lead us to accept Hypothesis 3, they should be interpreted with caution, as further investigation is necessary in analysing 3D lung sexual dimorphism from both morphological and physiological approaches. Such analyses will clarify the physiological implications of these breathing kinematic differences, and they will shed light on important questions of form–function relations in the human respiratory system.
Conclusions
Our findings reveal 3D lung sexual dimorphism, as well as breathing kinematic differences between sexes. Males have greater lungs than females in both FI and FE, and they show greater size and shape changes during breathing. This is likely due to a different contribution of respiratory muscles, which is observed in the more pyramidal lung shapes in males and the more prismatic lung shapes observed in females. Likewise, these lung size and shape sex‐related differences give rise to breathing kinematics differences, whose physiological implications need to be investigated in future research.
Author contributions
Conceived and designed the experiments: MB. Acquisition of data: NTT, DGM, ITS, FGR. Data analysis/interpretation: NTT, DGM, MB. Drafting of the manuscript: NTT, MB. Critical revision of the article: NTT, DGM, SLZ, ITS, FGR, MB. Approval of the article: MB.
Supporting information
Video S1. Lung deformation associated to PC1 in frontal view, from FI to FE. Grids were warped from the overall mean shape to the mean shape at FI (negative values of PC1) and FE (positive values of PC1).
Video S2. Lung deformation associated to PC1 in lateral view.
Video S3. Lung deformation associated to PC2 in frontal view. Grids were warped from overall mean shape to the pyramidal mean shape of males (negative values of PC2), and the prismatic mean shape of females (positive values of PC2).
Video S4. Lung deformation associated to PC2 in lateral view.
Table S1. Results of normality and homoscedasticity tests. K–S, Kolmogorov–Smirnov; FI, forced inspiration; FE, forced expiration.
Table S2. Percentage of variance and cumulative variance explained by each PC of the PCA.
Acknowledgements
This research is funded by CGL‐2012‐37279, CGL‐2015‐63648‐P (Ministry of Economy, Industry and Competitiveness, Spain) and PI10/02089 (Fondo de Investigación Sanitaria) Ministry of Health, Social Services and Equality, Spain.
References
- Adams DC, Rohlf FJ, Slice DE (2004) Geometric morphometrics: ten years of progress following the ‘revolution’. It J Zool 71, 5–16. [Google Scholar]
- Agostoni E, Mognoni P, Torri G, et al. (1965) Relation between changes of rib cage circumference and lung volume. J Appl Physiol 20, 1179–1186. [Google Scholar]
- Aiello L, Dean C (1990) An Introduction to Human Evolutionary Anatomy. London: Academic Press Harcourt Brace & Company. [Google Scholar]
- Aliverti A (2008) Lung and chest wall mechanics during exercise: effects of expiratory flow limitation. Respir Physiol Neurobiol 163, 90–99. [DOI] [PubMed] [Google Scholar]
- Aliverti A, Macklem PT (2001) How and why exercise is impaired in COPD. Respiration 68, 229–239. [DOI] [PubMed] [Google Scholar]
- Bastir M, Godoy P, Rosas A (2011) Common features of sexual dimorphism in the cranial airways of different human populations. Am J Phys Anthropol 146, 414–422. [DOI] [PubMed] [Google Scholar]
- Bastir M, García‐Martínez D, Recheis W, et al. (2013) Differential growth and development of the upper and lower human thorax. PLoS ONE 8, e75128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastir M, Higuero A, Ríos L, et al. (2014) Three‐dimensional analysis of sexual dimorphism in human thoracic vertebrae: implications for the respiratory system and spine morphology. Am J Phys Anthropol 155(4), 513–521. [DOI] [PubMed] [Google Scholar]
- Bastir M, García‐Martínez D, Torres‐Tamayo N, et al. (2017) In vivo 3D analysis of thoracic kinematics: changes in size and shape during breathing and their implications for respiratory function in recent humans and fossil hominins. Anatom Rec 300, 255–264. [DOI] [PubMed] [Google Scholar]
- Bellemare F, Jeanneret A, Couture J (2003) Sex differences in thoracic dimensions and configuration. Am J Respir Crit Care Med 168, 305–312. [DOI] [PubMed] [Google Scholar]
- Beyer B, Feipel V, Coupier J, et al. (2013) Thorax 3D modelling from costovertebral joint complex kinematics: preliminary results. In Proceedings of the XXIVth congress of the International Society of Biomechanics, Natal, pp.185.
- Beyer B, Jan SVS, Chèze L, et al. (2016) Relationship between costovertebral joint kinematics and lung volume in supine humans. Respir Physiol Neurobiol 232, 57–65. [DOI] [PubMed] [Google Scholar]
- Binazzi B, Lanini B, Bianchi R, et al. (2006) Breathing pattern and kinematics in normal subjects during speech, singing and loud whispering. Acta Physiol 186, 233–246. [DOI] [PubMed] [Google Scholar]
- Bookstein FL (1991) Morphometric Tools for Landmark Data: Geometry and Biology. Cambridge: Cambridge University Press. [Google Scholar]
- Campbell E (1955) The role of the scalene and sternomastoid muscles in breathing in normal subjects: an electromyographic study. J Anat 89, 378. [PMC free article] [PubMed] [Google Scholar]
- Campbell E, Agostoni E, Newsom Davis J (1970) The intercostal muscles and other muscles of the rib cage In: The Respiratory Muscles: Mechanics and Neural Control, 2nd edn pp. 161–174. Philadelphia: WB Saunders. [Google Scholar]
- Carey MA, Card JW, Voltz JW, et al. (2007) It's all about sex: gender, lung development and lung disease. Trends Endocrinol Metab 18, 308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T, Beyer B, Sholukha V, et al. (2017) How different are the Kebara 2 ribs to modern humans? J Anthropol Sci 95, 1–20. [DOI] [PubMed] [Google Scholar]
- Contreras G, Gutiérrez M, Beroíza T, et al. (1991) Ventilatory drive and respiratory muscle function in pregnancy. Am Rev Respir Dis 144, 837–841. [DOI] [PubMed] [Google Scholar]
- Crapo R, Morris A, Clayton P, et al. (1981) Lung volumes in healthy nonsmoking adults. Bulletin européen de physiopathologie respiratoire 18, 419–425. [PubMed] [Google Scholar]
- De Troyer A, Estenne M (1984) Coordination between rib cage muscles and diaphragm during quiet breathing in humans. J Appl Physiol 57, 899–906. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Kirkwood PA, Wilson TA (2005) Respiratory action of the intercostal muscles. Physiol Rev 85, 717–756. [DOI] [PubMed] [Google Scholar]
- Decramer M (1989) Effects of hyperinflation on the respiratory muscles. Eur Respir J 2, 299–302. [PubMed] [Google Scholar]
- Decramer M (1997) Hyperinflation and respiratory muscle interaction. Eur Respir J 10, 934–941. [PubMed] [Google Scholar]
- Dryden IL, Mardia KV (1998) Statistical Shape Analysis. Chichester: J. Wiley. [Google Scholar]
- Enlow DH, Hans MG (1996) Essentials of Facial Growth. Philadelphia: WB Saunders. [Google Scholar]
- Feltrim MIZ (1994) Estudo do padrao respiratorio e da configuracao toraco‐abdominal em individuos normais, nas posicoes sentada, dorsal e laterais, com o uso de plestismografia repiratori Universidade Federal de São Paulo. UNIFESPa por indutancia: Universidade Federal de São Paulo (UNIFESP. [Google Scholar]
- Franciscus RG, Churchill SE (2002) The costal skeleton of Shanidar 3 and a reappraisal of Neandertal thoracic morphology. J Hum Evol 42(3), 303–356. [DOI] [PubMed] [Google Scholar]
- Frappell PB, MacFarlane PM (2005) Development of mechanics and pulmonary reflexes. Respir Physiol Neurobiol 149, 143–154. [DOI] [PubMed] [Google Scholar]
- García‐Martínez D, Recheis W, Bastir M (2016a) Ontogeny of 3D rib curvature and its importance for the understanding of human thorax development. Am J Phys Anthropol 159, 423–431. [DOI] [PubMed] [Google Scholar]
- García‐Martínez D, Torres‐Tamayo N, Torres‐Sánchez I, et al. (2016b) Morphological and functional implications of sexual dimorphism in the human skeletal thorax. Am J Phys Anthropol 161, 467–477. [DOI] [PubMed] [Google Scholar]
- García‐Río F (2005) Importancia del atrapamiento aéreo en la EPOC. Archivos de Bronconeumología 41, 1–8. [Google Scholar]
- García‐Río F, Pino JM, Gómez L, et al. (1996) Regulation of breathing and perception of dyspnea in healthy pregnant women. Chest 110, 446–453. [DOI] [PubMed] [Google Scholar]
- Gayzik FS, Mao MY, Danelson KA, et al. (2008) Quantification of age‐related shape change of the human rib cage through geometric morphometrics. J Biomech 41, 1545–1554. [DOI] [PubMed] [Google Scholar]
- Gea J, Gáldiz JB, Comtois N, et al. (2009) Modificaciones en la actividad del diafragma inducidas por laparotomía media y cambios en la rigidez de la pared abdominal. Archivos de Bronconeumología 45, 30–35. [DOI] [PubMed] [Google Scholar]
- Goodyear M, Krleza‐Jeric K, Lemmens T (2007) The declaration of Helsinki. BMJ 335, 736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray H (2009) Gray's Anatomy: with Original Illustrations by Henry Carter. London: Arcturus Publishing. [Google Scholar]
- Guenette JA, Witt JD, McKenzie DC, et al. (2007) Respiratory mechanics during exercise in endurance‐trained men and women. J Physiol 581, 1309–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunz P, Mitteroecker P (2013) Semilandmarks: a method for quantifying curves and surfaces. Hystrix It J Mammal 24, 103–109. [Google Scholar]
- Gunz P, Mitteroecker P, Bookstein FL (2005) Semilandmarks in three dimensions In: Slice D. E. (Ed.), Modern morphometrics in physical anthropology, 73–98. New York: Kluwer Academic/Plenum Publishers. [Google Scholar]
- Hall RL (2005) Energetics of nose and mouth breathing, body size, body composition, and nose volume in young adult males and females. Am J Hum Biol 17, 321–330. [DOI] [PubMed] [Google Scholar]
- Hopkins SR, Harms CA (2004) Gender and pulmonary gas exchange during exercise. Exerc Sport Sci Rev 32, 50–56. [DOI] [PubMed] [Google Scholar]
- Howes MK, Hardy WN, Beillas P (2013) The effects of cadaver orientation on the relative position of the abdominal organs. Ann Adv Automotive Med 57, 209. [PMC free article] [PubMed] [Google Scholar]
- Johnson BD, Saupe KW, Dempsey JA (1992) Mechanical constraints on exercise hyperpnea in endurance athletes. J Appl Physiol 73, 874–886. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Suzuki T, Kaneko C, et al. (2003) Expression of androgen receptor and 5α‐reductase types 1 and 2 in early gestation fetal lung: a possible correlation with branching morphogenesis. Clin Sci 105, 709–713. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP (2011) MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Resour 11, 353–357. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP, Marugán‐Lobón J (2013) Evolutionary covariation in geometric morphometric data: analyzing integration, modularity, and allometry in a phylogenetic context. Syst Biol 62, 591–610. [DOI] [PubMed] [Google Scholar]
- Konno K, Mead J (1967) Measurement of the separate volume changes of rib cage and abdomen during breathing. J Appl Physiol 22, 407–422. [DOI] [PubMed] [Google Scholar]
- Layton AM, Garber CE, Thomashow BM, et al. (2011) Exercise ventilatory kinematics in endurance trained and untrained men and women. Respir Physiol Neurobiol 178, 223–229. [DOI] [PubMed] [Google Scholar]
- Levene H (1960) Robust tests for equality of variances1. Contributions to probability and statistics: essays in honor of Harold Hotelling 2, 278–292. [Google Scholar]
- LoMauro A, Aliverti A (2015) Respiratory physiology of pregnancy: physiology masterclass. Breathe 11, 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoMauro A, Pochintesta S, Romei M, et al. (2012) Rib cage deformities alter respiratory muscle action and chest wall function in patients with severe osteogenesis imperfecta. PLoS ONE 7, e35965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorensen WE, Cline HE (1987) Marching cubes: a high resolution 3D surface construction algorithm In: ACM siggraph computer graphics 21(4), 163–169. New York, NY: USA. [Google Scholar]
- McClaran SR, Harms CA, Pegelow DF, et al. (1998) Smaller lungs in women affect exercise hyperpnea. J Appl Physiol 84, 1872–1881. [DOI] [PubMed] [Google Scholar]
- Mead J (1980) Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity 1–3. Am Rev Respir Dis 121, 339–342. [DOI] [PubMed] [Google Scholar]
- Mitteroecker P, Gunz P (2009) Advances in geometric morphometrics. Evol Biol 36, 235–247. [Google Scholar]
- Ochs M, Nyengaard JR, Jung A, et al. (2004) The number of alveoli in the human lung. Am J Respir Crit Care Med 169(1), 120–124. [DOI] [PubMed] [Google Scholar]
- O'Higgins P (2000) The study of morphological variation in the hominid fossil record: biology, landmarks and geometry. J Anat 197, 103–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parreira VF, Bueno CJ, França DC, et al. (2010) Breathing pattern and thoracoabdominal motion in healthy individuals: influence of age and sex. Braz J Phys Ther 14, 411–416. [PubMed] [Google Scholar]
- Ragnarsdóttir M, Kristinsdóttir EK (2006) Breathing movements and breathing patterns among healthy men and women 20–69 years of age. Respiration 73, 48–54. [DOI] [PubMed] [Google Scholar]
- Ratnovsky A, Elad D, Halpern P (2008) Mechanics of respiratory muscles. Respir Physiol Neurobiol 163, 82–89. [DOI] [PubMed] [Google Scholar]
- Rohlf FJ, Slice D (1990) Extensions of the Procrustes method for the optimal superimposition of landmarks. Systematic zoology 39(1), 49–50. [Google Scholar]
- Romei M, Mauro AL, D'angelo, M , et al. (2010) Effects of gender and posture on thoraco‐abdominal kinematics during quiet breathing in healthy adults. Respir Physiol Neurobiol 172, 184–191. [DOI] [PubMed] [Google Scholar]
- Rosas A, Bastir M (2002) Thin‐plate spline analysis of allometry and sexual dimorphism in the human craniofacial complex. Am J Phys Anthropol 117, 236–245. [DOI] [PubMed] [Google Scholar]
- Roussos C, Koutsoukou A (2003) Respiratory failure. Eur Respir J 22, 3s–14s. [DOI] [PubMed] [Google Scholar]
- Roussos C, Macklem PT (1982) The respiratory muscles. N Engl J Med 307, 786–797. [DOI] [PubMed] [Google Scholar]
- Sharp JT, Goldberg NB, Druz WS, et al. (1975) Relative contributions of rib cage and abdomen to breathing in normal subjects. J Appl Physiol 39, 608–618. [DOI] [PubMed] [Google Scholar]
- Sheel AW, Guenette JA (2008) Mechanics of breathing during exercise in men and women: sex versus body size differences? Exerc Sport Sci Rev 36, 128–134. [DOI] [PubMed] [Google Scholar]
- Shi X, Cao L, Reed MP, et al. (2014) A statistical human rib cage geometry model accounting for variations by age, sex, stature and body mass index. J Biomech 47, 2277–2285. [DOI] [PubMed] [Google Scholar]
- Sokal R, Rohlf F (1998) Biometry (4th printing). New York: Freeman. [Google Scholar]
- Stahl WR (1967) Scaling of respiratory variables in mammals. J Appl Physiol 22, 453–460. [DOI] [PubMed] [Google Scholar]
- Stockert B (2003) Pulmonary ventilation teaching aid. Adv Physiol Educ 27, 41–42. [DOI] [PubMed] [Google Scholar]
- Thurlbeck WM (1982) Postnatal human lung growth. Thorax 37, 564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin MJ, Chadha TS, Jenouri G, et al. (1983) Breathing patterns. 1 Normal subjects. Chest J 84, 202–205. [DOI] [PubMed] [Google Scholar]
- Torres‐Tamayo N, García‐Martínez D, Bastir M, et al. (2015) Estudio de la morfología pulmonar en pacientes con EPOC, y su influencia en el patrón de movimiento respiratorio.
- Verschakelen JA, Demedts MG (1995) Normal thoracoabdominal motions. Influence of sex, age, posture, and breath size. Am J Respir Crit Care Med 151, 399–405. [DOI] [PubMed] [Google Scholar]
- Wade O (1954) Movements of the thoracic cage and diaphragm in respiration. J Physiol 124, 193–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward ME, Ward JW, Macklem PT (1992) Analysis of human chest wall motion using a two‐compartment rib cage model. J Appl Physiol 72, 1338–1347. [DOI] [PubMed] [Google Scholar]
- Weaver AA, Schoell SL, Stitzel JD (2014) Morphometric analysis of variation in the ribs with age and sex. J Anat 225, 246–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JB (2012) Respiratory Physiology: the Essentials. 9th ed. Philadelphia: Wolters Kluwer. [Google Scholar]
- Zach M (2000) The physiology of forced expiration. Paediatr Respir Rev 1, 36–39. [DOI] [PubMed] [Google Scholar]
- Zelditch ML, Swiderski DL, Sheets HD (2012) Geometric Morphometrics for Biologists: a Primer. 2nd edition: New York and London: Academic Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Lung deformation associated to PC1 in frontal view, from FI to FE. Grids were warped from the overall mean shape to the mean shape at FI (negative values of PC1) and FE (positive values of PC1).
Video S2. Lung deformation associated to PC1 in lateral view.
Video S3. Lung deformation associated to PC2 in frontal view. Grids were warped from overall mean shape to the pyramidal mean shape of males (negative values of PC2), and the prismatic mean shape of females (positive values of PC2).
Video S4. Lung deformation associated to PC2 in lateral view.
Table S1. Results of normality and homoscedasticity tests. K–S, Kolmogorov–Smirnov; FI, forced inspiration; FE, forced expiration.
Table S2. Percentage of variance and cumulative variance explained by each PC of the PCA.


