Abstract
Among the various pharmacological options to decrease peri-operative bleeding, tranexamic acid appears to be one of the most interesting. Several trials have consistently documented the efficacy of this synthetic drug in reducing the risk of blood loss and the need for allogeneic blood transfusion in patients undergoing total hip and knee arthroplasty. The safety of intravenous tranexamic acid in major orthopaedic surgery, particularly regarding the risk of venous thromboembolism, was systematically analysed in this review. A systematic search of the literature identified 73 randomised controlled trials involving 4,174 patients and 2,779 controls. The raw overall incidence of venous thromboembolism was 2.1% in patients who received intravenous tranexamic acid and 2.0% in controls. A meta-analytic pooling showed that the risk of venous thromboembolism in tranexamic acid-treated patients was not significantly different from that of controls (risk difference: 0.01%, 95% confidence interval [CI]: −0.05%, 0.07%; risk ratio: 1.067, 95% CI: 0.760–1.496). Other severe drug-related adverse events occurred very rarely (0.1%). In conclusion, the results of this systematic review and meta-analysis show that intravenous tranexamic acid is a safe pharmacological treatment to reduce blood loss and transfusion requirements in patients undergoing major orthopaedic surgery.
Keywords: tranexamic acid, orthopaedic surgery, safety, thromboembolic events
Introduction
Tranexamic acid (TXA) is a synthetic amino acid which competitively blocks the lysine binding sites on plasminogen, thereby slowing the conversion of plasminogen to plasmin1,2. Thanks to its ability to inhibit fibrinolysis and clot degradation, TXA, which can be administered intravenously, orally or topically, has been successfully used to prevent or decrease blood loss in a variety of clinical conditions characterised by excessive bleeding in patients with or without inherited bleeding disorders3,4. For instance, the early administration of TXA has been found to confer a survival benefit in the settings of severe trauma5,6 and of post-partum haemorrhage without an increase in thromboembolic events7. In addition, this haemostatic agent has been used successfully to decrease blood loss in numerous surgical specialties, particularly cardiac surgery8,9. More recently, TXA has been widely administered to minimise bleeding and exposure to allogeneic blood transfusion in major orthopaedic surgery and several large randomised clinical trials and meta-analyses have consistently confirmed that the intravenous administration of TXA could effectively and safely reduce perioperative blood loss and transfusion requirements in total hip and knee arthroplasty10–14. In spite of this sound evidence, however, some concerns still remain among physicians over the hypothetical increased risk of thromboembolic complications (i.e., venous thromboembolism [VTE], including deep vein thrombosis and pulmonary embolism) following the systemic infusion of this anti-fibrinolytic agent in the setting of orthopaedic surgery. Thus, to refute this unjustified, non-evidence-based perception, we conducted a systematic review and meta-analysis aimed at assessing the safety of intravenous TXA in patients undergoing major orthopaedic surgery.
Materials and methods
This systematic review was conducted according to the recommended PRISMA checklist guidelines15.
Search methods
A computer-assisted literature search of the Medline, Embase and Scopus electronic databases was performed to identify randomised clinical trials on the use of intravenous TXA in major orthopaedic surgery. The following search strategy was used to maximise the search specificity and sensitivity: “total knee arthroplasty OR total knee replacement OR total hip arthroplasty OR total hip replacement OR total shoulder arthroplasty OR total shoulder replacement OR major orthopaedic surgery OR hip fracture surgery” and “tranexamic acid”. In addition, we hand-searched the reference lists of the most relevant items (original studies and reviews) in order to identify potentially eligible studies not captured by the initial literature search.
Study selection
Study selection was performed independently by two reviewers (MF and CM), with disagreements resolved through discussion and on the basis of the opinion of a third reviewer (GML). The eligibility assessment was based on the title or abstract and on the full text if required. Articles were eligible if they reported either in the title or in in the abstract the use of intravenous TXA in patients undergoing major orthopaedic surgery. Studies on the use of TXAgiven by other routes of administration (i.e., oral or topical) were considered for the analysis only if they included an arm using intravenous TXA. Only randomised clinical trials published in full in English between January 1990 and July 2017 were included in this systematic review and meta-analysis16–88.
Data extraction
For each study included in the systematic review, the following data were extracted by two reviewers (MF and CM) independently: study design, type of intervention, sample size (TXA and control groups), protocol (dose administered of intravenous TXA), thromboprophylaxis and TXA-related adverse events (i.e., VTE and other drug-related severe adverse events). The follow-up period for each trial was also recorded, when available.
Outcome measures
According to the study design of the different trials, the primary outcome measured was the safety TXA administered intravenously in major orthopaedic surgical procedures. The safety included mainly the incidence of venous thromboembolic complications (i.e., pulmonary embolism and deep vein thrombosis) following the intravenous administration of the anti-fibrinolytic agent. All the other drug-related severe adverse events were, however, also recorded.
Statistical evaluation and meta-analysis
In each study, the risk of VTE was evaluated as risk difference (RD) and risk ratio (RR) between a group of TXA-treated patients and a group of control patients. The meta-analysis was done, as usual, by pooling these effect indexes. A fixed effect, inverse variance pooling of the RD was performed. This index enables the calculation of an important pharmaco-economic index, the number needed to treat in order to avoid an event. The heterogeneity χ2 was also calculated, as the I2 for the variation of RD due to heterogeneity. The same approach was used for the RR.
Since many of the studies provide zero-events, the estimate of the pooled RD is less biased than that of the pooled RR, although it is not very accurate. Indeed, even the RD method cannot avoid recourse to correction for lack of continuity, to adjust for zero. Moreover, some authors view the use of the RD unfavourably, since this method yields wide confidence intervals when events are rare, with poor statistical power89,90. A method recently developed by Tian and colleagues91 for the RD, suitable for rare-event meta-analysis, appears appealing. It performs exact fixed effect meta-analysis for rare-events data without the need for an artificial continuity correction. This procedure combines the confidence intervals for the RD obtained from all studies. For the classical RD and RR we used Stata v15.0 (StataCorp LLC, College Station, TX, USA), whereas for Tian’s RD we used the R package “exactmeta” (https://cran.r-project.org/web/packages/exactmeta/index.html).
Results
Literature search and study characteristics
In total, 824 articles were identified from the initial electronic and manual search, which was concluded on 7 September 2017 (Figure 1). Of these, 572 were excluded because they focused on other topics. Thus, 244 potentially relevant articles were selected; the next screening led to the exclusion of 171 additional studies (case-reports, reviews, protocols of randomised clinical trials, duplicates, studies containing no informative data). The remaining 73 randomised studies16–88
Figure 1.
Flow chart of the inclusion of the studies.
RCTs: randomised controlled trials.
were included in the systematic review (see Online Supplementary Table SI for the main characteristics and results of the included studies), while six further studies57,73,76,78,80,86 were excluded from meta-analytical pooling because they were uncontrolled. Overall, 4,174 patients who received intravenous TXA and 2,779 controls who received no treatment were enrolled in the 73 studies evaluated. With regards to the type of major surgery, 46 studies16–18, 20–22,24,25,27,33–35,38,39,42,43,45–49,51–53,56–62,66–70,73–78,84–87 (2,960 cases and 1,924 controls) were focused on total hip arthroplasty, 20 studies19,23,26,28–32,37,41,44,50,55,63,64,71,79,80,82,83 (924 cases and 564 controls) on total knee arthroplasty, six studies36,40,54,65,72,81 (237 cases and 242 controls) on hip fracture surgery and one study88 (53 cases and 49 controls) on shoulder arthroplasty. The dosing and timing of intravenous TXA varied from study to study. However, in the majority of cases (45/73 studies, 61.6%) it was given before or during and after surgery at a bolus dose ranging between 10 and 20 mg/kg. In 12 of the 73 studies (16.4%) TXA was also administered as a continuous intravenous infusion during surgery and/or postoperatively at a dose ranging between 1 and 10 mg/kg/hour. Sixty-four of the 73 (87.7%) selected trials also reported informative data on the administration of thromboprophylaxis. In the majority of the studies (50/64, 78.1%), thromboprophylaxis consisted of low molecular weight heparin given at various dosages (see Online Supplementary Table SI) and for a period ranging between 5 days and 6 weeks postoperatively. In six studies, low molecular weight heparin was followed by the direct oral anticoagulant rivaroxaban administered at a dose of 10 mg/day. In seven other studies rivaroxaban was given alone at the same daily dose for 7–14 days post-operatively. Only one study used unfractionated heparin, while thromboprophylaxis with warfarin or aspirin alone was performed in one and three studies, respectively. In two other studies only mechanical antithrombotic prophylaxis was provided.
Outcome analysis
The primary outcome analysed was the incidence of VTE. The VTE screening method performed was reported in 67/73 (91.8%) studies. In the majority of the cases (40/67 studies, 59.7%) VTE screening was based only on clinical examination (presence of symptoms and signs of thromboembolism), while in 24 additional studies (35.8%) an investigation with ultrasound or venography was also routinely associated. An additional laboratory screening for VTE (measurement of D-dimer levels) was also performed in four studies. The post-operative follow-up period ranged between three days and three months.
The overall incidence of VTE was 86 in 4,174 (2.1%) patients who received intravenous TXA and 55 in 2,779 (2.0%) control patients. A raw RD of 0.0008 and a raw RR of 1.0411 could thus be calculated. Excluding the six uncontrolled studies, the incidence of VTE was 77 in 3,417 TXA patients (2.2%), and the related raw indexes were RD=0.0027 and RR=1.1386.
With regards to the type of VTE, six (6/86, 7.0%) patients in the TXA group and five (5/55, 9.1%) patients in the control group had a pulmonary embolism, while the remaining patients (80/86 [93.0%] in the TXA group and 50/55 [91.9%] in the control group) experienced deep vein thrombosis.
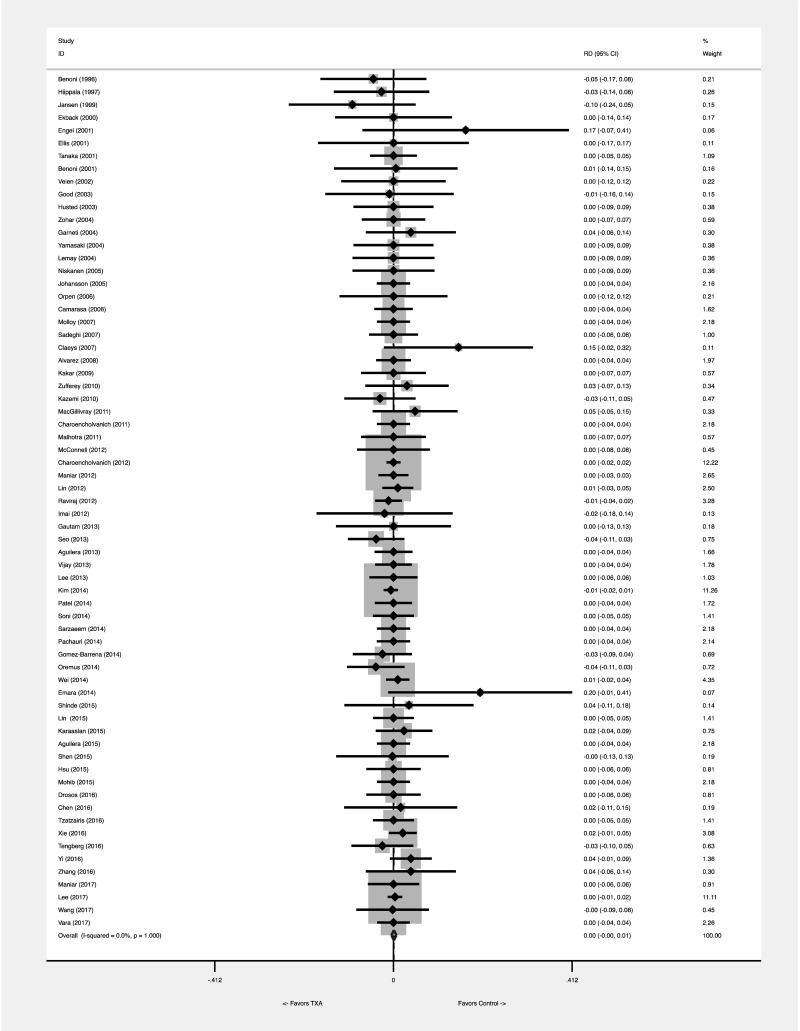
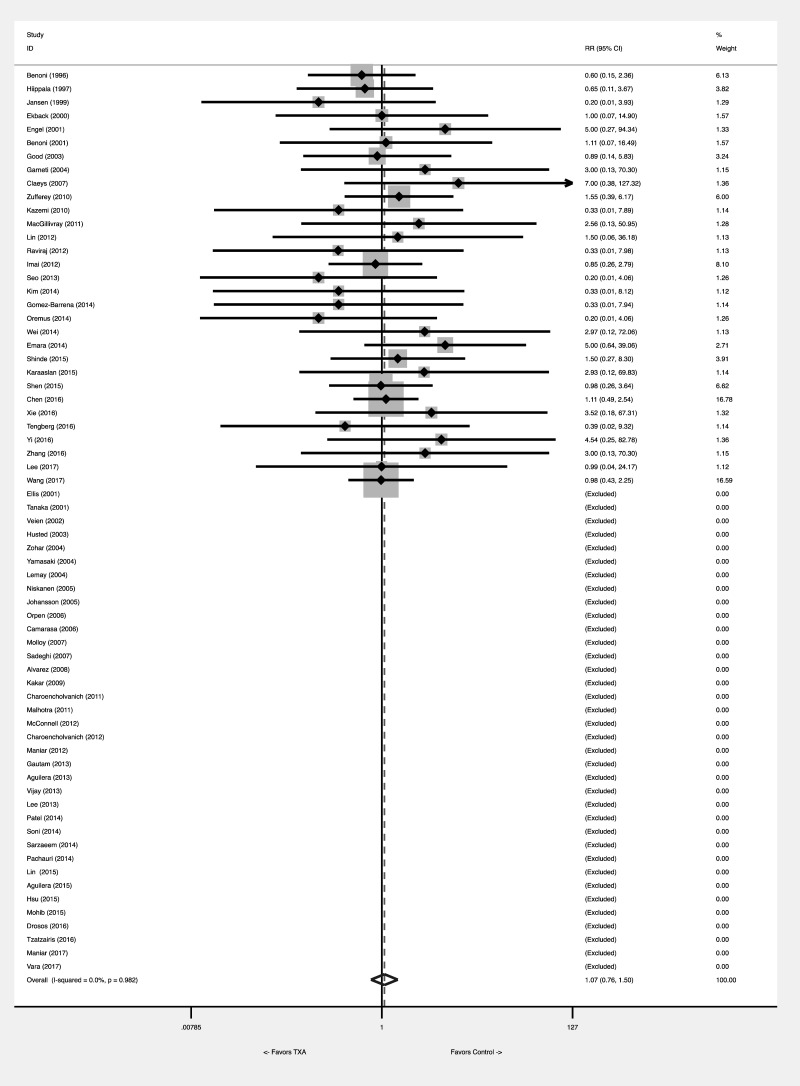
The pooled meta-analytical RD was 0.001 (95% CI: −0.005 to 0.007). No heterogeneity was found, with an I2=0.0%. The statistical significance of the test of RD=0 was p=0.745, so the evidence against the null hypothesis of no difference between the two groups was very modest (Figure 2A). The number needed to treat was 1,000. The calculation of the RR was disturbed by many zero-event studies, which could not be evaluated. The pooled meta-analytical RR was 1.067 (95% CI: 0.760 to 1.496). No heterogeneity was found, with an I2=0.0%. The statistical significance of RR=1 was p=0.708 (Figure 2B). Tian’s RD was 0 (95% CI: −0.007, 0.009), with a p=1.
Figure 2A.
Effect of tranexamic acid on VTE risk: meta-analytical pooling. The effect was comparatively measured as risk difference (Figure 2A) and risk ratio (Figure 2B). Squares denote the risk difference and risk ratio, with their size being proportional to the weight assigned to the study. Horizontal bars indicate the 95% CI for each study. The diamonds represent the aggregate effect, with their width representing the 95% CI of the total effect.
CI: confidence interval; RD: risk difference; TXA: tranexamic acid; VTE: venous thromboembolism.
Figure 2B.
Effect of tranexamic acid on VTE risk: meta-analytical pooling. The effect was comparatively measured as risk difference (Figure 2A) and risk ratio (Figure 2B). Squares denote the risk difference and risk ratio, with their size being proportional to the weight assigned to the study. Horizontal bars indicate the 95% CI for each study. The diamonds represent the aggregate effect, with their width representing the 95% CI of the total effect.
CI: confidence interval; RR: risk ratio; TXA: tranexamic acid; VTE: venous thromboembolism.
Other severe adverse events possibly related to the administration of TXA occurred so rarely (5/3, 396, 0.1%) that a statistical analysis could not be performed.
Discussion
Major orthopaedic surgical procedures, in particular total hip and knee arthroplasties, are continuously increasing worldwide due to population aging. For instance, in the USA alone, nearly 1,000,000 total knee and hip arthroplasties are performed each year92. Despite advances in surgical techniques, intra- and post-operative blood loss remains one of the most common and important complications associated with such operations, exposing patients to increased impairment of functional ability, longer stays in hospital and, ultimately, increased morbidity and mortality93. Allogeneic blood transfusion, a life-saving procedure used to correct post-operative anaemia, can, albeit rarely, be associated with serious adverse events94. In this context, a restrictive red blood cell transfusion strategy95,96 and the implementation of a patient blood management programme(PBM)97–101 in order to correct pre-operative anaemia have become the mainstay of management of patients undergoing major orthopaedic surgery. With regards to the latter issue, one of the most interesting methods of decreasing peri-operative blood loss and reducing the need for post-operative transfusion is based on the use of TXA and several hundreds of trials have assessed the potential beneficial effect of this pharmacological agent given topically and/or intravenously102,103. While the benefit of intravenous TXA in significantly reducing the risk of post-operative blood loss and the need for allogeneic blood transfusion has been demonstrated consistently by several systematic reviews and meta-analyses11,14,104,105, some concerns remain still among orthopaedic surgeons regarding its safety, in particular the risk of VTE. Thus, in order to contribute to a definitive response to these important concerns, we performed a systematic review and meta-analysis focused on the safety of intravenous TXA in major orthopaedic surgery, particularly regarding its association with VTE risk.
After a systematic electronic and manual search, we identified 73 randomised clinical trials including almost 7,000 patients. Such a large number of studies and patients enrolled, much larger than any other systematic review or meta-analysis previously published, is due to the pooling of all the randomised clinical trials evaluating the intravenous administration of TXA in the setting of any type of major orthopaedic surgery (i.e., total hip, knee and shoulder arthroplasties and hip fracture surgery).
After meta-analysis, we found that the risk of VTE, measured as RD and RR, in patients who received intravenous TXA was not significantly different from that in untreated controls. The negligible effect size of TXA on thromboembolic adverse events was also confirmed using the Tian’s RD (which was zero), a statistical method developed to overcome the limitations due to rare events (such as the thrombotic events recorded in this meta-analysis). In addition, no significant difference in the distribution of the different types of VTE (i.e., pulmonary embolism and deep vein thrombosis) was recorded in both cases and controls.
While the large number of studies evaluated represents the main strength of our systematic review, it must be pointed out that a significant proportion of these randomised clinical trials differed from each other with regards to study design and, in particular, TXA dosing and timing of administration. In addition, different antithrombotic prophylaxis regimens and VTE screening methods were applied in the various studies. Moreover, the baseline risk of thromboembolic events varies considerably depending on whether major orthopaedic surgery is performed on the upper or lower limbs.
All these variables could have influenced the occurrence and detection of VTE. Nevertheless, in spite of such limitations, no heterogeneity in the randomised clinical trials was detected by the I2 index (0%), which further validates the results of our study.
In conclusion, this systematic review and meta-analysis demonstrates the safety of the intravenous administration of TXA in patients undergoing major orthopaedic surgery. Our results, together with those from other previously published meta-analyses which document the efficacy of TXA and support the beneficial effect of this agent, TXA should, therefore, be used in orthopaedic surgery in the frame of PBM programmes.
Supplementary Information
Footnotes
Disclosure of conflicts of interest
GML is the Editor-in-Chief of Blood Transfusion and this manuscript has undergone additional external review as a result. The other Authors declare no conflicts of interest.
References
- 1.Mannucci PM. Hemostatic drugs. N Engl J Med. 1998;339:245–53. doi: 10.1056/NEJM199807233390407. [DOI] [PubMed] [Google Scholar]
- 2.Franchini M, Mannucci PM. Adjunct agents for bleeding. Curr Opin Hematol. 2014;21:503–8. doi: 10.1097/MOH.0000000000000084. [DOI] [PubMed] [Google Scholar]
- 3.Mannucci PM, Levi M. Prevention and treatment of major blood loss. N Engl J Med. 2007;356:2301–11. doi: 10.1056/NEJMra067742. [DOI] [PubMed] [Google Scholar]
- 4.Schulman S. Pharmacologic tools to reduce bleeding in surgery. Hematology Am Soc Hematol Educ Program. 2012;2012:517–21. doi: 10.1182/asheducation-2012.1.517. [DOI] [PubMed] [Google Scholar]
- 5.Shakur H, Roberts I, Bautista R, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376:23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 6.Ker K, Roberts I, Shakur H, Coats TJ. Antifibrinolytic drugs for acute traumatic injury. Cochrane Database Syst Rev. 2015;5:CD004896. doi: 10.1002/14651858.CD004896.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–16. doi: 10.1016/S0140-6736(17)30638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerstein NS, Brierley JK, Windsor J, et al. Antifibrinolytic agents in cardiac and noncardiac surgery: a comprehensive overview and update. J Cardiothorac Vasc Anesth. 2017 doi: 10.1053/j.jvca.2017.02.029. S1053-0770(17)30079-4. [DOI] [PubMed] [Google Scholar]
- 9.Henry DA, Carless PA, Moxey AJ, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2011;1886;3:CD00. doi: 10.1002/14651858.CD001886.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang X, Ma XL, Ma JX. Efficiency and safety of intravenous tranexamic acid in simultaneous bilateral total knee arthroplasty: a systematic review and meta-analysis. Orthop Surg. 2016;8:285–93. doi: 10.1111/os.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moskal JT, Capps SG. Meta-analysis of intravenous tranexamic acid in primary total hip arthroplasty. Orthopedics. 2016;39:e883–92. doi: 10.3928/01477447-20160526-02. [DOI] [PubMed] [Google Scholar]
- 12.Lin ZX, Woolf SK. Safety, efficacy, and cost-effectiveness of tranexamic acid in orthopedic surgery. Orthopedics. 2016;39:119–30. doi: 10.3928/01477447-20160301-05. [DOI] [PubMed] [Google Scholar]
- 13.He P, Zhang Z, Li Y, et al. Efficacy and safety of tranexamic acid in bilateral total knee replacement: a meta-analysis and systematic review. Med Sci Monit. 2015;21:3634–42. doi: 10.12659/MSM.895027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei Z, Liu M. The effectiveness and safety of tranexamic acid in total hip or knee arthroplasty: a meta-analysis of 2720 cases. Transfus Med. 2015;25:151–62. doi: 10.1111/tme.12212. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and metaanalyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br. 1996;78:434–40. [PubMed] [Google Scholar]
- 17.Hiippala ST, Strid LJ, Wennerstrand MI, et al. Tranexamic acid radically decreases blood loss and transfusions associated with total knee arthroplasty. Anesth Analg. 1997;84:839–44. doi: 10.1097/00000539-199704000-00026. [DOI] [PubMed] [Google Scholar]
- 18.Jansen AJ, Andreica S, Claeys M, et al. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth. 1999;83:596–601. doi: 10.1093/bja/83.4.596. [DOI] [PubMed] [Google Scholar]
- 19.Ekback G, Axelsson K, Ryttberg L, et al. Tranexamic acid reduces blood loss in total hip replacement surgery. Anesth Analg. 2000;81:1124–30. doi: 10.1097/00000539-200011000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Engel JM, Hohaus T, Ruwoldt R, et al. Regional hemostatic status and blood requirements after total knee arthroplasty with and without tranexamic acid or aprotinin. Anesth Analg. 2001;92:775–80. doi: 10.1097/00000539-200103000-00041. [DOI] [PubMed] [Google Scholar]
- 21.Ellis MH, Fredman B, Zohar E, et al. The effect of tourniquet application, tranexamic acid, and desmopressin on the procoagulant and fibrinolytic systems during total knee replacement. J Clin Anesth. 2001;13:509–13. doi: 10.1016/s0952-8180(01)00319-1. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka N, Sakahashi H, Sato E, et al. Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br. 2001;83:702–5. doi: 10.1302/0301-620x.83b5.11745. [DOI] [PubMed] [Google Scholar]
- 23.Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty: a randomized, double-blind study in 40 primary operations. Acta Orthop Scand. 2001;72:442–8. doi: 10.1080/000164701753532754. [DOI] [PubMed] [Google Scholar]
- 24.Veien M, Sørensen JV, Madsen F, Juelsgaard P. Tranexamic acid given intraoperatively reduces blood loss after total knee replacement: a randomized, controlled study. Acta Anaesthesiol Scand. 2002;46:1206–11. doi: 10.1034/j.1399-6576.2002.461007.x. [DOI] [PubMed] [Google Scholar]
- 25.Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90:596–9. doi: 10.1093/bja/aeg111. [DOI] [PubMed] [Google Scholar]
- 26.Husted H, Blond L, Sonne-Holm S, et al. Tranexamic acid reduces blood loss and blood transfusions in primary total hip arthroplasty: a prospective randomized double-blind study in 40 patients. Acta Orthop Scand. 2003;74:665–9. doi: 10.1080/00016470310018171. [DOI] [PubMed] [Google Scholar]
- 27.Zohar E, Ellis M, Ifrach N, et al. The postoperative blood-sparing efficacy of oral versus intravenous tranexamic acid after total knee replacement. Anesth Analg. 2004;99:1679–83. doi: 10.1213/01.ANE.0000136770.75805.19. [DOI] [PubMed] [Google Scholar]
- 28.Garneti N, Field J. Bone bleeding during total hip arthroplasty after administration of tranexamic acid. J Arthroplasty. 2004;19:488–92. doi: 10.1016/j.arth.2003.12.073. [DOI] [PubMed] [Google Scholar]
- 29.Yamasaki S, Masuhara K, Fuji T. Tranexamic acid reduces blood loss after cementless total hip arthroplasty: prospective randomized study in 40 cases. Int Orthop. 2004;28:69–73. doi: 10.1007/s00264-003-0511-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemay E, Guay J, Côté C, Roy A. Tranexamic acid reduces the need for allogeneic red blood cell transfusions in patients undergoing total hip replacement. Can J Anaesth. 2004;51:31–7. doi: 10.1007/BF03018543. [DOI] [PubMed] [Google Scholar]
- 31.Niskanen RO, Korkala OL. Tranexamic acid reduces blood loss in cemented hip arthroplasty. A randomized, double blind study of 39 patients with osteoarthritis. Acta Orthop. 2005;76:829–32. doi: 10.1080/17453670510045444. [DOI] [PubMed] [Google Scholar]
- 32.Johansson T, Pettersson LG, Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money: a randomized, double-blind study in 100 patients. Acta Orthop. 2005;76:314–9. [PubMed] [Google Scholar]
- 33.Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee. 2006;13:106–10. doi: 10.1016/j.knee.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Camarasa MA, Olle G, Serra-Prat M, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth. 2006;96:576–82. doi: 10.1093/bja/ael057. [DOI] [PubMed] [Google Scholar]
- 35.Molloy DO, Archbold HA, Ogonda L, et al. Comparison of topical fibrin spray and tranexamic acid on blood loss after total knee replacement: a prospective, randomised controlled trial. J Bone Joint Surg Br. 2007;89:306–9. doi: 10.1302/0301-620X.89B3.17565. [DOI] [PubMed] [Google Scholar]
- 36.Sadeghi M, Mehr-Aein A. Does a single bolus dose of tranexamic acid reduce blood loss and transfusion requirements during hip fracture surgery? A prospective randomized double blind study in 67 patients. Acta Med Iran. 2007;45:437–42. [Google Scholar]
- 37.Claeys MA, Vermeersch N, Haentjens P. Reduction of blood loss with tranexamic acid in primary total hip replacement surgery. Acta Chir Belg. 2007;107:397–401. doi: 10.1080/00015458.2007.11680081. [DOI] [PubMed] [Google Scholar]
- 38.Alvarez JC, Santiveri FX, Ramos I, et al. Tranexamic acid reduces blood transfusion in total knee arthroplasty even when a blood conservation program is applied. Transfusion. 2008;48:519–25. doi: 10.1111/j.1537-2995.2007.01564.x. [DOI] [PubMed] [Google Scholar]
- 39.Kakar P, Gupta N, Govil P, et al. Efficacy and safety of tranexamic acid in control of bleeding following TKR: a randomized clinical trial. Ind J Anaesthesia. 2009;53:667–71. [PMC free article] [PubMed] [Google Scholar]
- 40.Zufferey PJ, Miquet M, Quenet S, et al. for the investigators of tranexamic acid in hip fracture surgery (THIF) study. Tranexamic acid in hip fracture surgery: a randomized controlled trial. Br J Anaesth. 2010;104:23–30. doi: 10.1093/bja/aep314. [DOI] [PubMed] [Google Scholar]
- 41.Kazemi SM, Mosaffa F, Eajazi A, et al. The effect of tranexamic acid on reducing blood loss in cementless total hip arthroplasty under epidural anesthesia. Orthopedics. 2010;33:17. doi: 10.3928/01477447-20091124-30. [DOI] [PubMed] [Google Scholar]
- 42.MacGillivray RG, Tarabichi SB, Hawari MF, et al. Tranexamic acid to reduce blood loss after bilateral total knee arthroplasty: a prospective, randomized double blind study. J Arthroplasty. 2011;26:24–8. doi: 10.1016/j.arth.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 43.Charoencholvanich K, Siriwattanasakul P. Tranexamic acid reduces blood loss and blood transfusion after TKA: a prospective randomized controlled trial. Clin Orthop Relat Res. 2011;469:2874–80. doi: 10.1007/s11999-011-1874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malhotra R, Kumar V, Garg B. The use of tranexamic acid to reduce blood loss in primary cementless total hip arthroplasty. Eur J Orthop Surg Traumatol. 2012;21:101–4. [Google Scholar]
- 45.McConnell JS, Shewale S, Munro NA, et al. Reducing blood loss in primary knee arthroplasty: a prospective randomized controlled trial of tranexamic acid and fibrin spray. Knee. 2012;19:295–8. doi: 10.1016/j.knee.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 46.Chareancholvanich K, Siriwattanasakul P, Narkbunnam R, Pornrattanamaneewong C. Temporary clamping of drain combined with tranexamic acid reduce blood loss after total knee arthroplasty: a prospective randomized controlled trial. BMC Musculoskelet Disord. 2012;13:124. doi: 10.1186/1471-2474-13-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maniar RN, Kumar G, Singhi T, et al. Most effective regimen of tranexamic acid in knee arthroplasty: a prospective randomized controlled study in 240 patients. Clin Orthop Relat Res. 2012;470:2605–12. doi: 10.1007/s11999-012-2310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin PC, Hsu CH, Huang CC, et al. The blood-saving effect of tranexamic acid in minimally invasive total knee replacement. Is an additional pre-operative injection effective? J Bone Joint Surg Br. 2012;94-B:932–6. doi: 10.1302/0301-620X.94B7.28386. [DOI] [PubMed] [Google Scholar]
- 49.Raviraj A, Anand A, Chakravarthy M, et al. Tranexamic acid reduces blood loss in simultaneous bilateral total knee arthroplasty: a randomized control trial. Eur J Orthop Surg Traumatol. 2012;22:381–6. [Google Scholar]
- 50.Imai N, Dohmae Y, Suda K, et al. Tranexamic acid for reduction of blood loss during total hip arthroplasty. J Arthroplasty. 2012;27:1838–43. doi: 10.1016/j.arth.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 51.Gautam VK, Sambandam B, Singh S, et al. The role of tranexamic acid in reducing blood loss in total knee replacement. J Clin Orthop Trauma. 2013;4:36–9. doi: 10.1016/j.jcot.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seo JG, Moon YW, Park SH, et al. The comparative efficacies of intra-articular and IV tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21:1869–74. doi: 10.1007/s00167-012-2079-2. [DOI] [PubMed] [Google Scholar]
- 53.Aguilera X, Martinez-Zapata MJ, Bosch A, et al. Efficacy and safety of fibrin glue and tranexamic acid to prevent postoperative blood loss in total knee arthroplasty: a randomized controlled clinical trial. J Bone Joint Surg Am. 2013;95:2001–7. doi: 10.2106/JBJS.L.01182. [DOI] [PubMed] [Google Scholar]
- 54.Vijai BS, Bedi V, Mitra S, Das B. Role of tranexamic acid in reducing postoperative blood loss and transfusion requirement in patients undergoing hip and femoral surgeries. Saudi J Anaesth. 2013;7:29–32. doi: 10.4103/1658-354X.109803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee YC, Park SJ, Kim JS, Cho CH. Effect of tranexamic acid on reducing postoperative blood loss in combined hypotensive epidural anesthesia and general anesthesia for total hip replacement. J Clin Anesth. 2013;25:393–8. doi: 10.1016/j.jclinane.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 56.Kim TK, Chang CB, Kang YG, et al. Clinical value of tranexamic acid in unilateral and simultaneous bilateral TKAs under a contemporary blood-saving protocol: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2014;22:1870–8. doi: 10.1007/s00167-013-2492-1. [DOI] [PubMed] [Google Scholar]
- 57.Huang Z, Ma J, Shen B, et al. Combination of intravenous and topical application of tranexamic acid in primary total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29:2342–6. doi: 10.1016/j.arth.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 58.Patel JN, Spanyer JM, Smith LS, et al. Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2014;29:1528–31. doi: 10.1016/j.arth.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 59.Soni A, Saini R, Gulati A, et al. Comparison between intravenous and intra-articular regimens of tranexamic acid I reducing blood loss during total knee arthroplasty. J Arthroplasty. 2014;29:1525–7. doi: 10.1016/j.arth.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 60.Sarzaeem MM, Razi M, Kazemian G, et al. Comparing efficacy of three methods of tranexamic acid administration in reducing hemoglobin drop following total knee arthroplasty. J Arthroplasty. 2014;29:1521–4. doi: 10.1016/j.arth.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 61.Pachauri A, Acharya KK, Tiwari AK. The effect of tranexamic acid on hemoglobin levels during total knee arthroplasty. Am J Ther. 2014;21:366–70. doi: 10.1097/MJT.0b013e318250f85a. [DOI] [PubMed] [Google Scholar]
- 62.Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, et al. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement. J Bone Joint Surg Am. 2014;96:1937–44. doi: 10.2106/JBJS.N.00060. [DOI] [PubMed] [Google Scholar]
- 63.Oremus K, Sostaric S, Trkulja V, Haspl M. Influence of tranexamic acid on postoperative autologous blood retransfusion in primary total hip and knee arthroplasty: a randomized controlled trial. Transfusion. 2014;54:31–41. doi: 10.1111/trf.12224. [DOI] [PubMed] [Google Scholar]
- 64.Wei W, Wei B. Comparison of topical and intravenous tranexamic acid on blood loss and transfusion rates in total hip arthroplasty. J Arthroplasty. 2014;29:2113–6. doi: 10.1016/j.arth.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 65.Emara WM, Moez KK, Elkhouly AH. Topical versus intravenous tranxamic acid as a blood conservation intervention for reduction ofmpost-operative bleeding in hemiarthoplasty. Anesth Essays Res. 2014;8:48–53. doi: 10.4103/0259-1162.128908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shinde A, Sobti A, Maniar S, et al. Tranexamic acid reduces blood loss and need of blood transfusion in total knee arthroplasty: a prospective, randomized, double-blind study in Indian population. Asian J Transfusion Sci. 2015;9:168–72. doi: 10.4103/0973-6247.154251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin SY, Chen CH, Fu YC, et al. The efficacy of combined use of intraarticular and intravenous tranexamic acid on reducing blood loss and transfusion rate in total knee arthroplasty. J Arthroplasty. 2015;30:776–80. doi: 10.1016/j.arth.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 68.Karaaslan F, Karaoglu S, Mermerkaya MU, et al. Reducing blood loss in simultaneous bilateral total knee arthroplasty: combined intravenous-intra-articular tranexamic acid administration. A prospective randomized controlled trial. Knee. 2015;22:131–5. doi: 10.1016/j.knee.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 69.Aguilera X, Martinez-Zapata MJ, Hinarejos P, et al. Topical and intravenous tranexamic acid reduce blood loss compared to routine hemostasis in total knee arthroplasty: a multicenter, randomized, controlled trial. Arch Orthop Trauma Surg. 2015;135:1017–25. doi: 10.1007/s00402-015-2232-8. [DOI] [PubMed] [Google Scholar]
- 70.Shen PF, Hou WH, Chen JB, et al. Effectiveness and safety of tranexamic acid for total knee arthroplasty: a prospective randomized controlled trial. Med Sci Monit. 2015;21:576–81. doi: 10.12659/MSM.892768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hsu CH, Lin PC, Kuo FC, Wang JW. Aregime of two intravenous injections of tranexamic acid reduces blood loss in minimally invasive total hip arthroplasty. Aprospective randomized double-blind study. Bone Joint J. 2015;97-B:905–10. doi: 10.1302/0301-620X.97B7.35029. [DOI] [PubMed] [Google Scholar]
- 72.Mohib Y, Rashid RH, Ali M, et al. Does tranexamic acid reduce blood transfusion following surgery for inter-trochanteric fracture? A randomized controlled trial. J Pak Med Assoc. 2015;65(Suppl 3):S17–20. [PubMed] [Google Scholar]
- 73.Nielsen CS, Jans O, Orsnes T, et al. Combined intra-articular and intravenous tranexamic acid reduces blood loss in total knee arthroplasty. A randomized, double-blind, placebo-controlled trial. J Bone Joint Surg Am. 2016;98:835–41. doi: 10.2106/JBJS.15.00810. [DOI] [PubMed] [Google Scholar]
- 74.Drosos GI, Ververidis A, Valkanis C, et al. A randomized comparative study of topical versus intravenous tranexamic acid administration in enhanced recovery after surgery (ERAS) total knee replacement. J Orthop. 2016;13:127–31. doi: 10.1016/j.jor.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen X, Cao X, Yang C, et al. Effectiveness and safety of fixed-dose tranexamic acid in simultaneous bilateral total knee arthroplasty: a randomized double-blind controlled trial. J Arthroplasty. 2016;31:2471–5. doi: 10.1016/j.arth.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 76.Xie J, Ma J, Yao H, et al. Multiple boluses of intravenous tranexamic acid to reduce hiden blood loss after primary total knee arthroplasty without tourniquet: a randomized clinical trial. J Arthroplasty. 2016;31:2458–64. doi: 10.1016/j.arth.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 77.Tzatzairis T, Drosos GI, Kotsios SE, et al. Intravenous vs topical tranexamic acid in total knee arthroplasty without tourniquet application: a randomized controlled study. J Arthroplasty. 2016;31:2465–70. doi: 10.1016/j.arth.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 78.Jain NP, Nisthane PP, Shah NA. Combined administration of systemic and topical tranexamic acid for total knee arthroplasty: can it be a better regimen and yet safe? A randomized controlled trial. J Arthroplasty. 2016;1:542–7. doi: 10.1016/j.arth.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 79.Xie J, Yue C, Kang P, Pei F. Combined use of intravenous and topical tranexamic acid following cementless total hip arthroplasty: a randomized clinical trial. Hip Int. 2016;26:36–42. doi: 10.5301/hipint.5000291. [DOI] [PubMed] [Google Scholar]
- 80.Wu YG, Zeng Y, Yang TM, et al. The efficacy and safety of combination of intravenous and topical tranexamic acid in revision hip arthroplasty: a randomized, controlled trial. J Arthroplasty. 2016;31:2548–53. doi: 10.1016/j.arth.2016.03.059. [DOI] [PubMed] [Google Scholar]
- 81.Tengberg PT, Foss NB, Palm H, et al. Tranexamic acid reduces blood loss in patients with extracapsular fractures of the hip. Results of a randomized controlled trial. Bone Joint J. 2016;98-B:747–53. doi: 10.1302/0301-620X.98B6.36645. [DOI] [PubMed] [Google Scholar]
- 82.Yi Z, Bin S, Jing Y, et al. Tranexamic acid administration in primary total hip arthroplasty. A randomized controlled trial of intravenous combined with topical versus single-dose intravenous administration. J Bone Joint Surg Am. 2016;98:983–91. doi: 10.2106/JBJS.15.00638. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y, Zhang L, Ma X, et al. What is the optimal approach for tranexamic acid application in patients with unilateral total hip arthroplasty? Orthopade. 2016;45:616–21. doi: 10.1007/s00132-016-3252-y. [DOI] [PubMed] [Google Scholar]
- 84.Maniar R, Singhi T, Patil A, et al. Optimizing efficacy of tranexamic acid in bilateral knee arthroplasty - a prospective randomized controlled study. Knee. 2017;24:100–6. doi: 10.1016/j.knee.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 85.Lee SY, Chong S, Balasubramanian D, et al. What is the ideal route of administration of tranexamic acid in TKA? Arandomized controlled trial. Clin Orthop Relat Res. 2017;475:1987–96. doi: 10.1007/s11999-017-5311-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lei Y, Xie J, Xu B, et al. The safety and efficacy of multiple-dose intravenous tranexamic acid on blood loss following total knee arthroplasty: a randomized controlled trial. Int Orthop. 2017;41:2053–9. doi: 10.1007/s00264-017-3519-x. [DOI] [PubMed] [Google Scholar]
- 87.Wang JW, Chen B, Lin PC, et al. The efficacy of the combined use of rivaroxaban an tranexamic acid on blood conservation in minimally invasive total knee arthroplasty a double-blind randomized controlled trial. J Arthroplasty. 2017;32:801–6. doi: 10.1016/j.arth.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 88.Vara AD, Koueiter DM, Pinkas DE, et al. Intravenous tranexamic acid reduces total blood loss in reverse total shoulder arthroplasty: a prospective, double-blinded, randomized, controlled trial. J Shoulder Elbow Surg. 2017;8:1383–9. doi: 10.1016/j.jse.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 89.Higgins JPT, Green S, editors. 16.9.5 Validity of methods of meta-analysis for rare events. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. 2011. [Accessed on 15/09/2017]. Available at: http://handbook.cochrane.org/chapter_16/16_9_5_validity_of_methods_of_meta_analysis_for_rare_events.htm.
- 90.Bradburn MJ, Deeks JJ, Berlin JA, Localio AR. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Statist Med. 2007;26:53–77. doi: 10.1002/sim.2528. [DOI] [PubMed] [Google Scholar]
- 91.Tian L, Cai T, Pfeffer MA, et al. Exact and efficient inference procedure for meta-analysis and its application to the analysis of independent 2×2 tables with all available data but without artificial continuity correction. Biostatistics. 2009;10:275–81. doi: 10.1093/biostatistics/kxn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96:624–30. doi: 10.2106/JBJS.M.00285. [DOI] [PubMed] [Google Scholar]
- 93.Carson JL, Duff A, Poses RM, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348:1055–60. doi: 10.1016/S0140-6736(96)04330-9. [DOI] [PubMed] [Google Scholar]
- 94.Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113:3406–17. doi: 10.1182/blood-2008-10-167643. [DOI] [PubMed] [Google Scholar]
- 95.Liumbruno GM, Vaglio S, Biancofiore G, et al. Transfusion thresholds and beyond. Blood Transfus. 2016;14:123–5. doi: 10.2450/2016.0008-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Franchini M, Marano G, Mengoli C, et al. Red blood cell transfusion policy: a critical literature review. Blood Transfus. 2017;15:307–17. doi: 10.2450/2017.0059-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guerra R, Velati C, Liumbruno GM, Grazzini G. Patient blood management in Italy. Blood Transfus. 2016;14:1–2. doi: 10.2450/2015.0171-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vaglio S, Prisco D, Biancofiore G, et al. Recommendations for the implementation of a patient blood management programme. Application to elective major orthopaedic surgery in adults. Blood Transfus. 2016;14:23–65. doi: 10.2450/2015.0172-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Franchini M, Muñoz M. Towards the implementation of patient blood management across Europe. Blood Transfus. 2017;15:292–3. doi: 10.2450/2017.0078-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vaglio S, Gentili S, Marano G, et al. The Italian regulatory guidelines for the implementation of patient blood management. Blood Transfus. 2017;15:325–8. doi: 10.2450/2017.0060-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Muñoz M, Gómez-Ramírez S, Liumbruno GM. Peri-operative anaemia management in major orthopaedic surgery: the need to find a pathway. Blood Transfus. 2017;15:289–91. doi: 10.2450/2017.0296-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Melvin JS, Stryker LS, Sierra RJ. Tranexamic acid in hip and knee arthroplasty. J Am Acad Orthop Surg. 2015;23:732–40. doi: 10.5435/JAAOS-D-14-00223. [DOI] [PubMed] [Google Scholar]
- 103.Poole D. Coagulopathy and transfusion strategies in trauma. Overwhelmed by literature, supported by weak evidence. Blood Transfus. 2016;14:3–7. doi: 10.2450/2015.0244-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu Q, Zhang HA, Liu SL, et al. Is tranexamic acid clinically effective and safe to prevent blood loss in total knee arthroplasty? A meta-analysis of 34 randomized controlled trials. Eur J Orthop Surg Traumatol. 2015;25:525–41. doi: 10.1007/s00590-014-1568-z. [DOI] [PubMed] [Google Scholar]
- 105.Gandhi R, Evans HM, Mahomed SR, Mahomed NN. Tranexamic acid and the reduction of blood loss in total knee and hip arthroplasty: a meta-analysis. BMC Res Notes. 2013;6:184. doi: 10.1186/1756-0500-6-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.