Abstract
Background
Leucoreduction of blood components, including platelet components, is strongly encouraged but not yet universal, especially outside high income countries. As both leucocytes and platelets secrete copious amounts of pro-inflammatory cytokines/chemokines under various conditions and during storage, we investigated the potential of the respective secretory programmes of these cells in order to evaluate their subsequent pathophysiological effects.
Material and methods
A total of 158 individual non-leucoreduced platelet components were obtained from Tunisian donors and tested for characteristic biological response modifiers (BRM) of leukocytes (IL-1β, IL-8), platelets (sCD62P, sCD40L) and both cell types (TNF-α, RANTES) in the presence or absence of thrombin stimulation and after different periods of storage (up to 5 days). BRM levels were determined using enzyme-linked immunosorbent assays and Luminex technology. Platelet-leucocyte aggregate formation during storage was analysed using flow cytometry.
Results
Leucocyte- and platelet-associated BRM had clearly distinct profiles both at the onset (day 0) and termination (day 5) of the observation period but altered during the intermediate period so that their respective importance was inverted; in fact, the profiles were merged and indistinguishable on days 2–3. The leucocyte-derived BRM largely dominated over platelet-derived ones and further altered the BRM platelet secretion programme.
Discussion
This study contributes substantial, new information on leucocyte/platelet interactions and their likely role in transfusion when leucodepletion cannot be performed or is only partially achieved.
Keywords: transfusion, biological response modifiers, leucocyte/platelet interaction
Introduction
Leucoreduction has become the standard for blood component processing in Europe and in many high income countries1–5. Systematic leucoreduction has demonstrated advantages of reducing alloimmunisation, carriage of viruses and transfusion-related immunomodulation6,7. Leucoreduction consistently lowers the amount of pro-inflammatory cytokines and chemokines, released predominantly from granulocytes and monocytes, which are largely responsible for transfusion-associated disorders (chills, moderate fever, rigors, etc.)8. However, platelets themselves, especially when ageing (stored for more than 3 days), also release substantial quantities of cytokines and chemokines9,10. Despite accounting for only 10% of transfused blood components, in settings of stringent leucoreduction, platelet transfusions are responsible for almost 25% of recorded adverse events and 50% of serious adverse events11. We and others have extensively evaluated the evolution of platelet-derived biological response modifiers (BRM) in leucoreduced platelet components (PC), and explored their involvement in the development of platelet transfusion adverse events12–14. The results cannot, however, be extrapolated to PC prepared from non-leucoreduced blood donations, as occurs in many lower income countries in which systematic leucoreduction is not performed. This is particularly the case when PC comprise whole blood-derived platelet-rich plasma (PRP), as in Tunisia, among other countries15. PC obtained from such a production process contain residual leucocytes. The coexistence of platelets and leucocytes in a potential platelet-activating environment initiates an interaction that can influence the secretory programmes of both types of cells. Certain cytokines/chemokines and, in general, BRM secreted by leucocytes and platelets in blood components are common, but the majority are acknowledged to be fairly specific to one or other of the types of cells16,17.
Through the assessment of BRM characteristic of leucocytes (interleukin-1β [IL-1β], interleukin 8 [IL-8]), platelets (sCD62P, sCD40L) and both cell types (tumour necrosis cell factor-α [TNF-α], regulated on activation, normal T cell expressed and secreted [RANTES]) and the measurement of platelet-leukocyte aggregates, we investigated the mutual influence of leucocyte- and platelet-released BRM in stored PC (individual whole blood-derived PRP), with two clearly distinct objectives: Firstly, to further explore the pathophysiological process that can lead to adverse events, including severe ones such as transfusion-related acute lung injury and severe febrile non-haemolytic transfusion reactions, as there are still gaps in the fine understanding of the pathogenic sequences18; and secondly to identify strategies that could reduce the impact of BRM in PC transfusions and that could be applied, despite limited resources, in medium-income countries.
Materials and methods
Platelet component preparation
The PC were prepared from whole blood donations collected from healthy donors. Informed consent was obtained from all participants and the ad hoc Ethics Committee of the Regional Blood Transfusion Centre at “F. Hached” Hospital (Sousse, Tunisia) approved the protocol. Blood was collected into triple blood bags (JMS Co. Ltd, Ang Mo Kio, Singapore) with acid-citrate-dextrose as the anticoagulant. The PC were isolated by a standard, two-step centrifugation method, as previously described by Bouslama et al., and could be issued if they met all required testing parameters and quality controls15. Plasma removal and platelet additive solutions were not used and the PC were stored as unitary PC (UPC).
Testing protocols and sampling procedures
The samples used in this study were obtained by methods that preserved the sterility of the UPC so that they remained valid for distribution to patients. For each studied UPC, two samples were obtained. The first one was obtained immediately after the production process (day 0) as follows: after 1 hour of stirring, a 3 mL sample was moved into a quick transfer bag (VSE 0000A, MacoPharma, Mouvaux, France) that was aseptically connected to the bag containing the PC using a sterile connection device (Terumo Europe, Middle East & Africa, Leuven, Belgium). The second sample was obtained on a specified day (day 1, 2, 3, 4 or 5) of the UPC storage using the “stripping” method19. Each sample was subsequently divided into two aliquots, one for functional stimulation and analysis and one for determining the final concentrations of platelets, residual leucocytes, and contaminating red blood cells using a Beckman Coulter AcT 10 Hematology Analyzer (Beckman Coulter Inc., Paris, France).
A 600 μL volume from each sample was added to an equal volume of fixative solution (Thrombofix® Platelet Stabilizer 6607130, Beckman Coulter Inc.). This solution stabilises platelets and prevents their activation. The mixture was incubated for 1 hour at room temperature and then centrifuged at 450 g for 10 minutes. The supernatants were stored at −80 °C (unstimulated samples).
To a second 600 μL aliquot, we added 50 μL of thrombin receptor activator peptide (TRAP; Peptide SFLLRN, Sigma-Aldrich Chemie, Saint-Quentin Fallavier, France; 66.85 μM) for 30 minutes at room temperature and then added 650 μL of Thrombofix. One hour later, the mixture was centrifuged and the supernatants were frozen at −80 °C until further use (stimulated samples) (Online Supplementary Figure S1).
Quantification of biological response modifiers
The frozen PC supernatants were assayed using human enzyme-linked immunosorbent assays (ELISA) to measure sCD62P and RANTES (R&D Systems Europe Ltd., Abingdon, UK). The absorbance at 450 nm was measured with an ELISA microplate reader (Multiskan, Thermo Scientific, Illkirch, France). sCD40L, IL-8, IL-1β and TNF-α were measured by the multiplex method using Luminex technology, according to the manufacturer’s instructions (Milliplex Map Kit Millipore, Darmstadt, Germany).
Analysis of platelet-leucocyte aggregates
Platelet-leucocyte aggregates were assessed in six supplementary UPC by means of flow cytometry. Samples of 5 mL, without volume replacement, were taken from each UPC on days 0, 1, 2, 3, and 5 for analysis. Samples were diluted 100-fold in phosphate-buffered saline and analysed under unstimulated conditions using Thrombofix® fixative solution. One hundred microlitres of each aliquot were incubated, for 15 minutes in the dark at room temperature, with 5 μL of a mouse monoclonal antibody to human CD41 conjugated to fluorescein isothiocyanate (FITC) (R&D system) and 5 μL of allophycocyanin (APC)-conjugated mouse monoclonal antibody to the human leucocyte common antigen CD45 (BD Biosciences, Paris, France). Mouse IgG1 FITC and APC isotype controls (BD Biosciences) were used at saturating concentrations as the negative controls. Stained samples were diluted 4-fold in phosphate-buffered saline and analysed in a flow cytometer (EPICS XL, Beckman Coulter). Platelet-leucocyte aggregates were identified as particles positive for both CD45 and CD41 and expressed as the percentage of total leucocytes.
Statistical analyses
XLSTAT (Addinsoft, Paris, France) and GraphPad Prism version 5.00 for Windows (San Diego, CA, USA) were used for statistical evaluation of the data. The Kolmogorov-Smirnov test was used to check for the normal distribution of the data. To compare paired or unpaired data of the PC samples during storage, we used the Wilcoxon signed-rank or Kruskal-Wallis tests, respectively, for non-parametric comparisons of cytokine levels at the different studied conditions. Principle component analysis for the visualisation of BRM correlations was performed using Spearman’s correlations. P-values less than 0.05 are considered statistically significant.
For statistical reasons, samples stored for 1 or 2 days were grouped together, as were those stored for 3 or 4 days.
Results
Characteristics of the unitary platelet components
All 158 UPC, which originated from an equal number of donations from healthy volunteers (52 males and 106 females), were assessed for changes in platelet concentration, leucocyte contamination and pH. No significant changes in these parameters were observed between day 0 and day 5 and the samples were comparable between units and over time (Online Supplementary Figure S2).
The release (secretion) of a pre-defined set of BRM was tested for each UPC at day 0 and at the time of the units’ issue. Soluble CD62P and sCD40L were selected for measurement because they are characteristic BRM of platelets; RANTES and TNF-α, because they are principally characteristic of platelets, although not completely absent from leucocytes; and IL-1β and IL-8, because they are chiefly characteristic of leucocytes. Online Supplementary Table SI shows the respective contributions of platelets and leucocytes to the overall amount of the six selected BRM. Importantly, platelets have been reported to secrete all six analysed BRM, although their release profiles probably differ from those of leucocytes17,20.
Kinetics of release of biological response modifiers depending on time of storage
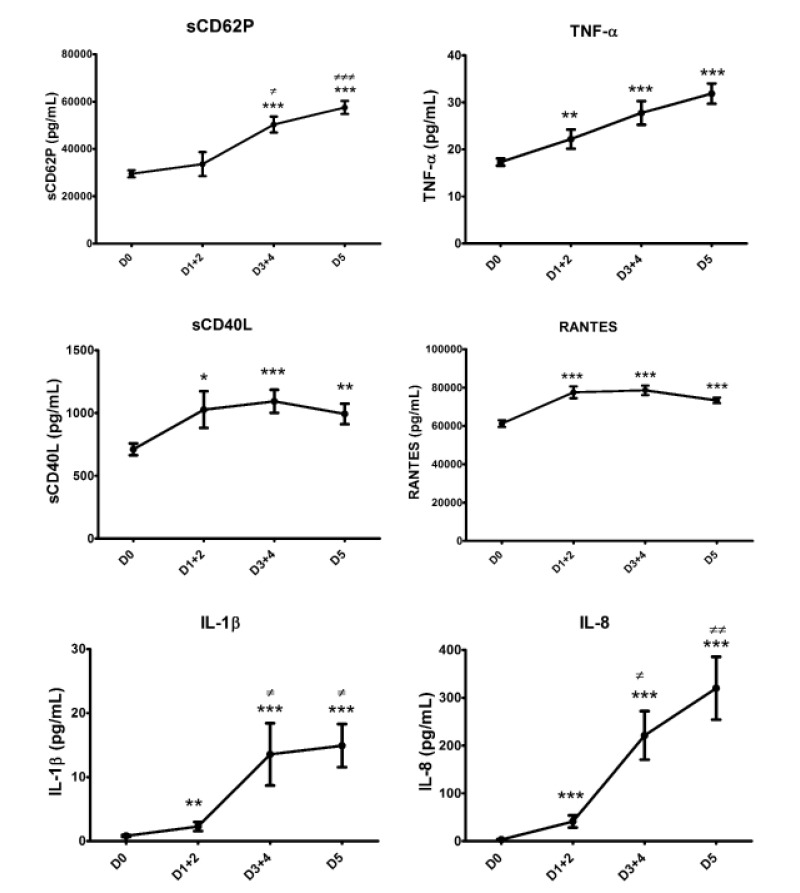
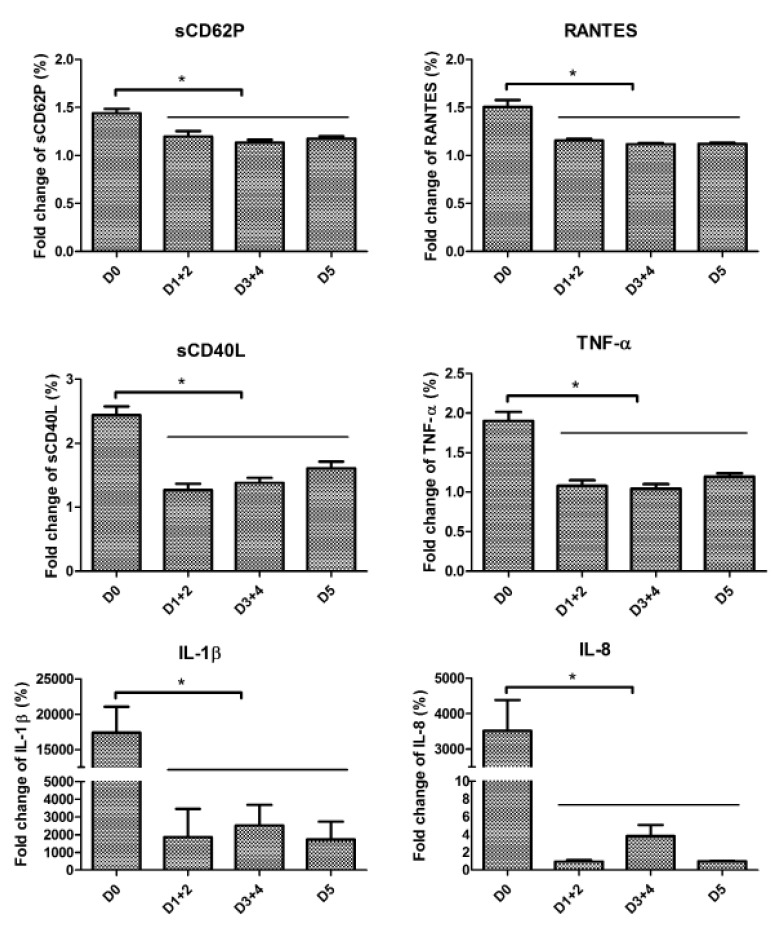
Figure 1 shows that the BRM secretion profiles can be grouped in pairs: sCD62P and TNF-α increased almost linearly over time, while sCD40L and RANTES peaked at days 3+4 and declined slightly, but not significantly, on day 5. IL-1β and IL-8 were nearly undetectable at day 0, began to be detectable on days 1+2, and were secreted fairly abundantly after day 2. There was a non-significant increase of IL-1β on day 5 over days 3+4, whereas there was a sustained and statistically significant increase of IL-8 on day 5 over days 3+4.
Figure 1.
Kinetics of BRM release depending on the day of storage.
* or ≠: p<0.05; ** or ≠≠: p<0.001; *** or ≠≠≠: p<0.0001. Asterisks and hashtags represent significant differences against D0 and D1, respectively, using the Kruskal-Wallis test with Dunn’s correction for multiple comparisons. Data are presented as mean ± SEM; n=158, 29, 65 and 64 for D0, D1+2, D3+4 and D5, respectively. D: day; TNF: tumour necrosis factor; RANTES: regulated on activation, normal T cell expressed and secreted; IL: interleukin; SEM: standard error mean.
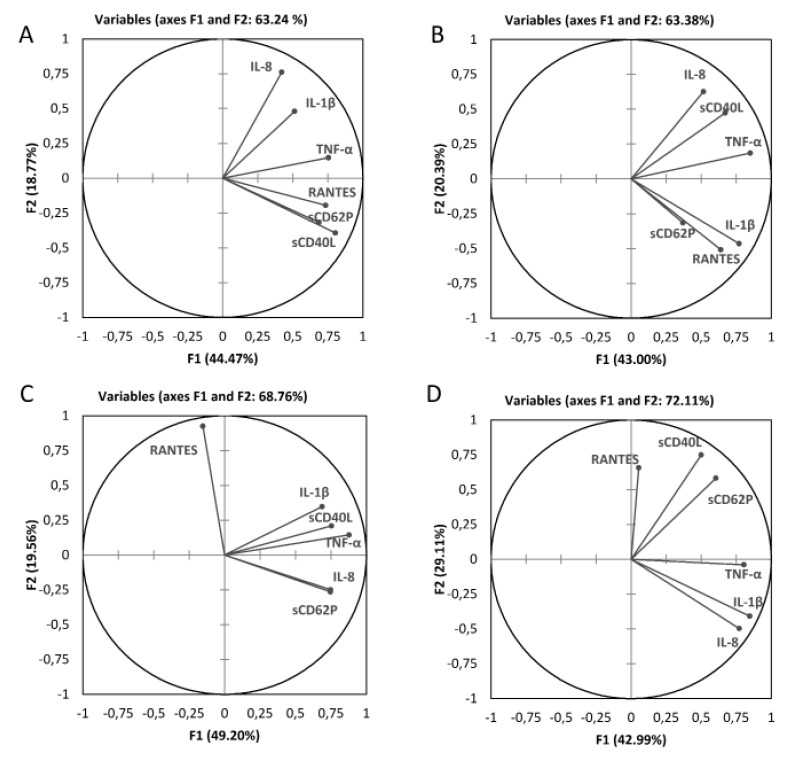
As reported, BRM are not specific but simply characteristic of certain cell types. Thus, we sought to investigate whether their secretion profiles could be determined. This could not be done based on the individual secretion assays for technical reasons, so we used a statistical (mathematical) model. Correlations between BRM release during storage were analysed through principle component analysis (Figure 2 and Table I). On day 0, two secretion profiles, separated by TNF-α, could be observed (Figure 2A): IL-1β and IL-8 in the upper right quadrant and RANTES, sCD62P and sCD40L below. There was a statistical correlation between TNF-α and the other five BRM (Table I). Moreover, IL-1β and IL-8 were statistically correlated but IL-8 did not correlate with sCD62P, RANTES and sCD40L, while IL-1β had no correlation with sCD62P but did exhibit a correlation with the other two BRM. On days 1+2, sCD62P dissociated from sCD40L and IL-1β dissociated from IL-8. The graphic representation (Figure 2B) clearly shows that sCD40L and IL-1β exchanged positions. On days 3+4, sCD62P re-associated with sCD40L, IL-1β and TNF-α, while RANTES dissociated from IL-1β and TNF-α. Indeed, RANTES secretion clearly polarised and opposed another polarisation: that of IL-8 and sCD62P (Figure 2C). Finally, on day 5, there were again two profiles with TNF-α returning to an intermediate position, similar to that on day 0, still dividing the RANTES+sCD40L+sCD62P and IL-1β+IL-8 secretion, but with these now in the opposite positions from day 0 (Figure 2D). This strongly suggests that platelet and leucocyte BRM secretion profiles are distinct from one another on days 1 and 5, while there are intricate associations in between, with possible cascade interactions (not assessed here).
Figure 2.
A two-dimensional correlation monoplot of the coefficients of the first two principal components, showing relationships between the BRM during storage.
The correlation monoplot has vectors pointing away from the origin to represent the original variables. The angle between the vectors is an approximation of the correlation between the variables. A small angle indicates that the variables are positively correlated, an angle of 90 degrees indicates the variables are not correlated, and an angle close to 180 degrees indicates the variables are inversely correlated. The length of the line and its closeness to the circle indicate how well the variable is represented in the plot. A, B, C and D illustrate correlations at D0, D1+2, D3+4 and D5, respectively. D: day; TNF: tumour necrosis factor; RANTES: regulated on activation, normal T cell expressed and secreted; IL: interleukin.
Table I.
Spearman’s correlations between BRM levels during PC storage.
| Variables | sCD62P D0 | RANTES D0 | CD40L D0 | IL-1β D0 | TNF-α D0 | IL-8 D0 |
|---|---|---|---|---|---|---|
| sCD62P D0 | 1/0 | 0.387/<0.0001 | 0.514/<0.0001 | 0.144/0.071 | 0.417/<0.0001 | 0.141/0.078 |
| RANTES D0 | 1/0 | 0.596/<0.0001 | 0.359/<0.0001 | 0.307/<0.0001 | 0.152/0.057 | |
| CD40L D0 | 1/0 | 0.208/0.009 | 0.552/<0.0001 | 0.063/0.428 | ||
| IL-1β D0 | 1/0 | 0.272/0.001 | 0.287/<0.0001 | |||
| TNF-α D0 | 1/0 | 0.392/<0.0001 | ||||
| IL-8 D0 | 1/0 | |||||
| Variables | sCD62P D1+2 | RANTES D1+2 | CD40L D1+2 | IL-1β D1+2 | TNF-α D1+2 | IL-8 D1+2 |
| sCD62P D1+2 | 1/0 | 0.144/0.453 | −068/0.723 | 0.347/0.065 | 0.155/0.419 | 0.268/0.159 |
| RANTES D1+2 | 1/0 | 0.275/0.147 | 0.579/0.001 | 0.382/0.041 | 0.00/1.00 | |
| CD40L D1+2 | 1/0 | 0.283/0.135 | 0.570/0.001 | 0.399/0.033 | ||
| IL-1β D1+2 | 1/0 | 0.560/0.002 | 0.099/0.608 | |||
| TNF-α D1+2 | 1/0 | 0.439/0.018 | ||||
| IL-8 D1+2 | 1/0 | |||||
| Variables | sCD62P D3+4 | RANTES D3+4 | CD40L D3+4 | IL-1β D3+4 | TNF-α D3+4 | IL-8 D3+4 |
| sCD62P D3+4 | 1/0 | −0.235/0.06 | 0.490/<0.0001 | 0.304/0.014 | 0.555/<0.0001 | 0.475/<0.0001 |
| RANTES D3+4 | 1/0 | 0.049/0.699 | 0.095/0.449 | −0.045/0.719 | −0.249/0.045 | |
| CD40L D3+4 | 1/0 | 0.361/0.003 | 0.647/<0.0001 | 0.396/0.001 | ||
| IL-1β D3+4 | 1/0 | 0.600/<0.0001 | 0.441/<0.0001 | |||
| TNF-α D3+4 | 1/0 | 0.526/<0.0001 | ||||
| IL-8 D3+4 | 1/0 | |||||
| Variables | sCD62P D5 | RANTES D5 | CD40L D5 | IL-1β D5 | TNF-α D5 | IL-8 D5 |
| sCD62P D5 | 1/0 | 0.244/0.052 | 0.644/<000.1 | 0.249/0.047 | 0.328/0.008 | 0.188/0.137 |
| RANTES D5 | 1/0 | 0.334/0.007 | −0.085/0.502 | −0.080/0.526 | −0.119/345 | |
| CD40L D5 | 1/0 | 0.085/0.501 | 0.418/0.001 | −0.0330.791 | ||
| IL-1β D5 | 1/0 | 0.586/<0.0001 | 0.875/<0.0001 | |||
| TNF-α D5 | 1/0 | 0.484/<0.0001 | ||||
| IL-8 D5 | 1/0 |
Values are presented as correlation matrix/p-value. The italic values were statistically different from 0 at a significance level of p<0.05. D: day; TNF: tumour necrosis factor; RANTES: regulated on activation, normal T cell expressed and secreted; IL: interleukin.
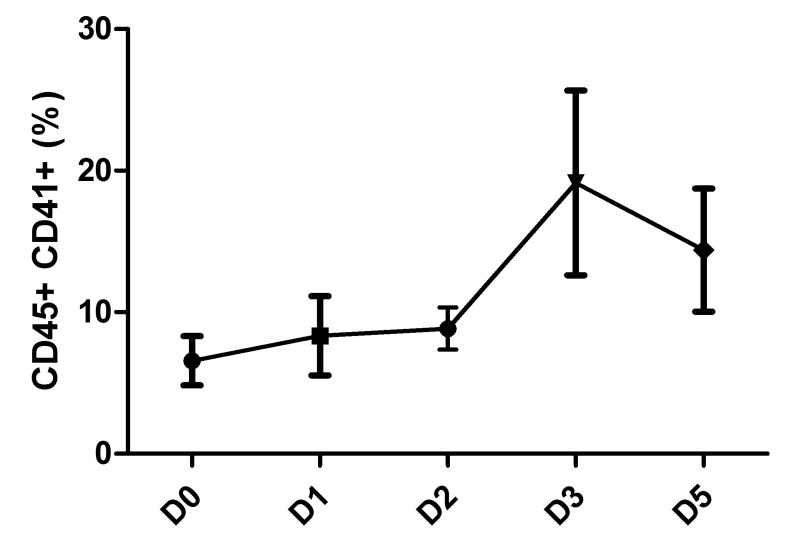
Kinetics of platelet-leucocyte aggregate formation
As shown in Figure 3, the formation of platelet-leucocyte aggregates varied very little during the first 2 days of PC storage, then increased on days 3 and 5 although the differences were not statistically significant.
Figure 3.
Leucocyte-platelet aggregate formation during PC storage. Complexes were detected by flow cytometry using allophycocyanin (APC)-conjugated anti-CD45 (BD Biosciences) and fluorescein isothiocyanate (FITC)-conjugated anti-CD41 (R&D system). Data are presented as mean ± SEM; n=6. PC: platelet concentrate; D: day; SEM: standard error mean.
Reactivity of platelets to PAR1 activation in a platelet-rich plasma suspension with leucocytes
We next examined whether the presence of leucocytes in PRP suspensions, representing UPC, altered platelet reactivity to a thrombin analogue, TRAP (SFLLRN), which signals through platelet-expressed platelet-activating receptor 1 (PAR1). Because the present investigation was focused on platelet/leucocyte interactions, we measured BRM secretion in response to non-leucodepleted UPC, comprising platelets and contaminating leucocytes.
There is little evidence from the literature that cells other than platelets express PAR1 and are sensitive to thrombin21–24. Despite our attempts, we failed to identify PAR1 on leucocytes in this series of experiments, but we do not rely unquestionably on these findings, as the working conditions (reagents) and controls appeared variable and did not lead to clear-cut data (not shown).
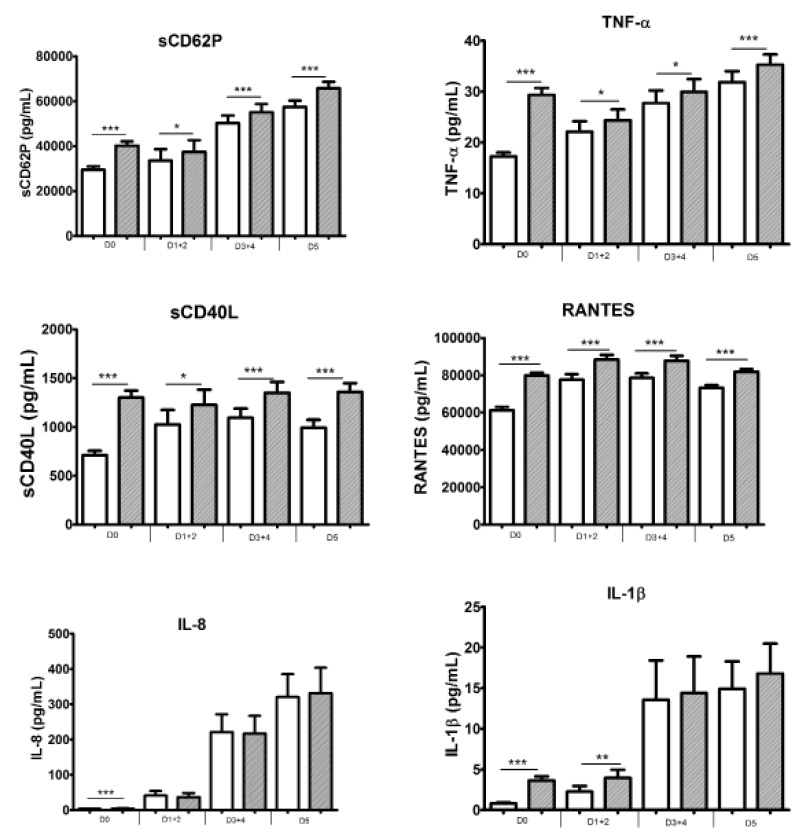
As shown in Figure 4, the exposure of platelets to TRAP during UPC storage still resulted in sustained production of sCD62P, TNF-α, sCD40L and RANTES. Furthermore, IL-8 and IL-1β secretion was elicited at the onset of the UPC process but only in minute amounts (although significantly different from the controls). However, while the secretion of these cytokines was more abundant beyond day 3, it was independent of TRAP stimulation; thus, one can hypothesise that minute mounts were mobilised from platelets early after stimulation, while the later, large amounts derived from the leucocytes in the stored PC. The responses of all BRM decreased after day 0 and remained stable (Figure 5).
Figure 4.
Secretory profiles of BRM in response to TRAP stimulation.
Comparisons used the mean of the Wilcoxon sign ranked test for paired samples. White boxes represent the unstimulated condition and grey boxes represent the TRAP-stimulated condition. Data are presented as mean ± SEM; n=158, 29, 65 and 64 for D0, D1+2, D3+4 and D5, respectively. * p<0.05; ** p<0.001; *** p<0.0001. BRM: biological response modifier; TRAP: thrombin receptor activator peptide; TNF: tumour necrosis factor; RANTES: regulated on activation, normal T cell expressed and secreted; IL: interleukin.
Figure 5.
Fold-changes of BRM levels in response to TRAP stimulation depending on storage time.
Data are presented as mean ± SEM; n=158, 29, 65 and 64 for D0, D1+2, D3+4 and D5, respectively. BRM: bilogical response modifier; TRAP: thrombin receptor activator peptide; RANTES: regulated on activation, normal T cell expressed and secreted; TNF: tumour necrosis factor; IL: interleukin; D: day; SEM: standard error mean.
Discussion
In this study, we evaluated the secretion of six BRM over time from platelets and residual leucocytes in non-leucodepleted individual PRP, constituting PC for transfusion in a medium income country (Tunisia). As was expected and in contrast to what is usually observed in PC obtained from leucodepleted-apheresis or from buffy coats, we found that there was a non-negligible production of BRM attributable to leucocytes, i.e., IL-1β and IL-8, and to a lesser extent, TNF-α. All three of these BRM can be produced by stored platelets in PC, but generally in less copious amounts17,25. Notably, the profile of platelet-secreted BRM, as demonstrated by the “standard” sCD62P and sCD40L as well as by RANTES, was largely comparable to what is commonly observed26–28. This led to the first impression that non-leucodeleted PC carry both platelet- and leucocyte-BRM-associated inflammatory risks13,29–31. Because a canonical property of cytokines/chemokines (and likely mediators, which largely constitute the BRM under investigation in this study) is their action in cascades (along with redundancy), we explored the influence - if any - of leucocyte-derived BRM on the platelet secretory programme to assess whether there is indeed a cumulative inflammatory risk. In fact, most leucocyte-derived BRM have ligands on platelets and most platelet-derived BRM have ligands on leucocytes8,32–34. We showed a clearly individualised BRM profile at the onset of PC processing, with sets of statistically associated leucocyte BRM and platelet BRM on day 0, with TNF-α, produced by both cell types, in an intermediate position and associated with both. On day 5, just prior to the expiry date of the PC, polarised sets of leucocyte- and platelet-BRM (with an intermediate association with TNF-α) were observed again, although in inverted positions from day 0 (leucocyte-BRM secretion was copious and seemed to dominate the picture). In the period between days 0 and 5, there was an interplay between the leucocyte- and platelet-BRM associations, suggesting that products of one cell type influence the secretion of products by the other type and vice versa. One limitation of this study is that BRM could not be measured along storage in the same PC. However, we found no significant variations in platelet and leucocyte contents between the PC groups analysed each day of storage.
Recently, there has been a flurry of publications highlighting the importance of platelet-leucocyte aggregates, which have proven to be essential in both physiology (haemostasis and clot stability35,36) and pathology (formation of neutrophil extracellular traps in sepsis, to cite one37). An intricate programme for platelet-leucocyte aggregate formation compared to the programmes for solely leucocytes or platelets would not be a complete surprise. We examined this issue closer, still in the context of UPC for transfusion. However, another limitation of this study is that this investigation could not be conducted on the series of samples described above, and data were obtained from pilot experiments carried out on an additional series of volunteer donations (n=6).
We obtained evidence that platelet-leucocyte aggregate formation increased between days 3 and 4, although differences were not statistically significant. As this increase paralleled the increase of sCD62p (indicating platelet activation) and the secretion of IL-1β and IL-8, our data may indicate not only the leucocyte origin of these BRM but also the probable mutual influence of both cell types (Figures 1 and 3). This platelet-leucocyte aggregate formation does not seem to alter the capacity of platelets to respond to external stimuli when needed and probably aids haemostasis.
We next examined the capacity of platelets, bathed in leucocyte-derived BRM, to react to haemostatic signals. The platelets maintained their secretory potential of platelet-associated BRM over time from day 0 to day 5 when they were exposed to TRAP (thrombin). On day 3 and later, IL-1β and IL-8 levels were high, irrespectively of TRAP stimulation, suggesting that these are platelet independent. While this specific point was not addressed in this study and it is likely that the ageing of PC is detrimental considering the cumulative risks posed by the inflammatory molecules that will be infused along with the therapeutic components (the platelets), ageing does seem compatible with continued reactivity of platelets to thrombin.
The present study examined the BRM responses of platelets in PRP as non-leucoreduced PC for transfusion. It confirmed that pro-inflammatory cytokines tend to accumulate in ageing PC stored under normal conditions; these cytokines create an inflammatory risk to the recipient and may also reduce the efficacy of the PC, as deduced from numerous other studies8,38–42. Furthermore, in non-leucodepleted conditions, leucocyte-derived BRM also accumulate with peaks reached gradually at the time the PC approaches its expiry date, but with considerable levels already by day 3 and onwards. These data confirm both data collected in the past by our group, albeit under different conditions, and those collected by others. In addition to the aforementioned evidence, we have contributed substantial novel information on leucocyte/platelet complexes and their likely role in transfusion when leucodepletion cannot be performed or is only partially achieved. Platelet-secreted and leucocyte-derived BRM are independent (as observed clearly on day 0), but their concomitant presence in the PC induces an interplay and a loss of independency was observed from day 1 to day 4. The variables became independent again by day 5, most likely because the amounts of leucocyte-derived BRM exceeded the platelet-secreted BRM so greatly that the leucocyte profile dominated.
Conclusions
This study confirms, in a novel transfusion circumstance, the interdependence of the BRM secretory profiles of leucocytes and platelets when bound in platelet-leucocyte aggregates. This interplay may have consequences for transfusion quality and safety, as already outlined. The data provide information for pathophysiological models of leucocyte/platelet interactions (at least via their secretory products). Leucocytes in PC are undesirable in general, and create higher risks as the shelf-life of the PC is prolonged. The leucocytes present in non-leucoreduced PC secrete BRM over time and are agonists with platelet-derived BRM. Strategies to reduce pro-inflammatory BRM should be sought. Alternatively, pathogen reduction technologies for whole blood are under development and hold some promise with regards to reducing both leucocyte-mediated effects (as deduced from early studies43) and, potentially, allo-immunisation (antigen presentation). To what extent this strategy could be cost-efficient compared to conventional PC processing involving leucoreduction remains to be evaluated.
Supplementary Information
Acknowledgements
This work was supported by grants from Erasmus Mundus Al-Idrisi (idri-1100823), the National French Blood Bank - EFS (grant APR), the Rhône-Alpes region (grant CMIRA), and the Association “Les Amis de Rémi”, Savigneux, France. We also thank Mr Charles-Antoine Arthaud and Jocelyne Fagan (EFS Auvergne-Loire, France) for technical help with the experimental procedures.
Footnotes
Authorship contributions
OG, SL and FC participated in the study design, study supervision, and data interpretation; CA, TC and SJY participated in sample collection, experiment analyses and data interpretation. CA and AP performed the statistical analysis. OG, SL and CA wrote the manuscript. All Authors approved the final content of the manuscript.
The Authors declare no conflicts of interest.
References
- 1.European Directorate for the Quality of Medicines & HealthCare (EDQM) Guide to the preparation, use and quality assurance of blood components. 18th ed. [Accessed on 12/02/2016]. Available at: https://www.edqm.eu/en/news/guide-preparation-use-and-quality-assurance-blood-components-18th-edition.
- 2.Beckman N, Sher G, Masse M, et al. Review of the quality monitoring methods used by countries using or implementing universal leukoreduction. Transfus Med Rev. 2004;18:25–35. doi: 10.1016/j.tmrv.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Bassuni WY, Blajchman MA, Al-Moshary MA. Why implement universal leukoreduction? Hematol Oncol Stem Cell Ther. 2008;1:106–23. doi: 10.1016/s1658-3876(08)50042-2. [DOI] [PubMed] [Google Scholar]
- 4.Mishima Y, Tsuno NH, Matsuhashi M, et al. Effects of universal vs bedside leukoreductions on the alloimmunization to platelets and the platelet transfusion refractoriness. Transfus Apher Sci. 2015;54:112–21. doi: 10.1016/j.transci.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Wortham ST, Ortolano GA, Wenz B. A brief history of blood filtration: clot screens, microaggregate removal, and leukocyte reduction. Transfus Med Rev. 2003;17:216–22. doi: 10.1016/s0887-7963(03)00023-3. [DOI] [PubMed] [Google Scholar]
- 6.Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev. 2007;21:327–48. doi: 10.1016/j.blre.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Refaai MA, Blumberg N. Transfusion immunomodulation from a clinical perspective: an update. Expert Rev Hematol. 2013;6:653–63. doi: 10.1586/17474086.2013.850026. [DOI] [PubMed] [Google Scholar]
- 8.Lannan KL, Sahler J, Spinelli SL, et al. Transfusion immunomodulation - the case for leukoreduced and (perhaps) washed transfusions. Blood Cells Mol Dis. 2013;50:61–8. doi: 10.1016/j.bcmd.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Refaai MA, Phipps RP, Spinelli SL, Blumberg N. Platelet transfusions: Impact on hemostasis, thrombosis, inflammation and clinical outcomes. Thromb Res. 2011;6:287–91. doi: 10.1016/j.thromres.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garraud O, Hamzeh-Cognasse H, Cognasse F. Platelets and cytokines: how and why? Transfus Clin Biol. 2012;19:104–8. doi: 10.1016/j.tracli.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Agence nationale de sécurité du médicament et des produits de santé (ANSM) Bilans et rapports d‘activité. [Accessed on 09/10/2015]. Available at: http://ansm.sante.fr/Mediatheque/Publications/Bilans-Rapports-d-activite-Bilans-et-rapports-d-activite.
- 12.Blumberg N, Gettings KF, Turner C, et al. An association of soluble CD40 ligand (CD154) with adverse reactions to platelet transfusions. Transfusion. 2006;46:1813–21. doi: 10.1111/j.1537-2995.2006.00979.x. [DOI] [PubMed] [Google Scholar]
- 13.Hamzeh-Cognasse H, Damien P, Nguyen KA, et al. Immune-reactive soluble OX40 ligand, soluble CD40 ligand, and interleukin-27 are simultaneously oversecreted in platelet components associated with acute transfusion reactions. Transfusion. 2014;54:613–25. doi: 10.1111/trf.12378. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen KA, Hamzeh-Cognasse H, Sebban M, et al. A computerized prediction model of hazardous inflammatory platelet transfusion outcomes. PloS One. 2014;9:e97082. doi: 10.1371/journal.pone.0097082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouslama M, Abdelkefi S, Houissa B, et al. [Quality control of platelet concentrates: experience of Sousse (Tunisia) blood centre]. Ann Biol Clin. 2004;62:115–9. [In French.] [PubMed] [Google Scholar]
- 16.Aye MT, Palmer DS, Giulivi A, Hashemi S. Effect of filtration of platelet concentrates on the accumulation of cytokines and platelet release factors during storage. Transfusion. 1995;35:117–24. doi: 10.1046/j.1537-2995.1995.35295125733.x. [DOI] [PubMed] [Google Scholar]
- 17.Jonnalagadda D, Izu LT, Whiteheart SW. Platelet secretion is kinetically heterogeneous in an agonist-responsive manner. Blood. 2012;26:5209–16. doi: 10.1182/blood-2012-07-445080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stolla M, Refaai MA, Heal JM, et al. Platelet transfusion - the new immunology of an old therapy. Front Immunol. 2015;6:28. doi: 10.3389/fimmu.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bégué S, Vidal-Obert M, Girard A, et al. [Quality control of labile blood products. Why and how to properly take a specimen? Labile Blood Products Group of the French Blood Transfusion Society]. Transfus Clin Biol. 1999;6:403–8. doi: 10.1016/s1246-7820(00)88985-5. [In French.] [DOI] [PubMed] [Google Scholar]
- 20.Blair P, Flaumenhaft R. Platelet α-granules: basic biology and clinical correlates. Blood Rev. 2009;23:177–89. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen KK, Saifeddine M, Hollenberg MD. Tethered ligand-derived peptides of proteinase-activated receptor 3 (PAR3) activate PAR1 and PAR2 in Jurkat T cells. Immunology. 2004;112:183–90. doi: 10.1111/j.1365-2567.2004.01870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colognato R. Differential expression and regulation of protease-activated receptors in human peripheral monocytes and monocyte-derived antigen-presenting cells. Blood. 2003;102:2645–52. doi: 10.1182/blood-2002-08-2497. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell JW, Baik N, Castellino FJ, Miles LA. Plasminogen inhibits TNFα-induced apoptosis in monocytes. Blood. 2006;107:4383–90. doi: 10.1182/blood-2005-07-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.López ML, Bruges G, Crespo G, et al. Thrombin selectively induces transcription of genes in human monocytes involved in inflammation and wound healing. Thromb Haemost. 2014;112:992–1001. doi: 10.1160/TH14-01-0034. [DOI] [PubMed] [Google Scholar]
- 25.Gear AR, Camerini D. Platelet chemokines and chemokine receptors: linking hemostasis, inflammation, and host defense. Microcirc. 2003;10:335–50. doi: 10.1038/sj.mn.7800198. [DOI] [PubMed] [Google Scholar]
- 26.Blumberg N, Spinelli SL, Francis CW, et al. The platelet as an immune cell-CD40 ligand and transfusion immunomodulation. Immunol Res. 2009;45:251–60. doi: 10.1007/s12026-009-8106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wadhwa M, Seghatchian MJ, Dilger P, et al. Cytokines in WBC-reduced apheresis PCs during storage: a comparison of two WBC-reduction methods. Transfusion. 2000;40:1118–26. doi: 10.1046/j.1537-2995.2000.40091118.x. [DOI] [PubMed] [Google Scholar]
- 28.Cognasse F, Boussoulade F, Chavarin P, et al. Release of potential immunomodulatory factors during platelet storage. Transfusion. 2006;46:1184–9. doi: 10.1111/j.1537-2995.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- 29.Garraud O, Cognasse F. Are platelets cells? And if yes, are they immune cells? Front Immunol. 2015;6:70. doi: 10.3389/fimmu.2015.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cognasse F, Hamzeh-Cognasse H, Lafarge S, et al. Human platelets can activate peripheral blood B cells and increase production of immunoglobulins. Exp Hematol. 2007;35:1376–87. doi: 10.1016/j.exphem.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 31.McKenzie CGJ, Kim M, Singh TK, et al. Peripheral blood monocyte-derived chemokine blockade prevents murine transfusion-related acute lung injury (TRALI) Blood. 2014;123:3496–503. doi: 10.1182/blood-2013-11-536755. [DOI] [PubMed] [Google Scholar]
- 32.Weyrich AS, Zimmerman GA. Platelets: signaling cells in the immune continuum. Trends Immunol. 2004;25:489–95. doi: 10.1016/j.it.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 33.André P, Nannizzi-Alaimo L, Prasad SK, Phillips DR. Platelet-derived CD40L: the switch-hitting player of cardiovascular disease. Circulation. 2002;106:896–9. doi: 10.1161/01.cir.0000028962.04520.01. [DOI] [PubMed] [Google Scholar]
- 34.Elstad MR, McIntyre TM, Prescott SM, Zimmerman GA. The interaction of leukocytes with platelets in blood coagulation. Curr Opin Hematol. 1995;2:47–54. doi: 10.1097/00062752-199502010-00007. [DOI] [PubMed] [Google Scholar]
- 35.Berndt MC, Metharom P, Andrews RK. Primary haemostasis: newer insights. Haemophilia. 2014;20(Suppl 4):15–22. doi: 10.1111/hae.12427. [DOI] [PubMed] [Google Scholar]
- 36.Pawelski H, Lang D, Reuter S. Interactions of monocytes and platelets: implication for life. Front Biosci. 2014;6:75–91. doi: 10.2741/s416. [DOI] [PubMed] [Google Scholar]
- 37.Koike Y, Tanaka K, Kobayashi M, et al. Dynamic pathology for leukocyte-platelet formation in sepsis model. J Surg Res. 2015;195:188–95. doi: 10.1016/j.jss.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 38.Shukla RV, Patel TG, Gupte SC. Time dependent release of interleukin-8 and tumor necrosis factor-α in platelet concentrate. Indian J Hematol Blood Transfus. 2015;31:259–63. doi: 10.1007/s12288-014-0435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Addas-Carvalho M, Origa AF, Saad ST. Interleukin 1 beta and tumor necrosis factor levels in stored platelet concentrates and the association with gene polymorphisms. Transfusion. 2004;44:996–1003. doi: 10.1111/j.1537-2995.2004.03257.x. [DOI] [PubMed] [Google Scholar]
- 40.Chudziak D, Sireis W, Pfeiffer HU, et al. Accumulation of soluble inflammatory mediators between blood donation and pre-storage leucocyte depletion. Vox Sang. 2009;96:163–6. doi: 10.1111/j.1423-0410.2008.01132.x. [DOI] [PubMed] [Google Scholar]
- 41.Plaza EM, Lozano ML, Guiu IS, et al. Evaluation of platelet function during extended storage in additive solution, prepared in a new container that allows manual buffy-coat platelet pooling and leucoreduction in the same system. Blood Transfus. 2012;10:480–9. doi: 10.2450/2012.0112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaufman J, Spinelli SL, Schultz E, et al. Release of biologically active CD154 during collection and storage of platelet concentrates prepared for transfusion. J Thromb Haemost. 2007;5:788–96. doi: 10.1111/j.1538-7836.2007.02412.x. [DOI] [PubMed] [Google Scholar]
- 43.Singh Y, Sawyer LS, Pinkoski LS, et al. Photochemical treatment of plasma with amotosalen and long-wavelength ultraviolet light inactivates pathogens while retaining coagulation function. Transfusion. 2006;46:1168–77. doi: 10.1111/j.1537-2995.2006.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.