Abstract
Background
Factor XI (FXI) deficiency is an autosomal bleeding disease associated with genetic defects in the F11 gene which cause decreased FXI levels or impaired FXI function. An increasing number of mutations has been reported in the FXI mutation database, most of which affect the serine protease domain of the protein. FXI is a heterogeneous disorder associated with a variable bleeding tendency and a variety of causative F11 gene mutations. The molecular basis of FXI deficiency in 14 patients from ten unrelated families in Turkey was analysed to establish genotype-phenotype correlations and inheritance of the mutations in the patients’ families.
Material and methods
Fourteen index cases with a diagnosis of FXI deficiency and family members of these patients were enrolled into the study. The patients’ F11 genes were amplified by polymerase chain reaction and subjected to direct DNA sequencing analysis. The findings were analysed statistically using bivariate correlations, Pearson’s correlation coefficient and the nonparametric Mann-Whitney test.
Results
Direct DNA sequencing analysis of the F11 genes revealed that all of the 14 patients had a F11 gene mutation. Eight different mutations were identified in the apple 1, apple 2 or serine protease domains, except one which was a splice site mutation. Six of the mutations were recurrent. Two of the mutations were novel missense mutations, p.Val522Gly and p.Cys581Arg, within the catalytic domain. The p.Trp519Stop mutation was observed in two families whereas all the other mutations were specific to a single family.
Discussion
Identification of mutations confirmed the genetic heterogeneity of FXI deficiency. Most of the patients with mutations did not have any bleeding complications, whereas some had severe bleeding symptoms. Genetic screening for F11 gene mutations is important to decrease the mortality and morbidity rate associated with FXI deficiency, which can be life-threatening if bleeding occurs in tissues with high fibrinolytic activity.
Keywords: FXI deficiency, F11 gene, rare bleeding disorders, coagulation deficiency
Introduction
Factor XI (FXI) is a homodimeric plasma glycoprotein essential for haemostasis. It is secreted by liver cells and circulates in plasma as a disulphide-bound homodimer (160 kDa) associated with high-molecular-weight kininogen. Each monomer of FXI is composed of four apple domains (A1 to A4) and a C-terminal trypsin-like catalytic domain (serine protease). Apple domains are homologous to each other and to the apple domains in prekallikrein1,2. They are involved in interactions with other molecules including factor IX and prothrombin. Interchain disulphide bonding at residue Cys321 and other non-covalent interactions within the A4 domain are required for formation of the dimer3–5. FIX is activated by proteolytic cleavage between Arg369 and Ile370 residues, which is performed mainly by thrombin5. Activated FXI is required for the activation of factor IX to generate thrombin during the coagulation process at the site of vascular injury1. The expression of FXI is variable and its plasma levels range from 70 to 150 U/dL in normal populations6,7.
Functional or quantitative abnormalities of FXI are associated with hereditary FXI deficiency. FXI deficiency is a rare bleeding disease first described in a Jewish population with a heterozygote frequency of 8% with FXI:C plasma levels less than 70 U/dL8. It is also observed in other populations with a worldwide frequency of 1:1,000,0009. Bleeding due to FXI deficiency is variable and does not correlate with the plasma FXI level or FXI coagulant activity10. Spontaneous bleeds are rare even in severely affected patients and patients may not bleed significantly even after surgical operations. However, bleeding after surgery or trauma in anatomical sites with high fibrinolytic activity, such as the nose and genitourinary tract, can be life-threatening. The diagnosis of FXI deficiency depends on FXI coagulation assays. However, the laboratory assessment of FXI deficiency by measuring FXI antigen and activity levels may not be adequate for the proper management of the disorder because of the poor correlation between FXI levels and coagulant activity and bleeding tendency6,7. However, studies are being carried out to develop a laboratory method to predict the bleeding phenotype, such as the thrombin generation assay11,12.
FXI deficiency is inherited as an autosomal trait associated with various mutations within the gene coding for FXI, the F11 gene. The F11 gene has been localised to chromosome 4 (4q35) and is mainly expressed by liver cells13. It is composed of 15 exons and the mature protein is encoded by exons 3 to 15. Exon 1 constitutes the 5′ untranslated region and exon 2 codes for the signal peptide which is cleaved during biosynthesis14. FXI deficiency has been associated with a wide range of F11 gene mutations including missense, nonsense, splice site, insertion, and deletion mutations. There is a degree of ethnic heterogeneity with higher prevalences of certain mutations in specific populations15,16. For example, p.Glu117* (p.Glu135*) and p.Phe283Leu (p.Phe301Leu) mutations are observed with high frequencies in the Ashkenazi Jewish population and p.Cys38Arg (p.Cys56Arg) is observed frequently in the French population17,18.
The molecular mechanism of FXI deficiency is heterogeneous although most of the mutations are missense mutations affecting the catalytic function of FXI. Mutations can cause selective degradation of the mutant transcript, intracellular retention of monomers, and intracellular retention of homodimers as well as heterodimers. Mutations can also impair dimer formation or decrease the secretion of the protein without affecting dimer formation19–21. Individuals with a heterozygous mutation have 20–70 U/dL FXI activity and are considered to have a partial deficiency, while individuals with homozygous or compound heterozygous mutations possess <1–20 U/dL FXI activity and have severe FXI deficiency. Both homozygotes and heterozygotes are at risk of bleeding. It is, therefore, important to identify people with a bleeding tendency prior to surgical operations and so on. Mutation screening of the F11 gene is important for the proper diagnosis and management of patients22,23.
FXI deficiency has been reported in Turkey and an increasing number of patients are being diagnosed by genetic analysis of the F11 gene24,25. In the present study we identified the molecular basis of FXI deficiency in 14 patients and 19 relatives from ten unrelated families in Turkey to establish genotype-laboratory phenotype correlations and the pattern of inheritance of the mutations in the patients’ families.
Materials and methods
Ethical approval
Ethical approval for this study was obtained from the local ethical committee of Hacettepe University Faculty of Medicine.
Study population
Fourteen index cases with a diagnosis of FXI deficiency and family members of these patients were enrolled into the study. Informed consent was obtained from each patient and his/her parents in accordance with the Declaration of Helsinki. Laboratory tests for activated partial thromboplastin time (aPTT), prothrombin time, international normalised ratio, in vitro bleeding time, and FXI:Ag were performed at Hacettepe University Faculty of Medicine, Coagulation Laboratory, Ankara, Turkey. Genomic DNA was isolated from leucocytes using the PureLINK Genomic DNA Kit (Invitrogen, Waltham, MA, USA).
Patients
Patients with a FXI level lower than the normal reference range (70–150 U/dL) were diagnosed as having FXI deficiency. Ten of the patients had been admitted to our hospital with bleeding symptoms, the four others had been diagnosed during preoperative screening by measuring their FXI levels. All the patients’ family members were asymptomatic except the mother of patient 24 who had a past history of bruising. All family members were screened for FXI level and F11 gene mutation in the process of the family screening.
The mean age, the mean FXI value and the mean aPTT level of the patients were 8.2 years, 25.65%, and 69.61 seconds, respectively. The average bleeding score of the patients was 2.1626. Four of the patients did not have any bleeding symptoms and ten of them had various bleeding symptoms including epistaxis, heavy menorrhagia, gastrointestinal bleeding, bleeding after tooth extraction, surgery, and haemarthrosis (Table I). The patients’ bleeding score was defined according to Rodeghiero et al.27.
Table I.
Phenotype and genotype in FXI deficient patients and family members.
| Family number | Individual ID | Bleeding symptoms | Age, years | Sex | aPTT, s | FXI, U/dL | Bleeding score | Mutation type | Protein sequence | Nucleotide sequence | Genotype |
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | 02** | AS | 26 | F | 33.9 | 78.4 | 0 | Missense | p.V522G | c.1619T>G | Heterozygote |
| II | 04**** | AS | 8 | F | 37.9 | 27 | 0 | Missense | p.G418V | c.1253G>T | Heterozygote |
| II | 06** | AS | 40 | F | uk | uk | 0 | No mutation | |||
| III | 08* | B, BMW, BTE, GIB, OB | 11 | F | 42.7 | 28.1 | 8 | Nonsense | p.W519* | c.1556G>A | Heterozygote |
| IV | 10* | E | 7 | M | 33 | 54 | 1 | Missense | p.T51P | c.151A>C | Heterozygote |
| IV | 12*** | AS | 40 | M | uk | uk | 0 | No Mutation | |||
| V | 14* | B, BMW | 3.5 | M | 118.2 | 5.1 | 2 | Missense | p.C581R | c.1741T>C | Homozygote |
| V | 16** | AS | 32 | F | 31.9 | 37.4 | 0 | Missense | p.C581R | c.1741T>C | Heterozygote |
| VI | 18** | AS | 45 | F | 30.4 | 59.1 | 0 | Nonsense | p.W519* | c.1556G>A | Heterozygote |
| VII | 20* | AS | 4 | M | 108 | 5.9 | - | Splice site | c.325+1G>A | Homozygote | |
| VII | 22*** | AS | 42 | M | 26.5 | 80.9 | - | Splice site | c.325+1G>A | Heterozygote | |
| VIII | 24* | E | 10 | F | 38.3 | 26.6 | 1 | Missense | p.T51P | c.151A>C | Heterozygote |
| VIII | 26*** | AS | 39 | M | 29.1 | 86.7 | - | No mutation | |||
| IX | 28** | AS | 28 | F | 32 | 75.5 | - | Missense | p.C416Y | c.1247G>A | Heterozygote |
| X | 30* | E | 6 | M | 36.3 | 61 | 0 | Nonsense | p.E135* | c.403G>T | Heterozygote |
| X | 32*** | AS | 60 | M | 27.3 | 67.9 | - | Nonsense | p.E135* | c.403G>T | Heterozygote |
Patient;
mother;
father;
sibling;
E: epistaxis; AS: asymptomatic; BTE: bleeding after tooth extraction; BO: bleeding after operation; B: bruising; BMW: bleeding from minor wounds; GIB: gastrointestinal bleeding; OB: oral bleeding; PPB: post-partum bleeding; CR: cerebral bleeding; H: haemarthrosis; * in p.W519*; p.E135*: nonsense mutation; uk: unknown; F: female; M: male; Men: menorrhagia.
DNA analysis
The F11 gene, including promoter region, exon 1, all the other 14 exons, and exons/intron junctions, was amplified by polymerase chain reaction (PCR). The purified PCR products were screened for mutations by direct DNA sequencing in two directions with reverse and forward primers. The chromatograms were read by two independent researchers. Primer sequences and PCR conditions are available on request. The DNAsequencing service was purchased from GENOKS (Ankara, Turkey).
Statistical analysis
A correlation analysis was performed starting with a bivariate correlation procedure. The pairwise associations were analysed using Pearson’s correlation coefficient. This method is especially useful for determining the strength and direction of relationships between two scale or ordinal variables. The nonparametric Mann-Whitney test was used to calculate the statistical significance of differences in FXI and aPTT values between homozygous and heterozygous patients. Some important findings from the correlation results were analysed further. Box plots and frequency plots were used for categorical data and linear/nonlinear regression prediction line-fitting methodologies (produced by Minitab statistical package, State College, PA, USA) were used if dependency between scale variables was observed from the correlation table.
Results
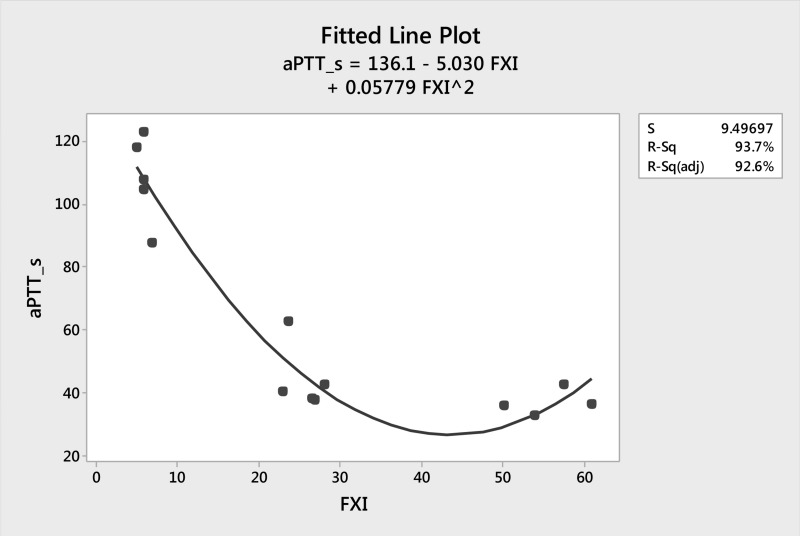
The F11 gene sequence was analysed in the ten symptomatic and four asymptomatic patients with FXI deficiency, who came from ten unrelated families. These patients had FXI levels ranging from 3 to 61 U/dL, with six having FXI levels below 10 U/dL and markedly prolonged aPTT (mean 115.50 seconds). Two of these patients with severe FXI deficiency had bleeding symptoms with a bleeding score of 2 while the others were asymptomatic and their disorder had been detected during preoperative screening or family screening due to high aPTT values. Eight of the patients had partial FXI deficiency with a mean FXI level of 39.71 U/dL and only five of them had bleeding symptoms with a mean bleeding score of 3.2. There is a strong relationship between patients’ aPTT values and FXI levels. As FXI level increases, aPTT decreases. The fitted non-linear regression line between these two variables, obtained from Minitab statistical software, is shown in Figure 1. The fitted line equation is: aPTT_s = 136.1−5.03 × FXI + 0.05779 FXI2. The resulting 92.6% adjusted R2 value is high enough for us to be confident about the appropriateness of the model (Figure 1).
Figure 1.
Correlation between the patients' aPTT values and FXI levels.
aPTT: activated partial thromboplastin time; FXI: factor IX; S: standard error of the regression; R-Sq: R-squared; R-Sq-(adj): R-squared adjusted.
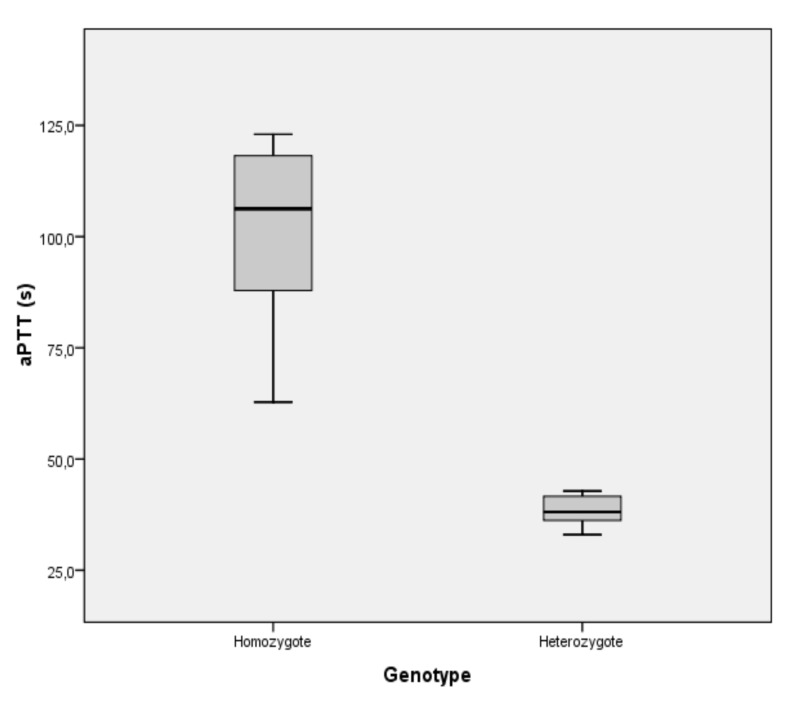
Statistical analysis also revealed that there is a strong relationship between patients’ aPTT scores and F11 genotype. aPTT values depend significantly on genotype. Analysis of variance (ANOVA) revealed a significance value of 0.001, which suggests that patients with a homozygous genotype have higher aPTT values than those with a heterozygous genotype (Figure 2).
Figure 2.
Correlation between the patients’ aPTT values and F11 genotype.
aPTT: activated partial thromboplastin time.
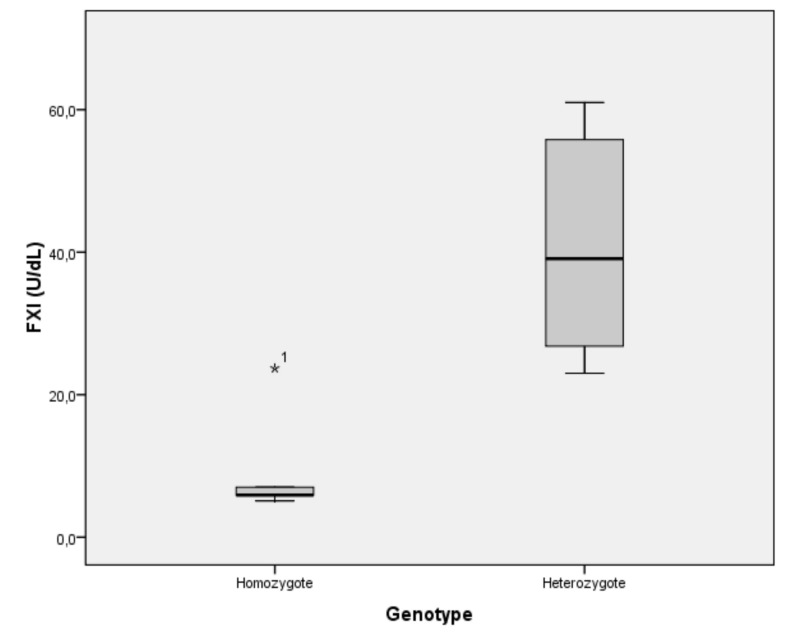
Furthermore, there is a strong relationship between patients’ FXI levels and genotype. Heterozygous individuals have higher FXI levels than homozygous individuals. Nonparametric Mann-Whitney testing showed that the difference between FXI levels of homozygous and heterozygous patients was statistically significant (p=0.001). Although there was an outlier (23.7 U/dL) for a homozygous patient this did not affect the outcome (Figure 3).
Figure 3.
Correlation between the patients’ FXI levels and F11 genotype.
FXI: factor IX.
The patients’ F11 genes were PCR-amplified and analysed by direct DNA sequencing of 11 amplicons containing the 5′ untranslated region, all exons, and exon/ intron boundaries. This analysis revealed that all of the 14 patients had F11 gene mutations. Eight different mutations were identified. Six of the mutations were recurrent mutations (p.Thr51Pro, p.Glu135X, p.Cys416Tyr, p.Gly418Val, p.Trp519X, and c.325+1G>A), while two were novel mutations (p.Val522Gly, and p.Cys581Arg) (Table I). All the mutations were specific to the families in which they were detected, except p.Thr51Pro and p.Trp519X which were detected in two families.
The p.Val522Gly mutation was detected in a 5.5-year old female patient with a FXI level of 23.7 U/dL, aPTT of 62.8 s and a bleeding score of 3. She was homozygous for the mutation which she had inherited from her asymptomatic, heterozygous parents who were first-degree consanguineous relatives. The p.Val522Gly mutation was caused by the substitution of c.1619T by G in exon 14 and located within the serine protease domain of FXI. The possible effect of the mutation on the phenotype was investigated using Polyphen-2 and SIFT analysis which are in silico tools that predict the possible impact of an amino acid substitution on the structure and function of a human protein (Polyphen-2: http://genetics.bwh.harvard.edu/pph2/; SIFT: http://sift.jcvi.org/). Although in vitro expression studies are required to prove an effect, Polyphen-2 and SIFT analysis of this mutation predicted that it can be tolerated.
The p. Cys581Arg mutation was identified in a homozygous state in 11-year-old and 3.5-year-old brothers. Both patients had severe FXI deficiency (FXI levels of 7 and 5.1 U/dL), prolonged aPTT (87.9 s and 118.2 s) with a bleeding score of 2. The F11 genes of the patients’ mother and another brother were also analysed for the presence of the mutation. Both the mother and the brother were heterozygous for the mutation. The mother was 32 years old, had partial FXI deficiency (FXI level: 37.4 U/dL) and was asymptomatic. The brother was 12 years old and had a partial FXI deficiency (FXI level: 50.1 U/dL); he had haemorrhagic symptoms with a bleeding score of 2. The p.Cys581Arg was caused by the substitution of c.1741T by C within exon 15 and located within the serine protease domain of FXI. Polyphen-2 and SIFT analysis of this mutation revealed that it is possibly damaging.
The p.Thr51Pro mutation, which was previously demonstrated to cause impaired secretion of FXI, was detected in two patients from unrelated families. Both of the patients were heterozygous for the mutation and they had mild bleeding symptoms with a modest reduction in FXI levels (Table I, individuals 10 and 24). Individual 10 inherited the mutant allele from his mother who was heterozygous for the mutation with a bleeding score of 19 and FXI level of 27 U/dL. Individual 24 also inherited the mutation from her heterozygous mother, had bleeding symptoms (easy bruising) and a FXI level of 28.9 U/dL.
The p.Gly418Val mutation was detected in two patients from family number II (daughter and father, individuals 5 and 7) with reductions in FXI levels (23 U/dL and 3 U/dL, respectively). The daughter patient was 15.5 years old, heterozygous for the mutation and had menorrhagia. She inherited the mutation from her heterozygous father patient who had bleeding symptoms and severe FXI deficiency. An 8-year old sister in the family was also heterozygous for the mutation but did not have any bleeding symptoms.
The p.Trp519* mutation, which disrupts catalytic domain structure, was identified in two unrelated patients, individual 8 from family III and individual 17 from family VI. Individual 8 was an 11-year old female heterozygote for the mutation with a FXI level of 28.1 U/dL, and had bleeding symptoms. Her mother was also heterozygous for the mutation with a FXI level of 37.7 U/dL but did not have any bleeding symptoms. The other patient was a 14.5-year old male with severe FXI deficiency (FXI level: 5.9 U/dL) who was homozygous for the mutation. He was diagnosed during pre-operative screening. He was asymptomatic and inherited the mutation from his heterozygous parents who were also asymptomatic.
The intronic mutation c.325+1G>A was detected in 4-year old and 12-year old brothers (individuals 20 and 23 in family VII) who are homozygous for the mutation, and have severe FXI deficiency but no bleeding symptoms. The 4-year old boy was diagnosed during preoperative screening; his 12-year old brother and parents were screened afterwards. The two brothers inherited the mutation from their heterozygous parents who are cousins and did not have any bleeding symptoms with FXI levels in the normal range.
p.Cys416Tyr was identified in the patient (individual 27 in family IX) who had a partial reduction in FXI level (57.6 U/dL) and no bleeding symptoms. The patient was diagnosed during preoperative screening. The patient’s mother was a heterozygote with a FXI level of 75.5 U/dL and did not have any bleeding symptoms.
p.Glu135Ter was identified in individual 30 in family X. He was 6 years old and heterozygous for the mutation with a FXI level of 61 U/dL. Although the patient had epistaxis, he was diagnosed as having FXI deficiency during pre-operative screening. Family screening revealed that his father and his sister are heterozygotes for the mutation. The father had a FXI level of 67.9 U/dL without bleeding symptoms.
Discussion
The molecular basis and haemorrhagic profile of FXI deficiency was analysed in 14 Turkish patients with FXI deficiency from ten unrelated families in this study. Eight different mutations including two novel mutations were identified in the patients. The fact that eight different mutations were found in ten unrelated families confirms the genetic heterogeneity of FXI deficiency in Turkey as well as in other populations in the world. The mean FXI level was 25.65 U/dL in the group of patients. Five of the patients had severe FXI deficiency (<10 U/dL) and their aPTT values are prolonged in correlation with their FXI values, but their bleeding symptoms are not correlated with their FXI values.
The mutations and the genotypes of the individuals are shown in Table I. The novel mutation p.Val522Gly was identified in one patient in family I. Val and Gly are both non-polar amino acids but Val is larger than Gly. It is located within the serine protease domain of the FXI dimer. The serine protease domain is located within the light chain containing the catalytic triad at His413, Asp462, and Ser557 of the protein and p.Val522Gly might affect the catalytic activity of activated FXI. SIFT and Polyphen-2 analysis indicated that this non-synonymous change is tolerated. However, due to the larger side chain of Gly, this variation might affect the three-dimensional structure of the catalytic domain sterically. Individuals with this mutation show a variable phenotype. Bleeding symptoms were observed in the patient, who was homozygous for the mutation, but not in the parents who were heterozygous for the mutation and did not have significantly decreased FXI levels.
The second novel mutation, p.Cys581Arg, was identified in two brothers (individuals 13 and 14 from family V). p.Cys581Arg is a non-conservative change from an uncharged polar amino acid (Cys) to a positively charged amino acid (Arg). This mutation disrupts the Cys553–Cys581 bond in the catalytic domain of activated FXI, thus affecting its catalytic activity. The two patients were homozygous for the mutation and had severe FXI deficiency with bleeding symptoms. The other family members were heterozygous for the mutation with partial reductions in FXI level. The change is located within the serine protease domain and in silico Polyphen and SIFT analysis revealed that it is possibly damaging. Although, Polyphen-2 and SIFT can predict the effect of variations on human protein structure and function, expression studies in cell culture are needed to demonstrate and prove the effect of the sequence variations.
The other six mutations (p.Thr51Pro, Glu135X, p.Cys416Tyr, p.Gly418Val, p.Trp519X, c.325+1G>A) have already been identified in patients with FXI deficiency. The molecular mechanisms of the pathogenicity of the p.Thr51Pro, p.Cys416Tyr, and p.Gly418Val mutations were analysed by in vitro expression studies. p.Thr33 lies in the first α-helix, in a buried region of the apple 1 domain which is involved in high-molecular-weight kininogen and prekallikrein and just next to a cysteine residue which forms the disulphide bond. On the other hand, the threonine residue is conserved in positions 33, 123, 213 and 304 of four apple domains, in the same position relative to the α-helix of each domain. In vitro expression studies have demonstrated that p.Thr51Pro impairs biosynthesis. The p.Cys416Tyr mutation is very closely located to the disulphide bond between Cys398 and Cys414 in the serine protease domain of activated FXI. In vitro expression analysis of p.Cys416Tyr in the 293 human kidney cell line demonstrated that the mutation affects the secretion of the protein with a dominant negative effect without affecting dimer formation. Similarly, the p.Gly418Val mutation was also shown to exert a dominant negative effect due to impaired secretion. However, individuals with a p.Cys416Tyr mutation were heterozygotes without severe reductions in FXI levels or bleeding symptoms. Individuals with p.Gly418Val had variable phenotypes. This mutation was identified in family II: the two heterozygous sisters inherited the mutation from their heterozygous father who had the most severe FXI deficiency and bleeding score.
The type II mutation (Glu117Stop) is the most common mutation in Jewish FXI-deficient patients. However, we identified it in our patients of non-Jewish origin. This mutation was also identified in two unrelated families from Turkey in a previous study21. The mutation was found in family X in which the patient and other carriers were heterozygotes with mild reductions in FXI levels without bleeding complications. The patients in the FXI mutation database had severe FXI deficiency when they were homozygous or compound heterozygous for the mutation. The heterozygotes did not have severe reductions of FXI levels. Although this mutation results in truncated protein production, it seems that heterozygotes for the mutation are not affected very much.
In conclusion, we have genetically characterised 14 patients with FXI deficiency in this study. Identification of family-specific mutations and novel mutations contributed to expanding the genetic heterogeneity of FXI deficiency. This study confirms the findings of previous studies that FXI level and the presence of mutation do not necessarily correlate with the bleeding phenotype in individuals. However, identification of the mutations in asymptomatic individuals demonstrates the importance of genetic diagnosis in the management of subjects with FXI deficiency, which can be a life-threatening disorder if bleeding occurs in tissues with high fibrinolytic activity.
Conclusions
Factor XI deficiency is a rare congenital bleeding disease. It is heterogeneous in clinical presentation and in genetic causality. Generally spontaneous bleeding is not commonly observed in FXI deficiency. However, mild to moderate bleeding is observed following haemostatic challenges. Accurate diagnosis of FXI deficiency is important for the clinical management of the disease since it can cause bleeding following circumcision, surgery or trauma28. It is especially important for women, since women with bleeding disorders frequently experience heavy menstrual bleeding and other gynaecological or obstetric bleeds associate with miscarriage, bleeding during pregnancy, and post-partum haemorrhage. It was reported that heavy menstrual bleeding is the most prevalent symptom in women with FXI deficiency (59%)29,30. Furthermore, studies have shown that women with FXI deficiency have an increased risk of menorrhagia, and of bleeding complications after miscarriage, termination of pregnancy and delivery31. Genetic diagnosis is, therefore, important to confirm the clinical diagnosis.
Five female and nine male patients with clinically diagnosed FXI deficiency were genetically screened for the presence of a F11 gene mutation. Four of the females and five of the males had bleeding problems. Causative mutations were identified in all the patients and the mutation was inherited within the family. Overall, 33 people (66 alleles) were screened for F11 gene mutations and mutations were identified in 28 of them (34 alleles) for an allele frequency of 51.5%. Two novel mutations (p.Val522Gly, and p.Cys581Arg) were identified. FXI deficiency with a causative type II mutation (p.Glu135*), previously identified in a Jewish population, was also identified among our patients who are not of Jewish origin. The other three mutations (p.T51P, p.W519* and c.325+1G>A) identified in this study had been previously described in other Turkish patients with FXI deficiency24,25. FXI levels were significantly lower in the homozygous individuals. However, bleeding was not associated with the genotype of the individuals in whom mutations were identified. Asymptomatic patients were identified during preoperative screening and three of them had severe reductions in FXI level (5.9 U/dL) and a homozygous F11 genotype. Carriers of F11 gene mutations in the families were detected upon family screening. The average FXI level in the mutation carriers was 60.32 U/dL which could be expected for F11 gene mutation heterozygotes. However, there were also heterozygotes with high FXI levels (>70 U/dL). There might be genetic and environmental factors affecting the mutant phenotype expression in these individuals, such as blood type, epigenetic modifications, and exercise, causing incomplete penetrance and variable expressivity as in the case of von Willebrand factor gene expression. It is, therefore, important to perform molecular genetic analysis for those individuals with FXI levels at the lower end of the normal range in order to diagnose them with FXI deficiency and take precautions to prevent bleeding, which could occur following an injury or during surgery. Furthermore, although FXI deficiency is considered a rare bleeding disorder, asymptomatic individuals with FXI levels at the lower end of the normal range might be overlooked during laboratory diagnosis. Widespread use of F11 gene molecular genetic analysis would increase the frequency of FXI deficiency detected in the world.
Footnotes
Funding and resources
This study was supported by Istanbul AREL University Research funding.
Authorship contributions
SC analysed the F11 gene mutations and sequencing data, and helped to write the manuscript; TB, BT, EYK, FG, MC and SA examined the patients and organised all the biochemical tests; VC performed the statistical analysis; EB analysed the data and wrote the manuscript.
The Authors declare no conflicts of interest.
References
- 1.Davie E, Fujikawa W. The coagulation cascade initiation, maintenance and regulation. Biochemistry. 1991;30:10363–70. doi: 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- 2.Gailani D, Broze GJ. Factor XI activation in a revised model of blood coagulation. Science. 1991;253:909–12. doi: 10.1126/science.1652157. [DOI] [PubMed] [Google Scholar]
- 3.Meijers JC, Davie EW, Chung DW. Expression of human blood coagulation factor XI: characterization of the defect in factor XI type III deficiency. Blood. 1992;79:1435–40. [PubMed] [Google Scholar]
- 4.O’Connell NM. Factor XI deficiency-from molecular genetics to clinical management. Blood Coagul Fibrinolysis. 2003;14:59–64. doi: 10.1097/00001721-200306001-00014. [DOI] [PubMed] [Google Scholar]
- 5.Renne T, Sugiyama A, Gailani D, et al. Fine mapping of the H-kininogen binding site in plasma prekallikrein apple domain 2. Int Immunopharmacol. 2002;2:1867–73. doi: 10.1016/s1567-5769(02)00170-4. [DOI] [PubMed] [Google Scholar]
- 6.Oliver JA, Monroe DM, Roberts HR, Hoffman M. Thrombin activates factor XI on activated platelets in the absence of FXII. Arterioscler Thromb Vasc Biol. 1999;19:170–7. doi: 10.1161/01.atv.19.1.170. [DOI] [PubMed] [Google Scholar]
- 7.Ragni MV, Sinha D, Seaman F, et al. Comparison of bleeding tendency, factor XI coagulant activity, and factor XI antigen in 25 factor XI-deficient kindreds. Blood. 1985;65:719–24. [PubMed] [Google Scholar]
- 8.Bolton-Maggs PH, Young Wan-Yin B, McCraw AH, et al. Inheritance and bleeding in factor XI deficiency. Br J Haematol. 1988;69:521–8. doi: 10.1111/j.1365-2141.1988.tb02409.x. [DOI] [PubMed] [Google Scholar]
- 9.Shpilberg O, Peretz H, Zivelin A, et al. One of the two common mutations causing factor XI deficiency in Ashkenazi Jews (type II) is also prevalent in Iraqi Jews, who represent the ancient gene pool of Jews. Blood. 1995;85:429–32. [PubMed] [Google Scholar]
- 10.Peyvandi F, Di Michele D, Bolton-Maggs PH, et al. Classification of rare bleeding disorders (RBD) based on the association between coagulant factor activity and clinical bleeding severity. J Thromb Haemost. 2012;10:1938–43. doi: 10.1111/j.1538-7836.2012.04844.x. [DOI] [PubMed] [Google Scholar]
- 11.Pike GN, Cumming AM, Hay CR, et al. Sample conditions determine the ability of thrombin generation parameters to identify bleeding phenotype in FXI deficiency. Blood. 2015;126:397–405. doi: 10.1182/blood-2014-12-616565. [DOI] [PubMed] [Google Scholar]
- 12.Livnat T, Shenkman B, Martinowitz U, et al. The impact of thrombin generation and rotation thromboelastometry on assessment of severity of factor XI deficiency. Thromb Res. 2015;136:465–73. doi: 10.1016/j.thromres.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 13.Kato A, Asakai R, Davie EW, Aoki N. Factor XI gene (F11) is located on the distal end of the long arm of human chromosome 4. Cytogenet Cell Genet. 1989;52:77–8. doi: 10.1159/000132844. [DOI] [PubMed] [Google Scholar]
- 14.Asakai R, Davie EW, Chung DW. Organization of the gene for human factor XI. Biochemistry. 1987;26:7221–28. doi: 10.1021/bi00397a004. [DOI] [PubMed] [Google Scholar]
- 15.Peretz H, Zivelin A, Usher S, Seligsohn U. A 14-bp deletion (codon 554 del AAGgtaacagagtg) at exon 14/intron N junction of the coagulation factor XI gene disrupts splicing and causes severe factor XI deficiency. Human Mutat. 1996;8:77–8. doi: 10.1002/(SICI)1098-1004(1996)8:1<77::AID-HUMU12>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 16.Asakai R, Chung DW, Ratnoff OD, Davie EW. Factor XI (plasma thromboplastin antecedent) deficiency in Ashkenazi Jews is a bleeding disorders that can result from three types of point mutations. Proc Natl Acad Sci USA. 1989;86:7667–71. doi: 10.1073/pnas.86.20.7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zivelin A, Bauduer F, Ducout L, et al. Factor XI deficiency in French Basques is caused predominantly by an ancestral Cys38Arg mutation in the factor XI gene. Blood. 2002;99:2448–54. doi: 10.1182/blood.v99.7.2448. [DOI] [PubMed] [Google Scholar]
- 18.Quelin F, Trossaert M, Sigaud M, et al. Molecular basis of severe factor XI deficiency in seven families from the west of France. Seven novel mutations, including an ancient Q88X mutation. J Thromb Haemost. 2004;2:71–6. doi: 10.1111/j.1538-7836.2004.00554.x. [DOI] [PubMed] [Google Scholar]
- 19.Kravtsov DV, Wu W, Meijers JC, et al. Dominant factor XI deficiency caused by mutations in the factor XI catalytic domain. Blood. 2004;104:128–34. doi: 10.1182/blood-2003-10-3530. [DOI] [PubMed] [Google Scholar]
- 20.Kravtsov DV, Monahan PE, Gailani D. A classification system for cross-reactive material-negative factor XI deficiency. Blood. 2005;105:4671–73. doi: 10.1182/blood-2004-05-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu W, Sinha D, Shikov S, et al. Factor XI homodimer structure is essential for normal proteolytic activation by factor XIIa, thrombin and factor XIa. J Biol Chem. 2008;283:18655–64. doi: 10.1074/jbc.M802275200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mannucci PM, Duga S, Peyvandi F. Recessively inherited coagulation disorders. Blood. 2004;104:1243–52. doi: 10.1182/blood-2004-02-0595. [DOI] [PubMed] [Google Scholar]
- 23.Wang HF, Tang L, Yang Y, et al. Genetic analysis in factor XI deficient patients from central China: identification of one novel and seven recurrent mutations. Gene. 2015;561:101–6. doi: 10.1016/j.gene.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 24.Berber E, Rimoldi V, Usluer S, et al. Characterization of the genetic basis of FXI deficiency in two Turkish patients. Haemophilia. 2010;16:564–6. doi: 10.1111/j.1365-2516.2009.02152.x. [DOI] [PubMed] [Google Scholar]
- 25.Keskin EY, Gürsel T, Kaya Z, et al. Molecular basis and bleeding manifestations of factor XI deficiency in 11 Turkish families. Blood Coagul Fibrinolysis. 2015;26:63–8. doi: 10.1097/MBC.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 26.Bowman M, Riddel J, Rand ML, et al. Evaluation of the diagnostic utility for von Willebrand disease of a pediatric bleeding questionnaire. J Thromb Haemost. 2009;7:1418–21. doi: 10.1111/j.1538-7836.2009.03499.x. [DOI] [PubMed] [Google Scholar]
- 27.Rodeghiero F, Tosetto A, Abshire T, et al. ISTH/SSC bleeding assessment tool: a standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J Thromb Haemost. 2010;8:2063–5. doi: 10.1111/j.1538-7836.2010.03975.x. [DOI] [PubMed] [Google Scholar]
- 28.James P, Salomon O, Mikovic D, Peyvandi F. Rare bleeding disorders-bleeding assessment tools, laboratory aspects and phenotype and therapy of FXI deficiency. Hemophilia. 2014;20:71–5. doi: 10.1111/hae.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Michele DM, Gibb C, Lefkowitz JM, et al. Severe and moderate hemophilia A and B in US families. Hemophilia. 2014;20:e136–43. doi: 10.1111/hae.12364. [DOI] [PubMed] [Google Scholar]
- 30.Kulkarni R. Improving care and treatment options for women and girls with bleeding disorders. Eur J Haematol. 2015;95:2–10. doi: 10.1111/ejh.12580. [DOI] [PubMed] [Google Scholar]
- 31.Wievel-Verschueren S, Arendz IJ, Knol MH, Meijer K. Gynaecological and obstetrical bleeding in women with factor XI deficiency-a systematic review. Hemophilia. 2016;22:188–95. doi: 10.1111/hae.12856. [DOI] [PubMed] [Google Scholar]