Abstract
Dietary partitioning often accompanies the increased morphological diversity seen during adaptive radiations within aquatic systems. While such niche partitioning would be expected in older radiations, it is unclear how significant morphological divergence occurs within a shorter time period. Here we show how differential growth in key elements of the feeding mechanism can bring about pronounced functional differences among closely related species. An incredibly young adaptive radiation of three Cyprinodon species residing within hypersaline lakes in San Salvador Island, Bahamas, has recently been described. Characterized by distinct head shapes, gut content analyses revealed three discrete feeding modes in these species: basal detritivory as well as derived durophagy and lepidophagy (scale‐feeding). We dissected, cleared and stained, and micro‐CT scanned species to assess functionally relevant differences in craniofacial musculoskeletal elements. The widespread feeding mode previously described for cyprinodontiforms, in which the force of the bite may be secondary to the requisite dexterity needed to pick at food items, is modified within both the scale specialist and the durophagous species. While the scale specialist has greatly emphasized maxillary retraction, using it to overcome the poor mechanical advantage associated with scale‐eating, the durophage has instead stabilized the maxilla. In all species the bulk of the adductor musculature is composed of AM A1. However, the combined masses of both adductor mandibulae (AM) A1 and A3 in the scale specialist were five times that of the other species, showing the importance of growth in functional divergence. The scale specialist combines plesiomorphic jaw mechanisms with both a hypertrophied AM A1 and a slightly modified maxillary anatomy (with substantial functional implications) to generate a bite that is both strong and allows a wide range of motion in the upper jaw, two attributes that normally tradeoff mechanically. Thus, a significant feeding innovation (scale‐eating, rarely seen in fishes) may evolve based largely on allometric changes in ancestral structures. Alternatively, the durophage shows reduced growth with foreshortened jaws that are stabilized by an immobile maxilla. Overall, scale specialists showed the most divergent morphology, suggesting that selection for scale‐biting might be stronger or act on a greater number of traits than selection for either detritivory or durophagy. The scale specialist has colonized an adaptive peak that few lineages have climbed. Thus, heterochronic changes in growth can quickly produce functionally relevant change among closely related species.
Keywords: adductor mandibulae, Cyprinodon, durophagy, functional morphology, jaw, lepidophagy, scale‐eating
Introduction
Adaptive radiations are characterized by lineages that have undergone rapid species diversification accompanied by significant morphological change within a short evolutionary time scale (Schluter, 2000). Trophic radiations can result in rapid change of the feeding apparatus associated with dietary divergence and specialization. African cichlids (Fryer & Iles, 1972; Kocher, 2005) and Darwin's finches provide two textbook examples of rapid trophic evolution and the concomitant changes in feeding mechanisms that are associated with such radiations. However, the evolutionary mechanisms responsible for significant functional changes within shorter time frames have been largely unexplored. Two such lesser known sympatric radiations have occurred within tropical pupfish Cyprinodon spp., a system that is appealing for studying incipient adaptive radiation due to both the microendemism of these radiations nested within the large range of Cyprinodon and their experimental tractability. For example, measurements of the empirical fitness landscape using F2 hybrid pupfish have demonstrated that multiple fitness peaks are driving adaptive radiation in this system (Martin & Wainwright, 2013a,2013b,2013c).
Across their range from Massachusetts to Venezuela, there are only two places where Cyprinodon species coexist sympatrically. Both places are tropical saline lakes with only one or two other competing fish species in which species flocks of Cyprinodon have specialized on novel dietary resources (Humphries & Miller, 1981; Martin & Wainwright, 2011). The first is a species flock of Cyprinodon thought to be 8000 years old (Covich & Stuiver, 1974) found in Laguna Chichancanab, Mexico. Stevenson (1992) published early accounts of diet within these species, noting significant overlap in diet especially due to high algal consumption by three of the species. Horstkotte & Strecker (2005) have quantified the diet of the six species that have evolved within this lake after invasion by Oreochromis and Astyanax. Although these authors provide a clear account of feeding ecology, the concomitant functional morphological differentiation that allowed for disparate feeding mechanisms was given less attention.
The second Cyprinodon species flock is endemic to hypersaline lakes within San Salvador Island, Bahamas. This Bahamian radiation, with clear trophic partitioning, has recently been described as containing the widespread Cyprinodon variegatus, and two endemics, Cyprinodon brontotheroides and Cyprinodon desquamator. Sampling from several hypersaline lakes/ponds on San Salvador, Martin & Wainwright (2013a,2013b,2013c) described clear trophic (Martin & Wainwright, 2013a; Martin, 2016) and genetic divergence (Martin & Feinstein, 2014; Lencer et al. 2017) among these three species. The widespread C. variegatus, thought to have given rise to this small species flock, enjoys a broad geographic distribution spanning from Maine to the West Indies (Hoese & Moore, 1977) and Venezuela (Turner et al. 2008) and feeds predominantly on detritus, algae, and other plant matter. Cyprinodon brontotheroides specializes on hard prey, consuming mostly ostracods in some locations while eating gastropods in others. Finally, the most specialized diet is seen in Cyprinodon desquamator, a specialized scale‐eater (Martin & Wainwright, 2013a,2013b,2013c). Not surprisingly, species with durophagous or lepidophagous diets exhibit morphological modifications as they diverge from more basal craniofacial features suited for detritivory.
Unlike cichlid radiations in African rift lakes and barb radiations in Lake Tana, in which older detailed functional descriptions of craniofacial features exist (Liem, 1973; de Graaf et al. 2008), to date there have been no functional morphological descriptions for radiations of Cyprinodon (but see Lencer et al. 2017 for an ontogenetic analysis). Although there are published data on the rapid rate of morphological evolution within key feeding structures (Martin & Wainwright, 2011; Martin, 2016), detailed reports of how such morphological changes may have led to trophic specialization are rare (Lencer et al. 2016). Examination of how subtle functional morphological changes can become compounded over time to allow for trophic partitioning in young adaptive radiations merits further attention.
There is little work describing functional morphological differences in the feeding apparatus among Cyprinodon spp. Martin & Wainwright (2011) and Lencer et al. (2016) measured and analyzed growth within several prominent feeding structures, but they did not investigate this group within a functional context considering changes in musculoskeletal and ligamentous attachments, structures whose subtle anatomical changes belie their functional importance. Although both the Mexican and Bahamian radiations involve trophic ecological diversification, there is a dearth of functional descriptions of these species. Morphological research on cyprinodontiforms (Ruell & Dewitt, 2005) in general and pupfish radiations specifically (Collyer et al. 2005; Tobler & Carson, 2010) has centered on morphometric analyses of changes in body shape. Such approaches are justified when the radiation examined is likely to be associated with swimming performance or overall body condition, but is less likely to be informative in teasing apart the reasons for trophic radiations.
Previous work on cyprinodontiforms has revealed that this group is best characterized by their ability to use picking as a specialized biting mode (Ferry‐Graham et al. 2008; Hernandez et al. 2008, 2009), a feeding mode made more efficient by several morphological novelties. In particular, a premaxillomandibular ligament allows for coordinated movements of the upper and lower jaw. Here we develop the argument that such coordinated movements of the jaws may allow for the evolution of several functional derivations on this basic plan, spanning the fine picking movements that characterize other cyprinodontiforms (which can readily be used to pick detritus or small, albeit hard, prey from the substrate as seen in the detritivorous and durophagous Cyprinodon spp. examined here) to the very specialized mechanism required for efficient scale‐feeding. This Cyprinodon radiation provides a test case of whether specializations in several ‘biting niches’ and the concomitant increased morphological and functional diversity can be brought about largely through heterochronic changes in growth rates within a very short evolutionary time scale.
Here we address the specific feeding mechanisms that are employed by these nascent Bahamian species in exploiting new prey items. Specifically, we hypothesize that the generalist phenotype of the basal detritivore evolved into a more rigid, short‐jawed morph in the durophage, while giving rise to a more flexible, long‐jawed morph within the scale specialist. Results show the degree of ecomorphological divergence that can evolve within a short timescale (less than 10 000 years), thus serving as a model of the type of anatomical analysis that is required to understand clearly how morphological evolution associated with rapid trophic radiations occurs. Moreover, as has been the case with cichlids, these data may provide clues as to the developmental mechanisms involved in the radiation of these trophic morphologies.
Methods
During July 2008, Cyprinodon species were collected in Little Lake, Osprey Lake, and Crescent Pond, San Salvador Island, Bahamas. Adult fishes were collected between 0.3 and 2 m depth using a monofilament hand net while snorkeling or by seine netting from the shore. At the surface, fishes were immediately euthanized with an overdose of MS‐222 (tricaine methanesulfonate; Argent Laboratories). Further digestion was prevented by an intraperitoneal injection of 15% formalin, followed by the preservation of the whole specimen in 15% formalin. At least 10 individuals from each species were examined for anatomical analyses.
MicroCT scans were reconstructed and segmented using amira (version 5.5) to make 3D reconstructions of cranial skeletal features. Morphological analyses entailed characterization and measurements of the following feeding structures: premaxilla, maxilla, articular, dentary, and palatine. The fixation used, allowed for the visualization of muscle, thus the muscular anatomy presented here represents actual reconstructions from microCT scans.
For the description of muscular and ligamentous anatomy, alcohol‐ and/or formalin‐fixed specimens were dissected and briefly immersed in iodine to facilitate identification of muscle fiber orientation. Eight specimens from each representative species were dissected to determine musculoskeletal architecture and to assess both inter‐ and intra‐individual variation in feeding morphology. Additional specimens were cleared and stained using a protocol presented in Dingerkus & Uhler (1977) with modifications by Potthoff (1984). Cleared and stained specimens were used to assess both the shape of the bones and cartilages of the anterior jaws, as well as to quantify differences in head elements among the different species.
Several osteological measurements of the skull from cleared and stained specimens were taken to test for significant differences in size. Ten individuals from each species were examined. Measurements taken included: length of maxilla, length of mandible, length of dentigerous arm of the premaxilla, dentary width at the widest point, width of the suspensorium at the point of jaw articulation, and standard length. ancova was used to determine significant differences among species using head length (HL) as the covariate.
Specimens from the three species used for quantitative analysis of the adductor mandibular complex were not significantly different in mass or standard length, thus we used anova to determine differences in the weight of different divisions of the adductor mandibulae complex. Adductor mandibulae (AM) A1, A2, and A3 were carefully separated and placed in 70% ethanol. Each muscle division was removed from the ethanol and briefly placed on absorbent tissue and then weighed. Each division was weighed three times and the mean was used as the weight. Weights for the three divisions of the AM were taken for C. variegatus (n = 5), C. brontotheroides (n = 5), and C. desquamator (n = 5).
While analyzing the anatomy of the oral jaws in cleared and stained specimens it was noted that there was noticeable variation in both gape of the jaw and rostrocaudal position of the ventral tip of the maxilla in relation to the jaw joint. Lateral pictures were taken and the distance between the posterior edge of the maxilla and the jaw joint (i.e. degree of rostral maxillary movement) were taken for C. variegatus (n = 21), C. brontotheroides (n = 31), and C. desquamator (n = 32). Correlations were run between degree of rostral maxillary movement and gape angle. Such correlations were performed to determine the extent to which maxillary movement may play a role during jaw opening and closing.
Results
Skeletal and ligamentous anatomy of C. variegatus
Osteological features of the anterior jaws of Cyprinodon spp. are like those described for cyprinodontiforms in general and cyprinodontids specifically (Fig. 1; Hernandez et al. 2009). Differences among the three species were based largely on size of elements with maxillary length, premaxillary length, as well as lower jaw length and width all showing interspecific differences (P < 0.05). Here we will first describe the trophic anatomy of the basal species within this species flock, C. variegatus. Emphasis throughout will be on the feeding structures of the oral jaws, the dentary, articular, premaxilla, suspensorium, maxilla, associated musculature, and ligamentous attachments. Preliminary data collected on structure of the pharyngeal jaws do not suggest any significant interspecific differences in the pharyngeal jaw apparatus, thus the pharyngeal jaw apparatus will not be discussed further here.
Figure 1.
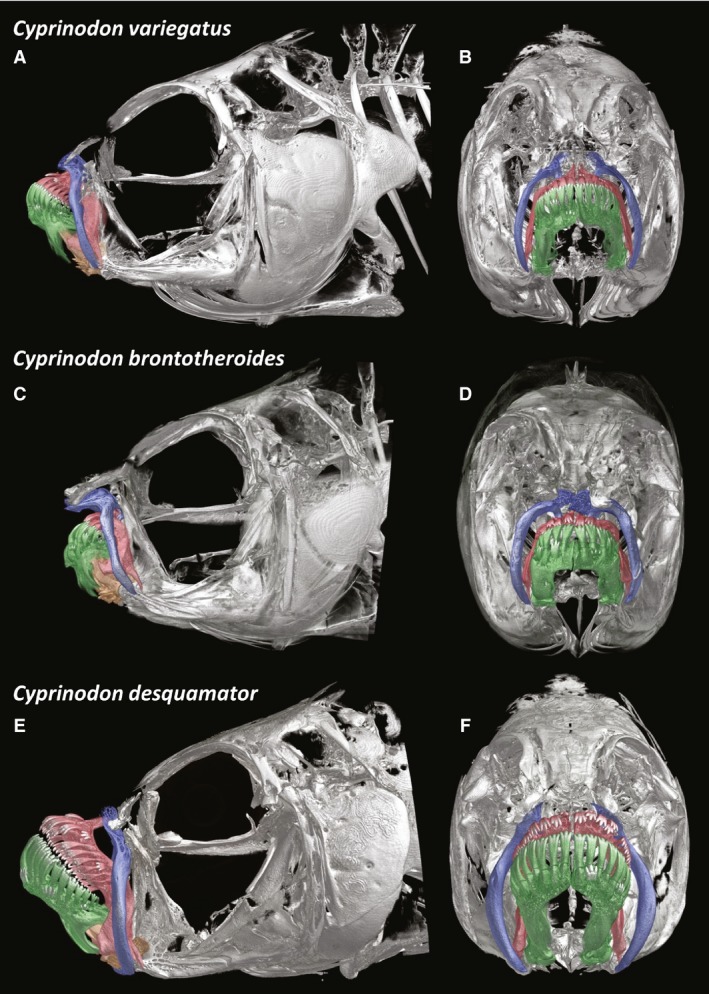
MicroCT scans of entire heads of the detritivore Cyprinodon variegatus (A,B), the durophage Cyprinodon brontotheroides (C,D), and the scale specialist Cyprinodon desquamator (E,F). Lateral (A,C,E) and frontal (B,D,F).
The oral jaws are inclined dorsally (approximately 40° from the horizontal) forming superiorly inclined jaws (Figs 1 and 2; terminal mouth characterization was based on external morphology alone, Martin & Wainwright, 2013a,2013b,2013c). The teeth on both the premaxilla and dentary comprise one peripheral row of tricuspid teeth (Fig. 2A,B). There is a pronounced dorsal coronoid process of the dentary as well as a ventral process forming a V‐shaped slot into which the rostral portion of the articular slides. Articular as used here refers to what is also known as the ‘angular complex’, the fused angular, articular, and retroarticular. The rostral process of the articular is quite long and inserts into the ‘V’ formed by the processes of the dentary, thus the articular and dentary are fairly tightly articulated (Fig. 3). The rostral portion of the articular is quite thin in frontal section (Fig. 3B).
Figure 2.
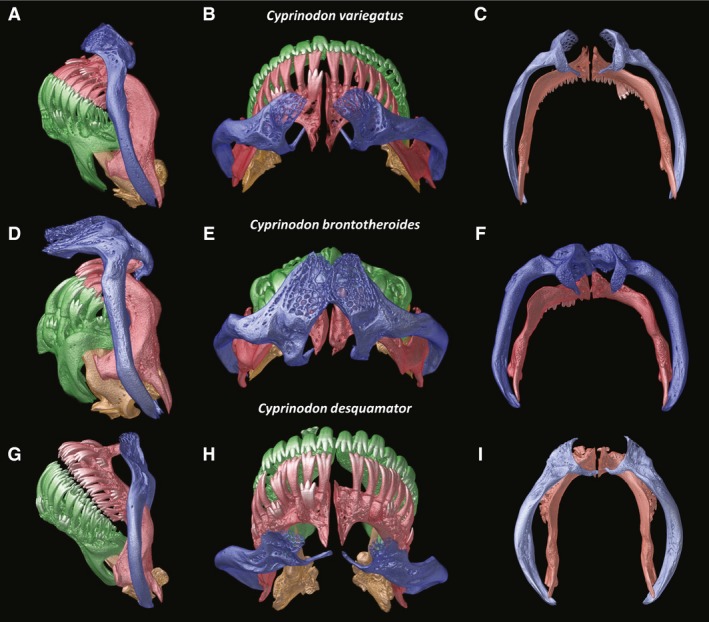
Close‐up of the oral jaw elements of the detritivore Cyprinodon variegatus (A–C), the durophage Cyprinodon brontotheroides (D–F), and the scale specialist Cyprinodon desquamator (G–I). Lateral (A,D,G), dorsal (B,E,H), posterior view of maxilla (blue) and premaxilla (red) showing degree of connection and surface for insertion of AM A1 on maxilla (C,F,I).
Figure 3.
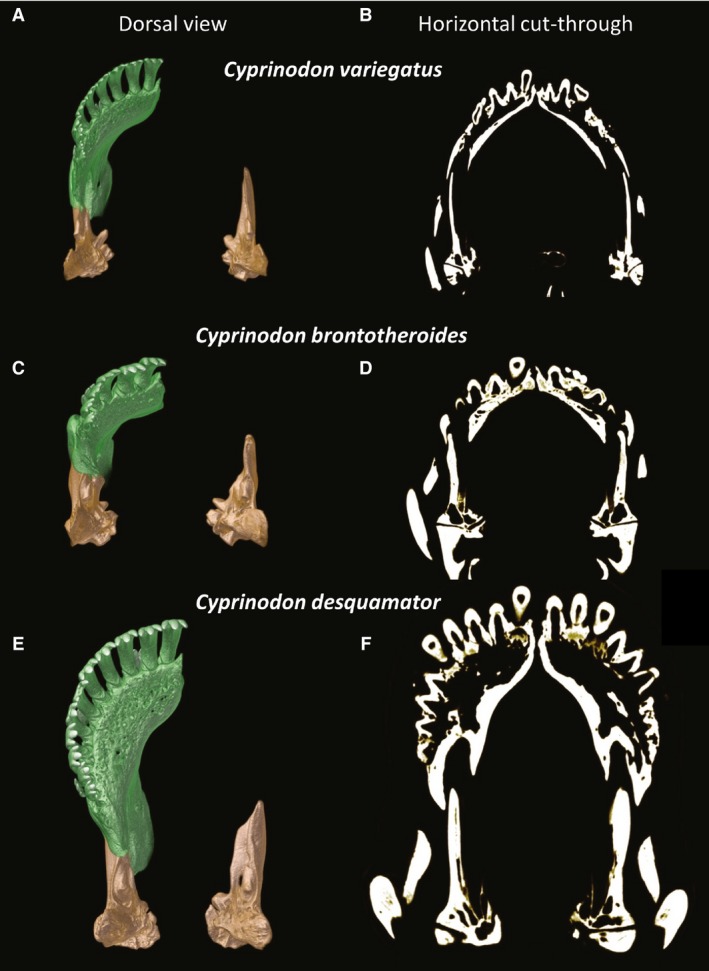
Dorsal view of the dentary (green) and articular (tan) of the detritivore Cyprinodon variegatus (A,B), the durophage Cyprinodon brontotheroides (C,D), and the scale specialist Cyprinodon desquamator (E,F). Frontal sections through the articular and dentary (B,D,E) showing relative thickness and mineralization.
In lateral view the dentigerous arms of the premaxillae have a strongly ventrally recurved shape (Fig. 2) forming a half moon. Such recurved elements of the oral jaws give the impression of a beak, a morphology well‐suited for picking. In lateral aspect the alveolar arm of the dentary and the dentigerous arm of the premaxilla are deeper than seen in the basal condition as exemplified by Kryptolebias (Hernandez et al. 2009). The premaxillae have patent ascending processes (largely obscured by the dorsal head of the maxilla in lateral view but clearly seen in dorsal view, Fig. 2B); however, these processes are shorter than those seen in more basal cyprinodontiforms. Previous work has suggested that these reduced ascending processes of the premaxillae are associated with a novel means of premaxillary protrusion (Hernandez et al. 2009).
Ventral to the point at which the premaxillae meet the lower jaw, the descending arm of the premaxilla has a long projecting spine (Fig. 2A,C). This spine, which constitutes the ventral‐most portion of the descending arm of the premaxilla, is adjacent to the ventromedial edge of the maxilla with both elements connected by a thick ligament. Manipulation of cleared and stained specimens revealed that this skeletal connection results in movement of these two bones in opposite directions, with forward rotation of the premaxilla when the arm of the maxilla is rotated caudally.
When the mouth is closed, the maxillae are tilted rostrally about 20° from a fully vertical position. Given the tilt of the jaws, portions of the lateral surface of the premaxillae are occluded by the thin maxillae, yet the dentary is completely exposed (Fig. 2A). The ventral end of each maxilla is relatively thin; however, the entire body of the maxilla is well‐mineralized and curved such that from a frontal aspect these bones frame the jaws (Fig. 1B). The dorsal ends of the maxillae are much more complex, with a highly trabeculated plate situated dorsal to the point at which the ascending process and dentigerous arm of the premaxillae meet. Thin medial hooks project from the ventral aspect of the dorsal head of the maxillae and pass under the ascending processes of the premaxillae as seen in dorsal view (Fig. 2B).
The head of the palatine resembles the head of a hammer with a broad flat cartilaginous articulating surface rostrally and a more tapered caudal head. A thick fibrous ligament connects the dorsal portion of the palatine to the dorsal head of the maxilla. The basal condition for cyprinodontids is met here with the head (and cartilaginous articular surface) angled rostrally.
As with other cyprinodontiforms, a discrete premaxillomandibular ligament connecting the caudolateral edge of the premaxilla (Fig. 4 lateral view) with the mediocaudal edge of the dentary (Fig. 4 medial view) characterizes this species. This ligament originates from a small process on the ventrolateral edge of the dentigerous arm of the premaxillae and wraps freely around the posterior edge of the premaxilla, before turning anteriorly to attach firmly to the mediocaudal aspect of the coronoid process of the dentary (Fig. 4 medial view). This ligament does not insert on the posterior margin of the premaxilla, but rather glides freely along the caudal edge of this bone when the lower jaw is depressed. Previous work on Cyprinodontiformes has shown that this ligament couples jaw depression with premaxillary protrusion (Hernandez et al. 2008, 2009). A premaxillomaxillary ligament connects the rostroventral edge of the maxilla to a caudoventral process on the dentigerous arm of the premaxilla (Fig. 4 lateral view).
Figure 4.
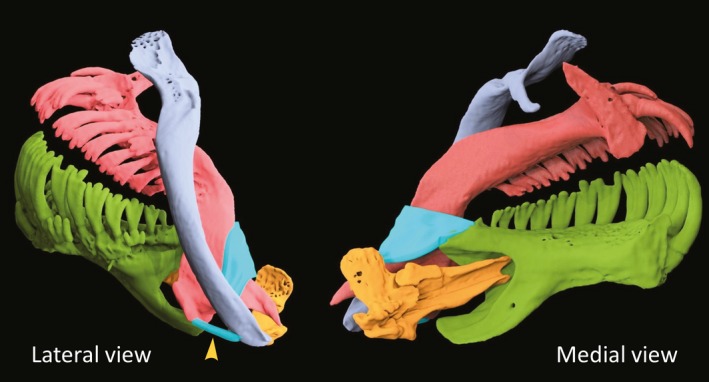
Lateral and medial views of the premaxillomandibular and premaxillomaxillary (arrowhead) ligament in aqua blue.
Musculoskeletal and ligamentous structures of C. brontotheroides and C. desquamator as compared with C. variegatus
Cyprinodon brontotheroides – hard prey specialist
Given the recent divergence of these species, all the basic elements of the cranial skeleton are quite similar, with individual elements varying largely in size of musculoskeletal elements and ligamentous connections. Cyprinodon brontotheroides, like C. variegatus, has a relatively deeper head as compared with C. desquamator. This has less to do with actual head depth than with the elongated suspensorium of C. desquamator (Fig. 1A,C vs. E).
In C. brontotheroides the jaws are even more superiorly positioned, approximately 50° to horizontal. More so than the mandibular architecture of C. variegatus, the mandibular symphysis is heavily reinforced in C. brontotheroides (Fig. 3D), with relatively thick walls especially compared with C. variegatus. Importantly, the dentary is much shorter than in the other species (Fig. 3). In the lower jaw, a patent coronoid process of the dentary and a ventral process of the articular, result in a firm articulation of the articular and dentary (Fig. 2D), with the articular deeply seated within the dentary, going nearly as far cranially as the rostrally limited teeth (Fig. 3C,D). A mediolateral expansion of the proximal portion of the articular likely provides increased structural stability during biting. Moreover, in frontal section the rostral arm of the articular is thicker than that of the basal form (Fig. 3B vs. D).
In C. brontotheroides when the mouth is closed the maxillae are nearly perpendicular, tilted rostrally about 10° from vertical. Although the ventral portion of the maxilla is like that of C. variegatus, the dorsal head of the maxilla is greatly expanded. A pronounced fleshy protuberance visible in fixed and live specimens sits upon the expanded shelf of trabeculated bone that constitutes this greatly expanded dorsal head of the maxilla. This dorsal elaboration may prevent movement of the maxillae. Indeed, the dorsal head of the maxilla is so expanded that the oral jaws are tucked in under the overhanging process (Fig. 2D).
Cyprinodon desquamator – scale‐eating specialist
Cyprinodon desquamator has a longer head with an elongated and deeper (mediolaterally) suspensorium (Fig. 1). Previous work (Martin & Wainwright, 2011; Lencer et al. 2016, 2017) has shown that C. desquamator has a significantly larger oral jaw apparatus as compared with other species, a finding verified here. The lower jaws (dentary and articular combined) are significantly longer in C. desquamator than the other species (P < 0.0001). Differences in shape and size of the suspensorium were consistent with hypertrophy of the adductor mandibulae complex (see below). The premaxilla and dentary of C. desquamator are significantly larger than that of the other species, particularly the dentary (Fig. 3).
Trabeculation of bone constituting the dentary is most pronounced in this species (Fig. 3E). Although the bony shell of this spatulate dentary is greatly enlarged as compared with the other two species, the dentary of C. desquamator is largely hollow (Fig. 3F). This thin‐walled construction coupled with the overall trabeculation of the dentary likely gives C. desquamator a relatively lighter jaw. Alternatively, the articular is more solid in construction (Fig. 3F), showing no trabeculation and a thickness even greater than that seen in C. brontotheroides. In addition to being thicker and more heavily mineralized, the proximal portion of the articular is mediolaterally expanded with an enlarged process at the insertion of AM A3.
There is a pronounced difference in the width of the lower jaw at its widest point and at the point of articulation with the quadrate in C. desquamator as compared with C. variegatus and C. brontotheroides (Fig. 3E vs. A,C). The relatively smaller width at the point of the jaw joint in C. desquamator is due to both a much deeper suspensorium (important for housing a much larger adductor musculature), which renders the jaw joint narrower, in comparison with a more cranially expanded spatulate jaw (Fig. 5).
Figure 5.
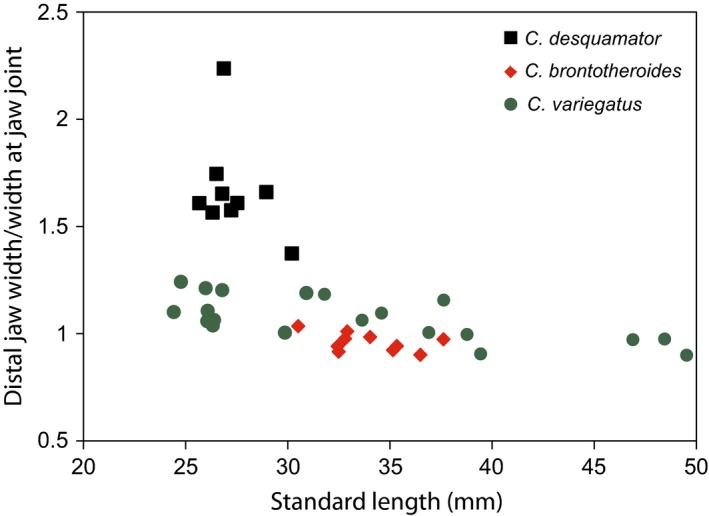
Interspecific differences in proportion of distal jaw width and width at point of articulation with quadrate. A much wider distal jaw characterizes the scale specialist, Cyprinodon desquamator.
In C. desquamator when the mouth is closed, the maxillae are only a few degrees from vertical. Importantly, the ventral tips of the laterally bowed and enlarged maxillae curve medially slightly, deep to the spine on the dentigerous arm of the premaxilla interlocking the ventral extremes of these two bones (Fig. 2C,F,I).
In lateral aspect, the maxillae are relatively narrow, belying the fact that this species has the thickest maxillae of this radiation, with a broad sculptured area where AM A1 inserts on this bone (Fig. 2I). The dorsal head of the maxilla is similar in shape in C. desquamator and C. variegatus, with significantly less elaboration of the dorsal process of the maxilla as compared with C. brontotheroides. Although the maxillae appear more caudally positioned due to the hypertrophied nature of the dentary, there is little difference in the basic architecture of the maxillae between C. variegatus and C. desquamator. However, upon closer inspection (made possible by microCT scans) it is apparent that the maxillae of this scale specialist have a more rounded and bowed shape (Fig. 2I vs. C,F), with a greatly expanded surface area for insertion of AM A1. When the mouth is closed, the maxillae are at a more acute angle in both C. variegatus and C. brontotheroides as compared with the more vertically inclined position seen in C. desquamator. Unlike the condition seen in most other cyprinodontiforms, the maxillae (especially in C. desquamator) are not strongly tied ventrally, thus the maxillae are free to pivot rostrally, since they are attached firmly only at the dorsal ligamentous attachment to the palatine. Such an orientation may have profound functional implications in C. desquamator (see below).
To test whether rostrocaudal movement of the maxillae at their ventral ends may be correlated with premaxillary protrusion and retraction, we examined whether gape angle (the angle between the upper and lower jaw) was correlated with rostrocaudal movement of the maxillae. There was a significant correlation for C. variegatus (r 2 = 0.282, P = 0.013) and a stronger correlation for the scale specialist (C. desquamator; r 2 = 0.687; P < 0.001), but no relationship between these two functional variables in the hard prey specialist (C. brontotheroides; r 2 = 0.001, P = 0.854; Fig. 6).
Figure 6.
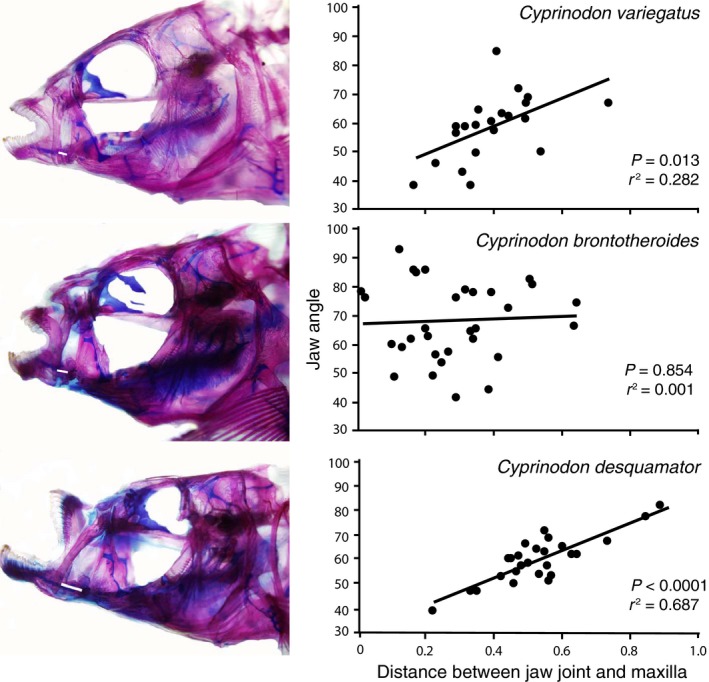
Gape/Jaw angle vs. distance between the ventral end of the maxilla and the jaw joint. There is a significant correlation between the angle of the dentary and premaxilla (jaw gape) with the distance between the jaw joint and ventral tip of the maxilla in Cyprinodon variegatus and Cyprinodon desquamator but not Cyprinodon brontotheroides.
Although the general shapes of the articular and dentary are largely conserved among species (but see below for the scale specialist), their type of articulation varies. Cyprinodon variegatus shows a typical cyprinodontid architecture whereby the articular is largely slotted into the medial aspect of the dentary. This general structure is seen in C. brontotheroides but the rostral tip of the articular is located slightly more rostrally into the dentary. Alternatively, in C. desquamator the rostral tip of the articular does not reach the level of the teeth as seen in C. brontotheroides, resulting in a looser connection (Fig. 3).
Palatine shape in both C. brontotheroides and C. desquamator varies slightly from that seen in C. variegatus. However, differences between C. variegatus and C. desquamator are relatively subtle, with both having a pronounced rostrally articulating surface with the maxilla. The maxilla has a discrete point of rotation with the palatine; however, that articulating surface is slightly more laterally inclined in the scale specialist. A thick ligament attaches the dorsal end of the palatine to the dorsolateral side of the head of the maxilla. The point of attachment of this ligament differs in C. brontotheroides, owing largely to the greatly hypertrophied dorsal head of the maxilla.
Overall architecture of the adductor mandibulae (AM) is largely conserved among the different species, but there are significant differences in size and subtle yet functionally significant differences in architecture. Although C. variegatus and C. brontotheroides do not have significantly different masses of the adductor mandibulae complex, that of the scale specialist is over four times that of the other species. Cyprinodon spp. examined here have a single, parallel‐fibered branch of adductor mandibulae A1 which inserts on the ventral one‐third (C. desquamator) or one‐fourth (C. brontotheroides and C. variegatus) of the maxilla via a broad, muscular insertion (Fig. 7). The bulk of the adductor mandibulae complex is composed of A1. A1 is significantly larger (P < 0.0001) in C. desquamator (6.93 mg ± 0.49) than in either C. brontotheroides (1.14 mg ± 0.49) or C. variegatus (1.66 mg ± 0.55). Indeed, the mass of A1 in C. desquamator is nearly five times the size of that in the other species. This significantly larger A1 contributed to a significantly larger adductor mandibulae complex overall in C. desquamator (P < 0.0001).
Figure 7.
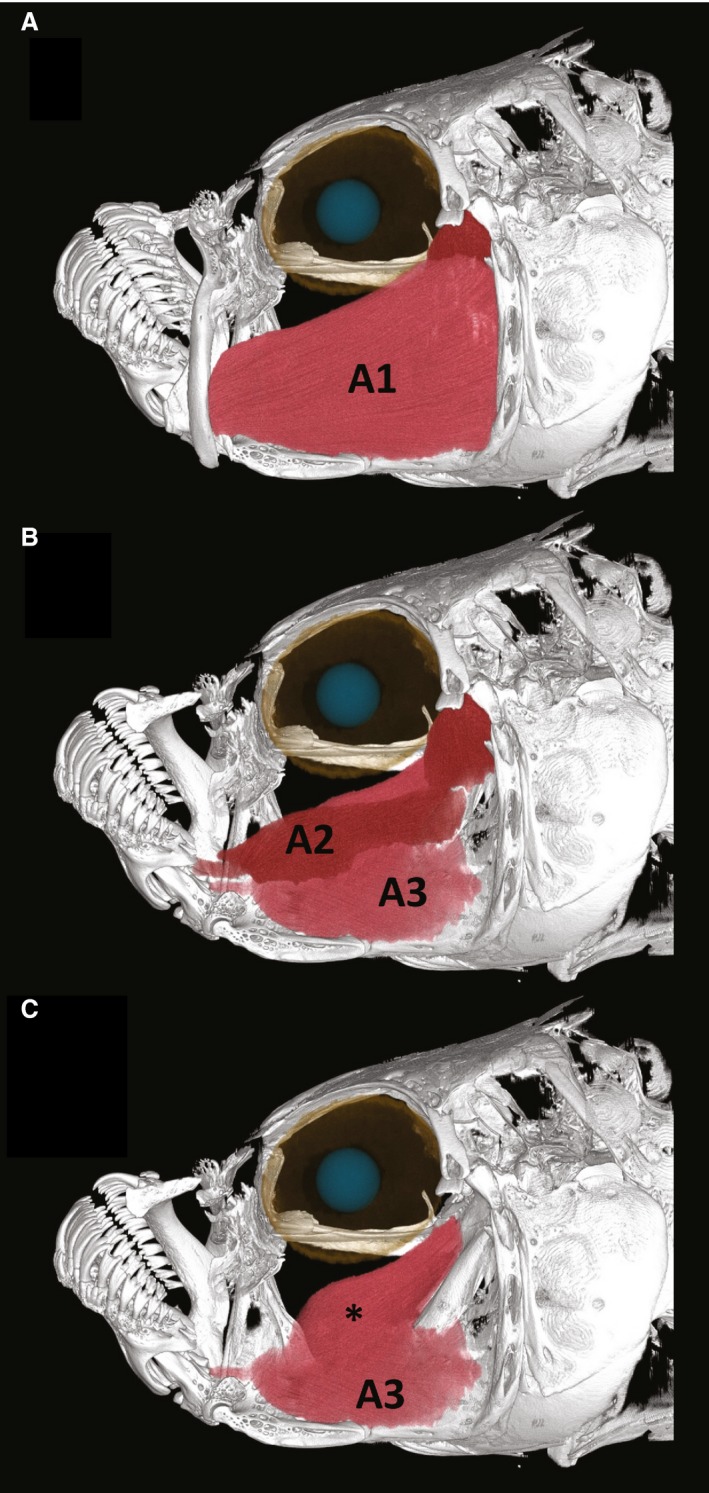
Adductor mandibulae (AM) complex in the scale specialist Cyprinodon desquamator. (A) AM A1 inserts on the maxilla and makes up most of the AM complex. (B) Maxilla and premaxilla removed to show AM A2 and A3. (C) AM A3 showing two major components, one more lateral and one more medial (*). The more medial section is better developed in the scale‐eater. Muscles were reconstructed using data from microCT scans.
Adductor mandibulae (AM) A2 and A3 are strongly attached in all species (but can be carefully separated), thus the belly of A2 is difficult to distinguish from the belly of A3 (Fig. 7B). AM A2 comprises two fairly discrete sections, an anteriorly compact (i.e. relatively short‐fibered) section as well as a more posterior longer, parallel‐fibered section. A2 inserts on the caudal edge of the coronoid process of the dentary and coronomeckelian, whereas A3 has a well‐developed tendon that inserts on a process on the medial face of the articular. There is a significant difference in the mass of A2 among species (P = 0.02); however, this difference is not as pronounced as that of A1. While there is no significant difference in mass of AM A2 between C. desquamator and C. brontotheroides and between C. brontotheroides and C. variegatus, there was a significant difference between C. desquamator and C. variegatus (P = 0.017). The mean size of A2 in the durophage was 0.854 mg, whereas that of the basal detritivore was 0.50 mg; likely due to the small sample size (n = 5) these were not significant. AM A3 is deep to AM A2 and has a bipennate division, inserting with a much less acute angle on to the articular. A small medial section of A3 is much better developed in C. desquamator than the other species (Fig. 7C*). There is a significant difference in the size of AM A3 among species (P = 0.0012), with C. desquamator having an A3 nearly five times the size of that in the other two species. This greatly hypertrophied mass is likely due to the expanded medial section described in C. desquamator (Fig. 7C).
Discussion
Niche partitioning within this adaptive radiation is reflected in differences in the feeding architecture of the oral jaws. Importantly, most of the functionally relevant changes appear to be a result of differential growth of key components of the feeding apparatus. Relatively minor shape changes coupled with significant differences in the size of the bony and muscular elements of the jaws has led to sympatric niche partitioning, illustrating the ecological changes that can result from closely related species undergoing heterochronic shifts in jaw development. Large changes in the size of some elements (e.g. size of adductor mandibulae A1 and A3) coupled with relatively subtle differences in skeletal anatomy (e.g. more medially recurved ventral spines of the maxillae), may have been key to building the most divergent feeding mechanism in the scale specialist.
Musculoskeletal differences
Clear interspecific differences in shape and architecture of the elements of the anterior jaws characterize these species. Indeed, this young species flock exhibits variation on a par with features that have been previously used to differentiate genera (Ghedotti, 2000).
In some teleosts the maxilla is not involved in premaxillary protrusion but rather acts as a brace against which the premaxilla is protruded (Schaeffer & Rosen, 1961; Motta, 1984). Within these species there was a very different relationship between the degree of maxillary rotation and jaw gape (Fig. 6). Such differences, especially between C. desquamator and C. brontotheroides, suggest that in the durophage the maxilla acts as a stable base from which the oral jaws may protrude. Alternatively, in the scale specialist, as the premaxilla is protruded via tension from the premaxillomandibular ligament during jaw opening, the maxilla is pulled forward due to the interlocked nature of the ventral ends of the maxilla and premaxilla (Fig. 2I). As the jaw is opened wide, as seen when the jaw is applied to the scaly flank of a target fish, the maxillae are translated rostrally.
Significant differences in size of the adductor mandibulae also play an important role in this trophic radiation. Not only do these differences characterize the different species of Cyprinodon, they also defy ecomorphological expectations given the functional challenges that these different prey categories pose. Given its important role in jaw adduction, AM A2 would be expected to show the most profound interspecific difference. Alternatively, both AM A3 and AM A1 would not be expected to vary as much as they do. Moreover, the hard prey specialist would be expected to have the largest AM A2 or A3, which it did not.
Striking differences among these species include both overall size of the jaw elements as well as the relative size of the dentary and articular (Fig. 3). Within C. brontotheroides the dentary is significantly shorter than in the other species (Lencer et al. 2016) and previous work (Martin, 2016) has found accelerated diversification rates for these jaw elements. Such diversification rates were especially pronounced in the scale‐eater, the species with the most greatly expanded dentary. Cyprinodon desquamator also showed the greatest difference in size of lower jaw elements, where the articular is dwarfed by the dentary. In other strong biters from different lineages, the articular is more comparable in size to the dentary (Hernandez & Motta, 1997). Such disparate sizes in lower jaw elements in which the dentary is the element varying greatly in size, may suggest one of several alternatives. There may be stronger functional constraints placed on the articular as it is the bone that must articulate with the quadrate. Alternatively, there may be permissive developmental pathways (potentially involving insulin‐like growth factors and/or cytokines, Lencer et al. 2017) that facilitate hypertrophy or reduction in the size of the dentary. The fact that previous work (Martin & Wainwright, 2011; Martin, 2016) has revealed a faster evolutionary rate of morphological change within the dentary would suggest permissive developmental factors. Moreover, recent work by Lencer et al. (2017) shows that the scale‐eater significantly overexpresses insulin‐like growth factor binding proteins, strongly implicating such growth factors in this divergent morphology of the jaw.
Laterally bowed maxillae are found in all three species but this phenotype is the most developed in the scale‐eater (Fig. 2), in which the surface for insertion of the adductor mandibulae is greatly expanded. Among the species, the greatest similarities in maxillary structure and underlying palatine support are between C. variegatus and the scale specialist. Both show a correlation between jaw gape and caudal movement of the maxilla. In contrast, the durophagous species has largely immobile maxillae, which seem to serve in stabilizing the jaws as opposed to playing a role in their closing. Bowed maxillae are not, however, shared by all cyprinodontids; different species of Cyprinodon and Jordanella previously investigated (Hernandez et al. 2009) did not have this strongly bowed shape. Indeed, Parenti (1981) noted that this bowed shape characterizes Fundulus (Eaton, 1935). Such a strongly bowed shape may have served as an important preadaptation for the unique scale‐feeding mechanism, as the strong bowing seen in the scale‐eater allows for an expanded surface area (Fig. 2I) for insertion of a hypertrophied AM A1.
The morphological evolution seen within this species flock is reminiscent of that previously described in loricarioids. Schaefer & Lauder (1996) document a gradual transformation of the anterior jaws from minimal premaxillary protrusion within primitive loricarioids to a derived condition in which the paired premaxillae are controlled independent of the lower jaw via the adductor mandibulae complex. That morphological transformation within loricarioids appeared to be associated with functional specialization for algal scraping. As in algal scraping, an important requirement for scraping scales is the ability to produce a large gape. Importantly, given that a fish is rather unlikely to allow for a second mouthful of scales to be taken, it seems important for scale‐eaters to maximize the number of scales taken per bite. Here this novel mechanism appears to allow for a much wider gape, since the only thing constraining maximum gape production is the degree of jaw depression (Hernandez et al. 2008). The architecture of the premaxillomandibular ligament (Fig. 4), as well as the ability to generate force at the anterior ends of the upper and lower jaws, are important during scraping – be it algae from the substrate or scales from brethren.
Making a flock of picky biters
Picking (a cyprinodontiform feeding mode previously described by Hernandez et al. 2008, 2009) constitutes a specialized type of biting in which the force produced may be secondary to the increased dexterity necessary to pick at food items. Here we see a functional elaboration of this system in two directions, one specialized for durophagy and one for lepidophagy. These two feeding niches are associated with different anatomical modifications. Hard prey are challenging because of the amount of force required to crush such prey items. Alternatively, effective lepidophagy requires both a fast strike and one that maximizes scales removed per bite. In C. brontotheroides the maxilla became stabilized, jaws were foreshortened, and the size of the adductor mandibulae complex was increased to allow for strong biting. Cyprinodon desquamator exploited the plesiomorphic cyprinodontiform feature of the premaxillomandibular ligament that allowed for coordinated jaw opening and coupled this with greatly hypertrophied jaws and adductor mandibulae A1 and A3 to allow for a rapid bite.
Foreshortened jaws and hypertrophied adductor musculature characterize strong biters. Here we see that not only does C. brontotheroides have the shortest jaws (P < 0.001), the jaws are stabilized by a largely immobile maxilla, and they possess the smallest AM A1. Jaw gape is not related to the position of the maxilla (Fig. 6; r 2 = 0.001), illustrating that contrary to the basal condition these largely immobile maxillae are a derived character. While several musculoskeletal elements are modified in these hard prey specialists, the scale specialist shows the most derived cranial morphology.
We infer three functional hurdles that must be cleared for effective scale‐feeding. First, jaw adduction must be rapid. Unlike algal grazing, a predator is unlikely to have an opportunity to leisurely take another bite, thus we assume that scale‐feeding requires a quick bite. Secondly, the bite must be strong enough to dislodge a large mouthful of scales. Thirdly, the range of upper and lower jaw motion needs to be extensive to allow the fish to engage the body of its prey with as many teeth as possible, maximizing the number of scales engaged and removed per bite. Thus, the scale‐feeding mechanism must provide a constructional solution to the classic tradeoff between speed and force, and must accommodate enhanced mobility. It is likely that the force required to pull off scales is not significant, rather speed and maximizing surface area scraped are likely more important. This functional challenge is made more difficult by a greatly hypertrophied lower jaw actuated by adductor mandibulae A2/A3 working at a pronounced mechanical disadvantage. Martin & Wainwright (2011) pointed out that this poor mechanical advantage could be compensated by hypertrophy of the adductor musculature, but they did not clarify how this would be accomplished. Curiously, the rapid rate at which adductor mandibulae A1 is evolving (Martin & Wainwright, 2011) may indicate a major and novel use of this hypertrophied muscle.
A strong ventral coupling of the maxillae and premaxillae (Fig. 2I), combined with hypertrophy of AM A1 (the muscle responsible for maxillary retraction), suggests that actions of A1 will be transmitted with little loss of motion through the maxilla to the premaxilla. Hypertrophy of A1 and its mechanical advantage in retraction of the maxilla suggest that the role of forceful adduction of the jaws is a particularly important aspect of feeding on scales. This important role of A1 is supported by enlarged toothed surfaces in both the upper and lower jaw (Martin & Wainwright, 2011), enhancing the potential of these jaws for engaging and dislodging scales. While a strong bite may be important to remove scales effectively, it is reasonable to assume that the speed of the bite is more important. The strong medial bowing of the maxilla at its ventral end curved around the laterally inclined spine of the premaxilla results in a capacity to bring the premaxilla forward quickly during lower jaw depression. The premaxillomandibular ligament that allows for coordinated movement during jaw opening, coupled with the connection of the maxilla and premaxilla via the premaxillomaxillary ligament (Fig. 4), will enhance synchrony of jaw movements during adduction. Once the large lower jaw has been partially adducted by the unimpeded action of AM A1, greatly improving mechanical advantage, both AM A2 and AM A3 can contract to bring about a more forceful bite. A unique skeletal connection between the premaxilla and maxilla, combined with a novel function of the adductor mandibulae complex, allows C. desquamator to defy the constructional constraints involved in rapidly moving a large jaw at a pronounced mechanical disadvantage.
In this functional model, AM A1 is involved in the rapid closure of the mouth while the jaws are maximally open, and AM A2 and A3 act later once a more favorable mechanical advantage has been restored. This decoupling of function within the adductor mandibulae complex may be especially well‐suited for feeding on scales. Liem & Stewart (1976) pointed out that scale‐eaters required strong yet variable application of the jaws to the flank of their prey. Such variable movement and concomitant force produced by the upper and lower jaw may be possible given this cyprinodont jaw mechanism. Thus, rapid evolution in size of the dentary and adductor mandibulae complex (Martin & Wainwright, 2011) reflects the specific challenges involved in dealing with the functional challenges of scale‐eating. Interestingly, Liem & Stewart (1976) describing the anatomy of a cichlid scale‐eater reported that while AM A3 is reduced in size a greatly enlarged dentary (as compared with the anguloarticular/articular) also characterizes lepidophagous cichlids. Thus, scale specialists from two different lineages share this specialization.
The classic paper of Fryer & Iles (1972) detailing the trophic morphospace occupied by East African cichlids, illustrates the great number of constructional solutions that have evolved for biting. Biting appears to inhabit a wide functional morphospace with many solutions (Collar et al. 2014). This Cyprinodon species flock arose from a ‘picky’ group of cyprinodontiform feeders (well built for fine manipulation – an overlooked form of biting) and secondarily evolved a capacity for scale‐eating and/or durophagy, suggesting an ability of this genus to provide abundant fodder for adaptive radiations. Importantly, this radiation illustrates the type of functionally relevant changes that is possible based largely on changes in growth rate. Within members of young species flocks, significant ecological segregation may result from allometric growth of key feeding structures.
Conclusions
Geological records suggest that the San Salvador radiation is only between 6000 and 10 000 years old (Turner et al. 2008; Martin & Wainwright, 2011). Younger adaptive radiations of this type may be characterized by divergent morphologies brought about largely by heterochronic differences in growth rate, whereas older radiations may show more qualitative differences. Investigating such young radiations affords us an opportunity to determine not only whether differences in growth rate may produce significant functional differences, but also how substantial these differences can become within a relatively short time. Importantly, at some point, quantitative differences evolving at an incredibly accelerated rate (such as those reported by Martin & Wainwright, 2011; Martin, 2016) may ultimately lead to adaptive peaks that serve to isolate these populations reproductively. Indeed, the scale‐eater appears to be isolated by a large fitness valley from the other two San Salvador species and is the most reproductively isolated (Martin & Wainwright, 2013a,2013b,2013c; Martin & Feinstein, 2014).
Author contributions
L.P.H. conceived the study, analyzed data, and wrote the initial draft. B.M. and M.D. performed microCT scans. D.A. segmented all CT scans, analyzed data, and produced the figures. C.H.M. collected, cleared and stained specimens, and provided expertise on the growth and genetics of this group. L.P.H., C.H.M., P.C.W., and D.A. were all involved in critical revision and final approval of the manuscript.
Acknowledgements
We are grateful to the reviewers and editor who greatly improved the quality of the manuscript. Grant support was provided by National Science Foundation Grant DEB‐1061981 to P.C.W., DEB‐1010849 to C.H.M. and P.C.W., and IOS‐1025845 to L.P.H. We thank the Gerace Research Centre and Rochelle Hanna for logistics in the Bahamas and the Bahamas BEST Commission for permission to collect and export specimens.
References
- Collar DC, Wainwright PC, Alfaro ME, et al. (2014) Biting disrupts integration to spur skull evolution in eels. Nat Commun 5, 5505. [DOI] [PubMed] [Google Scholar]
- Collyer ML, Novak JM, Stockwell CA (2005) Morphological divergence of native and recently established populations of White Sands Pupfish (Cyprinodon tularosa). Copeia 2005, 1–11. [Google Scholar]
- Covich A, Stuiver M (1974) Changes in oxygen 18 as a measure of long‐term fluctuations in tropical lake levels and molluscan populations. Limnol Oceanogr 19, 682–691. [Google Scholar]
- Dingerkus G, Uhler LD (1977) Enzyme clearing and staining of alcian blue stained small vertebrates for demonstration of cartilage. Stain Technol 52, 229–232. [DOI] [PubMed] [Google Scholar]
- Eaton TH (1935) Evolution of the upper jaw mechanism in teleost fishes. J Morphol 58, 157–172. [Google Scholar]
- Ferry‐Graham LA, Gibb AC, Hernandez LP (2008) Premaxillary movements in cyprinodontiform fishes: an unusual protrusion mechanism facilitates ‘picking’ prey capture. Zoology (Jena) 111, 455–466. [DOI] [PubMed] [Google Scholar]
- Fryer G, Iles TD (1972) The Cichlid Fishes of the Great Lakes of Africa: Their Biology and Evolution. Edinburgh: Oliver and Boyd. [Google Scholar]
- Ghedotti MJ (2000) Phylogenetic analysis and taxonomy of the poecilioid fishes (Teleostei: Cyprinodontiformes). Zool J Linn Soc 130, 1–53. [Google Scholar]
- de Graaf M, Dejen E, Osse JWM, et al. (2008) Adaptive radiation of Lake Tana's (Ethiopia) Labeobarbus species flock (Pisces, Cyprinidae). Mar Freshw Res 59, 391–407. [Google Scholar]
- Hernandez LP, Motta PJ (1997) Trophic consequences of differential performance: ontogeny of oral jaw crushing performance in the sheepshead, Archosargus probatocephalus (Teleostei: Sparidae). J Zool 243, 737–756. [Google Scholar]
- Hernandez LP, Ferry‐Graham LA, Gibb AC (2008) Morphology of a picky eater: a novel mechanism underlies premaxillary protrusion and retraction within Cyprinodontiforms. Zoology (Jena) 111, 442–454. [DOI] [PubMed] [Google Scholar]
- Hernandez LP, Gibb AC, Ferry‐Graham LA (2009) Trophic apparatus in cyprinodontiform fishes: functional specializations for picking and scraping behaviors. J Morphol 270, 645–661. [DOI] [PubMed] [Google Scholar]
- Hoese HD, Moore RH (1977) Fishes of the Gulf of Mexico, Texas, Louisiana, and Adjacent Waters. College Station, TX: Texas A&M University Press. [Google Scholar]
- Horstkotte J, Strecker U (2005) Trophic differentiation in the phylogenetically young Cyprinodon species flock (Cyprinodontidae, Teleostei) from Laguna Chichancanab (Mexico). Biol J Linn Soc 85, 125–134. [Google Scholar]
- Humphries JM, Miller RR (1981) A remarkable species flock of pupfishes, genus Cyprinodon, from Yucatan, Mexico. Copeia 1981, 52–64. [Google Scholar]
- Kocher TD (2005) Fish models for studying adaptive evolution and speciation (Special Feature – Roundtable Discussion). Zebrafish 2, 147–156. [DOI] [PubMed] [Google Scholar]
- Lencer ES, Riccio ML, McCune AR (2016) Changes in growth rates of oral jaw elements produce evolutionary novelty of Bahamian pupfish. J Morphol 277, 935–947. [DOI] [PubMed] [Google Scholar]
- Lencer ES, Warren WC, Harrison R, et al. (2017) The Cyprinodon variegatus genome reveals gene expression changes underlying differences in skull morphology among closely related species. BMC Genom 18, 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem KF (1973) Evolutionary strategies and morphological innovations: cichlid pharyngeal jaws. Syst Zool 22, 425–441. [Google Scholar]
- Liem KF, Stewart DJ (1976) Evolution of the scale‐eating cichlid fishes of Lake Tanganyika: a generic revision with a description of a new species. Bull Mus Comp Zool 147, 319–350. [Google Scholar]
- Martin CH (2016) The cryptic origins of evolutionary novelty: 1000‐fold faster trophic diversification rates without increased ecological opportunity or hybrid swarm. Evolution 70, 2504–2519. [DOI] [PubMed] [Google Scholar]
- Martin CH, Feinstein LC (2014) Novel trophic niches drive variable progress toward ecological speciation within an adaptive radiation of pupfishes. Mol Ecol 23, 1846–1862. [DOI] [PubMed] [Google Scholar]
- Martin CH, Wainwright PC (2011) Trophic novelty is linked to exceptional rates of morphological diversification in two adaptive radiations of Cyprinodon pupfishes. Evolution 65, 2197–2212. [DOI] [PubMed] [Google Scholar]
- Martin CH, Wainwright PC (2013a) On the measurement of ecological novelty: scale‐eating pupfish are separated by 168 my from other scale‐eating fishes. PLoS One 8, e71164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CH, Wainwright PC (2013b) Multiple fitness peaks on the adaptive landscape drive adaptive radiation in the wild. Science 339, 208–211. [DOI] [PubMed] [Google Scholar]
- Martin CH, Wainwright PC (2013c) A remarkable species flock of Cyprinodon pupfishes endemic to San Salvador Island, Bahamas. Bull Peabody Mus Nat Hist 54, 231–240. [Google Scholar]
- Motta PJ (1984) Mechanics and functions of jaw protrusion in teleost fishes: a review. Copeia 1984, 1–18. [Google Scholar]
- Parenti LR (1981) A phylogenetic and biogeographic analysis of cyprinodontiform fishes (Teleostei, Atherinomorpha). Bull Am Mus Nat Hist 168, 335–557. [Google Scholar]
- Potthoff T (1984) Clearing and staining techniques In: Ontogeny and Systematics of Fishes. (ed. Moser HG.), pp. 35–37, Allen Press, Lawrence, KS: Special Publication of the American Society of Ichthyologists and Herpetologists. [Google Scholar]
- Ruell CB, Dewitt TJ (2005) Trophic plasticity and fine‐grained resource variation in populations of western mosquitofish, Gambusia affinis . Evol Ecol Res 7, 801–819. [Google Scholar]
- Schaefer SA, Lauder GV (1996) Testing historical hypotheses of morphological change: biomechanical decoupling in loricarioid catfishes. Evolution 50, 1661–1675. [DOI] [PubMed] [Google Scholar]
- Schaeffer B, Rosen DE (1961) Major adaptive levels in the evolution of the actinopterygian feeding mechanism. Am Zool 1, 187–204. [Google Scholar]
- Schluter D (2000) The Ecology of Adaptive Radiation. Oxford: Oxford University Press. [Google Scholar]
- Stevenson MM (1992) Food habits within the Laguna Chichancanab Cyprinodon (Pisces: Cyprinodontidae) species flock. Southwest Nat 37, 337–343. [Google Scholar]
- Tobler M, Carson EW (2010) Environmental variation, hybridization, and phenotypic diversification in Cuatro Ciénegas pupfishes. J Evol Biol 23, 1475–1489. [DOI] [PubMed] [Google Scholar]
- Turner BJ, Duvernell DD, Bunt TM, et al. (2008) Reproductive isolation among endemic pupfishes (Cyprinodon) on San Salvador Island, Bahamas: microsatellite evidence. Biol J Linn Soc 95, 566–582. [Google Scholar]


