Abstract
Previous work, almost four decades ago, showed that hydrocortisone (HC) treatment reduces the number of skeletogenic condensations that give rise to the scleral ossicles in the chicken eye. The scleral ossicles are a ring of overlapping intramembranous bones, the sclerotic ring, and are present in most reptiles, including birds. The scleral condensations that give rise to the scleral ossicles are induced by a series of overlying thickenings (or papillae) of the conjunctival epithelium. Here, we further explore the effects of altering the dosage and timing of HC treatment on the morphology and number of skeletogenic condensations and conjunctival papillae. We show that high doses can completely obliterate the entire sclerotic ring. Significantly, the reduction in papillae number we observed was less extreme than that of the scleral condensations, indicating that additional factors contribute to the observed skeletogenic condensation loss. Via immunohistochemical analyses, we show that HC treatment alters the spatial expression pattern of several extracellular matrix components (tenascin‐C, decorin and procollagen I) and also alters the vasculature network within the sclera. This research provides important insights into understanding the role of the scleral tissue components in ossicle development within the vertebrate eye.
Keywords: bone development, collagen I, decorin, extracellular matrix, scleral ossicles, skeleton, tenascin‐C
Introduction
The vertebrate ocular skeleton is present within most reptiles and many teleost fish (Walls, 1942; Nakamura & Yamaguchi, 1991; Franz‐Odendaal & Hall, 2006; Franz‐Odendaal, 2011). In birds, the ocular skeleton consists of a posterior cup of hyaline cartilage and an anterior ring of scleral ossicles, the sclerotic ring (for review, see Franz‐Odendaal & Vickaryous, 2006). The ocular skeleton is thought to provide structural support and to function in visual accommodation (Walls, 1942). Scleral ossicles have been lost several times throughout the course of evolution, including in amphibians, snakes, crocodiles and placental mammals (Walls, 1942; Franz‐Odendaal & Hall, 2006; Franz‐Odendaal, 2011; Atkins & Franz‐Odendaal, 2016). The developmental mechanisms underlying this loss are not well understood.
The development of the scleral ossicle system is best known from research conducted in the chicken, Gallus gallus. A series of evenly spaced thickenings (papillae) develop from the conjunctival epithelium in a ring along the corneal–scleral limbus (Murray, 1941; Coulombre et al. 1962; Franz‐Odendaal, 2008a). The development of the full ring of 14–16 conjunctival papillae is prolonged and begins at Hamburger and Hamilton's (HH) stage 30 [6.5 days post‐fertilization (dpf)] with the appearance of the first papilla above the ciliary artery. The last papilla in the ring forms by HH 34 (8 dpf; Hamburger & Hamilton, 1951). These conjunctival papillae induce the underlying neural crest‐derived mesenchyme within the sclera to form aggregates of preosteogenic cells (the scleral condensations); this occurs in a 1 : 1 ratio (Coulombre et al. 1962; Hall, 1981; Pinto & Hall, 1991). By HH 37 (11 dpf), the scleral condensations are well established and the conjunctival papillae have begun to degenerate, and by HH 38 (12 dpf), the scleral ossicles are mineralized and distinct (Franz‐Odendaal, 2008a,b). The scleral ossicles then expand in size and ultimately overlap one another to form the sclerotic ring.
Previous research has shown that when a single conjunctival papilla is disrupted either mechanically or molecularly, the corresponding scleral ossicle does not form (Coulombre et al. 1962; Franz‐Odendaal, 2008a; Duench & Franz‐Odendaal, 2012). Interestingly, in these experiments, the adjacent scleral ossicles will increase in size to fill in the gap in the ring, demonstrating the presence of a lateral inhibition mechanism that establishes ossicle size. Although this aspect of the scleral ossicle system is fascinating, it makes studying experimentally induced scleral ossicle loss challenging.
Vasculature is known to play a crucial role in bone development, including in the chick scleral ossicles (Jourdeuil & Franz‐Odendaal, 2012; Jabalee & Franz‐Odendaal, 2015). We showed that vascular endothelial growth factor (VEGF)‐A knockdown at a single papilla results in large avascular zones within the sclera (Jabalee & Franz‐Odendaal, 2015). Although the vasculature recovers quickly, morphological defects are observed in the later developing scleral condensations.
In addition to these localized disruption methods, there are a few examples in the literature where a reduction of multiple scleral ossicles has been observed. Following an experimental reduction in eye size, Coulombre et al. (1962) observed that the overall number of conjunctival papillae and scleral ossicles was reduced, thus demonstrating a relationship between the space available in the eye to evenly space papillae/ossicles and the number of papillae/ossicles that ultimately form. In the scaleless mutant chick, epithelial derivatives including scales and feathers are significantly reduced, as are the conjunctival papillae. These few conjunctival papillae induce a few, abnormally large scleral ossicles in each eye (Stuart et al. 1972; Hall & Miyake, 2000; Hall, 2005). This mutant has a fibroblast growth factor 20 (fgf20) mutation as well as a defect in the matrix proteoglycan tenascin (Shames et al. 1991, 1994; Wells et al. 2012). Stuart et al. (1972) compared the scaleless mutant chick with embryos treated with hydrocortisone (HC; i.e. the steroid stress hormone cortisol) and observed similar phenotypes. This research was expanded upon a year later by Johnson (1973), who showed: (i) that there was a reduction in both conjunctival papillae and scleral ossicle counts after HC treatment; (ii) that two injections given 24 h apart (at 6 and 7 days of incubation) produced a greater effect on counts than single injections; (iii) that injecting consecutively at days 7 and 8 of incubation produced milder effects than injecting on days 6 and 7; and (iv) that eye size was not a factor contributing to the reduction in these numbers. Johnson (1973) thus concluded that the observed reductions in numbers were due to the chemical treatment affecting papillae development and consequently the ossicles in a 1 : 1 manner, and that this effect was specifically correlated with the time of injection.
The present study examines in more detail the effects of HC treatment on papillae and scleral condensation formation. Johnson (1973), for example, did not examine the effect of administering HC twice on the same day. First, we confirm the previous findings that two HC injections given at two consecutive stages of development affect the number of papillae and scleral condensations that develop. We then examined both quantitatively and qualitatively, via different combinations of dose and timing, whether a similar effect can be achieved with the same total dose given consecutively (twice) at just one of these two stages. We show that it cannot. Furthermore, after careful analysis of the number of papillae and number of ossicles after each treatment, we show that the effect on the number of papillae is not to the same degree as the effect on the number of scleral condensations, it is less severe, suggesting that other factors are contributing to the observed defects in the scleral condensations. To further understand what these factors might be, we examined the scleral tissue, scleral vasculature and several extracellular matrix (ECM) components after HC treatment. Our results show a distinct reduction in scleral vasculature following HC treatment, an extended dense connective tissue region within the scleral mesenchyme and altered expression of tenascin‐C, decorin and procollagen I following treatment. Collectively, this research identifies new components within the scleral mesenchyme that can affect development of the scleral ossicle system and that could possibly help us understand why the sclera of some vertebrates does not support ossicle development.
Materials and methods
Materials
Fertilized chicken eggs were purchased from the Dalhousie University Agricultural Campus (Truro, Nova Scotia, Canada). Eggs were incubated at 37 °C with 40% humidity in a Brinsea Ova‐Easy Advance incubator. Eggs were turned twice daily until treatment.
HC treatment
Prior to injections, two to three embryos from each batch of eggs were staged following Hamburger & Hamilton (1951). The HC injection protocol was modified from Johnson (1973). In this latter study, 0.2 mg mL−1 HC was injected twice, once at the end of the sixth day and once at the end of the seventh day of incubation; however, HH stages were not reported. Therefore, we first needed to repeat these results. We used three major treatment groups for all experiments: HC injections, control injections (CI) and controls without injections (CNI). CI embryos were injected with 3% ethanol in Howard's Ringer solution at the same time point(s) as the corresponding HC‐treated embryos. A total volume of 0.1 mL of either control or injection solution was injected directly into the air sac on one end of the egg using a P100 pipette after removal of about 6 mL of albumin with a 19‐gauge needle and 10‐mL syringe.
In order to optimize the HC treatment, different combinations of injection timings at HH 29 (6.0–6.5 days) and/or at HH 30 (6.5–7.0 days) were conducted using two different HC concentrations (summarized in Fig. 1E). Briefly, eggs were injected either once or twice (on the same day or on consecutive stages) with a low dose (0.2 mg mL−1, similar to Johnson, 1973) or with a high dose (0.4 mg mL−1) of HC (Sigma H4001) made up in 3% ethanol in Howard's Ringer solution. For CI and CNI, 6–14 embryos were examined for each control corresponding to each of the HC treatments. In order to determine the HC dose with the greatest effect, embryos were raised until HH 37 and then stained with alkaline phosphatase (AP; described below). Both left and right eyes were examined. The optimum HC treatment was determined as the dose and injection time that resulted in the most significant effect on scleral condensation morphology and number (described below). This was the high dose delivered at HH 29 and again at HH 30.
Figure 1.
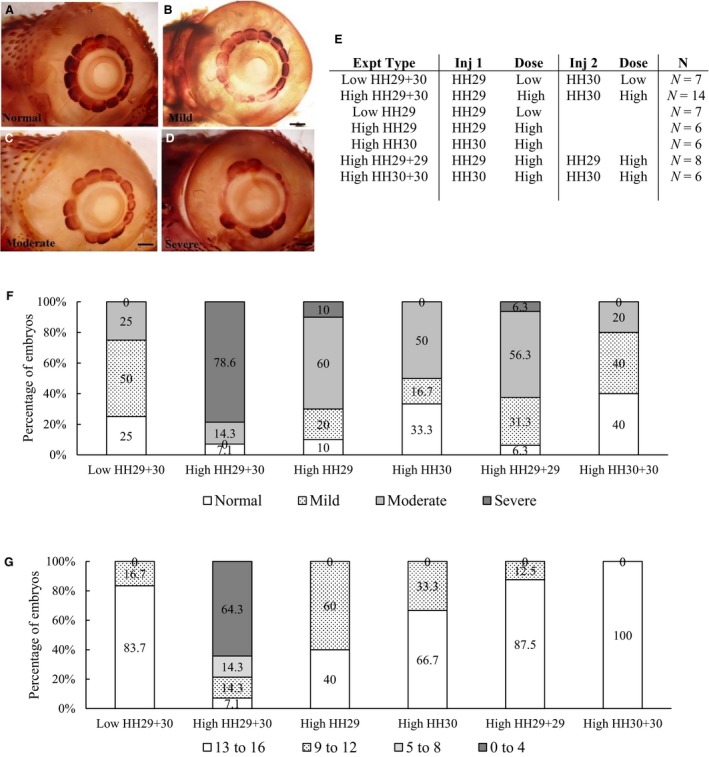
The effect of hydrocortisone (HC) injections at Hamburger and Hamilton (HH) 29 and HH 30 on scleral condensations. (A–D) Examples of the categories of morphological defects used to classify the scleral condensations of HH 37 embryos injected with a high dose of HC. All embryos are stained for alkaline phosphatase (AP); images are oriented with the beak to the right. (A) Normal embryos have no defects in scleral condensation morphology. (B) Mild defects of blurring (indistinct) condensation borders. (C) Moderate defects with abnormal condensation sizes and a few gaps in the ring. (D) Severe defects with large gaps in the condensation ring. Scale bars: 1 mm. (E) Summary of HC injection time points, doses and numbers of embryos used to determine the optimum dosage for maximum effect. Control no injection (CNI) and control injections (CI) were conducted for each HC treatment (n = 6–14 embryos per control group). These embryos were raised to HH 37 to evaluate the effect on scleral condensation development. (F,G) Graphs showing the percentage of embryos injected with different HC treatments, and the effects on the morphology of scleral condensations (F) and on the number of scleral condensations (G).
Alkaline phosphatase (AP) staining
Embryos were fixed at HH 37 in 4% paraformaldehyde (PFA) in 1 × phosphate‐buffered saline (pH 7.4) at 4 °C overnight. Embryo heads were then stained using a standard AP staining protocol (Jabalee & Franz‐Odendaal, 2015) in order to visualize the scleral condensations. Briefly, tissue samples were transferred to tris‐maleate buffer (pH 8.3) for 1 h, and then incubated in an AP substrate solution for 1 h in the dark. Saturated sodium borate water was used to wash the samples and stop the reaction. Eye pigment was bleached overnight. Finally, embryos were processed through a graded series of glycerol with 1% potassium hydroxide and stored in 80% glycerol at 4 °C.
Scleral condensation morphology and number
Following AP staining, the morphology of the scleral condensations was qualitatively assessed using four categories: normal; mild; moderate; and severe (Fig. 1A–D, modified from Jabalee & Franz‐Odendaal, 2015). Normal morphology reflects the typical staining pattern of CNI eyes, in which each scleral condensation is clearly visible without any shape abnormalities. Mild defects in morphology included one or two of the following: a small gap in the ring of scleral condensations; abnormal blurring (indistinct edges) of less than three adjacent condensations; and/or one condensation with an atypical size, shape or staining. Moderate defects included one or two of the following: more than three mild defects; missing one or two condensations; or abnormal blurring of more than three adjacent condensations. Severe defects included more than two moderate defects or a complete absence of scleral condensations. The percentage of normal, mild, moderate, severe eyes was determined for each HC treatment and each control group (i.e. CNI and CI).
The number of scleral condensations in each eye was also quantified following AP staining. Due to some asymmetry of the sclerotic rings between the left and right eye (Franz‐Odendaal, 2008a,b), the data were quantified by eye rather than by embryos. Samples were placed into one of four groups reflecting the numbers of osteogenic condensations per eye as follows: 13–16 condensations (i.e. the normal range); 9–12 condensations; 5–8 condensations; and 0–4 condensations. The percentage of eyes in each size category was determined for each HC treatment and control group. These data were then plotted in Microsoft Excel. Outliers were identified using Dixon's Q‐test with a 95% confidence interval. Three embryos, each from a different HC treatment group, were considered as outliers and were removed from subsequent analyses. minitab (version 16) was used to conduct two‐tailed t‐tests with a 95% confidence interval to compare numbers of ossicles and/or papillae in control vs. treated groups.
Based on this assessment of AP‐stained scleral condensations at HH 37, the high‐dose HC with injections at HH 29 and again at HH 30 had the greatest effect on scleral condensation development compared with the other treatments. This dosage was therefore considered as optimum and was used for all further analyses described below.
Conjunctival papillae assessment
In order to determine the effect of HC treatment earlier in development, specifically at the stages corresponding to conjunctival papillae development, embryos were treated and then incubated to HH 34, the stage at which the entire ring of papillae is present. The number of embryos examined is given in Table 1. Embryos were fixed overnight in 4% PFA at 4 °C. After fixation, eyelids were removed and the conjunctival papillae were counted under a Nikon SMZ 1000 dissecting microscope.
Table 1.
Samples used in papillae, tissue and vasculature analyses after a high dose of HC administered at HH 29 and again at HH 30.
| Injection type | Papillae analyses (at HH 34) | Vasculature analyses (at HH 36) | Histological analyses (at HH 37) |
|---|---|---|---|
| CNI | N = 6 | N = 8 | N = 6 |
| CI | N = 6 | N = 7 | N = 6 |
| HC treatment | N = 18 | N = 8 | N = 7 |
CI, control injection; CNI, control no injection; HC, hydrocortisone; HH, Hamburger and Hamilton; N, number of embryos.
Highlighter ink vasculature injections
In order to determine the effect of HC treatment on vasculature development, embryos were incubated to HH 36, a stage at which the scleral vasculature network is well established (Jabalee & Franz‐Odendaal, 2015). Prior to vascular injections, eggs were windowed at 2–3 dpf. Embryos were treated with 40 μL of penicillin streptomycin (5000 units penicillin streptomycin, Sigma: P4458) to prevent infection. In order to visualize scleral vasculature, highlighter ink (from Pilot Spotlighters) was injected directly into the vitelline artery of the chorioallantoic membrane prior to fixation (following Jabalee & Franz‐Odendaal, 2015) using a microcapillary tube (OD 1.5 mm, ID 0.86 mm; Sutter Instrument BF150‐86‐10). After ink injection, the embryo was returned to the incubator for 10 min and then fixed overnight in 4% PFA at 4 °C. Sample numbers are given in Table 1. Vasculature was visualized using a Nikon fluorescent SMX 1500 microscope with a Nikon Intensilight C‐HGFI illuminator under a GFP filter. Photographs were taken with a Nikon camera and the nis elements software package. imagej software was used to quantify the vasculature.
Histological analyses
Histological analyses were performed at HH 37 following HC treatment (Table 1). The dorsal group of scleral condensations was selected for sectioning because the conjunctival papillae have not yet completely degenerated in this region at HH 37. Dissected tissue was embedded in low‐melting point paraffin wax and sectioned at 6 μm thickness using a Leitz 1512 microtome. Every 10th–12th section was stained with Hall and Brunt's Quadruple stain (adapted from Hall, 1986). Cartilage stains blue, nuclei black, and calcified bone red with this stain. Uncalcified connective tissue stains turquoise in colour. All stained slides were observed and used to formulate a representative description of the histological tissue. Slides were observed using a Nikon Eclipse 50i compound microscope. Photographs were taken with a Nikon camera and the nis elements Software package.
Immunohistochemistry (IHC)
In order to investigate the ECM components of the sclera, IHC was used to examine the expression of tenascin‐C, decorin and procollagen I in eye tissue sections. The monoclonal antibodies for chick tenascin‐C (M1‐B4), decorin (CB‐1) and procollagen‐I (SP1.D8) were developed by Fambrough, D.M., Carrino, D.A. and Furthmayr, H., respectively. All primary antibodies were obtained as supernatants from the Developmental Studies Hybridoma Bank, created by the National Institute for Child Health and Human Development of the National Institute of Health, and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242, USA.
In control embryos, approximately 75–120 consecutive sections (approximately 450–720 μm) can be made through a single scleral condensation. Most HC‐treated embryos lacked distinct scleral condensations (five out of six embryos), as such representative sections in an equivalent eye region to the control embryos was used.
The IHC protocol used to determine the distribution of tenascin‐C, decorin and procollagen I was adapted from Franz‐Odendaal (2008a) and Delorme et al. (2012). Briefly, after dewaxing the sections, sections were bleached for 20 min with 3% hydrogen peroxide in a humidity chamber. A 0.1% sodium citrate buffer was used for antigen retrieval. After a serum blocking step, primary antibody was applied to the appropriate slides (1 : 2 dilution for tenascin‐C, decorin and procollagen I). The secondary antibody used was rabbit anti‐mouse‐horseradish peroxidase (HRP; 1 : 200). Two types of negative control experiments were conducted: one type that included experiments without the addition of the primary antibody; and a second experiment type that omitted only the secondary antibody. Detection with 3, 3′‐diaminobenzidene (DAB) peroxidase substrate (DAB tablets, Sigma D0426) was used to visualize the protein expression. Sections were observed using a Nikon Eclipse 50i compound microscope. Photographs were taken with a Nikon camera and the nis elements Software package.
Results
High HC dosages reduce scleral condensation number and alter condensation morphology
The scleral condensations of CNI embryos are relatively large and trapezoidal in shape at HH 37 (Fig. 1A, n = 11). CNI embryos had 14 or 15 normal scleral condensations per eye. Overall, CI embryos (from all treatment groups) had predominately normal scleral condensation morphology (78.6–91.7%), with the remainder having mild scleral condensation defects. Moderate and severe morphological defects were never observed in CNI or CI embryos. The CI embryos had 13–16 scleral condensations per eye, which is within the normal range for this species (Franz‐Odendaal, 2008a,b). Asymmetry between the left and right eye of up to two condensations was observed in less than 36.4% of control embryos. Franz‐Odendaal (2008a) reported that a difference of one ossicle is somewhat common between the left and right eye (42% of cases, n = 35), a difference of two ossicles can occur but is rare (0.03% of cases, n = 35).
In embryos that were injected with low‐dose HC at HH 29 and HH 30, moderate to severe morphological defects were observed in 25% of embryos (Fig. 1F). Most eyes (83.7%) had a normal condensation number (13–16 condensations per eye); however, 16.7% had slightly fewer condensations (11 or 12 condensations per eye; Fig. 1G). In embryos that were injected with a single low‐dose HC at HH 29 only, all eyes have a normal shape, size and arrangement of scleral condensations (data not shown).
In order to determine whether a higher concentration of HC would have a greater effect on scleral condensation development, embryos were injected with a HC dose two times higher than in the previous experiment, at HH 29 and again at HH 30. Moderate to severe morphological defects were observed in 92.9% of the eyes (Fig. 1F). Only two embryos had the normal scleral condensation morphology, no embryos had mild defects. The normal range of numbers of scleral condensations per eye (13–16 per eye) was observed in only 7.1% of embryos, while 64.3% of embryos had less than four scleral condensations per eye (Fig. 1G). Strikingly, 17.9% of embryos completely lacked scleral condensations after this treatment. These data show that the high dose of HC can severely affect scleral condensation formation, and to a much greater extent than the low‐dose HC treatment.
Scleral condensation number after high‐dose HC treatment averaged 4.5 ± 4.6 scleral condensations per eye. This result is in stark contrast to the comparative CNI and CI groups (14.4 ± 0.5 and 14.4 ± 0.6 condensations per eye, respectively). Condensation number in HC‐treated embryos differed significantly from CNI embryos (t‐test; t = 11.39; P < 0.001; df = 27). Condensation number also differed significantly between HC‐treated embryos and CI embryos (t‐test; t = 11.37; P < 0.001; df = 28). Unsurprisingly, CNI and CI embryos showed no statistical difference in condensation number (t‐test; t = −0.39; P = 0.699; df = 27). Interestingly, 71.4% of the affected embryos develop at least one scleral condensation in the temporal region of the eye, above the ciliary artery. This corresponds to the first condensation to form during normal scleral condensation development. Overall, these results show that a high HC dose delivered at HH 29 and HH 30 results in a significant reduction in scleral condensation number and morphology in the vast majority of embryos.
We next wanted to determine whether it was the increased concentration of HC that caused these results or whether timing of the injections was more important. For example, could the same dose given twice at one stage produce the same results? To address this, we injected embryos with a high dose at HH 29 once, with a high dose at HH 29 twice, with a high dose at HH 30 once and with a high dose at HH 30 twice (Fig. 1E–G). In embryos injected with a high dose at HH 29 (or at HH 30) once, 50–60% of the eyes had moderate defects and 0–10% had severe defects. Condensation numbers ranged 10–15 per eye. These data indicate that this dosage can affect scleral condensation formation. Double injections at one stage (i.e. high dose given twice at HH 29 or twice at HH 30) resulted in 20–62.6% of eyes with moderate to severe defects and 87.5–100% of eyes with normal condensation counts; the double injections at HH 29 had a slightly greater effect than at HH 30 (Fig. 1F,G). These results confirm that these combinations of injection time and dose have a less dramatic effect on condensation development than a high HC dose given once at HH 29 and again at HH 30. We thus conclude that the high dose (i.e. 0.4 mg mL−1 HC) administered at consecutive stage (HH 29 and again at HH 30) is an optimum treatment for disrupting the chick scleral ossicle system.
HC treatment affects conjunctival papillae number less than scleral condensation number
In order to gain further insights into how HC treatment affects the scleral ossicle development, conjunctival papillae number was assessed at HH 34, after HC treatment (high HC at HH 29 and HH 30; Fig. 2). CNI embryos have an average of 14.1 ± 0.5 conjunctival papillae per eye (n = 6 embryos), whereas CI embryos have a similar average of 13.6 ± 0.5 papillae per eye (n = 6 embryos). These papillae are arranged in a complete ring around the cornea (Fig. 2A). The difference in conjunctival papillae number between CNI and CI embryos was statistically significant (t‐test: t = 2.21; P = 0.037; df = 23); however, both are within the normal numbers of papillae reported for this species (Franz‐Odendaal, 2008a).
Figure 2.
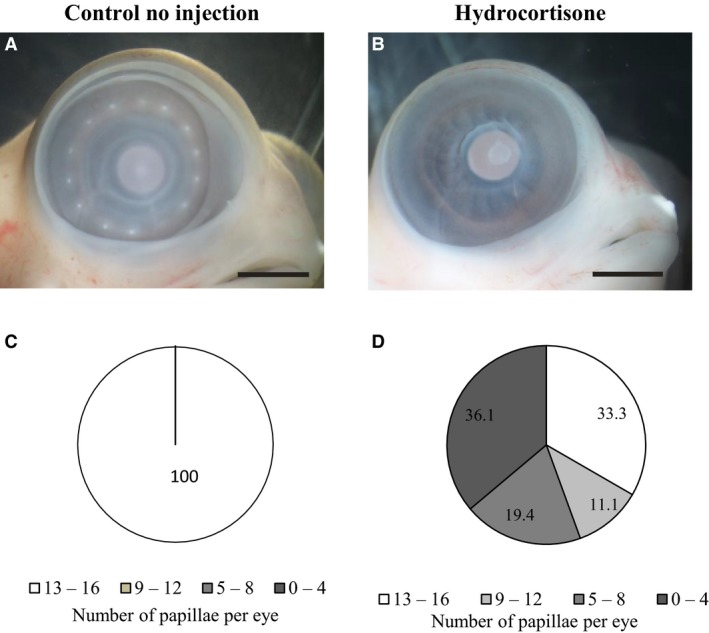
The effect of hydrocortisone (HC) treatment [high HC dose at Hamburger and Hamilton (HH) 29 and HH 30] on conjunctival papillae number at HH 34. (A) A control no injection (CNI) embryo with a normal ring of papillae. (B) An HC‐injected embryo in which no conjunctival papillae developed. In this particular embryo, the eyelids are also severely reduced. Scale bars: 2 mm. (C,D) Percentages of eyes at HH 34 that have 0–4, 5–8, 9–12 or 13–16 papillae per eye in CNI (C) embryos or after high HC treatment at HH 29 and HH 30 (D).
Hydrocortisone‐treated embryos had on average 7.7 ± 5.4 papillae per eye (n = 36 eyes); 36.1% of eyes had less than four conjunctival papillae per eye. Two of these embryos were completely missing the entire ring of conjunctival papillae (Fig. 2B). In this analysis, 19.4% of the eyes had 5–8 conjunctival papillae and 11.1% had 9–12 conjunctival papillae. Thus, there was a wide range of papillae numbers per eye after HC treatment (Fig. 2C). Embryos injected with HC had a significant reduction in papillae number when compared with both CNI embryos (t‐test: t = 7.03; P < 0.001; df = 36) and CI embryos (t‐test: t = 6.56; P < 0.001; df = 36). These results collectively show that HC treatment has a significant effect on papillae development at HH 34.
We next compared the effect of HC treatment on papillae number (at HH 34) with the effect on condensation number (at HH 37). Our results show HC‐treated embryos have more papillae on average per eye (7.7 ± 5.4; n = 36 eyes) than the average number of condensations at HH 37 (4.5 ± 4.6; n = 28 eyes); 33.3% of the treated eyes had a normal papillae number after HC treatment compared with only 7.1% of eyes with normal scleral condensation number. At the other end of the spectrum, 36.1% of treated eyes had less than four papillae, which is in stark contrast to 64.3% of treated eyes with less than four scleral condensations. These results were unexpected considering the 1 : 1 ratio between papillae and ossicles during development (Coulombre et al. 1962; Hall, 1981; Franz‐Odendaal, 2008a). These data indicate that HC treatment affects other component(s) in the sclera during later development. In order to gain some insights into what these might be, we investigated the scleral vasculature, tissue histology and three ECM components after HC treatment.
HC treatment reduces scleral vasculature
In order to determine whether vasculature is disrupted following HC injections, vasculature was visualized using highlighter ink injected directly into the blood vessels at HH 36 prior to fixation. This stage was selected because previous research shows vasculature is well established with distinct avascular zones located beneath the conjunctival papillae (Jabalee & Franz‐Odendaal, 2015). No morphological differences in vasculature were observed between CI and CNI embryos (n = 7 and n = 8, respectively) at HH 36; a normal vascular network was observed (Fig. 3A,C). In 75% of HC‐treated embryos, there was a noticeable reduction in vasculature compared with controls (Fig. 3B,D); these embryos also lacked several conjunctival papillae. The remaining HC‐treated eyes had either a normal number of papillae and normal vasculature (n = 2), or had an unusual vasculature network that was aggregated into clumps within the sclera (n = 2; data not shown).
Figure 3.
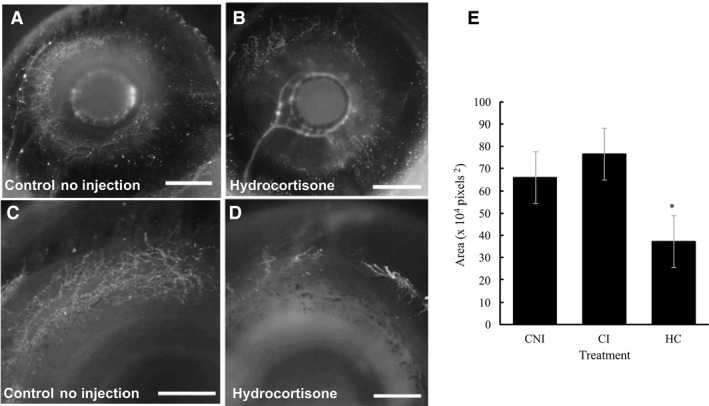
Scleral vasculature at Hamburger and Hamilton (HH) 36 following hydrocortisone (HC) injection (high dose) compared with control embryos. Fluorescent images were converted to greyscale. (A and B) show the entire eye, while (C and D) show a close up of the dorsal group of condensations. (A, C) Control no injection (CNI) eyes; (B, D) reduced scleral vasculature following HC injection. (E) The average area of vasculature (divided by 104, in pixels2) for HC‐treated eyes compared with controls (CI). The sample numbers are CNI (n = 16), CI (n = 14) and HC (n = 16). The asterisk indicates that statistical significance between HC‐treated eyes and each of the control groups was found using two‐tailed t‐tests. Scale bars: 2 mm (A, B); 1 mm (C, D).
To gain further insight into these differences, the scleral vasculature was quantified (Fig. 3E). There was no statistical difference between the amount of vasculature in CNI and CI eyes (t‐test: t = −1.61; P = 0.119; df = 27). However, statistical differences were detected when comparing HC‐treated embryos with CNI embryos (t‐test: t = 4.21; P < 0.001; df = 29) or with CI embryos (t‐test: t = 5.77; P < 0.001; df = 27). Collectively, these results show that high HC treatment at HH 29 + 30 affects the development of the scleral vasculature network at HH 36.
Denser scleral connective tissue after HC treatment
In order to determine whether thickening of the sclera prevented scleral condensation formation, histological analyses were conducted. At HH 37, there were no notable histological differences between the CI and CNI eyes (Fig. 4A,B). The conjunctival epithelium is generally 1–2 cell layers wide, and is thicker at the site of the degenerating conjunctival papillae. Mesenchymal cells aggregate directly beneath the conjunctival papillae in the superficial mesenchyme. The scleral condensations form beneath this zone, and have begun to deposit osteoid at HH 37 (Fig. 4B). Underneath the condensations, there is a region of denser connective tissue that eventually connects with the scleral cartilage. In all HC samples lacking condensations, the deep dense scleral mesenchyme was wider than in controls (Fig. 4C). The border between the presumptive condensation region and the underlying dense connective tissue in HC‐treated tissue is not distinct as in controls. Overall, these histological data indicate that the scleral mesenchyme is more densely arranged in HC‐treated tissue than in control tissue; this is similar to the effect noted in the skin after HC treatment (Démarchez et al. 1984).
Figure 4.
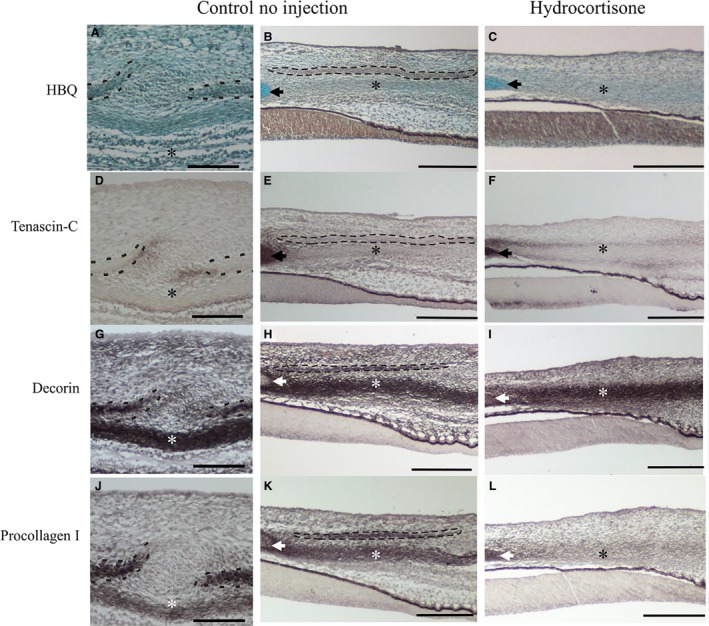
The effect of hydrocortisone (HC) injections on scleral extracellular matrix (ECM) composition, Hamburger and Hamilton (HH) 37. (A–C) stained with Hall and Brunt's Quadruple stain to visualize the histology; (D–F) tenascin‐c expression; (G–I) decorin expression; (J–L) procollagen 1 expression. Control no injection (CNI; A, B; D, E; G, H; J, K) showing the inter‐ossicle and ossicle regions; HC‐treated results (C, F, I, L) are shown. Scleral condensations are outlined with a black dashed line. The scleral cartilage is indicated by an arrowhead. The dense connective tissue that underlies the condensations in control embryos is marked with an asterisk. Scale bars: 200 μm.
HC treatment alters the scleral ECM
Hydrocortisone injections can alter the ECM composition and arrangement in a variety of tissues, including skin, bone and tendons (Schäcke et al. 2002; Hengge et al. 2006; Dean et al. 2014). Therefore, it was hypothesized that an ECM disruption following HC treatment might affect scleral condensation development. Three ECM components were assessed in this study; tenascin‐C, decorin and procollagen I. Tenascin‐C has previously been described in association with the scleral ossicle system (Fyfe et al. 1988). Procollagen I is the precursor of collagen I and the primary constituent of bone, therefore collagen I is likely to be present within the developing scleral ossicles (Peltonen et al. 1985; Mackie et al. 1987; Canty‐Laird et al. 2012). Decorin is closely associated with collagen I as it is involved in organizing collagen fibrils (Bianco et al. 1990; Iozzo et al. 1999; Zagris et al. 2011).
We observed some spatial variation in tenascin‐C expression at HH 37. In CNI and CI eye tissue (n = 6 embryos per group), tenascin‐C was most often present surrounding the scleral condensations, particularly in the regions of overlap with adjacent condensations (Fig. 4D). In some sections, tenascin‐C was observed within the condensations themselves or within the superficial mesenchyme directly beneath a degenerating papilla, extending down towards the condensation. Tenascin‐C was also abundant within the scleral cartilage (Fig. 4E). Overall, there were no obvious morphological differences between CI and CNI samples with regards to tenascin‐C expression. Following HC treatment, tenascin‐C was somewhat reduced throughout the scleral tissue compared with controls, except surrounding the dense scleral mesenchyme where it was more abundant (compare Fig. 4E and F). No primary antibody and no secondary antibody IHC control results show no expression (not shown).
Decorin is expressed strongly throughout the scleral mesenchyme in all CNI and CI samples (n = 6 embryos each; Fig. 4G,H). It is abundant within the scleral condensation, within the scleral cartilage, and in the deep dense connective tissue beneath the condensations. In sections containing degenerating conjunctival papillae, decorin was expressed strongly in the superficial mesenchyme directly beneath the papilla (not shown). Overall, no morphological difference in decorin expression was detected between CI and CNI embryos. In the HC‐treated embryos, decorin was present in a similar region to the controls (except for the scleral condensation region, which was not present after treatment; Fig. 4I).
Procollagen I is expressed in the scleral condensations, the scleral cartilage and in the deeper dense scleral mesenchyme in all CNI and CI embryos (Fig. 4J,K). When a degenerating papilla was present, procollagen I was also expressed directly beneath the epithelium in this region (not shown). In the affected HC samples, procollagen I was present in the dense scleral mesenchyme, similar to controls (CNI and CI); however, it was diffuse compared with controls (Fig. 4L). Overall, these results suggest that after HC treatment, procollagen I expression is reduced throughout the mesenchyme unlike in controls.
In summary, control embryos at HH 37 have distinct expression of tenascin‐C, decorin and procollagen I in association with scleral condensation development. These ECM components are typically found between adjacent condensations, within scleral condensations (decorin and procollagen I), and within the deep scleral mesenchyme. Following HC injections, most embryos do not have scleral condensations. Thus, the ECM arrangement typically observed within or surrounding scleral condensations is absent. Procollagen 1 was less abundant in the scleral mesenchyme after treatment, whereas tenascin‐C was more abundant surrounding the deeper scleral mesenchyme but less abundant overall in the sclera; decorin showed little change. These results further our understanding of the ECM composition during normal scleral condensation development, and indicate that HC treatment does affect (at least) some ECM components of the scleral mesenchyme.
Discussion
HC has a time‐ and concentration‐dependent effect on scleral condensations
Scleral condensation development was assessed using two different HC concentrations (0.2 mg mL−1 and 0.4 mg mL−1) at HH 29 and HH 30. Embryos treated with a low‐dose HC (0.2 mg mL−1 HC at HH 29 and again at HH 30) have 11–15 condensations per eye. These results are in contrast to the previous study by Johnson (1973) that found that 0.2 mg mL−1 HC injections delivered on both the end of the sixth day (approx. HH 29) and seventh day (approx. HH 30/31) of development resulted in less than five papillae/ossicles per eye. The difference between that study and ours may be the result of slightly different treatment times (Johnson, 1973; did not report embryonic stages), slightly different incubation temperatures (Hall, 1981, a change of 0.5 °C can dramatically affect staging) or the result of animal strain differences.
We analysed the effect of dose and timing on the number and morphology of scleral ossicles after HC treatment, thus extending the study by Johnson (1973), who only examined numbers. We confirmed that the effect on scleral condensation formation of two HC injections delivered over two stages (HH 29 and HH 30) was greater than that of a single injection delivered at only one developmental stage. We also show that a double injection given on the same day at either of these stages has less of an effect than when the dose is administered over two stages. These data suggest that the effect of injected HC is short term. Indeed, the average half‐life of HC injections in humans was found to be between 1 and 2 h (Peterson & Wyngaarden, 1955; Hindmarsh & Charmandari, 2015). Therefore, injections at only HH 29 or only at HH 30 disrupt the system for a short period of time only, and the embryo is able to recover quickly to form the conjunctival papillae and scleral condensations. When two injections occur over two stages, however, the embryo does not have sufficient time to recover, preventing the formation of a complete ring of conjunctival papillae and scleral condensations.
Although the first papilla is not visible until HH 30, induction may have occurred earlier and may have occurred at HH 29, a mere 12 h earlier. We hypothesize that by HH 29, when the first injection is conducted, the induction of the first few papillae is already underway and has passed a threshold time point in development beyond which it is unaffected by treatment. We recently showed that the conjunctival papillae undergo a placodal stage of development (similar to feathers, hair and teeth) involving β‐catenin (Jourdeuil & Franz‐Odendaal, 2016). β‐Catenin is currently the only marker known to label the very early stages of conjunctival papillae development; it is not expressed at HH 29 but is expressed at HH 30. More research is thus needed to determine the developmental mechanisms operating between HH 29 and HH 30, including whether β‐catenin expression is altered following HC treatment. An alternative hypothesis is that the ECM composition of the scleral mesenchyme at HH 29 is important for supporting the development of the papilla and subsequent condensation development (discussed below). These hypotheses should be tested once we understand more about the process of papillae induction, the spatiotemporal pattern of the molecular signals involved and the ECM composition of the developing sclera.
ECM and vasculature disruption contribute to scleral condensation loss
Glucocorticoids, including HC, are known to disrupt the formation of blood vessels (Schäcke et al. 2002; Hengge et al. 2006; Boller et al. 2015), and HC treatment disrupts feather development in chicks (at 6.5 dpf; Stuart et al. 1972; Démarchez et al. 1984; El‐Shabaka, 1986; Desbiens et al. 1992; Turque et al. 1997). The effect on feather patterning is also time specific (Sengel & Züst, 1968). Démarchez et al. (1984) provided two hypotheses to explain this disruption. The first hypothesis was that HC thickens and matures the skin tissue earlier than normal, making the tissue lose competency and become unable to respond to inductive signals to form feathers (Stuart et al. 1972; Desbiens et al. 1992; Turque et al. 1997). The second hypothesis was that HC disrupts the ECM, which in turn prevents feather formation (Démarchez et al. 1984; El‐Shabaka, 1986; Turque et al. 1997). Indeed, glucocorticoids have been reported to alter ECM composition and arrangement in various connective tissues, including tendons, cartilage and bone (Delany et al. 1995; Schäcke et al. 2002; Hengge et al. 2006; Dean et al. 2014).
In the present study, tenascin‐C expression in control samples was similar to that previously reported (Fyfe et al. 1988; Kaplony et al. 1991). Tenascin‐C is known to play a role in the boundary formation of mesenchymal condensations (reviewed in Hall & Miyake, 2000), and was observed at the edges of the developing scleral condensation. Altered tenascin‐C expression may play a role in the observed malformed scleral condensations following HC treatment; however, more research is needed to fully evaluate this finding.
Decorin is a small chondroitin sulphate proteoglycan (Bianco et al. 1990; Zagris et al. 2011) that plays a role in cellular proliferation (Iozzo et al. 1999) and can alter angiogenesis (Buraschi et al. 2013; Järveläinen et al. 2015). Decorin binds to several angiogenetic factors, such as VEGF receptor 2, and can thereby reduce the formation of vasculature (Buraschi et al. 2013). Decorin is also known to modify collagen fibrillogenesis, and bind with collagen I (Bianco et al. 1990; Iozzo et al. 1999; Zagris et al. 2011). As such, decorin is typically present in connective tissues rich in collagen I, including bones, tendons and dermis of the skin (Bianco et al. 1990). Therefore, it is possible that the disruption of decorin within the sclera following HC treatment is partially responsible for the observed vasculature defects.
Although the factors regulating the size and location of the avascular zones beneath conjunctival papillae are largely unknown, Jabalee & Franz‐Odendaal (2015) proposed they could partially be due to the ECM molecule thrombospondin‐4, which Tucker et al. (1995) reported at HH 36 in scleral condensations. Although other thrombospondins are known to regulate angiogenesis, the role of thrombospondin‐4 has not yet been determined (Bornstein, 2009; Adams & Lawler, 2011). Interestingly, Jabalee & Franz‐Odendaal (2014) showed that the vasculature recovers quickly after VEGF‐A knockdown yet still alters scleral ossicle morphology, whereas here we show that HC treatment has a longer sustained effect on the vasculature that significantly affects the number of scleral ossicles that develop. Future studies are needed to tease apart the potential cause and effect relationship between the ECM and vasculature defects observed in the chick sclera following HC treatment.
Procollagen I is the precursor for collagen I, which is the main extracellular component of bone (Peltonen et al. 1985; Mackie et al. 1987; Canty‐Laird et al. 2012). Glucocorticoids, such as HC, can reduce osteoblast proliferation, increase apoptosis of osteoblasts, and increase osteoclast activity (Weinstein et al. 1998; Silvestrini et al. 2000; Canalis & Delany, 2002; Schäcke et al. 2002; O'Brien et al. 2004; Shipunova et al. 2013). In this study, HC treatment resulted in reduced procollagen I expression in the sclera, which may have been insufficient to support osteoblast differentiation.
Our results show that both thickening of the scleral mesenchymal tissue and ECM disruption in the scleral mesenchyme follows HC treatment. These changes in the sclera could thus affect the later development of the scleral ossicles. A proposed mechanism for HC‐induced ECM disruption is through altering matrix metalloproteinase (MMP) expression and activity (Hillegass et al. 2007, 2008). Weidenfeller et al. (2005) stated that glucocorticoids can increase levels of MMPs in a tissue. Indeed, administration of HC in zebrafish has resulted in an increase in expression and activity of MMPs 2, 9 and 13, which together cleave collagens I–IV (Hillegass et al. 2007, 2008). Altered MMP expression following HC could also explain the effect on scleral vasculature as MMPs are essential for regulating angiogenesis (Haas et al. 2000; Stickens et al. 2004). Various fragments of ECM molecules have anti‐angiogeneic effects, which likely increase in abundance following ECM degradation caused by MMP upregulation (Neve et al. 2014). Thus, upregulation of MMPs could decrease angiogenesis, and in turn play a role in reducing scleral vasculature following HC treatment. HC treatment is known to particularly increase MMP13 in humans and zebrafish, and is known to degrade interstitial collagens in tissue (for review, see Hillegass et al. 2008; Buckley & Jessen, 2015; Small & Crawford, 2016). A similar mechanism may be present in the chick sclera following HC treatment, resulting in an increase in collagen degradation. MMP13 is present in the chick embryo, although little research has focused on the role of MMPs in the developing chick sclera (Lei et al. 1999; Schippert et al. 2006).
Conclusions
Our data extend previous work conducted in the 1970s to show that HC not only has a dose‐ and timing‐dependent effect on the development of the conjuctival papillae, but that it also has an effect on scleral tissue composition (cellular arrangements, ECM and vasculature). Previous studies investigating the loss of scleral ossicles have successfully disrupted only one or two scleral ossicles (Coulombre et al. 1962; Franz‐Odendaal, 2008a; Duench & Franz‐Odendaal, 2012; Jabalee & Franz‐Odendaal, 2015). Here we show that HC treatment can cause a global and total disruption of the scleral ossicles, and that this occurs via a combination of effects on the conjunctival papillae, the ECM components and the scleral vasculature. These effects may be direct or indirect. Importantly, HC reduces conjunctival papilla number to a lesser degree than it reduces scleral condensation number. Scleral vasculature was reduced following HC treatment, indicating that HC may have an anti‐angiogenic effect that contributes to the subsequent disruption of scleral ossicle development. The effect on osteoblasts, osteoclasts, MMP13 and other ECM components, if any, still needs to be investigated. This research emphasizes the importance of the ECM and vasculature components of the sclera in intramembranous bone development within the eye.
Author contributions
TFO designed the study and CH conducted the experiments. Both TFO and CH wrote and edited the manuscript. Both authors approved the final article.
Acknowledgements
The authors would like to thank the Developmental Study Hybridoma Bank for the primary antibodies used in this study. Additionally, CH thanks her thesis committee for their input. The authors would like to acknowledge all members of the Franz‐Odendaal Bone Development Lab for their support. This research was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC Discovery Grant #328376 to TFO) and a NSERC graduate scholarship to CH.
References
- Adams JC, Lawler J (2011) The thrombospondins. Cold Spring Harb Perspect Biol 3, a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins JB, Franz‐Odendaal TA (2016) The sclerotic ring of squamates: an evo‐devo‐eco perspective. J Anat 229, 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P, Fisher LW, Young MF, et al. (1990) Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non‐skeletal tissues. J Histochem Cytochem 38, 1549–1563. [DOI] [PubMed] [Google Scholar]
- Boller C, Prado M, de Toledo M, et al. (2015) The anti‐angiogenic effect of Chamomila recutita aqueous extract determined using a modified chicken chorioallantoic membrane ex ovo assay. Int J Curr Microbiol Appl Sci 4, 231–243. [Google Scholar]
- Bornstein SR (2009) Predisposing factors for adrenal insufficiency. N Engl J Med 360, 2328–2339. [DOI] [PubMed] [Google Scholar]
- Buckley JJ, Jessen JR (2015) Matrix metalloproteinase function in non‐mammalian model organisms. Front Biosci (Scholar Edition) 7, 168–183. [DOI] [PubMed] [Google Scholar]
- Buraschi S, Neill T, Goyal A, et al. (2013) Decorin causes autophagy in endothelial cells via Peg3. Proc Natl Acad Sci 110, E2582–E2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canty‐Laird EG, Lu Y, Kadler KE (2012) Stepwise proteolytic activation of type I procollagen to collagen within the secretory pathway of tendon fibroblasts in situ . Biochem J 441, 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canalis E, Delany AM (2002) Mechanisms of glucocorticoid action on bone. Annals of the New York Academy of Sciences 966, 73–81. [DOI] [PubMed] [Google Scholar]
- Coulombre AJ, Coulombre JL, Mehta H (1962) The skeleton of the eye: I. Conjunctival papillae and scleral ossicles. Dev Biol 5, 382–401. [DOI] [PubMed] [Google Scholar]
- Dean BJF, Lostis E, Oakley T, et al. (2014) The risks and benefits of glucocorticoid treatment for tendinopathy: a systematic review of the effects of local glucocorticoid on tendon. Semin Arthritis Rheum 43, 570–576. [DOI] [PubMed] [Google Scholar]
- Delany AM, Gabbitas BY, Canalis E (1995) Cortisol downregulates osteoblast α1 (I) procollagen mRNA by transcriptional and posttranscriptional mechanisms. J Cell Biochem 57, 488–494. [DOI] [PubMed] [Google Scholar]
- Delorme SL, Lungu IM, Vickaryous MK (2012) Scar‐free wound healing and regeneration following tail loss in the leopard gecko, Eublepharis macularius . Anat Rec 295, 1575–1595. [DOI] [PubMed] [Google Scholar]
- Démarchez M, Mauger A, Herbage D, et al. (1984) Effect of hydrocortisone on skin development in the chick embryo: ultrastructural, immunohistological, and biochemical analysis. Dev Biol 106, 15–25. [DOI] [PubMed] [Google Scholar]
- Desbiens X, Turque N, Vandenbunder B (1992) Hydrocortisone perturbs the cell proliferation pattern during feather morphogenesis: evidence for disturbance of cephalocaudal orientation. Int J Dev Biol 36, 373–380. [PubMed] [Google Scholar]
- Duench K, Franz‐Odendaal TA (2012) BMP and Hedgehog signaling during the development of scleral ossicles. Dev Biol 365, 251–258. [DOI] [PubMed] [Google Scholar]
- El‐Shabaka HA (1986) Effect of hydrocortisone on the skin of the developing chick embryo. Qatar Univ Sci Bull 1986, 181–197. [Google Scholar]
- Franz‐Odendaal TA (2008a) Toward understanding the development of scleral ossicles in the chicken, Gallus gallus . Dev Dyn 237, 3240–3251. [DOI] [PubMed] [Google Scholar]
- Franz‐Odendaal TA (2011) The ocular skeleton through the eye of evo‐devo. J Exp Zool B Mol Dev Evol 316, 393–401. [DOI] [PubMed] [Google Scholar]
- Franz‐Odendaal TA, Hall BK (2006) Skeletal elements within teleost eyes and a discussion of their homology. J Morphol 267, 1326–1337. [DOI] [PubMed] [Google Scholar]
- Franz‐Odendaal TA, Vickaryous MK (2006) Skeletal elements in the vertebrate eye and adnexa: morphological and developmental perspectives. Dev Dyn 235, 1244–1255. [DOI] [PubMed] [Google Scholar]
- Fyfe DM, Ferguson MW, Chiquet‐Ehrismann R (1988) Immunocytochemical localisation of tenascin during the development of scleral papillae and scleral ossicles in the embryonic chick. J Anat 159, 117. [PMC free article] [PubMed] [Google Scholar]
- Haas TL, Milkiewicz M, Davis SJ, et al. (2000) Matrix metalloproteinase activity is required for activity‐induced angiogenesis in rat skeletal muscle. Am J Physiol Heart Circ Physiol 279, 1540–1547. [DOI] [PubMed] [Google Scholar]
- Hall BK (1981) Specificity in the differentiation and morphogenesis of neural crest‐derived scleral ossicles and of epithelial scleral papillae in the eye of the embryonic chick. J Embryol Exp Morphol 66, 175–190. [PubMed] [Google Scholar]
- Hall BK (1986) The role of movement and tissue interactions in the development and growth of bone and secondary cartilage in the clavicle of the embryonic chick. J Embryol Exp Morphol 93, 133–152. [PubMed] [Google Scholar]
- Hall BK (2005) Consideration of the neural crest and its skeletal derivatives in the context of novelty/innovation. J Exp Zool B Mol Dev Evol 304, 548–557. [DOI] [PubMed] [Google Scholar]
- Hall BK, Miyake T (2000) All for one and one for all: condensations and the initiation of skeletal development. BioEssays 22, 138–147. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88(1), 49–92. [PubMed] [Google Scholar]
- Hengge UR, Ruzicka T, Schwartz RA, et al. (2006) Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol 54, 1–15. [DOI] [PubMed] [Google Scholar]
- Hillegass JM, Villano CM, Cooper KR, et al. (2007) Matrix metalloproteinase‐13 is required for zebra fish (Danio rerio) development and is a target for glucocorticoids. Toxicol Sci 100, 168–179. [DOI] [PubMed] [Google Scholar]
- Hillegass JM, Villano CM, Cooper KR, et al. (2008) Glucocorticoids alter craniofacial development and increase expression and activity of matrix metalloproteinases in developing zebrafish (Danio rerio). Toxicol Sci 102, 413–424. [DOI] [PubMed] [Google Scholar]
- Hindmarsh PC, Charmandari E (2015) Variation in absorption and half‐life of hydrocortisone influence plasma cortisol concentrations. Clin Endocrinol 82, 557–561. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Moscatello DK, McQuillan DJ, et al. (1999) Decorin is a biological ligand for the epidermal growth factor receptor. J Biol Chem 274, 4489–4492. [DOI] [PubMed] [Google Scholar]
- Jabalee J, Franz‐Odendaal TA (2015) Vascular endothelial growth factor signaling affects both angiogenesis and osteogenesis during the development of scleral ossicles. Dev Biol 406, 52–62. [DOI] [PubMed] [Google Scholar]
- Järveläinen H, Sainio A, Wight TN (2015) Pivotal role for decorin in angiogenesis. Matrix Biol 43, 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LG (1973) Development of chick embryo conjunctival papillae and scleral ossicles after hydrocortisone treatment. Dev Biol 30, 223–227. [DOI] [PubMed] [Google Scholar]
- Jourdeuil K, Franz‐Odendaal TA (2012) Vasculogenesis and the induction of skeletogenic condensations in the avian eye. Anat Rec 295, 691–698. [DOI] [PubMed] [Google Scholar]
- Jourdeuil K, Franz‐Odendaal TA (2016) Gene expression analysis during the induction and patterning of the conjunctival papillae in the chick embryonic eye. Gene Expr Patterns 22, 30–36. [DOI] [PubMed] [Google Scholar]
- Kaplony A, Zimmermann DR, Fischer RW, et al. (1991) Tenascin Mr 220,000 isoform expression correlates with corneal cell migration. Development 112, 605–614. [DOI] [PubMed] [Google Scholar]
- Lei H, Furth EE, Kalluri R, et al. (1999) Induction of matrix metalloproteinases and collagenolysis in chick embryonic membranes before hatching. Biol Reprod 60, 183–189. [DOI] [PubMed] [Google Scholar]
- Mackie EJ, Thesleff I, Chiquet‐Ehrismann R (1987) Tenascin is associated with chondrogenic and osteogenic differentiation in vivo and promotes chondrogenesis in vitro . J Cell Biol 105, 2569–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PDF (1941) Epidermal papillae and dermal bones of the chick sclerotic. Nature 148, 471. [Google Scholar]
- Nakamura K, Yamaguchi H (1991) Distribution of scleral ossicles in teleost fishes. Memoirs Fac Fish, Kagoshima University 40, 1–20. [Google Scholar]
- Neve A, Cantatore FP, Maruotti N, et al. (2014) Extracellular matrix modulates angiogenesis in physiological and pathological conditions. Biomed Res Int 2014, 756078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CA, Jia D, Plotkin LI, et al. (2004) Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology 145, 1835–1841. [DOI] [PubMed] [Google Scholar]
- Peltonen L, Halila R, Ryhänen L (1985) Enzymes converting procollagens to collagens. J Cell Biochem 28, 15–21. [DOI] [PubMed] [Google Scholar]
- Peterson RE, Wyngaarden JB (1955) The physiological disposition and metabolic fate of hydrocortisone in man. Ann N Y Acad Sci 61, 297–305. [DOI] [PubMed] [Google Scholar]
- Pinto CB, Hall BK (1991) Toward an understanding of the epithelial requirement for osteogenesis in scleral mesenchyme of the embryonic chick. J Exp Zool 259, 92–108. [DOI] [PubMed] [Google Scholar]
- Schäcke H, Döcke WD, Asadullah K (2002) Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther 96, 23–43. [DOI] [PubMed] [Google Scholar]
- Schippert R, Brand S, Schaeffel F, et al. (2006) Changes in scleral MMP2, TIMP‐2, and TGFB‐2 m RNA expression after imposed myopic and hyperopic defocus in chicken. Exp Eye Res 82, 710–719. [DOI] [PubMed] [Google Scholar]
- Sengel P, Züst B (1968) Malformations du plumage obtenues par l'injection d'hydrocortisone a l'embryon de Poulet. CR Acad Sci Ser D 267, 1304–1307. [PubMed] [Google Scholar]
- Shames RB, Jennings AG, Sawyer RH (1991) The initial expression and patterned appearance of tenascin in scutate scales is absent from the dermis of the scaleless (sc/sc) chicken. Dev Biol 147, 174–186. [DOI] [PubMed] [Google Scholar]
- Shames RB, Bade BC, Sawyer RH (1994) Role of epidermal–dermal tissue interactions in regulating tenascin expression during development of the chick scutate scale. J Exp Zool 269, 349–366. [DOI] [PubMed] [Google Scholar]
- Shipunova NN, Petinati NA, Drize NI (2013) Effect of hydrocortisone on multipotent human mesenchymal stromal cells. Bull Exp Biol Med 155, 159–163. [DOI] [PubMed] [Google Scholar]
- Silvestrini G, Ballanti P, Patacchioli FR, et al. (2000) Evaluation of apoptosis and the glucocorticoid receptor in the cartilage growth plate and metaphyseal bone cells of rats after high‐dose treatment with corticosterone. Bone 26, 33–42. [DOI] [PubMed] [Google Scholar]
- Small CD, Crawford BD (2016) Matrix metalloproteinases in neural development: a phylogenetically diverse perspective. Neural Regen Res 11, 357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickens D, Behonick DJ, Ortega N, et al. (2004) Altered endochondral bone development in matrix metalloproteinase 13‐deficient mice. Development 131(23), 5883–5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart ES, Garber B, Moscona AA (1972) An analysis of feather germ formation in the embryo and in vitro, in normal development and in skin treated with hydrocortisone. J Exp Zool 179, 97–118. [DOI] [PubMed] [Google Scholar]
- Tucker RP, Adams JC, Lawler J (1995) Thrombospondin‐4 is expressed by early osteogenic tissues in the chick embryo. Dev Dyn 203, 477–490. [DOI] [PubMed] [Google Scholar]
- Turque N, Buttice G, Beuscart A, et al. (1997) Hydrocortisone modulates the expression of c‐ets‐1 and 72 kDa type IV collagenase in chicken dermis during early feather morphogenesis. Int J Dev Biol 41, 103–109. [PubMed] [Google Scholar]
- Walls GL (1942) The Vertebrate Eye and Its Adaptive Radiation. Bloomfield Hills, MI: Cranbrook Institute of Science. [Google Scholar]
- Weidenfeller C, Schrot S, Zozulya A, et al. (2005) Murine brain capillary endothelial cells exhibit improved barrier properties under the influence of hydrocortisone. Brain Res 1053, 162–174. [DOI] [PubMed] [Google Scholar]
- Weinstein RS, Jilka RL, Parfitt AM, et al. (1998) Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest 102, 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells KL, Hadad Y, Ben‐Avraham D, et al. (2012) Genome‐wide SNP scan of pooled DNA reveals nonsense mutation in FGF20 in the scaleless line of featherless chickens. BMC Genom 13, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagris N, Gilipathi K, Soulintzi N, et al. (2011) Decorin developmental expression and function in the early avian embryo. Int J Dev Biol 55, 633. [DOI] [PubMed] [Google Scholar]


