Abstract
More frequent utilization of non‐heart‐beating donor (NHBD) organs for lung transplantation has the potential to relieve the shortage of donor organs. In particular with respect to uncontrolled NHBD, concerns exist regarding the risk of ischaemia/reperfusion (IR) injury‐related graft damage or dysfunction. Due to their immunomodulating and tissue‐remodelling properties, bone‐marrow‐derived mesenchymal stem cells (MSCs) have been suspected of playing a beneficial role regarding short‐ and long‐term survival and function of the allograft. Thus, MSC administration might represent a promising pretreatment strategy for NHBD organs. To study the initial effects of warm ischaemia and MSC application, a large animal lung transplantation model was generated, and the structural organ composition of the transplanted lungs was analysed stereologically with particular respect to the blood–gas barrier and the surfactant system. In this study, porcine lungs (n = 5/group) were analysed. Group 1 was the sham‐operated control group. In pigs of groups 2–4, cardiac arrest was induced, followed by a period of 3 h of ventilated ischaemia at room temperature. In groups 3 and 4, 50 × 106 MSCs were administered intravascularly via the pulmonary artery and endobronchially, respectively, during the last 10 min of ischaemia. The left lungs were transplanted, followed by a reperfusion period of 4 h. Then, lungs were perfusion‐fixed and processed for light and electron microscopy. Samples were analysed stereologically for IR injury‐related structural parameters, including volume densities and absolute volumes of parenchyma components, alveolar septum components, intra‐alveolar oedema, and the intracellular and intra‐alveolar surfactant pool. Additionally, the volume‐weighted mean volume of lamellar bodies (lbs) and their profile size distribution were determined. Three hours of ventilated warm ischaemia was tolerated without eliciting histological or ultrastructural signs of IR injury, as revealed by qualitative and quantitative assessment. However, warm ischaemia influenced the surfactant system. The volume‐weighted mean volume of lbs was reduced significantly (P = 0.024) in groups subjected to ischaemia (group medians of groups 2–4: 0.180–0.373 μm³) compared with the sham control group (median 0.814 μm³). This was due to a lower number of large lb profiles (size classes 5–15). In contrast, the intra‐alveolar surfactant system was not altered significantly. No significant differences were encountered comparing ischaemia alone (group 2) or ischaemia plus application of MSCs (groups 3 and 4) in this short‐term model.
Keywords: lung transplantation, mesenchymal stem cells, non‐heart‐beating donor, stereology, surfactant system
Introduction
During the last two decades, great progress has been made regarding safety as well as short‐ and long‐term results of lung transplantation. Subsequently, the number of lung transplantations has increased substantially, but a further expansion of transplantation programmes is limited by the scarcity of suitable donor organs (De Meester et al. 2001; Christie et al. 2011; Kotloff & Thabut, 2011; Yeung & Keshavjee, 2014; Yusen et al. 2015). To date, the vast majority of transplanted lungs have been retrieved from brain‐dead donors. Utilization of lungs from non‐heart‐beating donors (NHBD) conveys a great potential to increase the number of organs from the donor pool (Halpern et al. 2013). It varies greatly between centres and countries, and accounts for < 1 to 32% of lung transplantations (Cypel et al. 2015). Due to concerns regarding an increased risk of ischaemia/reperfusion (IR) injury, transplantation programmes presently include almost exclusively Maastricht category III and IV NHBD, while reports about utilization of category II donors remain rare (Kootstra et al. 1995; Gomez‐de‐Antonio et al. 2012; Cypel et al. 2015).
Ischaemia/reperfusion injury leading to primary graft dysfunction (PGD) represents the main cause of death in the early post‐transplant period (Yusen et al. 2015). Long‐term success is constrained by the development of chronic lung allograft dysfunction (CLAD), including bronchiolitis obliterans syndrome (BOS) and restrictive allograft syndrome, thus limiting survival rates to 54% at 5 years and 31% at 10 years (Sato et al. 2011; Warnecke & Haverich, 2012; Yusen et al. 2015).
Mesenchymal stem cells (MSCs) have been suspected of playing a potential role in both short‐ and long‐term viability and reorganization of the lung allograft. A main mode of action in different forms of acute lung injury was shown to be through secretion of anti‐inflammatory and reparatory paracrine factors (Zhen et al. 2010; Ionescu et al. 2012; Li et al. 2016). Furthermore, transplanted MSCs were able to integrate into the alveolar wall and differentiate into surfactant producing type II alveolar epithelial cells (Wong et al. 2007). This seems of particular significance, as impairment of the intra‐alveolar and cellular surfactant system has been identified as an important pathogenetic step in IR injury development (Mühlfeld et al. 2009; Knudsen et al. 2011, 2012). A prerequisite for potential long‐term effects of transplanted MSCs and an influence on BOS development is initial cell tolerance and the absence of adverse effects on blood–air barrier (bab) integrity.
In our study we aimed to evaluate the immediate effects of MSC application in a large animal NHBD model. MSCs were administered either by an endobronchial or pulmoarterial route. In a previous publication, deposition of MSCs was demonstrated in the respective lung compartments (Wittwer et al. 2014). The objective of this study was to identify the initial steps of lung injury in warm ischaemia with particular emphasis on the surfactant system, and to recognize a possible influence of MSCs on initial damage. Analysis was performed stereologically at light (LM) and electron microscopical (EM) levels to detect qualitative and quantitative cellular alterations.
Materials and methods
Experimental model
In a porcine NHBD model for lung transplantation, 20 donor animals (female domestic pigs, 20–22 kg body weight) and 15 recipient animals (female domestic pigs, 28–32 kg body weight) were used. The animal experiments were conducted according to the European Communities Council Directive 2010/63/EU for the protection of animals used for experimental purposes and the German Animal Protection law, and approved by the responsible authority (North Rhine‐Westphalia State Office for Nature, Environment and Consumer Protection).
The donor animals were assigned to four experimental groups, each n = 5: Sham control (group 1); NHBD (group 2); MSCvasc (group 3); MSCbronch (group 4). Details of the surgical procedures were described previously (Wittwer et al. 2014). Briefly, pigs in group 1 were sham‐operated, and the lungs were explanted and processed for histological and ultrastructural assessment without ischaemia, transplantation or any other treatment. In groups 2–4, cardiac arrest was induced, and the asystolic animals were continued to be ventilated for 3 h. In groups 3 and 4, MSCs were administered 10 min before the end of the 3‐h warm ischaemic period via the pulmonary artery and endobronchially, respectively. The left lungs of groups 2–4 were transplanted into recipient animals, reperfused and ventilated for 4 h. Then, lungs were retrieved and processed for histology and EM.
During reperfusion, functional data were collected. These as well as samples of both lungs had been used for different investigations in a previous study (Wittwer et al. 2014).
Bone‐marrow‐derived MSCs
Mesenchymal stem cells were derived from human bone marrow aspirates as isolation and large‐scale expansion of porcine MSCs is not established. Sternal bone marrow aspirates were collected from patients undergoing elective coronary artery bypass grafting surgery. The intervention was approved by the ethics committee of the University of Cologne, and written informed consent was obtained from all patients. Isolation, expansion and characterization were performed as previously described (Neef et al. 2012; Wittwer et al. 2014). Briefly, mononuclear cells were isolated from bone marrow aspirates by Ficoll density‐gradient centrifugation. Cells from the interphase cell layer were cultured in T75 cell culture flasks (Becton Dickson Biosiences, Heidelberg, Germany) in specific human MSC media (PAN Biotech, Aidenbach, Germany) at 37 °C and 5% CO2 in a humidified incubator (Binder, Tuttlingen, Germany). For further large‐scale expansion, MSCs were grown in multi‐layered cell culture vessels (Corning, Wiesbaden, Germany). After 14 days of expansion, cells were collected, fluorescence‐labelled (DiI, Life Technologies, Darmstadt, Germany), and the MSC phenotype was confirmed as CD45−, CD73+, CD90+, CD105+ using PE‐ and FITC‐conjugated antibodies (Miltenyi Biotech, Bergisch Gladbach, Germany; Becton Dickson Biosciences; Beckmann Coulter, Krefeld, Germany) and FACS analysis (FACS Calibur; Becton Dickson Biosciences).
Anaesthesia and surgery
Anaesthetical and surgical procedures were performed as described previously (Wittwer et al. 2014). In brief, both donor and recipient animals were premedicated i.m. with ketamine (20 mg kg−1 body weight), azaperone (2 mg kg−1) and atropine (0.04 mg kg−1). Anaesthesia was induced with propofol (3 mg kg−1 i.v.), and maintained by continuous infusion of fentanyl (0.3 μg kg−1 min−1), midazolam (20 μg kg−1 min−1), propofol (67–100 μg kg−1 min−1) and pancuronium (10 μg kg−1 min−1). Additionally, the pigs were heparinized (200 IU kg−1). The anaesthetized animals were ventilated in a pressure‐controlled mode [18 breaths per min; peak inspiratory pressure (PIP) 20 mm Hg; positive end‐expiratory pressure (PEEP) 8 mm Hg; FiO2 = 0.5].
Donor operation and warm ischaemia
Lungs and heart were exposed by median sternotomy in the donor animals of groups 2–4. Cardiac arrest was induced with electrical fibrillation. The non‐heart‐beating animals were kept for 3 h at room temperature (warm ischaemia), but ventilated continuously with the above parameters during that period. During the last 10 min of the warm ischaemic time, MSCs [50 × 106 cells (Dahlke et al. 2009) in 10 mL 0.9% saline] were given to groups 3 and 4. Group 3 received the cells via the pulmonary artery. In group 4, MSCs were administered endobronchially with an ultrasonic nebulizer (Nebu‐tec, Elsenfeld, Germany). After termination of warm ischaemia, flush preservation with 1.8 L of cold low‐potassium dextran solution was performed (max. perfusion pressure 14 mm Hg), lungs were clamped in an end‐inspiratory state and the heart lung‐bloc was recovered followed by storage at 4 °C for 3 h.
Sham control and recipient operation
The animals of the sham‐operated control group (group 1) and the recipient pigs of groups 2–4 were subjected to a lateral thoracotomy in the left 5th intercostal space. In all animals, tissue around the tracheal bifurcation, the main bronchi and both pulmonary arteries was dissected. In groups 2–4, left native lungs were removed and left donor lungs were transplanted. After reperfusion and reventilation (18 breaths per min; pressure‐controlled mode; PIP 30 mm Hg; PEEP 10 mm Hg; FiO2 = 0.5) for 15 min, the right lungs were clamped in all groups. Perfusion and ventilation of the left lungs were continued for 4 h. At the end of the experiment, the animals were killed by exsanguination in deep anaesthesia.
Fixation, sampling and tissue processing
The whole left lungs were perfusion fixed in situ with 2 L fixation solution (4% paraformaldehyde/0.1% glutaraldehyde in 0.2 m Hepes buffer) in an antegrade fashion via the pulmonary artery. During fixation, perfusion pressure was kept at 15 cm H2O and airway pressure at 12 cm H2O. After fixation, the lungs were explanted and organ volume was determined according to the Archimedes principle (Scherle, 1970). In order to obtain unbiased results representative for the whole organ, tissue samples for histology and EM were collected by systematic uniform random sampling (SURS; Howard & Reed, 2010; Tschanz et al. 2014). As a first step of the sampling cascade, lungs were cut into 20‐mm‐thick slices. A point grid was placed randomly on every slice and samples were excised where the points hit tissue (minimum five samples for LM and six samples for EM per lung). Both LM and EM samples were post‐fixed in osmium tetroxide (1% in 0.1 m sodium cacodylate buffer) and stained en bloc with uranyl acetate (half‐saturated). LM samples were embedded in glycolmethacrylate (Technovit 8100; Heraeus Kulzer, Wehrheim, Germany), sectioned at 1.5 μm and stained with toluidine blue. EM samples were embedded in Epon (Serva, Heidelberg, Germany). Ultrathin sections (60 nm) were counterstained with uranyl acetate and lead citrate.
Stereological evaluation
Following the principles of the American Thoracic Society and European Respiratory Society for quantitative assessment of lung structure (Hsia et al. 2010), design‐based stereology was employed on LM and transmission electron microscopical (TEM) levels in a multistage stratified analysis (Ochs, 2006a; Tschanz et al. 2014).
Evaluation of LM sections was conducted with a Leica 6000 LM (Leica; Wetzlar, Germany) equipped with a motorized microscope stage (MAC 6000 System; Ludl, New York, NY, USA) and the computer‐assisted stereology system newCAST (Visiopharm, Hoersholm, Denmark). In every section, fields of vision to be analysed were identified by SURS at an objective magnification of 5 × and 20 ×. Using the point counting method, volume densities of parenchyma, alveolar septum and intra‐alveolar oedema were determined (Ochs, 2006b; Howard & Reed, 2010; Schneider & Ochs, 2013).
Transmission electron microscopy analysis was performed with a Morgagni 268 TEM (FEI, Eindhoven, The Netherlands) including a digital camera (Veleta CCD; Olympus SIS, Münster, Germany) and the image analysis software iTEM (Olympus SIS). TEM micrographs were assessed stereologically using the stereology tool STEPanizer (Tschanz et al. 2011). A combined point and line grid was superimposed on micrographs with a primary magnification of 11 000 × to estimate volume densities (V V) of alveolar septa components, and intra‐alveolar surfactant subtypes and surface densities (S V) of alveolar epithelium and capillary endothelium (Howard & Reed, 2010; Schneider & Ochs, 2013). Absolute volumes of organ structures were calculated by multiplying volume fractions with total organ volume. From surface densities and volume densities, the arithmetic mean blood air–barrier thickness (τ̅bab) was calculated according to Eq. (1) (Ochs, 2006b; Hsia et al. 2010).
| (1) |
Further analysis of the intracellular surfactant pool within type 2 cells included estimation of volume density, volume‐weighted mean volume () and size distribution of lamellar bodies (lbs). Type 2 cells were sampled by SURS at an original magnification of 7100 × and images were taken from all sampled cells. To assess the latter two lb parameters, the point sampled intercepts method was employed (Gundersen & Jensen, 1985). A test system with lines and points was superimposed on sampled type 2 cells. Whenever a point hit a lb, its linear intercept was measured with a 15 class ruler (Braendgaard & Gundersen, 1986). The of lbs was then calculated according to Eq. (2) (Gundersen & Jensen, 1985).
| (2) |
Additionally, the frequency distribution of lbs in the profile size classes was determined.
The lung of one sham control group animal was perforated during organ recovery and fixation and collapsed; it had to be excluded from stereological analysis.
Statistics
Statistical analysis was performed with IBM spss statistics 23 (IBM Deutschland GmbH, Ehningen, Germany). Data were tested for normality with the Shapiro–Wilk test. When displaying a normal distribution, values are presented as mean ± standard deviation, and group differences were analysed using anova and post hoc Tukey test. For parameters not complying with a normal distribution, median ± interquartile range are given and the Kruskal–Wallis test including post hoc multi‐comparisons with stepwise down adjustment were used. In both cases, a P‐value < 0.05 was considered significant.
Results
Lung structure and ultrastructure after warm ischaemia and MSC pretreatment
Figures 1 and 2 show typical findings of histological and ultrastructural lung conditions in the four experimental groups. In sham‐operated control animals (group 1) as well as after warm ischaemia (group 2) and warm ischaemia plus MSC treatment (groups 3 and 4), lung parenchyma was well preserved with inflated alveoli and slender alveolar septa. Signs of IR injury were largely missing. Peribronchovascular space, alveolar septa and intra‐alveolar space were almost free from oedema. The bab was continuous, and none of its components was swollen or fragmented.
Figure 1.
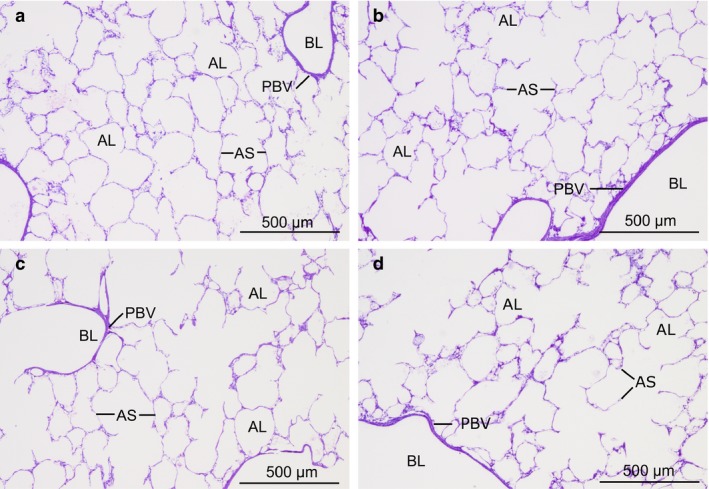
Light micrographs of lung sections of sham‐operated control group (a), non‐heart‐beating donor (NHBD) group (b), mesenchymal stem cell (MSC)vasc group (c) and MSCbronch group (d). Toluidine blue staining. Images show intact lung parenchyma with air‐filled alveoli free from oedema and slender alveolar septa. Peribronchovascular space was narrow without oedema fluid accumulation. AL, alveolar lumen (i.e. central lumina of ductus alveolares or sacculi alveolares or lumina of alveoli); AS, alveolar septum; BL, bronchiolar lumen; PBV, peribronchovascular space.
Figure 2.
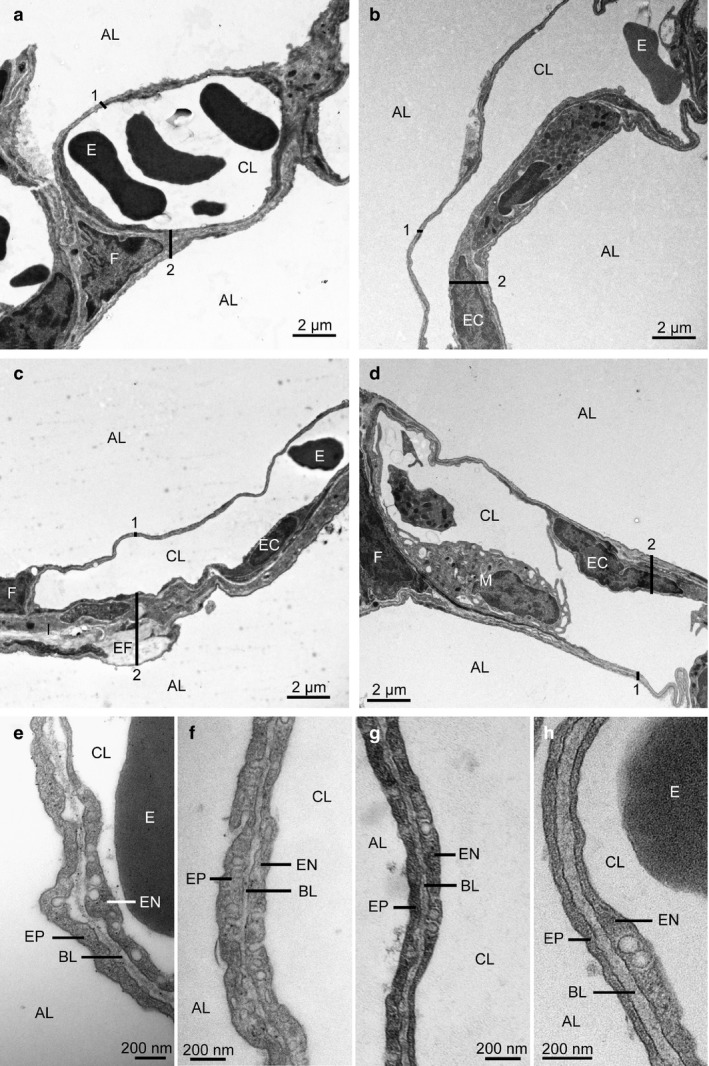
Transmission electron micrographs showing details of the alveolar septum. Sham‐operated control group (a, e), non‐heart‐beating donor (NHBD) group (b, f), mesenchymal stem cell (MSC)vasc group (c, g), MSCbronch group (d, h). (a–d) Alveolar septum with two adjacent alveoli. The alveoli are free from oedema fluid. The alveolar septum contains an open capillary, which is separated from the alveolar air space by the blood–air barrier (bab). Both the thin and the thick part of the bab are continuous. (e–h) Thin part of the bab. No swelling is seen in the epithelium, basal lamina or endothelium. AL, alveolar lumen; CL, capillary lumen; EC, endothelial cell (nucleus); 1, thin part of bab; 2, thick part of bab; I, interstitial layer of bab; F, fibroblast (nucleus); EF, elastic fibre; E, erythrocyte; M, intravascular macrophage; EP, alveolar epithelium; BL, basal lamina; EN, capillary endothelium.
Results of the stereological analysis are summarized in Table 1. The left lungs had a mean volume between 350 mL (sham control group) and 422 mL (MSCbronch group). Volume densities of structural organ components did not differ significantly between groups. Consistent with qualitative findings of parenchymal ventilation, air content made up 71–77% of total lung volume (range of group means). Little intra‐alveolar fluid/oedema was detected, amounting to 1.0–1.7% of lung volume, which corresponded to an absolute volume of 4.4–5.8 mL (range of group means). The alveolar septum made up between 6.7% of lung volume (22 mL) in the sham control group and 12.0% (50 mL) in the MSCbronch group. The difference in absolute volume between those groups reached the significance level (P = 0.021). No significant group differences were encountered in relative alveolar septum composition. Capillary lumina comprised approximately half of alveolar septum volume, while tissue components comprised the other half of the volume. In contrast, absolute values of alveolar septum composition showed significant differences for type 1 cells, endothelium and capillary lumina between the sham group and the MSCbronch group. Arithmetic mean thickness of the whole bab amounted to 0.863 μm in the MSCbronch group and 1.167 μm in the NHBD group (P = 0.030). Alveolar septum interstitium contributed the largest part to bab thickness in all groups. Alveolar epithelium and capillary endothelium were approximately of equal thickness, with group means ranging from 0.230 to 0.325 μm and 0.238 to 0.299 μm, respectively. Individual bab layers did not show statistically significant group differences.
Table 1.
Structural lung composition
| Parameter | Sham control | NHBD | MSCvasc | MSCbronch |
|---|---|---|---|---|
| VV(npar/lung) | 0.158 ± 0.052 | 0.132 ± 0.045 | 0.100 ± 0.022 | 0.158 ± 0.027 |
| VV(par/lung) | 0.841 ± 0.053 | 0.868 ± 0.045 | 0.879 ± 0.043 | 0.842 ± 0.027 |
| VV(air/lung) | 0.753 ± 0.056 | 0.777 ± 0.054 | 0.772 ± 0.066 | 0.711 ± 0.056 |
| VV(ed/lung) | 0.017 ± 0.002 | 0.011 ± 0.007 | 0.011 ± 0.006 | 0.010 ± 0.003 |
| VV(alvsept/lung) | 0.067 ± 0.026 | 0.078 ± 0.036 | 0.094 ± 0.025 | 0.120 ± 0.039 |
| VV(type1/sept) | 0.071 ± 0.010 | 0.079 ± 0.026 | 0.056 ± 0.009 | 0.065 ± 0.007 |
| VV(type2/sept) | 0.055 ± 0.016 | 0.067 ± 0.014 | 0.071 ± 0.030 | 0.047 ± 0.012 |
| VV(intas/sept) | 0.228 ± 0.047 | 0.244 ± 0.026 | 0.194 ± 0.030 | 0.200 ± 0.012 |
| VV(endo/sept) | 0.109 ± 0.024 | 0.127 ± 0.013 | 0.119 ± 0.024 | 0.112 ± 0.016 |
| VV(caplum/sept) | 0.538 ± 0.071 | 0.481 ± 0.059 | 0.559 ± 0.052 | 0.576 ± 0.041 |
| VV(lb/typ2) | 0.165 ± 0.023 | 0.135 ± 0.043 | 0.122 ± 0.016 | 0.123 ± 0.028 |
| V(lung) (mL) | 350 ± 57 | 417 ± 107 | 402 ± 86 | 422 ± 78 |
| V(npar/lung) (mL) | 55 ± 21 | 52 ± 11 | 41 ± 18 | 66 ± 7 |
| V(par/lung) (mL) | 295 ± 55 | 365 ± 107 | 354 ± 79 | 356 ± 54 |
| V(air/lung) (mL) | 265 ± 58 | 327 ± 104 | 312 ± 77 | 301 ± 55 |
| V(ed/lung) (mL) | 5.83 ± 1.29 | 4.34 ± 2.08 | 4.34 ± 2.24 | 4.41 ± 1.34 |
| V(alvsept/lung) (mL) | 22.32 ± 5.49§ | 32.02 ± 14.22 | 37.05 ± 8.52 | 50.24 ± 15.03† |
| V(type1/lung) (mL) | 1.54 ± 0.23† | 2.31 ± 0.85 | 2.10 ± 0.71 | 3.20 ± 0.85† |
| V(type2/lung) (mL) | 1.24 ± 0.52 | 2.08 ± 0.86 | 2.54 ± 0.97 | 2.31 ± 0.74 |
| V(intas/lung) (mL) | 5.29 ± 2.29 | 7.97 ± 3.85 | 7.26 ± 2.58 | 10.01 ± 2.81 |
| V(endo/lung) (mL) | 2.52 ± 1.09§ | 4.09 ± 1.74 | 4.37 ± 1.14 | 5.49 ± 1.20† |
| V(caplum/lung) (mL) | 11.72 ± 1.72§ | 15.57 ± 7.75§ | 20.76 ± 5.14 | 29.22 ± 9.91† , ‡ |
| V(lb/lung) (mL) | 0.209 ± 0.114 | 0.275 ± 0.150 | 0.310 ± 0.133 | 0.279 ± 0.098 |
| SV(alvepi/lung) (1/mm) | 30.4 ± 15.4§ | 36.1 ± 15.4 | 43.5 ± 10.0 | 59.7 ± 11.6† |
| S(alvepi/lung) (m²) | 10.0 ± 3.7§ | 14.9 ± 6.3 | 17.3 ± 5.4 | 23.6 ± 4.2† |
| τ (bab) (μm) | 1.056 ± 0.186 | 1.167 ± 0.163§ | 1.000 ± 0.120 | 0.863 ± 0.098‡ |
| τ (alvepi) (μm) | 0.267 ± 0.057 | 0.325 ± 0.070 | 0.298 ± 0.074 | 0.230 ± 0.033 |
| τ (intas) (μm) | 0.509 ± 0.075 | 0.566 ± 0.096 | 0.483 ± 0.086 | 0.427 ± 0.267 |
| τ (endo) (μm) | 0.239 ± 0.051 | 0.299 ± 0.048 | 0.289 ± 0.052 | 0.238 ± 0.032 |
V, volume; VV, volume density; npar, non‐parenchyma; par, parenchyma, air pulmonary air content; ed, intra‐alveolar oedema fluid; alvsept, alveolar septum; type1, Type 1 alveolar epithelial cells; type2, Type 2 alveolar epithelial cells; intas, interstitial layer of the alveolar septum; endo, capillary endothelium of the alveolar septum; caplum, lumina of alveolar septal capillaries; lb, lamellar bodies; bab, blood–air barrier; SV, surface density; τ, arithmetic mean thickness; alvepi, alveolar epithelium; MSC, mesenchymal stem cell; NHBD, non‐heart‐beating donor.
Mean ± standard deviation. Statistical significance of group differences was calculated by anova and post hoc Tukey test. Significant differences (P < 0.05) are marked by superscripts indicating significance vs.: †sham control group, ‡NHBD group and §MSCbronch group.
Intracellular surfactant pool
Alveolar epithelial type 2 cells (Fig. 3) represent the cellular part of the surfactant system. The mean total volume of type 2 cells in the left lung ranged between 1.24 mL in the sham control group and 2.54 mL in the MSCvasc group (Table 1). Within type 2 cells, surfactant is contained in lbs. These organelles comprised 12.2% (MSCbronch) to 16.5% (sham control) of type 2 cell volume, amounting to a total volume of 0.21–0.31 mL (group means; Table 1). Group differences of these values were not statistically significant.
Figure 3.
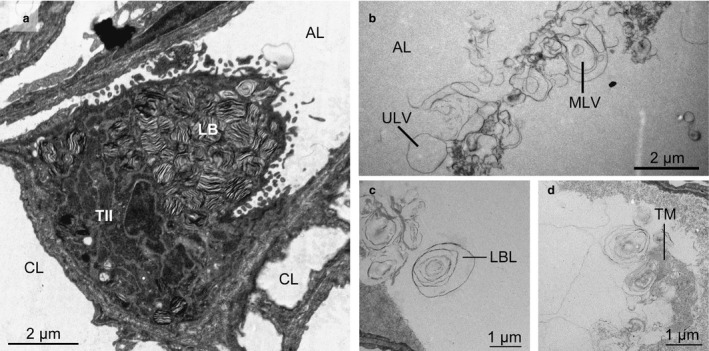
Transmission electron micrographs of cellular and intra‐alveolar surfactant system. (a) Type II cell, sham group. The cytoplasm contains numerous lamellar bodies (lbs). (b–d) Intra‐alveolar surfactant subtypes. AL, alveolar lumen; CL, capillary lumen; TII, type II cell; LB, lamellar body; LBL, lamellar body‐like form; TM, tubular myelin; MLV, multilamellar vesicle; ULV, unilamellar vesicle.
Furthermore, lb size was analysed: the (Fig. 4) differed significantly between groups (P = 0.024), with the sham control group harbouring larger lbs (median 0.814 μm³) compared with the other groups (group medians 0.180–0.373 μm³). Looking more closely at the distribution of lb profile size classes (Fig. 5) revealed that after warm ischaemia and MSC application (groups 2–4), type 2 cells contained significantly less large profiles of lbs (sum of classes 5–15) compared with the sham control group. A lower number of counts in classes 2–4 was not statistically significant.
Figure 4.
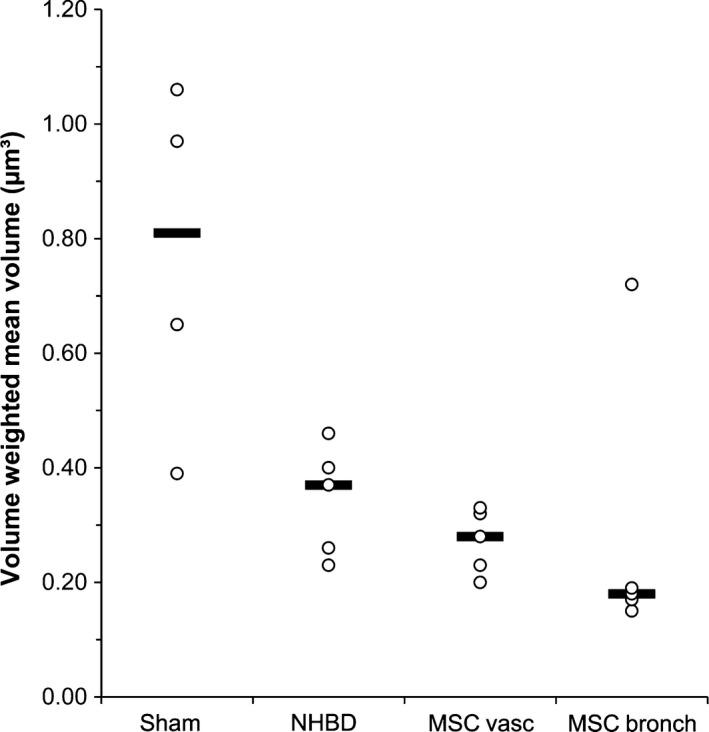
Volume weighted mean volume of lamellar bodies (lbs). Individual data points and group medians. The sham control group differed significantly from all other groups (P = 0.024). Sham, sham control group; NHBD, non‐heart‐beating donor group; MSCvasc, pulmoarterial application of mesenchymal stem cells; MSCbronch, endobronchial application of mesenchymal stem cells.
Figure 5.
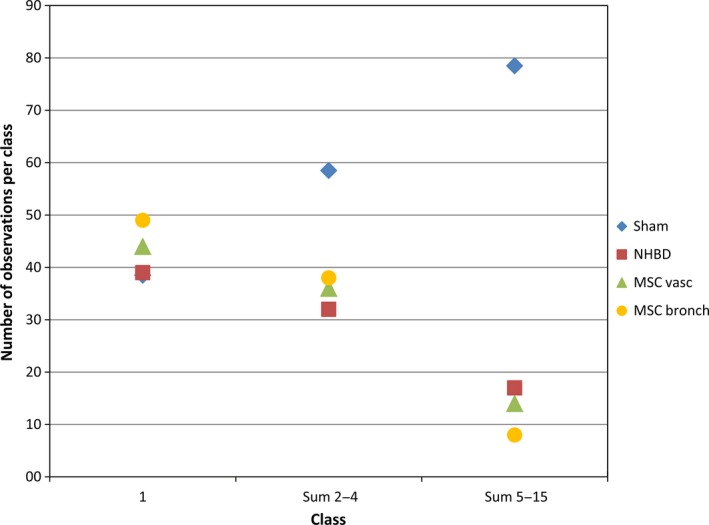
Profile size distribution of lamellar bodies (lbs). Lb size was analysed using the point sampled intercepts method. For all sampled lbs, the profile intercept length was measured and assigned to one of 15 profile size classes. The group medians of observation numbers in class 1, sum of classes 2–4 and sum of classes 5–15 are depicted. Sum of classes 5–15 differed significantly between sham control group and all other groups (P = 0.031). Sham, sham control group; NHBD, non‐heart‐beating donor group; MSCvasc, pulmoarterial application of mesenchymal stem cells; MSCbronch, endobronchial application of mesenchymal stem cells.
Intra‐alveolar surfactant pool
Electron microscopy sections were screened for intra‐alveolar surfactant at a magnification of 11 000 ×. All surfactant subtypes were detected, i.e. active [lamellar body‐like forms (lbl); tubular myelin (tm)], intermediate [multilamellar vesicles (mlv)] and inactive [unilamellar vesicles (ulv)] forms (Fig. 3). All surfactant subtypes together amounted to 0.313–0.858 mL (group medians; Table 2). Thus, the intra‐alveolar surfactant pool was slightly larger than the intracellular pool (Table 1). Ulvs were by far the most abundant surfactant subtype in all groups. Group differences in subtype distribution were not statistically significant. A remarkable phenomenon was a large individual variability in all surfactant parameters.
Table 2.
Quantitative analysis of the intra‐alveolar surfactant pool
| Parameter | Sham control | NHBD | MSCvasc | MSCbronch |
|---|---|---|---|---|
| V(surf/lung) (mL) | 0.313 ± 0.238 | 0.858 ± 0.631 | 0.424 ± 1.095 | 0.597 ± 0.527 |
| VV(lbl/surf) (%) | 0.00 ± 1.31 | 0.00 ± 1.90 | 0.00 ± 0.56 | 0.00 ± 0.00 |
| VV(tm/surf) (%) | 0.95 ± 1.71 | 2.58 ± 4.01 | 1.96 ± 3.86 | 0.00 ± 1.38 |
| VV(mlv/surf) (%) | 4.80 ± 8.38 | 7.19 ± 15.39 | 4.95 ± 30.44 | 29.64 ± 17.16 |
| VV(ulv/surf) (%) | 94.25 ± 11.36 | 91.46 ± 18.68 | 92.63 ± 29.69 | 70.36 ± 15.78 |
V, volume; VV, volume density; surf, surfactant; lbl, lamellar body‐like forms; tm, tubular myelin; mlv, multilamellar vesicles; ulv, uni‐lamellar vesicles; MSC, mesenchymal stem cell; NHBD, non‐heart‐beating donor.
Median ± interquartile range. No statistically significant group differences were found.
Discussion
Large animal NHBD model
In this study, a large animal NHBD model was developed to evaluate the initial steps of IR injury in warm ischaemia and the possible influence of pulmoarterial or endobronchial application of MSCs. After induction of cardiac arrest, a non‐heart beating, i.e. warm ischaemic, time period of 3 h was observed (except sham control group) during which the pigs were continuously ventilated.
In a clinical context, organs from NHBD are classified according to Maastricht categories I‐IV (Kootstra et al. 1995). Today, almost only organs from category III and IV donors are utilized. In these controlled donors cardiac arrest is awaited and donor procedures including cooling are started immediately or within minutes after cardiac arrest to keep warm ischaemic time as short as possible (Cypel et al. 2015). Category II donors are defined as ‘unsuccessful resuscitation’, i.e. cardiac arrest is followed by a period of constricted perfusion and ventilation of variable length during attempted resuscitation and, after cessation of resuscitation, by a NHB period of variable length until commencement of donor procedures (warm ischaemia). Depending on legislation, that time period might be bridged by ventilation and/or other preparatory handlings (Kootstra et al. 1995). Thus, the donor lungs of our model can be regarded as representing a subset of category II organs, but under well‐defined experimental conditions. In groups 3 and 4, MSCs were administered during the last 10 min of warm ischaemia. This would conform to a time point after consent has been obtained and at commencement of donor procedures in a clinical setting.
The aim of our study was to identify the initial steps of IR injury development in a NHBD setting and to evaluate possible immediate effect of MSC application. Therefore, a reperfusion time of 4 h was chosen. Within this time period, primary alterations of IR injury have been shown to commence (Eppinger et al. 1997; Fehrenbach et al. 2000; Fischer et al. 2000a,b; Quadri et al. 2005; Zhao et al. 2006; Mühlfeld et al. 2009; Yang et al. 2009) and at the same time avoiding too much secondary progression of the organ damage cascade.
Qualitative and quantitative organ structure after 3 h of warm ischaemia and MSC application
As revealed by qualitative microscopy, transplanted lungs in our model tolerated 3 h of ventilated warm ischaemia as well as MSC application without developing histological or ultrastructural signs of IR injury (Figs 1 and 2). In addition to qualitative evaluation of LM and EM sections, we employed stereology to obtain representative quantitative data. These aimed at detecting subtle changes in organ structure at the onset of IR injury. Stereology revealed morphofunctionally highly relevant specifics regarding blood–gas barrier condition as well as intracellular and intra‐alveolar surfactant pool in rodent IR injury models (Dreyer et al. 2008; Mühlfeld et al. 2009, 2010; Knudsen et al. 2011).
A key feature in IR injury is the development of oedema (Fehrenbach et al. 1999; Mühlfeld et al. 2009). Stereology enables the discrimination of peribronchovascular, septal and alveolar oedema by estimating volume fraction and absolute volume of non‐parenchyma, components of the alveolar septum and intra‐alveolar fluid, respectively (Ochs, 2006b). Functionally most significant is alveolar oedema, which showed a strong correlation with impairment of perfusate oxygenation (Fehrenbach et al. 1999). Volume densities of oedema‐related parameters did not display significant group differences (Table 1). In particular, the fraction of intra‐alveolar fluid remained low in all groups with group means of 1.0% (MSCbronch) to 1.7% (sham control). Also none of the other volume density parameters listed in Table 1 showed statistically significant group differences.
Additionally, absolute volumes of organ components were calculated. This can be of particular significance for the lung as volume densities depend heavily on ventilation. To account for this, all animals were ventilated in a stringently controlled mode intra‐operatively and ventilation pressure was maintained continuously during organ fixation. Still differences in lung compliance could influence pulmonary air content and subsequently volume densities of tissue components. Also looking at absolute volumes, no indications of peribronchovascular or intra‐alveolar oedema were found. Total intra‐alveolar fluid amounted to 4.3–5.8 mL (group means; Table 1). In contrast, differences in alveolar septum volume between sham control and MSCbronch group exceeded the significance level (Table 1). Tissue components of the alveolar septum and intravascular capillary volume were affected almost equally and surpassed statistical significance for type 1 cells, capillary endothelium and capillary lumen volume. An increase in alveolar septum volume and its components is conspicuous of septal oedema. However, the bab was not thicker in the MSCbronch group, due to a higher surface density of the alveolar septum. Additionally, no qualitative signs of septal oedema were detected. Furthermore, neither qualitative nor quantitative alterations suggestive of peribronchovascular oedema were found. Peribronchovascular oedema formation generally precedes septal oedema (Ochs, 2006b). Thus, we concluded that a higher volume of the alveolar septum and some of its components was not indicative of septal oedema formation but due to a higher amount of regular alveolar septa.
Alveolar surface density as well as absolute alveolar surface was significantly higher in the MSCbronch group compared with the sham control group (Table 1). Concordant alterations of those two parameters and an (insignificantly) higher air volume exclude malventilation or air loss as a cause for higher surface density in the MSCbronch group. Generation of new alveolar surface by sprouting, growth or ramification of alveolar septa in the MSCbronch group is quite unlikely due to the short duration of the experiment. Another possibility would be reduced surface tension, which could allow greater stretch of septal tissues and subsequently greater surface area. Interestingly, the MSCbronch group had a, even though insignificantly, differing composition of intra‐alveolar surfactant, i.e. a higher fraction of mlvs and lower fraction of ulvs (Table 2). However, the definite cause of a higher alveolar surface density and absolute alveolar surface in the MSCbronch group remains unclear, but must also take into account the small number of animals per group in this explorative study.
Considering qualitative and quantitative structural findings, it can be concluded that 3 h of ventilated warm ischaemia was tolerated well in our model. Functional data were also available for the same animals (Wittwer et al. 2014). Sham operated pigs presented with physiological oxygenation (mean PaO2/FiO2 472 mm Hg), good dynamic compliance (12.6 mL mbar−1) and pulmonary vascular resistance (507 dyn. s cm−5) at the end of the observation period post‐transplantation and depending only on the transplanted left lung. Values of the NHBD group did not differ significantly. In a comparable porcine NHBD model including 7 h of ventilated warm ischaemia, Wittwer et al. observed high mortality in the NHBD groups due to right heart failure (Wittwer et al. 2013).
The local oxygen supply to parenchyma/alveolar septa can be maintained and the tissues of the bab can be supported via the alveolar air space due to ventilation in the NHBD model. Also in vivo functional as well as nutritive oxygen supply is mainly provided from this source. The Vasa privata of the lung support bronchi, proximal bronchiole, larger blood vessels and surrounding peribronchovascular connective tissue. Additionally, some supernumerary arteries branch off and anastomose with the parenchymal pulmonary artery vascular bed (Ochs & O'Brodovich, 2012). In contrast to oxygen, the supply with all other nutrients is interrupted and metabolites accumulate during ventilated warm ischaemia also in parenchymal tissues. Additionally, cessation of perfusion eliminates shear stress on vascular endothelium and initiates complex mechanosensitive signalling. Through mechanotransduction, ischaemia independent of hypoxia can lead to generation of reactive oxygen species (ROS) through an NADPH oxidase (NOX) 2‐dependent pathway cascade (Chatterjee et al. 2014). ROS initiates activation of alveolar macrophages and recruitment of neutrophils, including subsequent induction of the inflammatory signalling cascade (den Hengst et al. 2010; Chatterjee et al. 2014). Tissue injury in ventilated warm ischaemia may arise from these pathways. The window between onset of detectable functional and structural organ impairment and irreversible tissue damage seems to be quite narrow in warm ischaemia, with a time frame between 3 and 7 h in the porcine model. In our model, lungs of clinically healthy animals at a juvenile age were used, which is typical for experimental lung transplantation studies. In contrast, clinical lung transplantation depends on lungs from a very heterogeneous donor population with potentially very heterogeneous reactivity towards ischaemic stress. Thus, the limiting frame for tolerable warm ischaemic time might differ to a great extent between individual lungs demanding individual testing of NHBD lungs. This could be achieved through the technique of ex vivo lung perfusion (Steen et al. 2001; Van Raemdonck et al. 2015).
In a previous study, deposition of MSC in alveolar septa vasculature after pulmoarterial application or in alveolar lumina after endobronchial application was demonstrated for the same animals (Wittwer et al. 2014). Looking at both qualitative and quantitative data from MSC groups in this study, MSC application had no detectable effect on lung structure or ultrastructure in the immediate post‐transplantation period. Both routes of application (vascular and endobronchial) were tolerated without eliciting qualitative or quantitative structural damage. A positive effect on structural preservation compared with the NHBD group was not detected. However, this does not preclude possible effects in a model conveying marked IR injury to the NHBD group.
Stereological data of the sham control group can also serve as a basis for the porcine lung in the respective age/weight category. Pigs with a body weight about 20–35 kg, i.e. approximately 8–14 weeks old, are commonly used in lung transplantation studies (Strüber et al. 2002; Wittwer et al. 2004; Cypel et al. 2009; Yeung et al. 2012). However, detailed and representative quantitative data regarding lung structure and ultrastructure have been missing so far. An earlier developmental study analysed lung ultrastructure in piglets aged 30–60 days (Winkler & Cheville, 1985). With respect to volume densities of bab components, equivalent values were found for capillary endothelium, alveolar septum interstitium and type I alveolar epithelial cells, while the volume density of type II alveolar epithelial cells was approximately twice as high in our older animals. Arithmetic mean bab thickness amounted to 1.55 μm in the younger piglets compared with 1.06 μm in our study, with septal interstitium contributing most of the difference (1.00 vs. 0.51 μm; Winkler & Cheville, 1985). These variations in quantitative organ composition of the porcine lung at ultrastructural level demonstrate the need for an age‐stratified data basis in this species.
Intracellular surfactant pool
The intracellular surfactant pool was affected by 3 h of warm ischaemia in a way that the of lbs decreased significantly (sham control: group median 0.814 μm³; groups 2–4: group medians 0.180–0.373 μm³) and the profile size distribution of lbs changed. MSC administration had no further influence on both parameters.
To characterize lbs, stereology offers two different possibilities of volume measurements: number weighted mean volume () and volume weighted mean volume () (Gundersen & Jensen, 1985; Ochs, 2006a; Howard & Reed, 2010; Mühlfeld & Ochs, 2013). addresses volume from the perspective of lbs and weighs each lb equally. In contrast, addresses this question from the perspective of contained surfactant and weighs each unit of surfactant material equally, asking: ‘A unit of surfactant is found on average in lbs of which volume?’. Thus, represents a value of the size condition of lbs that harbour available surfactant. Intracellular surfactant material forms the pool from which intra‐alveolar surfactant can be replenished (Ochs, 2010). In case lb size is of functional relevance for contained surfactant quality (e.g. maturity, composition, conformation), it is of interest to characterize lbs from the surfactant perspective.
was determined by the point sampled intercept method (Gundersen & Jensen, 1985). In point sampling, the sampling probability of lbs in sections is directly proportional to their volume (Braendgaard & Gundersen, 1986). The length of the intercept of every sampled lb was classified using a 15 class ruler (Braendgaard & Gundersen, 1986). Any lb can have a section profile maximally of the size class of its largest diameter. Additionally, it can produce profiles in all smaller profile size classes. For example, a reduction of very large lbs (maximal diameter class 15) in number leads not only to a smaller number of counts in class 15 but also to fewer counts in classes 1–14. In our study, all groups subjected to warm ischaemia (NHBD, MSCvasc and MSCbronch) had fewer counts in all classes beginning at class 2, which became statistically significant for the sum of classes 5–15. This leads to the assumption that during warm ischaemia mainly larger lbs released their surfactant content into the intra‐alveolar space. Interestingly, the number of profiles in class 1 was not reduced. This could be due to a compensatory formation of new small lbs to restore the intracellular surfactant pool.
The amount of total surfactant in type 2 cells did not differ significantly between groups despite the changes in lb. Regarding the differential profile size distribution of lbs between groups with probable extrusion of large and formation of small lbs, these two opposing effects seemed to balance the volume of the intracellular surfactant pool.
Intra‐alveolar surfactant pool
Changes in intra‐alveolar surfactant composition have been found to be related to IR injury after lung transplantation in an experimental as well as clinical context (Ochs et al. 1999; Strüber et al. 2007; Mühlfeld et al. 2010). Functional properties of surfactant are clearly associated with morphologically discernible surfactant subtypes. In particular, surface active large aggregates correspond to tm and ‘used’, inactivated small aggregates correspond to ulv. Directly after exocytosis, surfactant material presents as lbl, which feeds the tm lattice, whereas mlv are seen as intermediate, partially active surfactant subtypes (Ochs, 2010; Knudsen et al. 2012).
The total amount of intra‐alveolar surfactant per lung did not differ significantly between groups. All surfactant forms present in human and rodent lungs (Brasch et al. 2004; Mühlfeld et al. 2010) were also encountered in the porcine organs.
However, the proportion of active surfactant forms was very low (group medians 0.0% lbl and 0.0–2.6% tm) in all groups. Reference values for porcine lungs are missing so far. Studies on rodent lungs identified 1% lbl, 33% tm, 41% mlv and 26% ulv in intra‐alveolar surfactant of untreated control lungs. IR injury resulted in a reduction of tm (1%) and an increase of ulv (65%; Mühlfeld et al. 2010). Differences between rodent and porcine surfactant form composition could be due to species characteristics. On the other hand, intra‐operative inactivation of intra‐alveolar surfactant also in the sham control group cannot be excluded. Possibly, porcine surfactant could be more susceptible to ventilation‐associated inactivation compared with rodent surfactant. In the MSCbronch group a tendency towards less ulv and more mlv was observed. Thus, the intra‐alveolar surfactant pool might profit from endobronchial MSC application, but further studies, also covering a longer time period, are needed to substantiate this finding.
Intra‐alveolar and intracellular surfactant pools cannot be regarded as self‐contained entities. In our model, the findings could be interconnected forming the following surfactant turnover cycle. During warm ischaemia and reperfusion, intra‐alveolar surfactant was inactivated, i.e. active forms were converted to inactive ulv. Lbl and subsequently tm were replenished from the intracellular pool by secretion of large, mature lbs. Ulv were cleared from the hypophase by reuptake into type II cells. Endocytosed ulv material was recycled in type II cells into newly formed small lbs. In this hypothesis, surfactant turnover differs only minimally from physiological turnover (Dietl & Haller, 2005; Andreeva et al. 2007; Ochs, 2010). Consumption of intra‐alveolar surfactant can still be compensated from the intracellular pool. In the intracellular pool, the same amount of surfactant is now contained in smaller lbs as an indication of increased turnover and possibly as a first sign of exceeded compensatory capacity. These very subtle changes in our model are likely to stand at the very beginning of IR injury pathogenesis. This is corroborated by findings of Thompson et al. who demonstrated a direct connection between surfactant exocytosis and alveolar fluid resorption via fusion‐activated cation entry (Thompson et al. 2013).
Limitations of the study
Due to its design, our model incorporates some limitations. We used porcine lungs derived from young and healthy animals in this study. Human donor lungs might have a different, maybe higher, susceptibility to IR injury after a warm ischaemic insult due to age or previous impairment. Thus, time lines and severity of IR injury‐related alterations cannot be translated directly into clinical lung transplantation.
Additionally, findings in our model are specific for the experimental procedures applied, i.e. 3 h of ventilated warm ischaemia, and cannot be extrapolated to category II donors in general. With respect to organ conservation, it seems of particular importance how the time between cardiac arrest and start of donor procedures is bridged, especially if the donor is ventilated and topical cooling is used (Rega et al. 2003; Gomez‐de‐Antonio et al. 2012; Pierre et al. 2015).
Mesenchymal stem cells administered in this study were derived from bone marrow. Evidence is accumulating that adult MSCs comprise non‐uniform subpopulations depending on their origin. Lung‐resident MSCs derived from adult lungs exhibited different gene expression, surface marker and cytokine expression profiles as well as different morphology and proliferative capacity compared with bone‐marrow‐derived MSCs (Rolandsson Enes et al. 2016). Thus, MSCs derived from other sources than bone marrow might induce differential pulmonary reactivity.
Furthermore, the model was designed to reveal initial effects of warm ischaemia and MSC application with particular respect to IR injury development. Beyond short‐term impairment of organ function, in worst case culminating in high‐grade PGD, IR injury also influences the long‐term outcome of lung transplantation. PGD has been identified as a risk factor that promotes development of BOS (Daud et al. 2007). Medium‐ and long‐term effects of warm ischaemia and MSC application were not studied in this model. Nevertheless, they are of great interest. BOS and non‐BOS forms of CLAD are the major impediments to long‐term survival of lung transplant recipients (Kotloff & Thabut, 2011; Warnecke & Haverich, 2012; Sato, 2013; Yusen et al. 2015). On the one hand, beneficial preventive and therapeutic effects on the genesis of CLAD have been associated with MSC application due to their immunomodulatory properties, cytokine secretion profiles, cell differentiation potential and anti‐apoptotic capacity (Selmani et al. 2009; Zhen et al. 2010; Wittwer et al. 2014; Ito et al. 2015; Urbanek et al. 2016). On the other hand, MSCs have been suspected of playing a role in BOS development (Badri et al. 2011). For further studies on the long‐term effects of MSC application, the absence of initial damaging effects is an essential prerequisite.
In summary, this study provides insight into the initial stages of IR injury pathogenesis. Very subtle alterations within the surfactant system that are likely to represent a departure point for IR injury development could be detected using stereological methods. MSCs were not able to execute a positive short‐term effect, maybe also due to the good tolerance of 3 h of warm ischaemia. However, the absence of immediate organ damage will allow further medium‐ to long‐term studies to explore possible benefits regarding CLAD prevention.
Author contributions
TWi, CM and MO designed the study. TW, YHC, MZ, SK, KN, ASK, MG, FH and AM performed mesenchymal stem cell preparation, anaesthesia, surgical procedures and procurement of tissue samples. LK, AC, AS and MO created the concept for stereological analysis. AC performed stereological analysis. AC, AS, LK, CM and MO were engaged in data analysis and interpretation. AS drafted the manuscript. AC, LK, CM, MO and TWa revised the manuscript.
Acknowledgements
The authors gratefully acknowledge the skilful technical assistance of Suse Faßbender, Karin Westermann, Sabine Fiedler and Andrea Herden, and thank Sheila Fryk for reading the English manuscript (all Institute of Functional and Applied Anatomy, Hannover Medical School, Hannover, Germany). The authors also thank Jens Randel Nyengaard (Stereology and Electron Microscopy Research Laboratory, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark) and Ute Hahn (Centre for Stochastic Geometry and Advanced Bioimaging, Department of Mathematics, Aarhus University, Aarhus, Denmark) for their fruitful discussion on the point sampled intercept method. This work was supported by the Deutsche Forschungsgemeinschaft (DFG, OC 23/10‐1, MU 3118/2‐1, WI 1625/2‐1). The authors have no conflicts of interest to disclose. All authors are very thankful for the special contribution of Prof. Dr Thorsten Wittwer, who was one of the founders of this specific research. He initiated these projects many years ago and has continuously made a tremendous contribution. He also played the main role in interacting with the Deutsche Forschungsgemeinschaft. Unfortunately Prof. Wittwer died suddenly in April 2015. Our thoughts are with his family.
References
- Andreeva AV, Kutuzov MA, Voyno‐Yasenetskaya TA (2007) Regulation of surfactant secretion in alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 293, L259–L271. [DOI] [PubMed] [Google Scholar]
- Badri L, Murray S, Liu LX, et al. (2011) Mesenchymal stromal cells in bronchoalveolar lavage as predictors of bronchiolitis obliterans syndrome. Am J Respir Crit Care Med 183, 1062–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braendgaard H, Gundersen HJ (1986) The impact of recent stereological advances on quantitative studies of the nervous system. J Neurosci Methods 18, 39–78. [DOI] [PubMed] [Google Scholar]
- Brasch F, Johnen G, Winn‐Brasch A, et al. (2004) Surfactant protein B in type II pneumocytes and intra‐alveolar surfactant forms of human lungs. Am J Respir Cell Mol Biol 30, 449–458. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Nieman GF, Christie JD, et al. (2014) Shear stress‐related mechanosignaling with lung ischemia: lessons from basic research can inform lung transplantation. Am J Physiol Lung Cell Mol Physiol 307, L668–L680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JD, Edwards LB, Kucheryavaya AY, et al. (2011) The Registry of the International Society for Heart and Lung Transplantation: Twenty‐eighth Adult Lung and Heart‐Lung Transplant Report – 2011. J Heart Lung Transplant 30, 1104–1122. [DOI] [PubMed] [Google Scholar]
- Cypel M, Rubacha M, Yeung J, et al. (2009) Normothermic ex vivo perfusion prevents lung injury compared to extended cold preservation for transplantation. Am J Transplant 9, 2262–2269. [DOI] [PubMed] [Google Scholar]
- Cypel M, Levvey B, Van Raemdonck D, et al. (2015) International society for heart and lung transplantation donation after circulatory death registry report. J Heart Lung Transplant 34, 1278–1282. [DOI] [PubMed] [Google Scholar]
- Dahlke MH, Hoogduijn M, Eggenhofer E, et al. (2009) Toward MSC in solid organ transplantation: 2008 position paper of the MISOT study group. Transplantation 88, 614–619. [DOI] [PubMed] [Google Scholar]
- Daud SA, Yusen RD, Meyers BF, et al. (2007) Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med 175, 507–513. [DOI] [PubMed] [Google Scholar]
- De Meester J, Smits JM, Persijn GG, et al. (2001) Listing for lung transplantation: life expectancy and transplant effect, stratified by type of end‐stage lung disease, the Eurotransplant experience. J Heart Lung Transplant 20, 518–524. [DOI] [PubMed] [Google Scholar]
- Dietl P, Haller T (2005) Exocytosis of lung surfactant: from the secretory vesicle to the air‐liquid interface. Annu Rev Physiol 67, 595–621. [DOI] [PubMed] [Google Scholar]
- Dreyer N, Mühlfeld C, Fehrenbach A, et al. (2008) Exogenous surfactant application in a rat lung ischemia reperfusion injury model: effects on edema formation and alveolar type II cells. Respir Res 9, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppinger MJ, Deeb GM, Bolling SF, et al. (1997) Mediators of ischemia‐reperfusion injury of rat lung. Am J Pathol 150, 1773–1784. [PMC free article] [PubMed] [Google Scholar]
- Fehrenbach H, Schepelmann D, Albes JM, et al. (1999) Pulmonary ischemia/reperfusion injury: a quantitative study of structure and function in isolated heart‐lungs of the rat. Anat Rec 255, 84–89. [DOI] [PubMed] [Google Scholar]
- Fehrenbach A, Ochs M, Warnecke T, et al. (2000) Beneficial effect of lung preservation is related to ultrastructural integrity of tubular myelin after experimental ischemia and reperfusion. Am J Respir Crit Care Med 161, 2058–2065. [DOI] [PubMed] [Google Scholar]
- Fischer S, Cassivi SD, Xavier AM, et al. (2000a) Cell death in human lung transplantation: apoptosis induction in human lungs during ischemia and after transplantation. Ann Surg 231, 424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Maclean AA, Liu M, et al. (2000b) Dynamic changes in apoptotic and necrotic cell death correlate with severity of ischemia‐reperfusion injury in lung transplantation. Am J Respir Crit Care Med 162, 1932–1939. [DOI] [PubMed] [Google Scholar]
- Gomez‐de‐Antonio D, Campo‐Canaveral JL, Crowley S, et al. (2012) Clinical lung transplantation from uncontrolled non‐heart‐beating donors revisited. J Heart Lung Transplant 31, 349–353. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB (1985) Stereological estimation of the volume‐weighted mean volume of arbitrary particles observed on random sections. J Microsc 138, 127–142. [DOI] [PubMed] [Google Scholar]
- Halpern SD, Hasz RD, Abt PL (2013) Incidence and distribution of transplantable organs from donors after circulatory determination of death in U.S. intensive care units. Ann Am Thorac Soc 10, 73–80. [DOI] [PubMed] [Google Scholar]
- den Hengst WA, Gielis JF, Lin JY, et al. (2010) Lung ischemia‐reperfusion injury: a molecular and clinical view on a complex pathophysiological process. Am J Physiol Heart Circ Physiol 299, H1283–H1299. [DOI] [PubMed] [Google Scholar]
- Howard CV, Reed MG (2010) Unbiased Stereology, 2nd edn Liverpool: QTP Publications. [Google Scholar]
- Hsia CC, Hyde DM, Ochs M, et al. (2010) An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med 181, 394–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu L, Byrne RN, van Haaften HT, et al. (2012) Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol 303, L967–L977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Uchida T, Makita K (2015) Interactions between rat alveolar epithelial cells and bone marrow‐derived mesenchymal stem cells: an in vitro co‐culture model. Intensive Care Med Exp 3, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen L, Waizy H, Fehrenbach H, et al. (2011) Ultrastructural changes of the intracellular surfactant pool in a rat model of lung transplantation‐related events. Respir Res 12, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen L, Boxler L, Mühlfeld C, et al. (2012) Lung preservation in experimental ischemia/reperfusion injury and lung transplantation: a comparison of natural and synthetic surfactants. J Heart Lung Transplant 31, 85–93. [DOI] [PubMed] [Google Scholar]
- Kootstra G, Daemen JH, Oomen AP (1995) Categories of non‐heart‐beating donors. Transplant Proc 27, 2893–2894. [PubMed] [Google Scholar]
- Kotloff RM, Thabut G (2011) Lung transplantation. Am J Respir Crit Care Med 184, 159–171. [DOI] [PubMed] [Google Scholar]
- Li J, Huang S, Zhang J, et al. (2016) Mesenchymal stem cells ameliorate inflammatory cytokine‐induced impairment of AT‐II cells through a keratinocyte growth factor‐dependent PI3K/Akt/mTOR signaling pathway. Mol Med Rep 13, 3755–3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlfeld C, Ochs M (2013) Quantitative microscopy of the lung: a problem‐based approach. Part 2: stereological parameters and study designs in various diseases of the respiratory tract. Am J Physiol Lung Cell Mol Physiol 305, L205–L221. [DOI] [PubMed] [Google Scholar]
- Mühlfeld C, Schaefer IM, Becker L, et al. (2009) Pre‐ischaemic exogenous surfactant reduces pulmonary injury in rat ischaemia/reperfusion. Eur Respir J 33, 625–633. [DOI] [PubMed] [Google Scholar]
- Mühlfeld C, Becker L, Bussinger C, et al. (2010) Exogenous surfactant in ischemia/reperfusion: effects on endogenous surfactant pools. J Heart Lung Transplant 29, 327–334. [DOI] [PubMed] [Google Scholar]
- Neef K, Choi YH, Weichel A, et al. (2012) The influence of cardiovascular risk factors on bone marrow mesenchymal stromal cell fitness. Cytotherapy 14, 670–678. [DOI] [PubMed] [Google Scholar]
- Ochs M (2006a) A brief update on lung stereology. J Microsc 222, 188–200. [DOI] [PubMed] [Google Scholar]
- Ochs M (2006b) Stereological analysis of acute lung injury. Eur Respir Rev 15, 115–121. [Google Scholar]
- Ochs M (2010) The closer we look the more we see? Quantitative microscopic analysis of the pulmonary surfactant system. Cell Physiol Biochem 25, 27–40. [DOI] [PubMed] [Google Scholar]
- Ochs M, O'Brodovich H (2012) The structural and physiologic basis of respiratory disease In: Kendig and Chernick's Disorders of the Respiratory Tract in Children, 8th edn (eds Wilmott RW, Boat TF, Bush A, Chernick V, Deterding RR, Ratjen F.), pp. 35–74. Philadelphia: Elsevier. [Google Scholar]
- Ochs M, Nenadic I, Fehrenbach A, et al. (1999) Ultrastructural alterations in intraalveolar surfactant subtypes after experimental ischemia and reperfusion. Am J Respir Crit Care Med 160, 718–724. [DOI] [PubMed] [Google Scholar]
- Pierre L, Lindstedt S, Ingemansson R (2015) Ventilation in situ after cardiac death improves pulmonary grafts exposed to 2 hours of warm ischemia. Scand Cardiovasc J 49, 293–298. [DOI] [PubMed] [Google Scholar]
- Quadri SM, Segall L, Perrot M de, et al. (2005) Caspase inhibition improves ischemia‐reperfusion injury after lung transplantation. Am J Transplant 5, 292–299. [DOI] [PubMed] [Google Scholar]
- Rega FR, Jannis NC, Verleden GM, et al. (2003) Should we ventilate or cool the pulmonary graft inside the non‐heart‐beating donor? J Heart Lung Transplant 22, 1226–1233. [DOI] [PubMed] [Google Scholar]
- Rolandsson Enes S, Andersson Sjoland A, Skog I, et al. (2016) MSC from fetal and adult lungs possess lung‐specific properties compared to bone marrow‐derived MSC. Sci Rep 6, 29 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M (2013) Chronic lung allograft dysfunction after lung transplantation: the moving target. Gen Thorac Cardiovasc Surg 61, 67–78. [DOI] [PubMed] [Google Scholar]
- Sato M, Waddell TK, Wagnetz U, et al. (2011) Restrictive allograft syndrome (RAS): a novel form of chronic lung allograft dysfunction. J Heart Lung Transplant 30, 735–742. [DOI] [PubMed] [Google Scholar]
- Scherle W (1970) A simple method for volumetry of organs in quantitative stereology. Mikroskopie 26, 57–60. [PubMed] [Google Scholar]
- Schneider JP, Ochs M (2013) Stereology of the lung In: Laboraroy Methods in Cell Biology: Imaging. (ed. Conn PM.), pp. 257–294. Amsterdam: Elsevier. [DOI] [PubMed] [Google Scholar]
- Selmani Z, Naji A, Gaiffe E, et al. (2009) HLA‐G is a crucial immunosuppressive molecule secreted by adult human mesenchymal stem cells. Transplantation 87, S62–S66. [DOI] [PubMed] [Google Scholar]
- Steen S, Sjoberg T, Pierre L, et al. (2001) Transplantation of lungs from a non‐heart‐beating donor. Lancet 357, 825–829. [DOI] [PubMed] [Google Scholar]
- Strüber M, Hohlfeld JM, Kofidis T, et al. (2002) Surfactant function in lung transplantation after 24 hours of ischemia: advantage of retrograde flush perfusion for preservation. J Thorac Cardiovasc Surg 123, 98–103. [DOI] [PubMed] [Google Scholar]
- Strüber M, Fischer S, Niedermeyer J, et al. (2007) Effects of exogenous surfactant instillation in clinical lung transplantation: a prospective, randomized trial. J Thorac Cardiovasc Surg 133, 1620–1625. [DOI] [PubMed] [Google Scholar]
- Thompson KE, Korbmacher JP, Hecht E, et al. (2013) Fusion‐activated cation entry (FACE) via P2X(4) couples surfactant secretion and alveolar fluid transport. FASEB J 27, 1772–1783. [DOI] [PubMed] [Google Scholar]
- Tschanz SA, Burri PH, Weibel ER (2011) A simple tool for stereological assessment of digital images: the STEPanizer. J Microsc 243, 47–59. [DOI] [PubMed] [Google Scholar]
- Tschanz S, Schneider JP, Knudsen L (2014) Design‐based stereology: planning, volumetry and sampling are crucial steps for a successful study. Ann Anat 196, 3–11. [DOI] [PubMed] [Google Scholar]
- Urbanek K, De Angelis A, Spaziano G, et al. (2016) Intratracheal administration of mesenchymal stem cells modulates tachykinin system, suppresses airway remodeling and reduces airway hyperresponsiveness in an animal model. PLoS One 11, e0158746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raemdonck D, Neyrinck A, Cypel M, et al. (2015) Ex‐vivo lung perfusion. Transpl Int 28, 643–656. [DOI] [PubMed] [Google Scholar]
- Warnecke G, Haverich A (2012) Lung re‐transplantation: review. Curr Opin Organ Transplant 17, 485–489. [DOI] [PubMed] [Google Scholar]
- Winkler GC, Cheville NF (1985) Morphometry of postnatal development in the porcine lung. Anat Rec 211, 427–433. [DOI] [PubMed] [Google Scholar]
- Wittwer T, Franke UF, Fehrenbach A, et al. (2004) Innovative pulmonary preservation of non‐heart‐beating donor grafts in experimental lung transplantation. Eur J Cardiothorac Surg 26, 144–150. [DOI] [PubMed] [Google Scholar]
- Wittwer T, Madershahian N, Rahmanian P, et al. (2013) Surfactant application in experimental lung transplantation. J Heart Lung Transplant 32, 355–359. [DOI] [PubMed] [Google Scholar]
- Wittwer T, Rahmanian P, Choi YH, et al. (2014) Mesenchymal stem cell pretreatment of non‐heart‐beating‐donors in experimental lung transplantation. J Cardiothorac Surg 9, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AP, Dutly AE, Sacher A, et al. (2007) Targeted cell replacement with bone marrow cells for airway epithelial regeneration. Am J Physiol Lung Cell Mol Physiol 293, L740–L752. [DOI] [PubMed] [Google Scholar]
- Yang Z, Sharma AK, Linden J, et al. (2009) CD4 + T lymphocytes mediate acute pulmonary ischemia‐reperfusion injury. J Thorac Cardiovasc Surg 137, 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung JC, Keshavjee S (2014) Overview of clinical lung transplantation. Cold Spring Harb Perspect Med 4, a015628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung JC, Cypel M, Machuca TN, et al. (2012) Physiologic assessment of the ex vivo donor lung for transplantation. J Heart Lung Transplant 31, 1120–1126. [DOI] [PubMed] [Google Scholar]
- Yusen RD, Edwards LB, Kucheryavaya AY, et al. (2015) The Registry of the International Society for Heart and Lung Transplantation: Thirty‐second Official Adult Lung and Heart‐Lung Transplantation Report–2015; Focus Theme: early Graft Failure. J Heart Lung Transplant 34, 1264–1277. [DOI] [PubMed] [Google Scholar]
- Zhao M, Fernandez LG, Doctor A, et al. (2006) Alveolar macrophage activation is a key initiation signal for acute lung ischemia‐reperfusion injury. Am J Physiol Lung Cell Mol Physiol 291, L1018–L1026. [DOI] [PubMed] [Google Scholar]
- Zhen G, Xue Z, Zhao J, et al. (2010) Mesenchymal stem cell transplantation increases expression of vascular endothelial growth factor in papain‐induced emphysematous lungs and inhibits apoptosis of lung cells. Cytotherapy 12, 605–614. [DOI] [PubMed] [Google Scholar]


