Abstract
Objective
There is little randomized controlled trial (RCT) evidence to guide treatment for anxiety after stroke. We systematically reviewed RCTs of anxiety interventions in acquired brain injury (ABI) conditions including stroke and traumatic brain injury (TBI) in order to summarize efficacy and key aspects of trial design to help guide future RCTs.
Methods
We searched the Cochrane trial register, Medline, Embase, PsychInfo and CINAHL systematically up to August 2017. Two independent reviewers systematically selected studies and extracted data. We summarized the effect size, key study characteristics and sources of potential bias in trial design.
Results
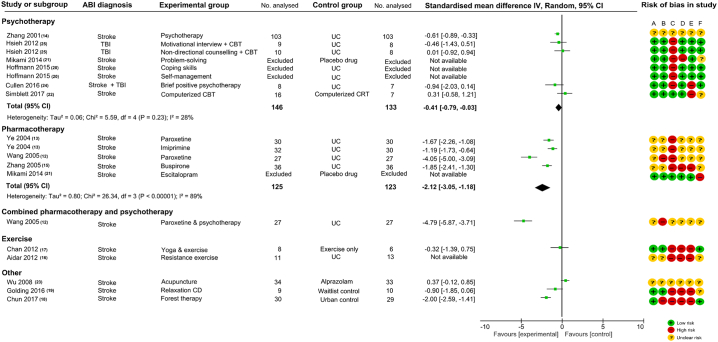
14 studies (12 stroke; one stroke & TBI; one TBI) with 928 participants were included. Meta-analysis of five psychotherapy comparisons favoured intervention over control (standardized mean difference (SMD): − 0.41 [− 0.79, − 0.03], I2 = 28%); Overall effect size of pharmacotherapy comparisons favoured intervention over control (SMD: − 2.12 [− 3.05, − 1.18], I2 = 89%). One comparison of mixed pharmacotherapy and psychotherapy favoured intervention over usual care (SMD: − 4.79 [− 5.87, − 3.71]). One comparison favoured forest therapy versus urban control (SMD: − 2.00 [− 2.59, − 1.41]). All positive studies carried high or unclear risk of bias. Sample sizes were small in all included studies.
Conclusions
There is low quality evidence to suggest that psychotherapy and pharmacotherapy may be effective interventions in the treatment of anxiety after stroke based on underpowered studies that carried high risk of bias. Large-scale well-designed definitive trials are needed to establish whether pharmacological or psychotherapy works. Our review highlighted key considerations for investigators wishing to design high quality trials to evaluate treatments for anxiety after stroke.
Keywords: Anxiety, Stroke, Neuropsychiatric, Intervention, Rehabilitation, Clinical trial
Highlights
-
•
A systematic review of trials of anxiety interventions for stroke and acquired brain injury.
-
•
Some evidence to suggest efficacy of psycho- and pharmacotherapy interventions.
-
•
Key aspects of trial design and sources of bias are summarized and discussed.
1. Introduction
Anxiety is a common neuropsychiatric complication of stroke with an estimated frequency between 20 and 25% [1]. There are two main subtypes of anxiety—phobic and generalized in non-stroke populations, requiring different treatment approaches. Phobic disorder is characterized by fear disproportionate to the threat posed by a well-defined situation, and marked avoidance of the situation [2]. Generalized anxiety disorder (GAD) presents with diffuse anxiety about events of daily life that is persistent and unremitting that the individual finds difficult to control [2]. In the general population, phobic disorder is treated with exposure techniques [3] whereas GAD responds to selective serotonin reuptake inhibitors (SSRI), short-term benzodiazepines and/or other cognitive behavioural therapy (CBT) techniques e.g. cognitive restructuring, problem solving [4], [5]. Randomized controlled trials (RCTs) of anxiety intervention in stroke have not yielded any definitive evidence in a recent Cochrane review—only three trials (2 pharmacological, 1 relaxation CD) with 196 participants were included [6]. These had high risk of bias and were of small sample size. Aware of the lack of RCT evidence in anxiety after stroke we aimed to review systematically the wider evidence base encompassing both stroke and traumatic brain injury (TBI). To date, there is no evidence to suggest that pathophysiological mechanism underlying anxiety disorders differs from one acquired brain injury (ABI) condition to another. The last systematic review of anxiety interventions in TBI in 2007 included three studies, providing some evidence for CBT in acute stress disorder, and in improving generalized anxiety symptomology but these studies had small sample sizes and were done in mild TBI only [7]. The current review would enable us to extrapolate from one ABI to the other as these conditions have abrupt onset, result in varying degrees of brain damage, and transient or long-term neurological and neuropsychiatric impairments. Furthermore, summarizing the key considerations in trial design (anxiety subtype targeted, setting and timing of intervention and outcome measure), and the sources of potential bias would help guide trialists to design high quality trials to evaluate anxiety treatments in the future.
1.1. Aims
To evaluate the efficacy of anxiety treatments and to summarize key aspects of trial design, we systematically reviewed RCTs of interventions—psychotherapy, pharmacotherapy or other types, for anxiety disorders in ABI conditions including stroke—ischaemic, haemorrhagic or subarachnoid haemorrhage (SAH), and TBI.
2. Methods
We followed a pre-defined protocol in conducting this systematic review and reported our review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) checklist [8].
2.1. Searches and information sources
We searched electronically for RCTs on Medline (1946-18/8/17), Embase (1980-17/8/17), PsychInfo (1940-17/8/17), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (inception-16/10/17), the Cochrane Stroke Register (16/10/17), and the Cochrane Central Register of Controlled Trials (CENTRAL) (inception-16/10/17) using search strategies supplied by the trials search co-ordinator of the Cochrane Stroke Group (Supplement B). We reviewed the reference list of key systematic reviews to date to identify additional titles [6], [7]. We contacted authors of eligible titles that were trial protocols, conference abstracts or trial register entries for published or unpublished primary data.
2.2. Inclusion criteria
We included RCTs that evaluated interventions designed to target anxiety symptoms/anxiety disorder as a primary outcome, with any comparator group (placebo, usual care, waitlist control, active comparator). We included RCTs that recruited participants aged 18 or over with ABI conditions: ischemic or haemorrhagic stroke; SAH, confirmed by brain imaging with or without a lumbar puncture; moderate-to-severe TBI as defined according to the Scottish Intercollegiate Guidelines Network [9]. We excluded mild TBI, a clinical group that is difficult to diagnose reliably [10]. Where studies were carried out in a mixed sample, we included only those that recruited over 70% of stroke/SAH/moderate-to-severe TBI. We excluded trials that recruited exclusively military veterans. No language restrictions were applied.
2.3. Data collection
Two reviewers (HYYC and RN) screened titles and abstracts independently and excluded ineligible titles. They assessed full text for eligibility and resolved discrepancies through discussion. A third reviewer (AJC) was consulted if a consensus could not be reached. They extracted data independently using an electronic data extraction form. HYYC collated final data. One reviewer (HYYC) assessed studies that were only available in Chinese.
2.4. Data extracted
We recorded key characteristics of the study population: ABI diagnosis, age, sex, exclusion of specific deficit, baseline anxiety level, and intervention type (e.g. psychotherapy, pharmacotherapy, other).
2.4.1. Quality assessment
We reported the level of bias across six domains of study design for the included studies: (A) random sequence generation, (B) allocation concealment, (C) blinding of participants and personnel, (D) blinding of outcome assessment (E) incomplete outcome data, and (F) selective reporting. We categorised the level of bias into ‘low’, ‘high’ or ‘unclear’ and recorded justification for our judgement for each domain in accordance with the Cochrane Risk of Bias Tool (http://methods.cochrane.org/bias/assessing-risk-bias-included-studies).
2.4.2. Efficacy of intervention
We estimated effect size for each comparison by calculating the standardized mean difference (SMD) with 95% confidence intervals (CI) using the mean and standard deviation (SD) of the post-intervention anxiety severity. Meta-analysis was carried out for studies of the same intervention type using inverse variance and random-effects models. All analysis was performed using the Cochrane Review Manager (RevMan) Version 5.3 [11]. Where data were not reported in study publication we contacted the corresponding authors for further information.
2.4.3. Key study characteristics and potential bias in trial design
We summarized the key study characteristics: anxiety type targeted, the setting and timing of intervention, outcome measures, the type of comparator, and ways that could have introduced or minimized potential bias in study design.
3. Results
The electronic searches yielded 8218 titles after removal of duplicates (Fig. 1). Of the 59 full text articles reviewed, we included 14 eligible studies with 928 participants. Sample size ranged from 17 to 206. Four studies were in Chinese [12], [13], [14], [15]. No clear evidence of publication bias on funnel plot (Supplement C).
Fig. 1.
PRISMA diagram of included studies.
Supplementary Fig. C.
Funnel plot for publication bias.
3.1. Characteristics of study population
Table 1 summarizes the characteristics of the 14 included studies. 12 studies recruited stroke patients only (ischaemic and primary haemorrhage) [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], one study recruited stroke and moderate-to-severe TBI [24], and one study recruited moderate-to-severe TBI only [25]. No study recruited patients with SAH. The mean age ranged from 48 to 72 years in studies of stroke patients only, and from 35 to 58 years in the two studies that included TBI patients. More men than women were recruited in all included studies. 12 studies excluded patients with communication difficulties due to aphasia or cognitive impairment [12], [13], [14], [16], [17], [18], [19], [20], [21], [22], [24], [25]; one yoga exercise intervention excluded participants who were unable to ambulate independently [17]. Seven studies required participants to have a baseline diagnosis of anxiety disorder or ‘emotional distress’ either made on standardized diagnostic criteria e.g. Diagnostic Statistical Manual (DSM-IV TR), or by meeting a defined cut-off on a rating scale [12], [13], [19], [22], [23], [24], [25]. Six studies did not specify a baseline anxiety level for inclusion [14], [15], [16], [17], [18], [20]. One study of a preventative intervention excluded the diagnosis of GAD on DSM-IV TR at baseline [21]. Studies used different anxiety rating scales at baseline and outcome assessment (Table 1): Hamilton Anxiety Rating Scale (HAMA) in five studies [12], [13], [15], [21], [23], Hospital Anxiety and Depression Scale-anxiety subscale (HADS-A) in three studies [19], [20], [25]; State-Trait Anxiety Inventory (STAI) in three studies [16], [17], [18]; Depression Anxiety Stress Scales (DASS) in one study [24]; Zung Self-rating Anxiety Scale (SAS) in one study [14]; Beck Anxiety Inventory (BAI) in one study [22].
Table 1.
Characteristics of included studies.
| Study (by year of publication) | ABI diagnosis | Anxiety disorder/type targeted | Eligible time since injury | Setting | Exclusion of specific deficit (e.g. speech) | Sample size | Type of intervention (I) and control (C), number randomized (n) (‘description’) |
Age (mean (SD)) |
Female (%) |
Baseline anxiety level (measure: mean (SD)) |
Time of intervention since injury (mean (SD)) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | C | I | C | I | C | I | C | ||||||||
| Zhang et al. [14] | Stroke | Unspecified | Not specified | Setting not given, China | NA | 206 | Psychotherapy (n = 103) ‘Weekly sessions, each lasting 20–30 min, for 5–6 weeks, delivered by trained researcher using in-house manual’ |
Usual care (n = 103) ‘Usual care’ |
NA | NA | NA | NA | SAS I) 34(8) C) 31 (8) |
NA | NA |
| Ye et al. [13] | Stroke | ‘Mixed anxiety and depression’ | Not specified | Neurology inpatient, China | Impairment of comprehension | 90 | 1) Paroxetine (n = 31) ‘20 mg daily for 12 weeks’ 2) Imipramine (n = 32) ‘incremental regime of 50-150 mg daily for 12 weeks’ |
Routine care (n = 30) ‘Routine care: for 12 weeks' |
1) 58.04 (8.28) 2) 56.9 (11.36) |
59.21 (9.52) | 1) 26 2) 37 |
43 | HAMA 1) 18.2 (4.6) 2) 18.9 (4.4) C) 17.9 (2.24) Required diagnosis of mixed anxiety and depression on CCMD |
NA | NA |
| Wang et al. [12] | Stroke | ‘Mixed anxiety and depression’ | ‘Acute’ stroke | Neurology inpatient, China | Aphasia; severe cognitive impairment | 81 | 1) paroxetine (n = 27) ‘20 mg daily for 6 weeks’ 2) paroxetine + psychotherapy (n = 27) ‘Paroxetine 20 mg daily + weekly psychotherapy session lasting 30–60 min, delivered by psychiatrist for 6 weeks’ |
Routine care (n = 27) ‘routine stroke care’ |
1) 62.4 (6.1) 2) 64.0 (5.3) |
63.2 (5.7) | 1) 48 2) 48 |
48 | HAMA 1) 14.0 (2.8) 2) 13.9 (2.9) C) 13.8 (2.8) Required diagnosis of mixed anxiety and depression on CCMD |
1) 21.7 days (4.9) 2) 22.0 days (4.7) |
21.4 days (5.0) |
| Zhang et al. [15] | Stroke | Unspecified | Not specified | Neurology inpatient, China | NA | 94 | Buspirone butylbromide (n = 47) ‘A 2-week course of buspirone butylbromide (first week 20-30 mg/day, second week 40-60 mg per day)’ |
Routine care (n = 47) ‘Routine care’ |
57.8 (6.4) | 59.2 (5.8) | 36 | 38 | HAMA I) 22.7 (5.2) C) 22.5 (4.3) |
NA | NA |
| Wu and Liu [23] | Stroke | ‘Post-stroke neurosis’ | Not specified | Outpatient, China | Aphasia; cognitive impairment | 67 | Acupuncture (n = 34) ‘acupuncture once a day for 2 courses with 15 times as one course’ |
Alprazolam (n = 33) ‘0.4–0.8 mg 3 times a day for 4 weeks’ |
48–72 | 49–70 | 44 | 48 | HAMA I) 22.31 (3.1) C) 22.3 (3.2) Required diagnosis of post-stroke neurosis on ICD-10 |
Range: 15–53 days | Range: 15–61 days |
| Aidar et al. [16] | Ischaemic stroke | Unspecified | ≥ 1 year | Community, Portugal | Aphasia | 29 | Resistance exercise training (n = 14) ‘4 familiarization sessions + 3 pre-treatment sessions + 12 treatment sessions delivered 3 times a week, focused on walking & strength training. Duration: each session lasted 45–60 min with minimum 48-hour rest between sessions.’ |
Usual care (n = 15) ‘continue normal daily activities’ |
51.7 (8.0) | 52.5 (7.7) | 45 | 31 | STAI (data not available) | NA | NA |
| Chan et al. [17] | Stroke | Unspecified | ≥ 6 months | Community, Australia | Unable to follow 2-stage commands; unable to ambulate for 10 m or more | 17 |
Yoga and exercise (YEX) (n = 9) ‘90-minute group yoga class once per week for 6 weeks plus 24 individual 40-min home practice sessions + Exercise (EX)’ |
Exercise only (EX) (n = 8) ‘50-minute exercise class, once per week for 6 weeks’ |
67.1 (15.4) | 71.7 (12.7) | 13 | 17 | STAI-state I) 36.8 (11.6) 2) 37 (5.8) |
6.4 years (3.0) | 11.2 years (5.8) |
| Hsieh et al. [25] | Moderate-to-severe TBI | Unspecified | Not specified | Community, Australia | Language impairment | 27 | 1) Motivational interviewing (MI) + Cognitive Behavioural Therapy (CBT) (n = 9) ‘3 weekly MI sessions + 9 weekly CBT sessions’ 2) Non-directional counselling (NDC) + CBT (n = 10) ‘3 weekly NDC sessions + 9 weekly CBT sessions’ Both delivered by clinical psychologist or clinical neuropsychologist |
Usual care and waitlist (n = 8) ‘offered CBT after waitlist period’ |
1) 41.8 (15.2) 2) 36.4 (14.1) |
35.6 (9.8) | 1) 22 2) 30 |
13 | HADS-A 1) 11.9 (3.3) 2) 13.0 (5.0) C) 11.8 (4.3) DSM-IV TR anxiety disorder or adjustment issues required |
1) 37.2 months (45.4) 2) 50.4 months (89.7) |
23.0 months (18.5) |
| Mikami et al. [21] | Stroke | Generalized anxiety disorder (GAD) | Within 3 months | Community, USA | Severe comprehension deficits | 149 | 1) Escitalopram (n = 47) ‘5 or 10 mg per day for 12 months’ 2) Problem solving therapy (PST) (n = 53) ‘manual-based, 6 treatment sessions (weeks 1, 2, 3, 4, 6 and 10), plus 6 reinforcement sessions (months 4, 5, 6, 8, 10 and 12)’ |
Placebo (n = 49) ‘Placebo pills’ |
1) 61.5 (13.7) 2) 68.3 (10.4) |
64.8 (13.5) | 1) 36 2) 45 |
33 | HAMA 1) 7.1 (5.6) 2) 8.3 (5.4) C) 6.8 (4.4) Excluded DSM-IV TR GAD diagnosis |
NA | NA |
| Hoffmann et al. [20] | Stroke | Unspecified | Not specified | Stroke unit inpatient & community, Australia | Communication difficulties/cognitive impairment | 33 | 1) Coping skills (n = 11) ‘cognitive and behavioural exercises, delivered by clinical psychologist’ 2) Self-management (n = 12) ‘Information provision and activities to learn problem solving skills, delivered by occupational therapist’ |
Usual care (n = 10) ‘multidisciplinary care on stroke unit’ Both interventions 1) and 2) consist of 8 one-hour face-to-face sessions, with first 2 sessions delivered pre-discharge, and remaining sessions at patient's home |
1) 63.6 (13.0) 2) 60.8 (11.7) |
57.0 (14.2) | 1)36 2) 25 |
40 | HADS-A 1) 5.3 (2.9) 2) 5.7 (0.5) C) 8.4 (3.1) |
NA | NA |
| Cullen et al. [24] | Stroke; moderate-severe TBI | ‘Emotional distress—anxiety and/or depression’ | 3–36 months | Outpatient clinic, UK | Significant communication impairments | 27 | Brief positive psychotherapy (n = 14) ‘One-to-one weekly sessions with psychologist for 8 weeks—Psychoeducation about ABI and positive psychology (Week 1), therapeutic exercises and homework (Weeks 2–7), midpoint review at (Week4), final review and plan for maintenance (Week 8)’ |
Usual care (n = 13) ‘Within clinical service’ |
Median 54.0 (IQR 46.0–59.0) | median 58.0 (IQR 56.0–68.0) | 36 | 39 | DASS-21 anxiety I) 17.6 (9.7) C) 21.1 (9.4) Had to score moderate-to-above on at least depression or anxiety subscale on DASS-21 |
Median: 5.8 months (IQR 3.5–8.2) | Median: 5.6 months (IQR 3.1–8.4) |
| Golding et al. [19] | Stroke | Unspecified | Not specified | Community, UK | Unable to complete telephone questionnaire | 21 | I: relaxation CD ‘self-help autogenic relaxation CD, five times per week for a month with diarly sheets; each session 20-min in length, instructions on body awareness’ |
Waitlist | 67.8 (7.5) | 62.4 (8.4) | 40 | 50 | HADS-A I) 10.9 (3.4) C) 10.5 (3.5) Had to score at least 6 on HADS-A |
118 months (101) | 70 months (70) |
| Chun et al. [18] | Stroke | Unspecified | At least 1 year after stroke onset | Community, Korea | Severe cognitive or communication impairment | 59 | I: Forest therapy ‘4-day and 3-night program at recreational forest area, consisting of 1) promoting positive emotion through mediation, 2) experiencing the forest through all five sense and 3) walking in the forest’ |
Urban group ‘stay in a hotel, with similar mediation and walking activities in the urban area’ |
62.1 (8.3) | 59.5 (9.7) | 37 | 28 | STAI I) 38.1 (11.0) C) 34.3 (12.1) |
140 months (90) | 153 months (84) |
| Simblett et al. [22] | Stroke | ‘Emotional distress—anxiety and/or depression’ | Within 5 years | Community, UK | Impairment of comprehension; visual or auditory problem that would interfere participation and could not be corrected | 28 | Computerised cognitive behavioural therapy (cCBT) (n = 19) ‘An 8-module online course-‘Beating the Blues', one module per week for 8 consecutive weeks’ |
Computerised Cognitive remediation therapy (cCRT) (n = 9) ‘An 8-module online course-‘ForamenRehab’, one module per week for 8 consecutive weeks’ |
62.1 (11.4) | 64.6 (8.1) | 47 | 11 | BAI I) 11.2 (7.6) C) 8.3 (6.2) Required ‘emotional distress’: BDI > 13 or BAI > 7 |
Median: 1.19 years (IQR 0.5–1.1) | Median: 0.89 years (IQR 0.6–4.1) |
| Both the intervention and active control are delivered via computer, facilitated by a researcher via telephone/email/face-to-face | |||||||||||||||
I indicates intervention; C, control; n, number; SD, standard deviation; IQR, interquartile range; NA, data not available; DASS, Depression Anxiety Stress Scales; DSM-IV, Diagnostic Statistical Manual of Mental Disorders, fourth edition; BDI, Beck Depression Inventory; BAI, Beck Anxiety Inventory; CCMD, Chinese Classification of Mental Disorders, third version; HAMA, Hamilton Anxiety Rating Scale; HAMD, Hamilton Rating Scale for Depression; SAS, Zung Self-Rating Anxiety Scale. STAI, State Trait Anxiety Inventory.
3.2. Quality assessment
None of the 14 studies scored ‘low’ risk of bias across all six domains (A-F) of study design (Fig. 2). Three studies scored ‘low’ risk across five domains [20], [21], [25]. Two studies scored ‘low’ risk across four domains [22], [24]. One studies scored ‘low’ risk across three domains [17]. Eight studies scored ‘low’ risk on fewer than three of the six domains [12], [13], [14], [15], [16], [18], [19], [23], including six studies that scored ‘high’ risk or ‘unclear’ risk across all six domains [12], [13], [14], [15], [16], [23].
Fig. 2.
Effect sizes, meta-analysis, and bias assessment for included studies.
ABI, acquired brain injury; IV, inverse variance; CI, confidence intervals; UC, usual care; TBI, traumatic brain injury; CBT, cognitive behavioural therapy; CRT, cognitive remediation therapy;
Risk of bias.
(A) Random sequence generation (selection bias).
(B) Allocation concealment (selection bias).
(C) Blinding of participants and personnel (performance bias).
(D) Blinding of outcome assessment (detection bias).
(E) Incomplete outcome data (attrition bias).
(F) Selective reporting (reporting bias).
3.3. Efficacy of intervention
The 14 included studies provided 19 comparisons: eight psychotherapy [14], [20], [21], [22], [24], [25], five pharmacotherapy [12], [13], [15], [21], one combined pharmacotherapy and psychotherapy [12], two exercise [16], [17], and three other interventions [18], [19], [23]. We carried out meta-analyses for psychotherapy and pharmacotherapy studies.
3.3.1. Psychotherapy
Six studies provided eight comparisons of psychotherapy interventions, the content of each is summarized in Table 1. Data were not available for three comparisons after contacting study authors. Meta-analysis of the five comparisons showed an overall positive effect favouring psychotherapy intervention over control (SMD: − 0.41 [95%CI -0.79, − 0.03]). I2 statistic of 28% suggests a low-to-moderate level of heterogeneity across studies (Fig. 2). The only study that demonstrated an effect favouring ‘psychotherapy’ over usual care [14] received ‘unclear’ risk of bias across all six domains of study design. The remaining four neutral comparisons (one ‘brief positive psychotherapy’ versus usual care(24), one ‘motivational interviewing & CBT’ versus usual care [25]; one ‘non-directional counselling & CBT’ versus usual care [25], one ‘computerised CBT’ versus computerised cognitive remediation therapy [22]) received ‘low’ risk of bias across at least three domains of study design; all had small sample sizes. One comparison not included in our analysis reported that group receiving placebo was four times more likely to develop GAD compared to ‘problem-solving’ therapy (adjusted hazard ratio: 4.00 [95%CI 1.84, 8.70]) [21]. The other two comparisons not included in our analysis reported a non-statistically significant reduction in adjusted mean HADS-anxiety score with psychotherapy: ‘coping skills’ vs usual care (− 0.5, [95%CI − 2.0, 1]); ‘self-management’ vs usual care (− 0.6, [95%CI − 2.0, 0.8]) [20].
3.3.2. Pharmacotherapy
Four studies provided five comparisons of pharmacotherapy versus control, data were not available in one comparison after contacting study author [21]. Meta-analysis of these four comparisons showed an overall effect favouring pharmacotherapy intervention over control (SMD: − 2.12 [95%CI − 3.05, − 1.18]). I2 statistic of 89% suggests a high level of heterogeneity across studies. Two of these comparisons were between paroxetine, an SSRI and usual care [12], [13]. One comparison was between imipramine, a tricyclic antidepressant and usual care [13]. One study compared buspirone, an azapirone anxiolytic with usual care [15]. All four comparisons are from three studies which scored ‘high’ risk or ‘unclear’ risk of bias across all domains of study design. The study without available data for analysis reported an increased reported that group receiving placebo was four times more likely to develop GAD compared to escitalopram (adjusted hazard ratio: 4.95 [95%CI 1.54–15.93]) [21].
3.3.3. Combined pharmacotherapy and psychotherapy
One comparison of combined paroxetine and psychotherapy with usual care demonstrated a large effect favouring combined therapy (SMD: − 4.79 [95%CI − 5.87, − 3.71]) [12]. This study scored ‘unclear’ and ‘high’ risk of bias across all six domains of study design.
3.3.4. Exercise intervention
Two studies evaluated exercise interventions, One study compared yoga and exercise with exercise only and showed a neutral effect [17]. One study on resistance exercise reported lower state anxiety favouring resistance exercise over usual care but data were unavailable for calculating SMD after contacting the study author [16]. Both studies had small sample sizes. The yoga study scored ‘low’ risk of bias across three domains of study design and the study on resistance exercise scored ‘high’ and ‘unclear’ risk of bias across all six domains.
3.3.5. Other therapies
One study compared acupuncture with alprazolam [23], one study compared relaxation CD with waitlist control [19]. Both of these studies were neutral. The study of acupuncture scored ‘unclear’ risk of bias across all six domains, and the study of relaxation CD scored ‘high’ risk of bias across more than three domains of study design. One study compared forest therapy with urban control and demonstrated an effect favouring forest therapy (SMD: − 2.00 [− 2.59, − 1.41]). This study scored ‘high’ risk of bias on four domains of study design. All three studies had small sample sizes.
3.4. Key study characteristics
3.4.1. Anxiety subtype targeted
One study specified GAD as the target of its interventions (escitalopram; problem solving therapy) [21]. No study targeted phobic disorder. Two studies of pharmacotherapy (SSRI, TCA), and combined pharmacotherapy (SSRI) and psychotherapy specified a diagnosis of ‘mixed anxiety and depression’ as an inclusion criterion and had positive results [12], [13]. Two studies of psychotherapy (brief positive psychotherapy; computerised CBT) targeted ‘emotional distress’—anxiety and/or depression and were neutral [22], [24]. One study of acupuncture and alprazolam targeted ‘post-stroke neurosis’ which is now a defunct diagnosis [23]. The remaining eight studies targeted ‘anxiety’ without subtyping [14], [15], [16], [17], [18], [19], [20], [25], three of them were positive [14], [15], [18].
3.4.2. Setting of intervention
Seven studies were carried out in the community [16], [17], [18], [19], [21], [22], [25], three studies in an inpatient setting [12], [13], [15], two in outpatient clinic [23], [24], and one commenced in an inpatient setting then continued in the community [20]. One study did not report setting of the intervention [14]. Only one community-based study was positive [18]. All three inpatient studies and the study with unknown setting were positive.
3.4.3. Timing of intervention since injury
Seven studies specified time since injury as an inclusion criterion: ‘acute stroke’ [12], within 3 months [21]; between 3 and 36 months [24]; anytime within 5 years [22]; at least 6 months [17]; at least one year [16], [18]. The actual time of intervention since injury in the studied sample ranged from 15 days to 13 years. Of the five positive studies, three did not report timing of intervention since injury in studied samples, one study reported intervention at 21 days from injury [12], and one reported intervention at 140–150 months [18].
3.4.4. Timing of outcome measures
Eight studies measured anxiety outcome at the end of the intervention [12], [13], [15], [16], [17], [18], [23], [25]. Other studies measured primary outcome at various time points post-intervention: 2 weeks; 8 weeks; 12 weeks; 12 months. Four of the five positive studies measured primary outcome at the end of intervention [12], [13], [15], [18] and one measured at two weeks post-intervention [14].
3.4.5. Comparator
‘Usual care’ was the most commonly used control condition. Four studies used an active comparator [17], [18], [22], [23] and one study used a placebo control [21]. Four of the five positive studies used ‘usual care’ as control conditions (12–15) and one used an active control [18].
3.5. A summary of sources of potential bias in study design
3.5.1. Random sequence generation
Studies scoring ‘unclear’ risk of bias in this domain only reported that patients were randomly allocated but did not give detail on how, and by whom the randomisation sequence was generated. Studies scoring ‘low’ risk reported the type of randomisation carried out e.g. computerised randomisation, stratified randomisation with blocking, random number generator, and by whom the randomisation was performed e.g. person external to the study/independent of the study.
3.5.2. Allocation concealment
Studies scoring ‘high’ risk of bias reported that it was the study personnel who performed randomisation and provided the treatment allocation. Studies scoring ‘low’ risk reported methods that would prevent the study team from knowing the allocation in advance e.g. allocation informed via mailed letters by external person who carried out randomization, study personnel were blinded to randomization block length with randomisation performed externally, use of opaque/sealed envelopes pre-filled by person independent of the study.
3.5.3. Blinding of participants and personnel
Most studies scored ‘high’ risk in this domain as blinding of participants was rarely attempted. The most common comparator group was ‘usual care’. We considered participant blinding sufficient in the study that used computerised CRT as a comparator of computerised CBT, and the study that used placebo as a comparator of escitalopram.
3.5.4. Blinding of outcome assessment
Studies scoring ‘high’ risk reported outcome assessment being performed by the same study personnel that delivered the interventions. Studies that scored ‘low’ risk reported methods to blind outcome assessment e.g. a second research assistant performed outcome assessment using a standard script to prevent unblinding, use of self-rated questionnaires and data entry by blinded assessor.
3.5.5. Incomplete outcome data
All studies scoring ‘high’ risk lost follow-up data (attrition ranged from 2 to 22%) and did not perform intention-to-treat analysis. Reasons for attrition were: personal reasons, additional health concerns/injury unrelated to intervention, improved mood, other commitments, lack of time, found it distressing to talk about difficulties, wish to discontinue involvement.
3.5.6. Selective reporting
We examined the published trial protocol, if available, for each included study to detect whether selective reporting was present. One study scoring ‘high’ risk reported results on anxiety from the same study in an earlier publication that evaluated intervention for depression prevention.
4. Discussion
Our findings suggest efficacy of psychotherapy and pharmacotherapy interventions in the treatment of anxiety after ABI. The positive effect sizes were driven entirely by studies of low quality. These findings alone are not definitive evidence to guide treatment of anxiety after stroke. Compared to previous systematic reviews in stroke and TBI [6], [7] we opted to include studies from a broader ABI population encompassing stroke (ischaemic, primary haemorrhage, SAH) and moderate-to-severe TBI, and included a wider continuum of baseline anxiety levels (i.e. not limited to patients with a baseline anxiety diagnosis). This approach led to more studies to be included in our review, and enabled us to meta-analyse results for the same type of anxiety interventions for the first time. Furthermore, we found studies that were better reported and of better quality which were excluded in the previous reviews. This enabled us to summarize key aspects of trial design and measures to minimize bias in order to help guide trialists in designing high quality RCTs in the future.
4.1. Intervention design
4.1.1. Anxiety subtype targeted
Studies have targeted ‘mixed anxiety and depression’, ‘emotional distress (anxiety and/or depressive symptoms)’, or ‘anxiety’. Only one study specified the prevention of GAD as the target of intervention. No studies targeted phobic disorder.
Phobic disorders e.g. agoraphobia may be more common than GAD after stroke [1]. Intervention design should reflect the treatment approaches known to be effective at treating these anxiety subtypes in non-stroke populations. Anxiety with a phobic element invariably requires some form of behavioural therapy with exposure work, while generalized anxiety is treated with other CBT techniques e.g. cognitive restructuring, problem solving, and/or medications e.g. SSRI.
Although the content of psychotherapy interventions varied across our included studies, the majority of interventions consisted of some form of, or a combination of psychoeducation, skills learning e.g. problem solving, positive psychology, therapeutic exercises, and CBT. Interventions for anxiety after stroke should encompass components that aim to address the symptomology of both phobic and generalized anxiety subtypes.
A variety of anxiety rating scales were used to assess primary outcome in our included studies. These are validated for generalized anxiety and none for the phobic subtype. The choice of outcome measures should reflect both types of anxiety symptomology given that phobic disorder is also common after stroke.
4.1.2. Setting and timing of intervention, and timing of outcome measures
Most of the positive studies were carried out in an inpatient setting and measured primary outcome immediately post-intervention. This approach does not address the consistent finding from other studies that anxiety continues to be frequent at six-months or more post-stroke [1] and cannot generalize to patients who have returned to living in the community. An anxiety intervention should aim to relieve anxiety and its debilitating impact on stroke patients in the long-term. Determining the best time of outcome measure should be based on this goal, and be balanced against the feasibility of study procedures to ensure completion of long-term follow-up. We suggest that outcome measures should be taken at the end of the intervention and then after a period with no treatment to see whether any benefits are sustained.
4.2. Measures to minimize bias
Most of the positive studies in our review were poorly reported across all aspects of study design on the Cochrane bias assessment tool. All trialists should adhere to standardized reporting guidelines e.g. CONSORT checklist on RCTs, and the TiDier (Template for Intervention Description and Replication) checklist when evaluating complex interventions, both of which can be found on the EQUATOR (Enhancing the QUAlity and Transparency of health Research network) website: http://www.equator-network.org/reporting-guidelines/consort/.
4.2.1. Participant blinding and control conditions
Most of our included studies did not attempt participant blinding. ‘Usual care’ was the commonest comparator in our review and in four out of the five positive studies. The description of what constituted ‘usual care’ was minimal across our included studies. ‘Usual care’ and waitlist controls have been shown to exaggerate effect size in meta-analyses of trials evaluating psychotherapy [26]. A recently published transparent decision framework help guide trialists select the appropriate type of control based on several factors: participants' interests (expected benefit, or harm or worsening of symptoms induced by the control condition), the researchers' interests (available resources, maximizing validity of findings), and trial purpose (e.g. phase 2, phase 4) [27]. Placebo is the gold-standard comparator for pharmacotherapy intervention. In a trial of psychotherapy or other non-pharmacological intervention, an active comparator or another established treatment that is known to be effective and widely available in the ‘real world’ would be more appropriate as a control in phase 3 or phase 4 (pragmatic/real world) trials [27].
4.2.2. Other measures to minimize bias
Some included studies provided examples of good practice in minimizing bias in other domains: external personnel to randomize patient; allocation concealment to ensure study personnel cannot foresee allocation while recruiting; use of outcome assessors blinded to allocation; use of standard script at telephone follow up to prevent unblinding; use of self-completed outcome measures; data input by blinded external assessor; reporting missing data and methods for handling missing data; intention-to-treat analysis; publishing protocol on trial registries. Studies should also provide detailed description of the experimental intervention and control condition to ensure standardized procedures are given to all participants of each arm e.g. use of manuals. Adherence to the allocated treatment and any deviation from standardized procedures should be recorded and reported.
4.3. Study limitations
Data for calculating SMDs were missing in four comparisons despite contacting corresponding authors. We included one mixed ABI (strokes in > 85% of intervention and control groups), and one TBI-only samples. Almost all studies excluded patients who had communication impairments e.g. dysphasia, cognitive impairment, and varied in settings, timing since injury, timing of outcome measures, limiting the generalizability of our findings.
4.4. Considerations for future studies
Compared to pharmacological interventions, psychological or behavioural interventions pose unique challenges in trial methodology, both in its execution and in bias minimization. While the current review cannot provide definitive evidence on efficacy of anxiety treatments in stroke due to poor study quality and small sample sizes of the included studies, we provided a summary of key considerations in trial design (anxiety type targeted, setting, timing of intervention and outcome measure, methods to minimize bias) to guide trialists and clinicians on what would constitute a high quality RCT. High quality definitive RCTs of sufficient sample size are now warranted to evaluate psychotherapy and pharmacotherapy interventions in the treatment of anxiety after stroke.
5. Conclusion
There is low quality evidence to suggest psychotherapy and pharmacotherapy may be effective interventions in the treatment of anxiety after stroke. However, the evidence is from underpowered studies that carried high risk of bias. Large-scale well-designed definitive trials are needed to establish whether pharmacotherapy or psychotherapy works. Our review highlighted key considerations for investigators wishing to design high quality trials to evaluate treatments for anxiety after stroke.
The following are the supplementary data related to this article.
Data extraction table.
Full search strategies.
Funding
HYYC received funding for a clinical academic fellowship from the Chief Scientist Office of Scotland (CAF/15/07) to conduct this research. The funder had no role in the study design, data collection, analysis or interpretation of the data in this study.
Disclosures
AJC is a paid associate editor of JNNP. He holds a small grant £10,000 from UK HTA to develop an app to deliver CBT after mild traumatic brain injury. This grant is shared with commercial partners. WNW, MD, GEM having nothing to disclose.
Acknowledgements
Princess Margaret Research Development Fellowship, funded by the Stroke AssociationPMF 2013/01, provided short-term early research support (2014–2015) to HYYC.
Cochrane Stroke Research Group for supplying the search strategies (Brenda Thomas) and the list of studies on the Cochrane stroke trial register (Josh Cheyne) for this systematic review.
References
- 1.Campbell Burton C.A., Murray J., Holmes J., Astin F., Greenwood D., Knapp P. Frequency of anxiety after stroke: a systematic review and meta-analysis of observational studies. Int. J. Stroke. 2013;8(7):545–559. doi: 10.1111/j.1747-4949.2012.00906.x. [DOI] [PubMed] [Google Scholar]
- 2.DSM-V . 5th edition. American Psychiatric Publishing; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders. (American Psychiatric Association). [Google Scholar]
- 3.Wolitzky-Taylor K.B., Horowitz J.D., Powers M.B., Telch M.J. Psychological approaches in the treatment of specific phobias: a meta-analysis. Clin. Psychol. Rev. 2008;28(6):1021–1037. doi: 10.1016/j.cpr.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Cuijpers P., Sijbrandij M., Koole S., Huibers M., Berking M., Andersson G. Psychological treatment of generalized anxiety disorder: a meta-analysis. Clin. Psychol. Rev. 2014;34(2):130–140. doi: 10.1016/j.cpr.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Stein M.B., Sareen J. Clinical practice. Generalized anxiety disorder. N. Engl. J. Med. 2015;373(21):2059–2068. doi: 10.1056/NEJMcp1502514. [DOI] [PubMed] [Google Scholar]
- 6.Knapp P., Campbell Burton C.A., Holmes J., Murray J., Gillespie D., Lightbody C.E. Interventions for treating anxiety after stroke. Cochrane Database Syst. Rev. 2017;5:Cd008860. doi: 10.1002/14651858.CD008860.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Soo C., Tate R. Psychological treatment for anxiety in people with traumatic brain injury. Cochrane Database Syst. Rev. 2007;3:Cd005239. doi: 10.1002/14651858.CD005239.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J. Clin. Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 9.SIGN. Scottish Intercollegiate Guidelines Network . SIGN publication; 2013. Brain Injury Rehabilitation in Adults. (no. 130) [Google Scholar]
- 10.Holm L., Cassidy J.D., Carroll L.J., Borg J. Summary of the WHO collaborating Centre for Neurotrauma Task Force on mild traumatic brain injury. J. Rehabil. Med. 2005;37(3):137–141. doi: 10.1080/16501970510027321. [DOI] [PubMed] [Google Scholar]
- 11.The Nordic Cochrane Centre TCC . 2014. Review Manager (RevMan) Version 5.3. (Copenhagen) [Google Scholar]
- 12.Wang X.H.Y., Xiao C.L.A. Clinical trial of paroxetine and psychotherapy in patients with poststroke depression and anxiety. Chin. Ment. Health J. 2005;19(8):564–566. [Google Scholar]
- 13.Ye L.X.W.H., Wang Y.D., Zhang L., Liang D.S., Guo Y. Effect of anti-depressive therapy on the rehabilitation of psychological and neurological function after stroke. Chin. J.Clin. Rehabil. 2004;8(31):6826–6828. [Google Scholar]
- 14.Zhang B.B.X., Chi Z. Effect of supportive psychological intervention on anxiety after stroke: a controlled prospective study. Chin. Ment. Health J. 2001;15(6):415–418. [Google Scholar]
- 15.Zhang Y.X., Zhang H.L., Wang H. Effects of buspirone hydrochloride on post-stroke affective disorder and neural function. Chin. J. Clin. Rehabil. 2005;9(12):8–9. [Google Scholar]
- 16.Aidar F.J., de Oliveira R.J., Silva A.J., de Matos D.G., Mazini Filho M.L., Hickner R.C. The influence of resistance exercise training on the levels of anxiety in ischemic stroke. Stroke Res. Treat. 2012;2012:298375. doi: 10.1155/2012/298375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan W., Immink M.A., Hillier S. Yoga and exercise for symptoms of depression and anxiety in people with poststroke disability: a randomized, controlled pilot trial. Altern. Ther. Health Med. 2012;18(3):34–43. [PubMed] [Google Scholar]
- 18.Chun M.H., Chang M.C., Lee S.J. The effects of forest therapy on depression and anxiety in patients with chronic stroke. Int. J. Neurosci. 2017;127(3):199–203. doi: 10.3109/00207454.2016.1170015. [DOI] [PubMed] [Google Scholar]
- 19.Golding K., Kneebone I., Fife-Schaw C. Self-help relaxation for post-stroke anxiety: a randomised, controlled pilot study. Clin. Rehabil. 2016;30(2):174–180. doi: 10.1177/0269215515575746. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann T., Ownsworth T., Eames S., Shum D. Evaluation of brief interventions for managing depression and anxiety symptoms during early discharge period after stroke: a pilot randomized controlled trial. Top. Stroke Rehabil. 2015;22(2):116–126. doi: 10.1179/1074935714Z.0000000030. [DOI] [PubMed] [Google Scholar]
- 21.Mikami K., Jorge R.E., Moser D.J., Arndt S., Jang M., Solodkin A. Prevention of post-stroke generalized anxiety disorder, using escitalopram or problem-solving therapy. J. Neuropsychiatry Clin. Neurosci. 2014;26(4):323–328. doi: 10.1176/appi.neuropsych.11020047. [DOI] [PubMed] [Google Scholar]
- 22.Simblett S.K., Yates M., Wagner A.P., Watson P., Gracey F., Ring H. Computerized cognitive behavioral therapy to treat emotional distress after stroke: a feasibility randomized controlled trial. JMIR Mental Health. 2017;4(2):e16. doi: 10.2196/mental.6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu P., Liu S. Clinical observation on post-stroke anxiety neurosis treated by acupuncture. J. Tradit. Chin. Med. 2008;28(3):186–188. doi: 10.1016/s0254-6272(08)60043-6. [DOI] [PubMed] [Google Scholar]
- 24.Cullen B., Pownall J., Cummings J., Baylan S., Broomfield N., Haig C. Positive PsychoTherapy in ABI Rehab (PoPsTAR): a pilot randomised controlled trial. Neuropsychol. Rehabil. 2016;(1):17. doi: 10.1080/09602011.2015.1131722. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh M.Y., Ponsford J., Wong D., Schonberger M., Taffe J., McKay A. Motivational interviewing and cognitive behaviour therapy for anxiety following traumatic brain injury: a pilot randomised controlled trial. Neuropsychol. Rehabil. 2012;22(4):585–608. doi: 10.1080/09602011.2012.678860. [DOI] [PubMed] [Google Scholar]
- 26.Mohr D.C., Ho J., Hart T.L., Baron K.G., Berendsen M., Beckner V. Control condition design and implementation features in controlled trials: a meta-analysis of trials evaluating psychotherapy for depression. Transl. Behav. Med. 2014;4(4):407–423. doi: 10.1007/s13142-014-0262-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gold SM, Enck P, Hasselmann H, Friede T, Hegerl U, Mohr DC, et al. Control conditions for randomised trials of behavioural interventions in psychiatry: a decision framework. Lancet Psychiatry.4(9):725–32. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data extraction table.
Full search strategies.