Summary
While it is appreciated that reactive oxygen species (ROS) can act as second messengers in both homeostastic and stress response signaling pathways, potential roles for ROS during early vertebrate development have remained largely unexplored. Here, we show that fertilization in Xenopus embryos triggers a rapid increase in ROS levels, which oscillate with each cell division. Furthermore, we show that the fertilization-induced Ca2+ wave is necessary and sufficient to induce ROS production in activated or fertilized eggs. Using chemical inhibitors, we identified mitochondria as the major source of fertilization-induced ROS production. Inhibition of mitochondrial ROS production in early embryos results in cell-cycle arrest, in part, via ROS-dependent regulation of Cdc25C activity. This study reveals a role for oscillating ROS levels in early cell cycle regulation in Xenopus embryos.
Keywords: mitochondria, reactive oxygen species, ROS, Xenopus, Cdc25C, cell cycle, fertilization, Ca2+ wave, HyPer, respiratory burst
Graphical Abstract

Highlights
-
•
ROS, including hydrogen peroxide, are produced after fertilization in Xenopus
-
•
Ca2+ signaling after fertilization induces ROS production in mitochondria
-
•
Mitochondria are the major source of oscillating ROS levels
-
•
ROS regulate Cdc25C activity and the early cell cycle
Han et al. show that the fertilization-triggered calcium wave induces reactive oxygen species production from mitochondria, which oscillate with each cell division in Xenopus embryos. Moreover, they demonstrate that inhibition of mitochondrial ROS production disrupts cell cycle progression.
Introduction
Reactive oxygen species (ROS), including the superoxide anion (O2•−), hydroxyl radical (OH•−), hydrogen peroxide (H2O2), and singlet oxygen (1O2), have various functions in cells and tissues. ROS have several sources, including monoamine oxidases, xanthine oxidase, NADPH oxidases (NOXs), and several mitochondrial complexes, which are part of the electron transport chain (ETC) (Bedard and Krause, 2007, Brand, 2016, Cantu-Medellin and Kelley, 2013). In addition, some forms of ROS are changed into others. For example, superoxide dismutase uses O2•− as a substrate to generate H2O2, a more stable, diffusible, and less harmful ROS (Burgoyne et al., 2013). Once generated, H2O2 modulates the activity of protein phosphatases via oxidizing their catalytic cysteine residues, which can, in turn, enhance kinase-driven signaling pathways (Östman et al., 2011).
Embryonic development is triggered by sperm entry during fertilization, which activates the egg by generating a Ca2+ wave and increasing oxygen uptake and ATP synthesis. This increase in oxygen consumption was first noted over 100 years ago in sea urchin eggs (Warburg, 1908), and it was later found to correlate with increased ROS production (Foerder et al., 1978). More recently, it was found that the dual oxidase protein (Udx1) is responsible for the generation of ROS following fertilization in sea urchins (Wong et al., 2004). Notably Wong and colleagues also showed that Udx1 is present throughout early development and that the inhibition of ROS generation using diphenyleneiodonium (DPI) inhibits cell division (Wong and Wessel, 2005). This suggested a role for ROS in the control of the early embryonic cell cycle, but the mechanisms by which ROS regulate the cell cycle and, moreover, whether ROS play similar roles during early vertebrate embryonic development remain unknown.
In early Xenopus embryos, the cell cycle is driven by an autonomous oscillator, which is cytokinesis independent (Hara et al., 1980, Murray and Kirschner, 1989). The cyclin B/cyclin-dependent kinase 1 (Cdk1) complex is a master regulator for entry into mitosis. Accumulating cyclin B levels activate Cdk1, which in turn activates Cdc25C phosphatase, which then dephosphorylates the inhibitory phosphorylated Thr 14 and Tyr 15 in Cdk1, resulting in activation of the cyclin B/Cdk1 complex. This positive feedback loop ensures entry into mitosis. Conversely, Cdk1 also generates a negative feedback loop by activating the anaphase-promoting complex (APC/CCdc20) that promotes degradation of cyclin B, thus ensuring the exit of mitosis. These positive and negative feedback loops are thought to constitute an ultrasensitive bistable circuit to generate the cell cycle oscillator (Ferrell, 2013).
Mitochondria are important organelles that generate ATP in aerobic eukaryotes and participate in other aspects of cellular metabolism and cell signaling. It has been thought that mitochondria produce ROS as a by-product; however, recent studies have shown that mitochondrial ROS (mtROS) can mediate intracellular signaling. For instance, mtROS generated in complex III was shown to be essential in antigen-specific T cell activation in vivo (Sena and Chandel, 2012). In fact, there are at least 11 sites in mitochondria that produce ROS (Brand, 2016, Mailloux, 2015). Although mitochondrial complexes I and III are thought to be the major sources of mtROS, their contributions to overall ROS production appear to differ among species, organs, tissues, and mitochondrial subpopulations. For example, complex III produces most of the ROS generated by heart and lung mitochondria, while complex I is responsible for most of the ROS produced in brain mitochondria in vitro (Barja and Herrero, 1998, Turrens and Boveris, 1980, Turrens et al., 1982). How or whether mtROS-producing enzymes affect cellular embryonic processes in vivo, however, has remained largely unknown.
Using a transgenic Xenopus line expressing an H2O2 indicator, HyPer, we found that fertilization induces a rapid increase in ROS levels in vivo. Using this assay, we then explored both the molecular mechanisms that trigger the burst of ROS and how these ROS effect early embryonic development. We show that Ca2+ induces ROS production after fertilization and that the major sources of ROS during the early embryonic development are the mitochondria. We then show that mtROS production oscillates with the cell cycle and this oscillation participates in the regulation of the cell cycle in early Xenopus embryos, at least partly through ROS-mediated modulation of the cell cycle phosphatase Cdc25C.
Results
Fertilization Induces Increased ROS Levels in Xenopus Oocytes
We previously showed that Xenopus tadpole tail amputation induces sustained ROS production, which is necessary for successful tail regeneration (Love et al., 2013). For that study, we generated a transgenic Xenopus laevis line that ubiquitously expressed the H2O2 sensor HyPer (Love et al., 2011, Love et al., 2013). Serendipitously, we found that HyPer was expressed maternally in eggs in the transgenic females. We subsequently found that fertilization induced an 85% increased HyPer ratio (n = 11; 1-cell stage compared to egg, p = 0.001, Wilcoxon matched-pairs signed-rank test), indicating an increased production of ROS that was sustained throughout early development (Figures 1A and 1B; Movie S1).
Figure 1.
Fertilization and Injury Trigger a Substantial Increase in ROS Levels
(A) HyPer ratio images (500/430 nm) showing a ROS production in transgenic embryos expressing HyPer. See also Movie S1.
(B) Quantification of HyPer ratio in (A). n = 11; p = 0.001, 1 cell compared to egg, Wilcoxon matched-pairs signed-rank test.
(C) Schematic diagram of oocytes experiments. Immature ovarian oocytes were injected with HyPer RNA, matured with 2 μM progesterone, and then pricked by a needle or laser wound activated.
(D) HyPer images of immature oocytes expressing HyPer were captured every 20 min after pricking. There is no increase in the HyPer ratio.
(E) Quantification of HyPer ratio in (D). n = 33; p = 0.2, 20 min compared to 0 min, paired t test.
(F) HyPer images of mature oocytes expressing HyPer were captured every 20 min after pricking. There is an increase in the HyPer ratio.
(G) Quantification of HyPer ratio in (F). n = 28; p < 0.0001, 20 min compared to 0 min, paired t test.
(H) SypHer images of mature oocytes expressing Sypher were captured every 20 min after pricking.
(I) Quantification of SypHer ratio in (H). n = 27; p < 0.0001, 20 min compared to 0 min, Wilcoxon matched-pairs signed-rank test.
Scale bars, 200 μm (A, D, F, and H). Data are from two independent experiments. Error bars represent mean ± SEM. ∗∗∗p ≤ 0.001 and ∗∗∗∗p < 0.0001; ns, not significant. See also Figure S1 and Movie S2.
To examine the mechanisms regulating fertilization-induced ROS production in Xenopus embryos, we injected non-transgenic, immature oocytes with HyPer mRNAs. We allowed these mRNAs to translate and induced maturation in a subset of these injected oocytes with progesterone (Figure 1C). While HyPer-expressing immature oocytes failed to activate following pricking and they did not show a change in their ROS levels (n = 33; p = 0.2, 20 min compared to 0 min, paired t test) (Figures 1D and 1E), HyPer-expressing mature oocytes were immediately activated by pricking, and they showed a dramatic increase in ROS levels (43.5% increase in ratio; n = 28; p < 0.0001, 20 min compared to 0 min, paired t test), mimicking the increase ROS levels following fertilization (Figures 1F and 1G). We also found that prick activation of unfertilized eggs obtained from transgenic HyPer-expressing females also resulted in a dramatic increase in ROS production (n = 60; p < 0.0001, 20 min compared to 0 min, paired t test) (Figures S1A and S1B). Since HyPer has also been shown to be pH sensitive, oocytes were also injected with SypHer RNA, which encodes a pH-sensitive but ROS-insensitive mutant version of HyPer (Poburko et al., 2011). While we detected a slight increase of SypHer ratio in prick-activated eggs (3.5% increase in ratio; n = 27; p < 0.0001, 20 min compared to 0 min, Wilcoxon matched-pairs signed-rank test) (Figures 1H and 1I), corresponding to a pH increase from 7.37 to 7.42 and consistent with a previous finding (Webb and Nuccitelli, 1981), the change was considerably less than that seen with the ROS-sensitive HyPer version. Therefore, we concluded that the change of HyPer ratio following fertilization is primarily due to an increase in ROS levels rather than a change in pH. Thus, both fertilization and prick activation of mature oocytes and eggs led to a significant increase in ROS production in the activated eggs/zygotes.
To further confirm whether egg activation resulted in elevated ROS levels, we turned to an alternative ROS-detecting assay based on Amplex Red (AR), a compound that can specifically react with H2O2 in the presence of horseradish peroxidase (HRP) to produce a fluorescent product, resorufin (Figure S1C) (Kalyanaraman et al., 2012). We injected AR into unfertilized albino eggs with or without HRP, and then we filmed the production of resorufin. We observed strong fluorescence in activated eggs injected with AR and HRP (Figure S1D; Movie S2). Taken together, these data indicate that H2O2 is generated in mature oocytes and eggs following activation or fertilization and that the ROS levels increased.
ROS Production Is Dependent on the Ca2+ Wave after Fertilization
Previous studies have shown that sperm entry induces a Ca2+ wave, which activates development, cytoskeletal changes, re-entry of the meiotic cell cycle, and subsequent initiation of the mitotic cell cycle (Nader et al., 2013). Oocyte maturation is associated with a rearrangement of Ca2+-signaling components, whereby oocytes acquire the ability to propagate a Ca2+ wave after fertilization (El-Jouni et al., 2005, Machaca, 2004). Since ROS were not induced in immature oocytes by pricking (Figures 1D and 1E), we hypothesized that Ca2+ signaling may act upstream of ROS production.
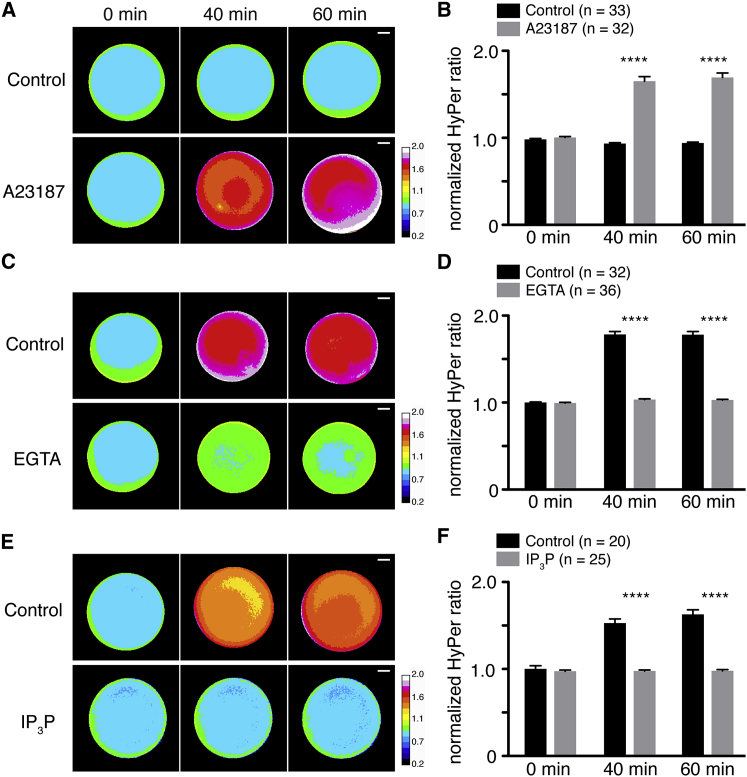
To test this hypothesis, first we confirmed that a Ca2+ wave was induced by laser wounding (mimicking pricking activation) in mature oocytes expressing R-GECO, a genetically encoded calcium sensor (Movie S3) (Zhao et al., 2011). A similar wave was not detected in laser-activated mCherry control immature or mature oocytes, nor in immature R-GECO-injected oocytes (Movie S3). Notably, the Ca2+ wave was also induced in mature oocytes by the addition of 10 μM A23187, a Ca2+ ionophore, but not following incubation with 0.1% ethanol, the final concentration of solvent used for the ionophore (Movie S4). Importantly, the Ca2+ ionophore also induced a 76.7% and 80% increase of HyPer ratio at 40 and 60 min (n = 32–33; p < 0.0001, compared to control, two-way ANOVA) (Figures 2A and 2B), suggesting that activation of the Ca2+ wave was sufficient to induce the increase in ROS production. To determine if Ca2+ influx was necessary for ROS production, we transferred oocytes to calcium-free OR2 medium containing the Ca2+ chelator EGTA (100 μM) for 5 min prior to activation. Extracellular Ca2+ has been shown to be required for fertilization in Xenopus (Wozniak et al., 2017). In the presence of calcium-free OR2 medium containing EGTA, the Ca2+ wave was completely inhibited in laser-activated oocytes (Movie S5), and ROS production was also inhibited 42% and 42.2% at 40 and 60 min after prick activation (n = 32–36; p < 0.0001, compared to control, two-way ANOVA) (Figures 2C and 2D).
Figure 2.
ROS Production Is Generated Downstream of Ca2+ Signaling
(A) HyPer images of control-treated or Ca2+ ionophore A23187 (10 μM) -treated mature oocytes injected with HyPer RNA.
(B) Quantification of HyPer ratio in (A). n = 32–33; p < 0.0001, compared to control at 40 and 60 min, two-way ANOVA and Sidak post hoc tests.
(C) Oocytes injected with HyPer RNA were matured and cultured in calcium-free OR2 medium with or without EGTA (100 μM), and then HyPer images were taken after prick activation.
(D) Quantification of HyPer ratio in (C). n = 32–36; p < 0.0001, compared to control at 40 and 60 min, two-way ANOVA and Sidak post hoc tests.
(E) Oocytes were injected with 20 ng of R-GECO as a control or inpp5a and HyPer RNAs, matured, and then imaged after prick activation.
(F) Quantification of HyPer ratio in (E). n = 20–25; p < 0.0001, compared to control at 40 and 60 min, two-way ANOVA and Sidak post hoc tests.
Scale bars, 200 μm (A, C, and E). Data are from three independent experiments. Error bars represent mean ± SEM. ∗∗∗∗p < 0.001. See also Movies S3, S4, S5, and S6.
It is known that the Ca2+ wave in Xenopus zygotes after fertilization is mediated by the IP3-signaling pathway (Larabell and Nuccitelli, 1992, Nuccitelli et al., 1993). Consistently, when IP3 signaling was inhibited by overexpressing the IP3 phosphatase (Inpp5a) in the oocytes, the Ca2+ wave was completely abolished (Movie S6) and production of ROS was not detected in prick-activated matured oocytes with 36% and 39.8% reduction in ratio at 40 and 60 min (n = 20–25; p < 0.0001, compared to control, two-way ANOVA) (Figures 2E and 2F). These results demonstrate that the fertilization/activation-induced Ca2+ wave is both necessary and sufficient to induce ROS production in mature Xenopus oocytes and eggs.
ROS Production Downstream of Ca2+ Wave Is Mediated by Mitochondria
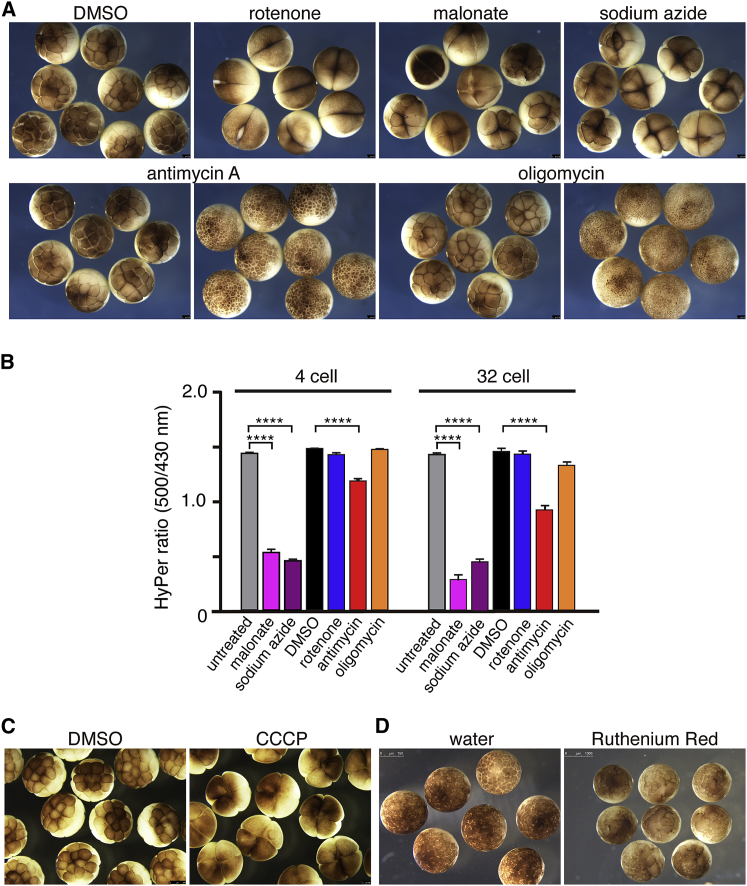
We next sought to identify the source of ROS production downstream of Ca2+ signaling. Cellular ROS are mainly produced by either NOX family enzymes or the ETC in mitochondria. We found that the addition of the NOX inhibitors, 10 μM DPI and 1 mM apocinin, had no effect on ROS production following prick activation in mature oocytes (n = 29–42) (Figure 3A). In addition, application of the mitochondrial complex I inhibitor rotenone (1 μM), which promotes ROS production in the forward direction and inhibits ROS production via reverse electron transport (RET) at complex I (Murphy, 2009), did not change HyPer ratios (n = 36; two-way ANOVA) (Figure 3B), suggesting that complex I is not involved in fertilization-induced ROS production, neither in the forward nor the reverse direction. Addition of high levels of 100 μM DPI, which also inhibits ROS production via RET from complex I (Lambert et al., 2008), also did not affect HyPer ratios in activated eggs (data not shown), providing further evidence that the mitochondrial complex I is not the primary source of increased ROS production following egg activation. In contrast, we found that incubation of mature oocytes with 5 mM malonate, which inhibits ROS production from complex II in both the forward and reverse directions (Quinlan et al., 2012), significantly reduced ROS levels both before (52.8% decrease in ratio) and after activation (68.1%–69.8% decrease in ratio) (n = 45; p < 0.0001, compared to controls at each time point, two-way ANOVA) (Figure 3C). A mitochondrial complex III inhibitor, 10 μM antimycin A, and a complex IV inhibitor, 1 mM sodium azide, also attenuated ROS production after prick activation (23.2%–25.9% and 28.7%–31.8%, respectively; n = 46 and 25; p < 0.0001, compared to control at 40 and 60 min, two-way ANOVA) (Figures 3D and 3E).
Figure 3.
Mitochondrial Inhibitors Malonate, Antimycin A, and Sodium Azide Impair ROS Production after Activation
(A) NOX inhibitors 10 μM DPI or 1 mM apocynin had no effect on HyPer ratio in mature oocytes from HyPer transgenic females after pricking. n = 29–42, two-way ANOVA and Tukey’s post hoc tests.
(B–F) HyPer ratio on oocytes treated with 1 μM rotenone (n = 36; B), 5 mM malonate (n = 45; p < 0.0001, compared to control at each time point, two-way ANOVA and Sidak post hoc tests; C), 10 μM antimycin A (n = 46; p < 0.0001, compared to control at 40 and 60 min, two-way ANOVA and Sidak post hoc tests; D), 1 mM sodium azide (n = 25; p < 0.0001, compared to control at 40 and 60 min, two-way ANOVA and Sidak post hoc tests; E), and 6 μM oligomycin (n = 42; F).
(G) Ca2+ wave was measured by fluorescent intensity of R-GECO in laser-activated mature oocytes. Ca2+ wave was not affected by any of the mitochondrial inhibitors. Each treatment, n = 6; two independent experiments; two-way ANOVA and Tukey’s post hoc tests. See also Movie S7.
(H) HyPer ratio on oocytes treated with 2 μM FCCP. n = 36; p < 0.0001, compared to control at 40 and 60 min, two-way ANOVA and Sidak post hoc tests.
Data for (A)–(F) and (H) are from three or four independent experiments. Error bars represent mean ± SEM; ns, not significant; ∗∗∗∗p < 0.0001.
We wondered whether the lack of ROS production following the addition of complex II, III, and IV inhibitors might be due to the depletion of ATP in the mature oocytes. Therefore, we also added the ATP synthase inhibitor oligomycin (6 μM), but this did not affect ROS production in the activated oocytes (n = 42; two-way ANOVA) (Figure 3F). Furthermore, we found that none of the ETC inhibitors decreased ATP levels in the activated oocytes (n = 3; unpaired t test and Mann-Whitney test) (Figure S2A). To exclude the possibility that the ETC inhibitors affected the Ca2+ wave, we tested the ETC inhibitors in R-GECO-expressing oocytes, and we found that none of the inhibitors affected the propagation of the Ca2+ wave following laser wounding (n = 6; two-way ANOVA) (Figure 3G; Movie S7). Finally, we assessed whether the ETC inhibitors affected the intracellular pH of the activated oocytes expressing SypHer, and we found that none of the inhibitors affected the SypHer ratio (Figures S3A–S3E), confirming that the increases in HyPer ratios were due to changes in ROS levels rather than changes in pH levels in the activated oocytes. In summary, these data show that mitochondria are largely responsible for ROS production following egg activation/fertilization and, interestingly, ROS production is primarily generated from complex II of the ETC. Furthermore, the data suggest that mitochondria are not a major source of ATP production following egg activation/fertilization.
To confirm whether the mitochondria are indeed responsible for ROS production after egg activation/fertilization, we employed a mitochondrial targeted HyPer construct (mitoHyPer) to more specifically detect ROS produced in mitochondria (Malinouski et al., 2011). Immature oocytes were injected with mitoHyPer RNA, and the oocytes were then matured and activated by pricking in the presence of the ETC inhibitors. Although the increases in mitoHyPer ratios were less apparent than those seen with the cytosolic version of HyPer, similar effects of inhibitors on ROS production were observed using mitoHyPer, in that 5 mM malonate (n = 46; 19.8%, 34.6%, and 35.7% at 0, 40, and 60 min), 10 μM antimycin A (n = 42; 14.5% and 16.5% at 40 and 60 min), and 1 mM sodium azide (n = 48; 13.1% and 14.9% at 40 and 60 min) decreased ROS production significantly in activated oocytes (p < 0.0001, compared to control, two-way ANOVA), while rotenone (n = 46) and oligomycin (n = 40) did not (Figures S3F–S3J). These findings suggest that, while ROS production is primarily generated in the mitochondria, the bulk of the ROS generated within the mitochondria rapidly diffuses into the cytoplasm.
ROS production in mitochondria is known to be regulated by the amplitude of the mitochondrial membrane potential (ΔΨm) (Andreyev et al., 2005). To examine if ROS production in oocytes depends on the ΔΨm, oocytes expressing HyPer were treated with the mitochondrial uncoupler carbonyl cyanide 3-chlorophenylhydrazone (CCCP). As shown in Figure 3H, ROS production was significantly reduced by CCCP treatment when compared with controls (39.5% and 39.1% decreases at 30 and 60 min; n = 36; p < 0.0001, compared to control at 30 and 60 min, two-way ANOVA). Taken together, these data suggest that mitochondria are responsible for the generation of ROS downstream of Ca2+ after fertilization/activation.
Ca2+ Wave Directly Mediates ROS Production by Mitochondria via MCU
Previous reports have shown that mitochondria uptake calcium from the cytosol via the mitochondrial calcium uniporter (MCU) (Szabadkai and Duchen, 2008, Xu and Chisholm, 2014). We therefore wondered whether MCU-mediated calcium import was essential for the ROS production following fertilization. To test this hypothesis, we injected oocytes with 0.4 pmol ruthenium red (RuR) (0.4 μM final concentration), an inhibitor of MCU, along with HyPer mRNA. Indeed, oocytes injected with RuR and HyPer mRNA failed to produce ROS after pricking (29.1%–30.5% decrease; n = 23–24; p < 0.0001, compared to control at each time point, Mann-Whitney test) (Figures 4A and 4B). To confirm that the inhibition of ROS production in oocytes injected with RuR was specific to MCU, we explored an independent way of inhibiting MCU. It was previously shown that the MCU functions as a tetramer. Furthermore, Raffaello and colleagues (Raffaello et al., 2013) showed that MCUb (also called Ccdc109b), encodes an MCU-related protein that acts as an endogenous dominant negative of MCU activity, disrupting the ability for MCU to form a functional tetramer. To exploit this inhibitory property of mcub, we injected mRNA encoding X. tropicalis mcub RNA with HyPer RNA, and we found that overexpressing mcub reduced ROS production in the prick-activated mature oocytes (9.4% and 11.5% decreases; n = 24; p < 0.01, compared to control at 30 min and p < 0.001 at 60 min, two-way ANOVA) (Figures 4C and 4D). Notably, the Ca2+ wave after laser activation was not affected by the overexpression of mcub (Movie S8). These data show that the increased Ca2+ after fertilization enters the mitochondria via the MCU and this results in an increase in mtROS production.
Figure 4.

Ca2+ Mediates ROS Production by Mitochondria via MCU
(A) HyPer images of oocytes injected with 0.4 pmol RuR (0.4 μM final concentration), MCU inhibitor, and 20 ng HyPer RNA.
(B) Quantification of HyPer ratio in (A). ROS production was reduced by MCU inhibition. Two independent experiments; ∗∗∗∗p < 0.0001, compared to control at each time point, Mann-Whitney test. Error bars represent mean ± SEM.
(C) HyPer images of oocytes injected with 20 ng of RNA for dominant-negative MCU, mcub, and 20 ng HyPer RNA compared to 20 ng HyPer RNA-injected control.
(D) Quantification of HyPer ratio in (C). Inhibition of MCU by overexpression of mcub impairs ROS production. Error bars represent mean ± SEM.
Two independent experiments; ∗∗p < 0.01 and ∗∗∗p < 0.001, compared to control at t30 and t60, two-way ANOVA and Sidak post hoc tests. See also Movie S8. Scale bars, 200 μm (A and D).
mtROS Inhibition Causes Cell Division Arrest during the Early Cleavage Stages of the Embryo
To elucidate the role of ROS produced by mitochondria in early development, one-cell-stage embryos were treated with various mitochondrial inhibitors toward the end of the first cell cycle. Figure 5A shows phenotypes of treated embryos observed at the 32-cell stage. Embryos treated with 1 μM rotenone stopped dividing at the 2-cell stage (100%; n = 92). 10 mM malonate and 3 mM sodium azide also caused cell division arrest at the 4- or 8-cell stage (100%; n = 40–58). Embryos treated with 10 μM antimycin A developed normally until the 64-cell stage (97.6%; n = 83); however, their division rates were delayed, and they ultimately underwent embryonic death at the blastula stage (100%; n = 83). Embryos treated with 6 μM oligomycin developed normally up to the blastula stage (n = 88) (Figure 5A).
Figure 5.
Inhibition of mtROS Induces Cell Division Arrest in Xenopus Early Development
(A) Embryos were treated at the one-cell stage with 0.1% DMSO (n = 36), 1 μM rotenone (n = 92), 10 mM malonate (n = 40), 3 mM sodium azide (n = 58), 10 μM antimycin A (n = 83), and 6 μM oligomycin (n = 88). Embryos treated with rotenone, malonate, and sodium azide were arrested at the 2- to 8-cell stage. By the blastula stage embryos treated with antimycin A were arrested.
(B) 1-cell stage embryos obtained from females expressing HyPer were treated with mitochondrial inhibitors, and HyPer ratio was measured at the 4-cell and 32-cell stages. n = 12–13; ∗∗∗∗p < 0.0001, two-way ANOVA and Tukey’s multiple comparisons tests. Error bars represent mean ± SEM.
(C) Embryos were treated with 0.1% DMSO or 2 μM CCCP (n = 66).
(D) Embryos were injected with water as a control and 50 pmol RuR into one cell at the 2-cell stage. Injection of RuR induced cell-cycle arrest (n = 56).
Pictures in (A), (C), and (D) are representative of at least three independent experiments.
To determine whether these phenotypes correlated with a reduction of ROS production, 1-cell-stage embryos obtained from transgenic females expressing HyPer were treated with the various mitochondrial inhibitors to monitor ROS levels. We observed an increase in HyPer ratio in untreated (n = 12) and DMSO (n = 13) control embryos at the 4-cell and 32-cell stages (Figure 5B). As seen in oocytes, malonate (n = 11) and sodium azide (n = 12) significantly reduced ROS levels 62.9% and 67.7%, respectively, in 4-cell-stage embryos in which cell division was arrested (p < 0.0001, compared to untreated control, two-way ANOVA). Antimycin A, which caused cell-cycle delay and eventual arrest only at the blastula stage, only slightly reduced ROS production (20.3% at 4-cell stage; n = 12; p < 0.0001, compared to DMSO control, two-way ANOVA). Oligomycin, an ATP synthase inhibitor, had no effect on either the cell division rates or the ROS levels (n = 11). Although rotenone induced rapid cell division arrest, ROS reduction in these embryos was not observed (n = 12). Given that rotenone has been shown to affect actin dynamics through modification of Rho-GTPase (Sanchez et al., 2008), which are known to be necessary for cytokinesis (Drechsel et al., 1997), we interpret this early cell cycle division defect as an inhibition of cytokinesis, rather than inhibiting of the cell cycle oscillator. ATP levels in the embryos treated with the various inhibitors were also measured, and we confirmed that there were no significant reductions in ATP levels in the inhibitor-treated embryos (n = 6; unpaired t test) (Figure S2B), and, thus, the cell-cycle arrest cannot be due to a decrease in ATP levels in the embryos. Consistent with ROS reduction in oocytes treated with CCCP (Figure 3H), embryos treated with 2 μM CCCP also exhibited cell division arrest at the 4-cell stage (100%; n = 66) (Figure 5C). Injection of 50 pmol RuR, the MCU inhibitor that caused a reduction of ROS in oocytes (Figure 4A), also induced cell division defects at the injection site (100%; n = 56) (Figure 5D). Taken together, these data suggest a strong link between ROS production from mitochondria and progression of the cell-cycle.
We then asked whether the defect of cell division could be rescued by the addition of either H2O2 or menadione, but neither was able to rescue the cell division arrest caused by malonate or sodium azide (data not shown). This is likely due to the difficulty in reconstituting the correct level of ROS and its dynamics via simple addition of these oxidants. However, given that malonate and sodium azide are reversible inhibitors of mitochondrial complex II and complex IV, respectively, we tested whether cell division and ROS production could be restored after the removal of these inhibitors. Thus, we treated fertilized embryos with the inhibitors, and once they had arrested at the 4-cell stage, we transferred the embryos into fresh medium without the inhibitors. The removal of inhibitor led to the re-initiation of the cell cycle in 48% of embryos treated with malonate and 93% of embryos treated with sodium azide (n = 42–44) (Figure 6A). Remarkably, most of the rescued embryos then developed to the swimming tadpole stage. Using embryos expressing HyPer, we confirmed that ROS production was restored within 30 min after the removal of inhibitors (Figure 6B; Movie S9). After treatment of inhibitors (0 min), HyPer ratios were 49.1% in malonate-treated embryos and 33.8% in sodium azide-treated embryos compared to control embryos, but they recovered to 84.9% and 78.9% within 40 min after removal of the inhibitors, respectively (n = 36). Taken together, these data confirm that the cell division defects observed in the embryos treated with malonate and sodium azide were primarily caused by the reduction in ROS levels and that the cell cycle is able to re-initiate once ROS levels are allowed to be restored following inhibitor removal.
Figure 6.
Removal of Inhibitors Restores Cell Division and ROS Production
(A) Embryos were treated with inhibitors, 10 mM malonate, 3 mM sodium azide, and 1 μM rotenone, for 40 min until cells stopped dividing (left column), and then they were transferred to a dish containing fresh medium without inhibitors. Embryos transferred after treatment with malonate and azide retrieved cell division and divided to 8- to 16-cell stages (middle), but not in medium with inhibitors (right). Pictures are representative of three independent experiments.
(B) Fertilized transgenic embryos expressing HyPer were treated with inhibitors for 40 min and imaged shown as 0 min. Then embryos were transferred to fresh medium without inhibitors, and imaged at 40 and 70 min (n = 34–36; three independent experiments).
Error bars represent mean ± SD. See also Movie S9.
Cdc25C-Dependent Cell Cycle Progression Is Regulated by ROS
Next we sought to investigate the molecular mechanisms by which ROS may regulate the cell cycle in the early Xenopus embryos. Given that H2O2 is a potent inhibitor of protein tyrosine phosphatases through the reversible oxidation of their catalytic cysteine residues (Lee et al., 2002, Salmeen et al., 2003), we decided to focus on the Cdc25C phosphatase, a key regulator of the cell cycle that, in yeast, has been shown to be redox sensitive (Rudolph, 2005, Seth and Rudolph, 2006, Sohn and Rudolph, 2003). We therefore endeavored to ask whether Cdc25C might be a target of ROS during the early Xenopus mitotic cycles, especially given that Xenopus Cdc25C is present at constant levels during early Xenopus development (Kumagai and Dunphy, 1992).
Cdc25C activates the cyclin B-Cdk1 complex to induce entry into mitosis by dephosphorylating the inhibitory-phosphorylated Thr 14 and Tyr 15 residues in Cdk-1 (Perdiguero and Nebreda, 2004). Cdc25C itself is also regulated by phosphorylation, and it has been shown that there are at least five sites whose phosphorylation correlates with increased phosphatase activity. In contrast, hypophosphorylation of those five sites and phosphorylation on Ser 287 (Ser216 in human) is associated with inactive Cdc25C (Matsuoka et al., 1998, Peng et al., 1997, Sanchez et al., 1997). To examine Cdc25C phosphorylation, we performed immunoblots using anti-Xenopus Cdc25C antibodies. Embryos treated with the various mitochondrial inhibitors were collected every 15 min and subjected to western blot analysis. Notably, extracts from DMSO control-treated embryos exhibited oscillating hyper- and hypophosphorylation states of Cdc25C, depending on the stage of the cell cycle, correlating with cycling Cdc25C activity (Figure 7A). Inhibitors that had no effect on ROS production, such as 1 μM rotenone and 6 μM oligomycin, did not change the oscillating state of Cdc25C phosphorylation during the cell cycle, suggesting that these inhibitors do not affect the cell cycle oscillator. In contrast, treatment with 10 mM malonate and 3 mM sodium azide, which cause a reduction in ROS levels, resulted in either a delay or total inhibition in the cycling Cdc25C hyper-/hypophosphorylation oscillations (asterisks in Figure 7A), suggesting that reduction in ROS levels affected the cycling activation/deactivation pattern of Cdc25 during the cell cycle.
Figure 7.
Inhibition of mtROS Causes Misregulation of Cdc25C Activity Resulting in Mitotic Arrest
(A) Immunoblots of Xenopus Cdc25C in embryos treated with mitochondrial inhibitors. 10 mM malonate and 3 mM sodium azide, which reduced ROS generation, caused misregulation of Cdc25C (asterisks in the blots).
(B) Embryos were treated with inhibitors at the 1-cell stage (90 min after fertilization) and fixed every 10 min for immunohistochemistry. The staining for α-tubulin, Lamin B1, and DNA was used to identify the phase of cell cycle. The numbers in parentheses indicate numbers of embryos examined. A defect in mitotic entry was observed in embryos treated with malonate and sodium azide. Data are from two to three independent experiments.
(C) Immunoblots of Xenopus cyclin B2 in embryos treated with 10 mM malonate or 3 mM sodium azide. Cyclin B2 degraded before cell-cycle arrest (asterisks in the blots), but its accumulation seemed to be impaired afterward.
(D) Immunoblots of Xenopus Cdc25C, Cdk-Y15, pan-Cdk, cyclin B2, and α-tubulin in activated eggs treated with 0.1% DMSO, 10 mM malonate, or 3 mM sodium azide at 0, 5, 30, and 80 min after prick activation.
(E) Immunoblots detecting human Cdc25C and human Cdc25C-pS216 in activated mature oocytes.
Oocytes injected with human Cdc25C WT or C330S/C377S RNA were treated with DMSO or mtROS inhibitors, prick-activated, and extracted at 0, 30, 60, and 90 min.
All blots are representative of at least three independent experiments.
(F) Plots of measurement of HyPer ratio shown in black (left y axis) and the raw fluorescence intensities at 500 nm of HyPer and YFP embryos shown in the plot as green and yellow lines, respectively (right y axis). Embryos were imaged every 30 s from the beginning of fertilization (0 min) throughout the cleavage stage in Movie S10. Data are representative of at least two independent experiments. See Movie S11.
(G) YFP (500 nm)/CFP (430 nm) ratio of embryos expressing HyPer (green) or SypHer (black and pink) obtained by nuclear transplantation of in vitro-matured oocytes. Embryos were imaged every 30 s when it started dividing (0 min) throughout the cleavage stage. Data are representative of at least two independent experiments (n = 2–4).
Rotenone inhibited cytokinesis but had no effect on either ROS production or the cell cycle oscillator, as evidenced by the continued cycling of Cdc25C hyper-/hypophosphorylation states (Figures 5A, 5B, and 7A). It is known that inhibition of cytokinesis does not affect the cell cycle oscillator in early Xenopus embryos (Gerhart et al., 1984). Thus, cytokinesis cannot be used as sole measure of whether the cell cycle oscillator is operational or not in early frog embryos, as evidenced by the lack of cytokinesis in embryos treated with rotenone, yet these embryos still show an oscillating Cdc25 hyper-/hypophosphorylation pattern.
To investigate which phase of the cell cycle was affected by the various treatments, immunohistochemistry was carried out on embryos using antibodies against α-tubulin and Lamin B1, alongside sytox DNA staining to visualize the chromosomes. As shown in Figure 7B, control embryos proceeded to anaphase of the second cell cycle at 40 min after treatment. On the other hand, embryos showing cell-cycle arrest at 40 min after treatment with malonate or sodium azide had nuclei and microtubules typically seen in prophase. Embryos treated with rotenone proceeded to anaphase similarly to control embryos (data not shown), confirming that the phenotype caused by rotenone is not due to a defect in the nuclear cell cycle. Thus, embryos treated with the mtROS inhibitors (malonate and sodium azide) lost Cdc25C cycling activity and failed to proceed through mitosis, resulting in cell-cycle arrest.
It is known that degradation of cyclin B is critical for mitotic exit. Therefore, we wished to ask whether cyclin B degradation was affected in embryos treated with the mtROS inhibitors. As shown in Figure 7C, cyclin B degraded at interphase in control embryos. In embryos treated with the mtROS inhibitors, cyclin B degradation was observed as expected in interphase before cell-cycle arrest at the 4-cell stage in azide and at the 8-cell stage in malonate (asterisks in Figure 7C). However, after cell-cycle arrest, the accumulation of cyclin B required for mitotic re-entry was not detected. This observation indicates that the failure of mitotic arrest caused by mtROS inhibitors is not due to disregulation of cyclin B degradation, although we did find a disregulation in cyclin B re-accumulation in the subsequent cell cycle.
To determine whether mtROS have an impact on Cdc25C phosphatase activity, the phosphorylation state of Tyr 15 of Cdk1, a direct target of Cdc25C, was examined by immunoblot using an anti-Cdk1-Y15 antibody (Tsai et al., 2014). To examine how Cdc25C activity is regulated by mtROS, unfertilized eggs were activated by pricking and collected at different time points. As shown in Figure 7D, endogenous Cdc25C was hyperphosphorylated before activation (t0), and it was enzymatically active since phosphorylation of Cdk1 at Tyr 15 was not detected. At 30 min after prick activation (t30), control mature oocytes exhibited hypophosphorylation, and, coincident with this, its specific substrate (Cdk1-Tyr15) was phosphorylated, suggesting diminished Cdc25C activity (Figure 7D). However, under the condition where mtROS were inhibited by either malonate or sodium azide, Cdc25C remained enzymatically active after 30 min, as suggested by undetectable Cdk1-Tyr 15 phosphorylation (Figure 7D). Intriguingly, sodium azide, which is the more potent inhibitor of the cell cycle, displayed continued Cdc25 activity and/or Wee1 inactivity, even after 80 min when control oocytes showed recovery of Cdk1-Tyr15 phosphorylation. In addition, activated oocytes treated with malonate, which delays progression through the cell cycle prior to complete inhibition, exhibited an intermediate level Cdk-Tyr15 phosphorylation, suggesting continued Cdc25 activity after 30 min and/or diminished Wee1 activity, relative to the control oocytes (Figure 7D). Consistent with our findings in embryos (Figure 7C), cyclin B degradation still occurred normally by t30 in the activated oocytes treated with malonate or sodium azide, but both inhibitors either delayed (malonate) or inhibited (sodium azide) the recovery of cyclin B levels at 80 min (t80) (Figure 7D). These data indicate that mtROS are involved in the rapid downregulation of Cdc25C activity at the meiotic exit and, in its absence, Cdc25 activity remains high, especially under continual sodium azide treatment.
Cdc25 has been proposed to be regulated by oxidation of the conserved cysteines in the catalytic domain (Rudolph, 2005, Seth and Rudolph, 2006). To test whether these cysteine residues are involved in the dynamic post-translational regulation of Cdc25C, we utilized the oocyte system to overexpress human Cdc25C containing mutations in the two critical redox-sensitive cysteine residues, C330S and C377S (Savitsky and Finkel, 2002). Monitoring the human Cdc25C protein also allowed us to assess the dynamics of the phosphorylation state of serine 216, which is inhibitory to its subcellular localization and activity (Takizawa and Morgan, 2000), as the available anti-Cdc25C (phospho S216) antibody reacts with the human isoform, but not the endogenous Xenopus Cdc25 protein at its homologous position at serine 287. Activation of Cdc25C induced by progesterone has been shown to be important for oocyte maturation, and overexpression of Cdc25C induces precocious oocyte maturation in Xenopus (Gautier et al., 1991, Perdiguero and Nebreda, 2004). Indeed, we noticed that injection of 5 ng wild-type human Cdc25C RNA induced oocyte maturation without progesterone treatment. Since overexpression of mutant human Cdc25C RNA did not inhibit maturation induced by progesterone, and co-injection of human Cdc25C mutant RNA (1–10 ng) with 5 ng wild-type human Cdc25C RNA did not prevent the precocious maturation by the wild-type Cdc25C, it is unlikely that mutant human Cdc25C acts as a dominant negative. We also confirmed that injection of 500 pg mutant RNA had no effect on embryonic development when it was injected at the 1-cell stage (data not shown). For our experiments, we injected 100 pg of wild-type or mutant human Cdc25C RNAs, which did not induce precocious oocyte maturation and did not disrupt maturation induced by progesterone.
We found that both the wild-type (WT) and the C330S/C377S mutant form of human Cdc25 were hyperphoshorylated in the oocytes prior to prick activation. Furthermore, at t0, no detectable phosphorylation at S216 was observed, suggesting that both the WT and mutant forms had the post-translational hallmarks of active Cdc25 (t0 in Figure 7E, top DMSO control-treated oocytes). At 30 min after prick activation, both the WT and mutant Cdc25 became fully hypophosphorylated, and both forms contained the inactive S216 phosphorylation state (t30 in Figure 7E, top DMSO control-treated oocytes). Inhibiting mtROS production following oocyte activation by treating them with malonate resulted in the retention of the hyperphosphorylated WT and mutant forms of Cdc25, as well as the sustained lack of phosphorylation of the inhibitory S216 site, suggesting that inhibition of mtROS via malonate resulted in the retention of post-translational modification of Cdc25 associated with a sustained active state (Figure 7E, middle malonate-treated oocytes). Furthermore, inhibiting mtROS with sodium azide resulted in a delay in the change from hyper- to hyperphosphorylation state of the WT and mutant forms of Cdc25, such that, at t30, there still remained significant amounts of hyperphosphorylated Cdc25, while DMSO-treated oocytes at t30 had no hyperphosphorylated Cdc25 left, and, interestingly, the mutant form was more delayed in acquiring its inhibitory phosphorylation at S216 than the WT form at t30, suggesting that the rate of deactivation in the mutant was slower when Cdc25 lacked the two ROS-sensitive cysteines (Figure 7E, bottom sodium azide-treated oocytes). These data suggest that mtROS facilitate the inactivation of Cdc25C through its ROS-sensitive cysteine residues.
ROS Oscillate with the Cell Cycle
Having seen that inhibiting ROS production leads to cell-cycle arrest, as well as the loss of the oscillatory phosphorylation and activity states of Cdc25, we hypothesized that ROS levels might also oscillate during the early Xenopus embryo, coincident with the cell cycle. Thus, we returned to HyPer imaging of early embryos and began imaging the embryos in greater detail. We found that ROS levels do fluctuate during the cell cycle (Figure 7F, black line; Movies S10 and S11). We also imaged embryos expressing YFP maternally, and fluorescence intensity at 500 nm was measured every 30 s. As shown in Figure 7F, while HyPer signal (green line) showed oscillatory fluctuations, YFP (yellow line) did not. To eliminate the possibility that an oscillating change in pH may be responsible for the oscillating HyPer pattern, we generated embryos expressing SypHer. While we observed a clear oscillation pattern in HyPer ratios (green line), we failed to observe oscillating SypHer ratios in the embryos (pink and black lines) (Figure 7G). These results strongly suggest that ROS levels oscillate through the cell cycle, thus explaining why the cell-cycle arrest could not be simply rescued by the addition of H2O2 or menadione, as these treatments would not have reconstituted the oscillating patterns of ROS necessary for restoring the cell cycle.
Discussion
We have previously shown that tail amputation in Xenopus tadpoles induces a sustained increase in ROS levels throughout tail regeneration and these increased levels are required for tail regeneration (Love et al., 2013). Intriguingly, we show here that fertilization also triggers increased ROS levels, which are sustained, yet oscillating with the cell cycle, during early embryonic development. Given that the events triggered by fertilization can be mimicked by prick activation or laser wounding, one might consider fertilization as a sort of injury, which triggers sustained ROS production and cell cycle progression and development, in the same way that tail amputation triggers sustained ROS production, which is essential for cell proliferation, growth factor signaling, and, ultimately, appendage regeneration. Indeed, we also know that both tissue injury and fertilization induce a rapid Ca2+ wave (Soto et al., 2013), which, in the context of fertilization, is necessary and sufficient for ROS production via the Ca2+ uniporter MCU. Intriguingly, however, we find that the source of ROS production is different in both scenarios. While NADPH oxidase activity is the primary source of ROS production following tail amputation (Love et al., 2013), ROS production following fertilization or egg activation is primarily dependent on the mitochondrial ETC, more specifically complexes II, III, and IV. Although we have used DPI as a potent NADPH oxidase inhibitor, DPI has also been shown to inhibit the FMN coenzyme of complex I (Majander et al., 1994) and ROS production from complex I via RET (Lambert et al., 2008). In our studies, neither high concentration of DPI (100 μM) nor rotenone affected ROS production in oocytes or embryos, indicating that complex I is not responsible for ROS production in early embryos. Thus, we find that complex I, the site traditionally viewed as the major source of mtROS production (Mailloux, 2015, Murphy, 2009), is not involved in ROS production in the early frog embryos, suggesting the mitochondria in early embryos do not behave in a canonical manner. This is further supported by our finding that inhibiting the mitochondrial ETC does not decrease ATP levels in the early embryos. It is thus fascinating to speculate whether mitochondria in early embryos are primarily used as signaling organelles, via ROS production, rather than ATP-producing organelles. The use of mitochondria-targeted antioxidants may provide further information for understanding the mechanism of ROS production by mitochondria during early embryogenesis.
Our previous study on the role of ROS production during appendage regeneration showed that inhibiting ROS production resulted in a significant decrease in proliferation following tail amputation (Love et al., 2013). However, in that study, we also found that both Wnt and FGF signaling were attenuated when ROS production was inhibited. Given that cell proliferation is often controlled via growth factor signaling, it was not clear from that study whether the cell proliferation defect was a consequence of attenuated growth signaling or whether ROS levels affected growth factor signaling and cell proliferation independently. This study provides evidence that ROS are capable of impacting cell proliferation independently from their effects on growth factors signaling. This is because the early cell cycle progression in frog embryos is not dependent on growth factor signaling. Indeed, growth factor signaling does not play a role in development until the mid-blastula stage, when zygotic transcription is initiated (Newport and Kirschner, 1982, Zhang et al., 2013), yet we find that attenuating ROS levels affects the cell cycle at the cleavage stages. Intriguingly, after a burst of ROS production following fertilization, we find that ROS levels then oscillate with the cell cycle and that the elevated, oscillating ROS levels are necessary for the cell cycle to progress. We were unable to rescue cell-cycle arrest caused by mtROS inhibitors by adding H2O2 or menadione. This is likely due to the fact that ROS levels oscillate during the cell cycle, an aspect that was not reproduced adequately in the attempted rescue experiments.
The control of the cell cycle in Xenopus early embryos involves positive and negative feedback loops, which constitute a bistable mitotic trigger (Thron, 1996). For generation of robust bistability, ultrasensitivity of Cdc25C has been suggested (Trunnell et al., 2011). In this study, we have shown that the conserved cysteine residues in Cdc25C help mediate a quick inactivation of Cdc25C, suggesting that ROS may supply Cdc25C with the necessary ultrasensitivity for robustness suggested by Trunnell and colleagues. Although here we have focused on the redox-sensitive Cdc25C, future studies will strive to identify other redox-sensitive proteins and how they utilize the fertilization-induced, oscillatory ROS production to ensure successful progression through early embryonic development.
Experimental Procedures
Further details and an outline of resources used in this work can be found in the Supplemental Experimental Procedures.
Experimental Animals
All experiments involving animals were approved by the local ethics committee and the Home Office. Unfertilized oocytes were obtained from X. laevis WT females by injecting with 250 units of human chorionic gonadotropin (HCG). Embryos were obtained from in vitro fertilization. Ovaries were isolated from females after they had been humanely sacrificed.
Manipulation of Xenopus Oocytes and Embryos
RNA synthesis using SP6 Message Machine was carried out following the manufacturer’s protocol (Life Technologies). Stage VI oocytes were isolated manually from ovaries of pregnant mare serum gonadotropin (PMSG)-primed female frogs, injected with 20 ng HyPer or SypHer RNA, and cultured in OR2 medium at 16°C for 24 hr. Then progesterone was added to the medium at a final concentration of 2 μM for 16 hr at 16°C. The 20 ng HyPer RNA was co-injected with 20 ng inpp5a phosphatase RNA or 20 ng mcub RNA, cultured, and matured as above. To inhibit MCU, oocytes were injected with 0.4 pmol RuR (0.4 μM final concentration) and 20 ng HyPer RNA, and they were cultured in OR2 medium at 16°C for 24 hr before in vitro maturation. Embryos were injected with 10 nL 5 mM RuR into one blastomere at the 2-cell stage and cultured at 22°C in 0.1X Marc’s modified ringers (MMR).
Oocytes injected with 100 pg human Cdc25c RNA for WT or cysteine mutants were treated with progesterone immediately after injections. Matured oocytes were identified by the presence of a white spot at the animal pole, associated with germinal vesicle breakdown. Oocytes were incubated with inhibitors for 20 min before prick activation.
Statistical Analysis
Statistical analyses were performed with Prism (GraphPad). Data were first checked for variance, and the appropriate statistical tests showed in each figure legend were used to generate p values; p values < 0.05 were considered significant as follows: ∗ p < 0.05, ∗∗ p < 0.01, ∗∗∗ p < 0.001, and ∗∗∗∗ p < 0.0001.
Acknowledgments
We thank Professor J.E. Ferrell, Jr. and Dr. J. Gannon for the anti-Xenopus Cdc25C antibodies and Dr. Peter March for his help with imaging. The Bioimaging Facility microscopes used in this study were purchased with grants from BBSRC, Wellcome Trust, and the University of Manchester Strategic Fund. This work was supported by two studentships and a grant from The Healing Foundation, now known as the Scar Free Foundation (Y.H., N.R.L., Y.C., and E.A.) and an MRC Research Project Grant (MR/L007525/1) (S.I., J.I.-G., and E.A.).
Author Contributions
S.I. and E.A. conceived the study. Y.C. and N.R.L. initially observed and imaged ROS generation in embryos. Y.H. designed and performed oocyte experiments and HyPer/calcium imaging. S.I. designed and carried out plasmid construction, AmplexRed assays, mcub and cell cycle experiments, immunoblots, and HyPer/SypHer imaging using nuclear transplantation. J.I.-G. imaged ROS oscillation in embryos. S.I., N.R.L., and E.A. wrote the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: January 2, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, three figures, and eleven movies and can be found with this article online at https://doi.org/10.1016/j.celrep.2017.12.042.
Supplemental Information
Signal was obtained using excitation filters of 490/20 nm (left) and 402/15 nm (middle) with an emission filter of BP525/36. The HyPer ratio was calculated using ImageJ (right).
Albino oocytes were injected with AR only (left) and AR with HRP, and time lapse movies were taken every 1 min after injection using Leica M205FA with DSR filter (pseudo color).
Image of immature (left) and mature (mature) oocytes injected with mCherry (3 s of movie) or R-GECO (3 s of movie). Ca2+ wave was observed in the R-GECO movie, but only in the mature oocyte (right).
Ethanol alone control does not induce Ca2+ wave (left).
Time lapse movies were taken every 30 s after 4-cell arrested embryos were transferred to medium without inhibitors.
Sperm solution was added to unfertilized oocyte expressing HyPer, and imaged every 30 s for 5 hr. Images were processed without smooth using ImageJ and Brightness/Contrast was set between 1.2 and 1.6.
A dividing embryo after fertilization was imaged every 30 s for 5 hr with 1,000 ms exposure for YFP500 and 500 ms for CFP430. Images were processed without smooth and Brightness/Contrast was set between 2.9 and 3.8.
References
- Andreyev A.Y., Kushnareva Y.E., Starkov A.A. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc.) 2005;70:200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- Barja G., Herrero A. Localization at complex I and mechanism of the higher free radical production of brain nonsynaptic mitochondria in the short-lived rat than in the longevous pigeon. J. Bioenerg. Biomembr. 1998;30:235–243. doi: 10.1023/a:1020592719405. [DOI] [PubMed] [Google Scholar]
- Bedard K., Krause K.-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Brand M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016;100:14–31. doi: 10.1016/j.freeradbiomed.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Burgoyne J.R., Oka S., Ale-Agha N., Eaton P. Hydrogen peroxide sensing and signaling by protein kinases in the cardiovascular system. Antioxid. Redox Signal. 2013;18:1042–1052. doi: 10.1089/ars.2012.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantu-Medellin N., Kelley E.E. Xanthine oxidoreductase-catalyzed reactive species generation: A process in critical need of reevaluation. Redox Biol. 2013;1:353–358. doi: 10.1016/j.redox.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsel D.N., Hyman A.A., Hall A., Glotzer M. A requirement for Rho and Cdc42 during cytokinesis in Xenopus embryos. Curr. Biol. 1997;7:12–23. doi: 10.1016/s0960-9822(06)00023-6. [DOI] [PubMed] [Google Scholar]
- El-Jouni W., Jang B., Haun S., Machaca K. Calcium signaling differentiation during Xenopus oocyte maturation. Dev. Biol. 2005;288:514–525. doi: 10.1016/j.ydbio.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Ferrell J.E., Jr. Feedback loops and reciprocal regulation: recurring motifs in the systems biology of the cell cycle. Curr. Opin. Cell Biol. 2013;25:676–686. doi: 10.1016/j.ceb.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerder C.A., Klebanoff S.J., Shapiro B.M. Hydrogen peroxide production, chemiluminescence, and the respiratory burst of fertilization: interrelated events in early sea urchin development. Proc. Natl. Acad. Sci. USA. 1978;75:3183–3187. doi: 10.1073/pnas.75.7.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J., Solomon M.J., Booher R.N., Bazan J.F., Kirschner M.W. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991;67:197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- Gerhart J., Wu M., Kirschner M. Cell cycle dynamics of an M-phase-specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J. Cell Biol. 1984;98:1247–1255. doi: 10.1083/jcb.98.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K., Tydeman P., Kirschner M. A cytoplasmic clock with the same period as the division cycle in Xenopus eggs. Proc. Natl. Acad. Sci. USA. 1980;77:462–466. doi: 10.1073/pnas.77.1.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman B., Darley-Usmar V., Davies K.J.A., Dennery P.A., Forman H.J., Grisham M.B., Mann G.E., Moore K., Roberts L.J., 2nd, Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic. Biol. Med. 2012;52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A., Dunphy W.G. Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell. 1992;70:139–151. doi: 10.1016/0092-8674(92)90540-s. [DOI] [PubMed] [Google Scholar]
- Lambert A.J., Buckingham J.A., Boysen H.M., Brand M.D. Diphenyleneiodonium acutely inhibits reactive oxygen species production by mitochondrial complex I during reverse, but not forward electron transport. Biochim. Biophys. Acta. 2008;1777:397–403. doi: 10.1016/j.bbabio.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Larabell C., Nuccitelli R. Inositol lipid hydrolysis contributes to the Ca2+ wave in the activating egg of Xenopus laevis. Dev. Biol. 1992;153:347–355. doi: 10.1016/0012-1606(92)90119-2. [DOI] [PubMed] [Google Scholar]
- Lee S.-R., Yang K.-S., Kwon J., Lee C., Jeong W., Rhee S.G. Reversible inactivation of the tumor suppressor PTEN by H2O2. J. Biol. Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- Love N.R., Thuret R., Chen Y., Ishibashi S., Sabherwal N., Paredes R., Alves-Silva J., Dorey K., Noble A.M., Guille M.J. pTransgenesis: a cross-species, modular transgenesis resource. Development. 2011;138:5451–5458. doi: 10.1242/dev.066498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love N.R., Chen Y., Ishibashi S., Kritsiligkou P., Lea R., Koh Y., Gallop J.L., Dorey K., Amaya E. Amputation-induced reactive oxygen species are required for successful Xenopus tadpole tail regeneration. Nat. Cell Biol. 2013;15:222–228. doi: 10.1038/ncb2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machaca K. Increased sensitivity and clustering of elementary Ca2+ release events during oocyte maturation. Dev. Biol. 2004;275:170–182. doi: 10.1016/j.ydbio.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Mailloux R.J. Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species. Redox Biol. 2015;4:381–398. doi: 10.1016/j.redox.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majander A., Finel M., Wikström M. Diphenyleneiodonium inhibits reduction of iron-sulfur clusters in the mitochondrial NADH-ubiquinone oxidoreductase (Complex I) J. Biol. Chem. 1994;269:21037–21042. [PubMed] [Google Scholar]
- Malinouski M., Zhou Y., Belousov V.V., Hatfield D.L., Gladyshev V.N. Hydrogen peroxide probes directed to different cellular compartments. PLoS ONE. 2011;6:e14564. doi: 10.1371/journal.pone.0014564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S., Huang M., Elledge S.J. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A.W., Kirschner M.W. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989;339:275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- Nader N., Kulkarni R.P., Dib M., Machaca K. How to make a good egg!: The need for remodeling of oocyte Ca(2+) signaling to mediate the egg-to-embryo transition. Cell Calcium. 2013;53:41–54. doi: 10.1016/j.ceca.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Newport J., Kirschner M. A major developmental transition in early Xenopus embryos: II. Control of the onset of transcription. Cell. 1982;30:687–696. doi: 10.1016/0092-8674(82)90273-2. [DOI] [PubMed] [Google Scholar]
- Nuccitelli R., Yim D.L., Smart T. The sperm-induced Ca2+ wave following fertilization of the Xenopus egg requires the production of Ins(1, 4, 5)P3. Dev. Biol. 1993;158:200–212. doi: 10.1006/dbio.1993.1179. [DOI] [PubMed] [Google Scholar]
- Östman A., Frijhoff J., Sandin A., Böhmer F.-D. Regulation of protein tyrosine phosphatases by reversible oxidation. J. Biochem. 2011;150:345–356. doi: 10.1093/jb/mvr104. [DOI] [PubMed] [Google Scholar]
- Peng C.Y., Graves P.R., Thoma R.S., Wu Z., Shaw A.S., Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- Perdiguero E., Nebreda A.R. Regulation of Cdc25C activity during the meiotic G2/M transition. Cell Cycle. 2004;3:733–737. [PubMed] [Google Scholar]
- Poburko D., Santo-Domingo J., Demaurex N. Dynamic regulation of the mitochondrial proton gradient during cytosolic calcium elevations. J. Biol. Chem. 2011;286:11672–11684. doi: 10.1074/jbc.M110.159962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan C.L., Orr A.L., Perevoshchikova I.V., Treberg J.R., Ackrell B.A., Brand M.D. Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. J. Biol. Chem. 2012;287:27255–27264. doi: 10.1074/jbc.M112.374629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaello A., De Stefani D., Sabbadin D., Teardo E., Merli G., Picard A., Checchetto V., Moro S., Szabò I., Rizzuto R. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J. 2013;32:2362–2376. doi: 10.1038/emboj.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph J. Redox regulation of the Cdc25 phosphatases. Antioxid. Redox Signal. 2005;7:761–767. doi: 10.1089/ars.2005.7.761. [DOI] [PubMed] [Google Scholar]
- Salmeen A., Andersen J.N., Myers M.P., Meng T.-C., Hinks J.A., Tonks N.K., Barford D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature. 2003;423:769–773. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- Sanchez Y., Wong C., Thoma R.S., Richman R., Wu Z., Piwnica-Worms H., Elledge S.J. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- Sanchez M., Gastaldi L., Remedi M., Cáceres A., Landa C. Rotenone-induced toxicity is mediated by Rho-GTPases in hippocampal neurons. Toxicol. Sci. 2008;104:352–361. doi: 10.1093/toxsci/kfn092. [DOI] [PubMed] [Google Scholar]
- Savitsky P.A., Finkel T. Redox regulation of Cdc25C. J. Biol. Chem. 2002;277:20535–20540. doi: 10.1074/jbc.M201589200. [DOI] [PubMed] [Google Scholar]
- Sena L.A., Chandel N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth D., Rudolph J. Redox control of cell cycle progression via Cdc25 phosphatase (Mih1p) in S. cerevisiae. Cell Cycle. 2006;5:2172–2173. doi: 10.4161/cc.5.18.3252. [DOI] [PubMed] [Google Scholar]
- Sohn J., Rudolph J. Catalytic and chemical competence of regulation of cdc25 phosphatase by oxidation/reduction. Biochemistry. 2003;42:10060–10070. doi: 10.1021/bi0345081. [DOI] [PubMed] [Google Scholar]
- Soto X., Li J., Lea R., Dubaissi E., Papalopulu N., Amaya E. Inositol kinase and its product accelerate wound healing by modulating calcium levels, Rho GTPases, and F-actin assembly. Proc. Natl. Acad. Sci. USA. 2013;110:11029–11034. doi: 10.1073/pnas.1217308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadkai G., Duchen M.R. Mitochondria: the hub of cellular Ca2+ signaling. Physiology (Bethesda) 2008;23:84–94. doi: 10.1152/physiol.00046.2007. [DOI] [PubMed] [Google Scholar]
- Takizawa C.G., Morgan D.O. Control of mitosis by changes in the subcellular location of cyclin-B1-Cdk1 and Cdc25C. Curr. Opin. Cell Biol. 2000;12:658–665. doi: 10.1016/s0955-0674(00)00149-6. [DOI] [PubMed] [Google Scholar]
- Thron C.D. A model for a bistable biochemical trigger of mitosis. Biophys. Chem. 1996;57:239–251. doi: 10.1016/0301-4622(95)00075-5. [DOI] [PubMed] [Google Scholar]
- Trunnell N.B., Poon A.C., Kim S.Y., Ferrell J.E., Jr. Ultrasensitivity in the Regulation of Cdc25C by Cdk1. Mol. Cell. 2011;41:263–274. doi: 10.1016/j.molcel.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai T.Y.-C., Theriot J.A., Ferrell J.E., Jr. Changes in oscillatory dynamics in the cell cycle of early Xenopus laevis embryos. PLoS Biol. 2014;12:e1001788. doi: 10.1371/journal.pbio.1001788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens J.F., Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem. J. 1980;191:421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens J.F., Freeman B.A., Levitt J.G., Crapo J.D. The effect of hyperoxia on superoxide production by lung submitochondrial particles. Arch. Biochem. Biophys. 1982;217:401–410. doi: 10.1016/0003-9861(82)90518-5. [DOI] [PubMed] [Google Scholar]
- Warburg O. Beobachtungen über die Oxydationsprozesse im Seeigelei. Hoppe Seylers Z. Physiol. Chem. 1908;57:1–16. [Google Scholar]
- Webb D.J., Nuccitelli R. Direct measurement of intracellular pH changes in Xenopus eggs at fertilization and cleavage. J. Cell Biol. 1981;91:562–567. doi: 10.1083/jcb.91.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J.L., Wessel G.M. Reactive oxygen species and Udx1 during early sea urchin development. Dev. Biol. 2005;288:317–333. doi: 10.1016/j.ydbio.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Wong J.L., Créton R., Wessel G.M. The oxidative burst at fertilization is dependent upon activation of the dual oxidase Udx1. Dev. Cell. 2004;7:801–814. doi: 10.1016/j.devcel.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Wozniak K.L., Mayfield B.L., Duray A.M., Tembo M., Beleny D.O., Napolitano M.A., Sauer M.L., Wisner B.W., Carlson A.E. Extracellular Ca2+ Is Required for Fertilization in the African Clawed Frog, Xenopus laevis. PLoS ONE. 2017;12:e0170405. doi: 10.1371/journal.pone.0170405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Chisholm A.D. C. elegans epidermal wounding induces a mitochondrial ROS burst that promotes wound repair. Dev. Cell. 2014;31:48–60. doi: 10.1016/j.devcel.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Li J., Lea R., Amaya E., Dorey K. A functional genome-wide in vivo screen identifies new regulators of signalling pathways during early Xenopus embryogenesis. PLoS ONE. 2013;8:e79469. doi: 10.1371/journal.pone.0079469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Araki S., Wu J., Teramoto T., Chang Y.-F., Nakano M., Abdelfattah A.S., Fujiwara M., Ishihara T., Nagai T., Campbell R.E. An expanded palette of genetically encoded Ca2+ indicators. Science. 2011;333:1888–1891. doi: 10.1126/science.1208592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Signal was obtained using excitation filters of 490/20 nm (left) and 402/15 nm (middle) with an emission filter of BP525/36. The HyPer ratio was calculated using ImageJ (right).
Albino oocytes were injected with AR only (left) and AR with HRP, and time lapse movies were taken every 1 min after injection using Leica M205FA with DSR filter (pseudo color).
Image of immature (left) and mature (mature) oocytes injected with mCherry (3 s of movie) or R-GECO (3 s of movie). Ca2+ wave was observed in the R-GECO movie, but only in the mature oocyte (right).
Ethanol alone control does not induce Ca2+ wave (left).
Time lapse movies were taken every 30 s after 4-cell arrested embryos were transferred to medium without inhibitors.
Sperm solution was added to unfertilized oocyte expressing HyPer, and imaged every 30 s for 5 hr. Images were processed without smooth using ImageJ and Brightness/Contrast was set between 1.2 and 1.6.
A dividing embryo after fertilization was imaged every 30 s for 5 hr with 1,000 ms exposure for YFP500 and 500 ms for CFP430. Images were processed without smooth and Brightness/Contrast was set between 2.9 and 3.8.