Abstract
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a heterogeneous upper airway disease with multiple etiologies. Clinically, CRSwNP can be classified into either eosinophilic or non-eosinophilic subtypes. The eosinophilic phenotype of CRSwNP is widely thought to be highly associated with recurrence of nasal polyps or surgical failure. Epithelial cells have a crucial role in the development of Th2-biased airway diseases. Recent studies have shown that a wide range of external stimuli such as allergens and microorganisms can elicit the release of epithelial-derived Th2-driving cytokines and chemokines. Protease activity is a feature common to these multiple environmental insults and there is growing evidence for the concept that an imbalance of proteases and protease inhibitors in the epithelial barrier leads to both the initiation and maintenance of chronic eosinophilic airway inflammation. In this review, we analyze recent work on the role of proteases in the development of the sinonasal mucosal type 2 immune response with an emphasis on the molecular pathways promoting adaptive Th2 cell immunity.
Keywords: chronic rhinosinusitis, nasal polyps, eosinophil, protease, epithelium
Introduction
Chronic rhinosinusitis is a chronic inflammatory upper airway disease characterized by 12 weeks of typical symptoms including nasal discharge, congestion, facial pressure or pain, and olfactory disorder (Fokkens et al., 2012). Chronic rhinosinusitis with nasal polyps (CRSwNP), a multifactorial and highly heterogeneous upper airway disease, is a severe phenotype of chronic rhinosinusitis and presents with distinct immunological and histopathological features compared with chronic rhinosinusitis without nasal polyps (CRSsNP).
Despite aggressive medical therapy or radical endoscopic sinus surgical treatment, many patients with CRSwNP tend to be poorly controlled and have a high recurrence rate (Wynn and Har-El, 2004; Mendelsohn et al., 2011; Baguley et al., 2014; DeConde et al., 2017). Several factors which associate with a worse outcome or recurrence risk have been identified, such as high tissue eosinophil infiltration, more severe preoperative disease (i.e., a higher CT score), and a series of comorbid disease (i.e., aspirin-exacerbated respiratory disease (AERD), allergic asthma and cystic fibrosis) (Desrosiers, 2004; Tosun et al., 2010; Mortuaire et al., 2015; Ta and White, 2015; Tipirneni and Woodworth, 2017; Wu et al., 2017).
Clinically, CRSwNP is classified into two phenotypes based on the dominant inflammatory cell type in tissues: eosinophilic CRSwNP (ECRSwNP) and non-eosinophilic CRSwNP (NECRSwNP) (Cao et al., 2009; Shah et al., 2016; Wu et al., 2016; Cho S.-W. et al., 2017). In western countries, the majority of patients with CRSwNP (80–88%) have prominent tissue eosinophilia, edema formation, and a type 2 helper T-cell (Th2) dominant immune response (Bateman et al., 2005; Fokkens et al., 2005; Van Zele et al., 2006). CRSwNP may be associated with asthma and aspirin intolerance (Fokkens et al., 2012; Stevens et al., 2017). However, at least half of patients with CRSwNP in East Asian countries including China, Korea and Japan have a non-eosinophilic phenotyps of nasal polyps characterized by Th1/Th17-dominant inflammation (Kim et al., 2007; Zhang et al., 2008; Cao et al., 2009; Ikeda et al., 2013).
The past decade has witnessed a change in the understanding of mechanisms underlying eosinophilic airway diseases from a paradigm in which allergen-independent, e.g., Th2 cells are the primary drivers, to one in which production of epithelial-derived chemokines and cytokines by dysfunctional respiratory epithelium are the primary orchestrators of the eosinophilic immune response (Hammad and Lambrecht, 2015; Pfeffer and Corrigan, 2017). A large range of both endogenous and extrinsic stimuli can activate the epithelial cell and elicit the release of epithelial-derived chemokines and cytokines which, in turn, induce the type 2 immune response (Hammad and Lambrecht, 2015; Schleimer and Berdnikovs, 2017). External stimuli, including allergen, fungus, Staphylococcus aureus and microbiome disturbance have been posited as significant contributing factors in CRSwNP pathophysiology and have been implicated in driving Th2-biased airway disease (Sachse et al., 2010; Clark et al., 2013; Madeo and Frieri, 2013; Ou et al., 2014; Lan et al., 2016; Orlandi et al., 2016; Tomassen et al., 2016; Schleimer, 2017).
Protease activity is a common unifying feature of many of these environmental insults suggesting an underlying common etiopathogenesis (Sokol et al., 2008; Gregory and Lloyd, 2011; Stentzel et al., 2017; Teufelberger et al., 2017). Airborn allergens, such mites, pollen, as well as microorganisms, such as bacteria, rhinovirus, and influenza virus, and fungi are major sources of exogenous proteases (Reed and Kita, 2004; Sokol et al., 2008; Costenaro et al., 2011; Takai and Ikeda, 2011; Kesic et al., 2012). The innate immune response to these exogenous proteases seems to play a crucial role during the development of Th2-biased immune response (Kamijo et al., 2013; Hara et al., 2014; Snelgrove et al., 2014; Teufelberger et al., 2017). It therefore follows that an imbalance of proteases and protease inhibitors in the epithelial barrier may lead to the initiation and maintainancc of eosinophilic inflammation in CRSwNP and therefore be a central driver of eosinophilic airway disease (Kouzaki et al., 2017; Pfeffer and Corrigan, 2017).
This review will summarize the current knowledge on the role of proteases during the development of the sinonasal mucosal type 2 immune response, with an emphasis on the molecular pathways initiating the innate type 2 cell response and then promoting adaptive Th2 cell immunity. This is followed by a discussion of the dysfunctional regulation of proteases and proteases inhibitors in the epithelial barrier.
Mechanisms of the activation of the airway epithelial cells upon external protease exposure
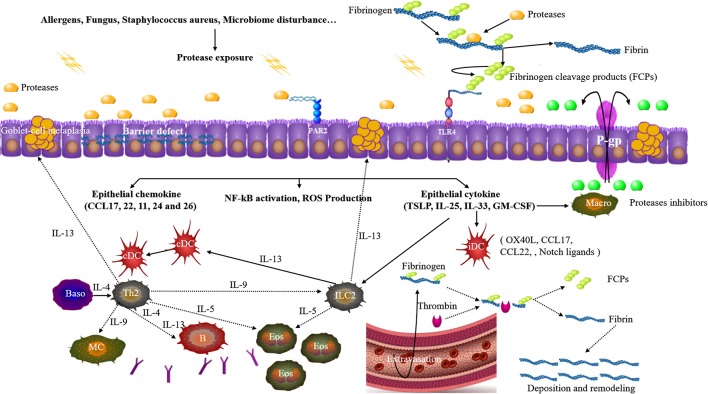
Cysteine and or serine proteases occur in some groups of airborne mite, pollen, cockroach, fungi, and Staphylococcus aureus (Asokananthan et al., 2002; Reed and Kita, 2004; Jacquet, 2011; Takai and Ikeda, 2011; Balenga et al., 2015; Kale et al., 2017; Stentzel et al., 2017; Teufelberger et al., 2017). Allergen derived proteases interact with epithelial cells through three principle pathways: direct effects on junctional proteins, reacting with cell surface protease-activated receptors (PARs), and toll-like receptor 4 (TLR4)-dependent epithelial activation. An integrated mechanism is summarized and illustrated in Figure 1.
Figure 1.
Upon allergen proteases exposure, junctional proteins among epithelial cells are disrupted. Allergen proteases can directly react with protease-activated receptor 2 (PAR2). Allergen proteases cleave the serum factor fibrinogen, thus releasing fibrinogen cleavage products (FCPs) which can activate toll-like receptor 4 (TLR4). Epithelial cells get activated to produce and release pro-Th2 cell chemokines and cytokines which instruct immature dendritic cells (iDC) and activate ILC2s. Additionally, the activation of these receptors will also induce NF-kB activation, ROS production. Th2 cells and ILC2s are activated and promote the eosinophilia, production of IgE and goblet-cell metaplasia. Allergen exposure is generally accompanied by fluid extravasation and thrombin also generates FCPs from fibrinogen, thus triggering TLR4. P-glycoproteins (P-gp) in the epithelial cells promote the efflux of protease inhibitors to suppress the allergen proteases. cDC classical DC, Macro macrophage, Baso basophils, MC mast cell.
Allergen source-derived proteases (both cysteine and serine protease) can directly degrade tight junctions in the epithelium (Wan et al., 1999, 2001; Tai et al., 2006; Runswick et al., 2007; Hirasawa, 2010; Kale et al., 2017) and increase the accessibility of microorganisms and antigens to the underlying lamina propria and connective tissue thereby triggering strong innate immune responses to allergens (Gregory and Lloyd, 2011). It has been reported that the levels of occludin, E-cadherin, and zonula occludens-1 (ZO-1) were all reduced in mature polyps derived from patients with CRSwNP. Moreover, aquaporin 5, a marker of epithelial differentiation, was obviously reduced in sinonasal samples of patients with CRSwNP when compared with levels in CRSsNP or control subjects (Shikani et al., 2014).
Apart from direct effects on junctional epithelial proteins, environmental proteases can interact with PARs in the airway to stimulate the proliferation and migration of innate and adaptive leukocytes (Reed and Kita, 2004). PARs are a novel family of seven-transmembrane G protein-coupled receptors that are widely expressed in human airway epithelium. There are four types of PARs (PAR1, PAR2, PAR3, and PAR4) which play an integral role in defending against environmental proteases (Coughlin and Camerer, 2003; Reed and Kita, 2004). Several reports have linked PAR activation to the allergic immune response (Kheradmand et al., 2002; Jacquet, 2011). Exogenous proteases from house dust mite (HDM), cockroach or Alternaria alternate were shown to play an important role in allergy development, partly by activating PAR-2 signaling in the epithelial cells (de Boer et al., 2014). In CRSwNP, airborne fungal proteases can activate both PAR-2 and PAR-3 leading to the proliferation and migration of inflammatory cells (Shin et al., 2006). Furthermore, the level of the PAR-2 in cultured primary nasal epithelial cells and nasal polyps from patients with ECRSwNP was significantly increased as compared with NECRSwNP and controls (Kouzaki et al., 2016). However, in patients with allergic fungal rhinosinusitis, only PAR-3 showed statistically significant differential expression compared to non-diseased controls (Ebert et al., 2014).
Staphylococcus aureus is a versatile bacteria frequently found colonizing patients with Th2-biased diseases such CRSwNP and asthma (Bachert et al., 2010; Sachse et al., 2010). Several endotypes of chronic rhinosinusitis have been identified based on the presence of S. aureus enterotoxin(SE)- specific IgE (Bachert and Akdis, 2016; Tomassen et al., 2016). The presence of SE-specific IgE associates with intense eosinophilic inflammation in CRSwNP, high IgE concentration and comorbid asthma (Bachert et al., 2010; Tomassen et al., 2016). Recently, serine protease like protein D (SplD) and other closely related proteases secreted by S. aureus have been identified as inducers of allergic asthma in both humans and mice (Stentzel et al., 2017). Furthermore, SplD-induced Th2-biased inflammatory response and IgE production in the airway inflammation were largely dependent on the IL-33/ST2 axis and independent of TLR4 and PAR-2 signaling (Teufelberger et al., 2017).
TLR activation has been the subject of intense study with respect to its role in protease mediated airway inflammation. The coagulation system has been implicated in eosinophilic airway diseases, such as asthma and CRSwNP as a result of collagen deposition and airway remodeling, (Shimizu et al., 2011; Lambrecht and Hammad, 2013; Kim et al., 2015). Millien et al. found that activation of the coagulation cascade by allergen-derived proteases is an important factor promoting asthma-like changes in mice. Allergen proteases can cleave the serum factor fibrinogen, thus releasing FCPs which directly activate TLR4 signaling (Millien et al., 2013). The development of an asthma-like condition caused by house-dust mites challenge relies on the expression of TLR4 on lower airway epithelial cells (Hammad et al., 2009). Furthermore, thrombin, the classic activator of coagulation, can also cleave fibrinogen into FCPs resulting in further upregulation of the TLR4 pathway. A recent study identifies a programmed cell death 1 ligand 2+ (PD-L2+) DC phenotype which accounts for the induction of Th2 cell response upon protease allergens exposure and fibrinogen-cleavage products can promote the generation of PD-L2+ DC through TLR4 (Cho M. et al., 2017). These studies suggest that TLR4 plays a critical role in the allergic response upon exposure to exogenous proteases.
A study by Seung-Heon Shin et al. showed that airborne fungi induced the activation of nasal polyp epithelial cell and TLR expression (TLR2, TLR3 and TLR4). Cytokine production was, in turn, suppressed by protease inhibitors and anti-TLR4 antibodies (Shin and Lee, 2010).
Imbalance and dysfunctional regulation of proteases and proteases inhibitors in the epithelial barrier of CRSwNP
Recently, a study showed that an imbalance of proteases and protease inhibitors within the epithelial barrier contributes to the pathogenesis of eosinophilic chronic rhinosinusitis (Kouzaki et al., 2017). Barrier defects might be induced by damage to key proteins that comprise tight or adherent junctions secondary to increased or unopposed protease activity. These findings suggest that individual susceptibility to protease mediated inflammation may arise from the inability to adequately mitigate exogenous protease mediated epithelial damage. A recent review (Schleimer and Berdnikovs, 2017) suggested that cystatin A and SPINK5 (a cysteine and serine protease inhibitor, respectively) possess important roles in protecting the airway epithelium against environmental proteases. Furthermore, SPINK5 can protect PARs which are expressed on multiple cell types in the nasal epithelium from environmental proteases (Hershenson, 2007). Furthermore, SPINK5 is thought to regulate the function of numerous proteases that might compromise the barrier (Tieu et al., 2009). What's more, both human and animal studies have showed that SPINK5 mutations are associated with chronic inflammation in epithelium (Cookson, 2004; Moffatt, 2004).
P-glycoprotein (P-gp) has been reported as a key immunoregulator of eosinophilic inflammation in both CRSwNP and CRSsNP (Bleier et al., 2013; Feldman et al., 2013; Cheng and Bleier, 2016). Protease inhibitors have been reported to induce the expression of P-gp suggesting that an imbalance in the protease system may further exacerbate inflammation through the induction of P-gp expression(Perloff et al., 2000; Huang et al., 2001; Chandler et al., 2003; Zastre et al., 2009). Additionally, some protease inhibitors have been shown to function as P-gp substrates further strengthening the link between protease inhibitors and P-gp (Chaillou et al., 2002; Meaden et al., 2002) (Zhang and Benet, 1998). While disequilibrium of both P-gp expression and proteases inhibitors within the nasal mucosa may play an interrelated role in CRSwNP, further studies are needed to explore this possible function.
Summary and perspectives
In patients with CRSwNP, exogenous allergen and microorganism derived proteases play a crucial role in the development of type 2 immune response at the mucosal surface. Through direct effects on junctional proteins, binding to cell surface PARs, TLR4-dependent epithelial activation, disruption of barrier function, and P-gp activation, proteases both initiate and maintain the inflammation characteristic of Th2 mucosal disease. It has been proposed that drugs targeting protease function (Verma et al., 2016) in nasal mucus to restore the balance between proteases and protease inhibitors (Pfeffer and Corrigan, 2017) may represent an important potential therapeutic strategy in patients with CRSwNP and other eosinophilic airway diseases. However, more studies are required to explore the exact role of the protease and protease inhibitor axis in CRSwNP.
Author contributions
DW drafted the manuscript. Both YW and BB reviewed the manuscript and provided revisions.
Conflict of interest statement
The senior author has a patent related to P-gp modulation in CRSwNP. The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
Abbreviations
- CRSwNP
Chronic rhinosinusitis with nasal polyps
- CRSsNP
chronic rhinosinusitis without nasal polyps
- ECRSwNP
eosinophilic chronic rhinosinusitis with nasal polyps
- NECRSwNP
non-eosinophilic chronic rhinosinusitis with nasal polyps
- Th2
type 2 helper T-cell
- PARs
protease-activated receptors
- TLR4
toll-like receptor 4
- ZO-1
Zonula occludens-1
- HDM
house dust mite
- SpID
serine protease like protein D
- FCPs
fibrinogen cleavage products
- P-gp
P-glycoprotein.
References
- Asokananthan N., Graham P. T., Stewart D. J., Bakker A. J., Eidne K. A., Thompson P. J., et al. (2002). House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: the cysteine protease allergen, Der p 1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. J. Immunol. 169, 4572–4578. 10.4049/jimmunol.169.8.4572 [DOI] [PubMed] [Google Scholar]
- Bachert C., Akdis C. A. (2016). Phenotypes and emerging endotypes of chronic rhinosinusitis. J. Allergy Clin. Immunol. Pract. 4, 621–628. 10.1016/j.jaip.2016.05.004 [DOI] [PubMed] [Google Scholar]
- Bachert C., Zhang N., Holtappels G., De Lobel L., van Cauwenberge P., Liu S., et al. (2010). Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J. Allergy Clin. Immunol. 126, 962–968.e6. 10.1016/j.jaci.2010.07.007 [DOI] [PubMed] [Google Scholar]
- Baguley C., Brownlow A., Yeung K., Pratt E., Sacks R., Harvey R. (2014). The fate of chronic rhinosinusitis sufferers after maximal medical therapy. Int. Forum Allergy Rhinol. 4, 525–532. 10.1002/alr.21315 [DOI] [PubMed] [Google Scholar]
- Balenga N. A., Klichinsky M., Xie Z., Chan E. C., Zhao M., Jude J., et al. (2015). A fungal protease allergen provokes airway hyperresponsiveness in asthma. Nat. Commun. 6:6763 10.1038/ncomms7763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman N., Shahi A., Feeley K. M., Woolford T. J. (2005). Activated eosinophils in nasal polyps: a comparison of asthmatic and non-asthmatic patients. Clin. Otolaryngol. 30, 221–225. 10.1111/j.1365-2273.2005.00969.x [DOI] [PubMed] [Google Scholar]
- Bleier B. S., Nocera A. L., Iqbal H., Hoang J. D., Feldman R. E., Han X. (2013). P-glycoprotein functions as an immunomodulator in healthy human primary nasal epithelial cells. Int. Forum Allergy Rhinol. 433–438. 10.1002/alr.21166 [DOI] [PubMed] [Google Scholar]
- Cao P.-P., Li H. B., Wang B. F., Wang S. B., You X. J., Cui Y. H., et al. (2009). Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J. Allergy Clin. Immunol. 124, 478–484.e2. 10.1016/j.jaci.2009.05.017 [DOI] [PubMed] [Google Scholar]
- Chaillou S., Durant J., Garraffo R., Georgenthum E., Roptin C., Clevenbergh P., et al. (2002). Intracellular concentration of protease inhibitors in HIV-1–infected patients: correlation with MDR-1 gene expression and low dose of ritonavir. HIV Clin. Trials 3, 493–501. 10.1310/0873-BVDP-AKAY-445U [DOI] [PubMed] [Google Scholar]
- Chandler B., Almond L., Ford J., Owen A., Hoggard P., Khoo S., et al. (2003). The effects of protease inhibitors and nonnucleoside reverse transcriptase inhibitors on p-glycoprotein expression in peripheral blood mononuclear cells in vitro. J. Acquir. Immune Defic. Syndr. 33, 551–556. 10.1097/00126334-200308150-00001 [DOI] [PubMed] [Google Scholar]
- Cheng Y. S., Bleier B. S. (2016). Influence of P-glycoprotein function on chronic rhinosinusitis/nasal polyps pathophysiology. Adv. Otorhinolaryngol. 79, 38–47. 10.1159/000445094 [DOI] [PubMed] [Google Scholar]
- Cho M., Lee J. E., Lim H., Shin H. W., Khalmuratova R., Choi G., et al. (2017). Fibrinogen cleavage products and Toll-like receptor 4 promote the generation of programmed cell death 1 ligand 2-positive dendritic cells in allergic asthma. J. Allergy Clin. Immunol. [Epub ahead of print]. 10.1016/j.jaci.2017.09.019 [DOI] [PubMed] [Google Scholar]
- Cho S.-W., Kim D. W., Kim J. W., Lee C. H., Rhee C. S. (2017). Classification of chronic rhinosinusitis according to a nasal polyp and tissue eosinophilia: limitation of current classification system for Asian population. Asia Pac. Allergy 7:121. 10.5415/apallergy.2017.7.3.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. W., Wenaas A., Luong A., Citardi M. J., Fakhri S. (2013). Staphylococcus aureus prevalence in allergic fungal rhinosinusitis vs other subsets of chronic rhinosinusitis with nasal polyps. Int. Forum Allergy Rhinol. 3, 89–93. 10.1002/alr.21090 [DOI] [PubMed] [Google Scholar]
- Cookson W. (2004). The immunogenetics of asthma and eczema: a new focus on the epithelium. Nat. Rev. Immunol. 4, 978–988. 10.1038/nri1500 [DOI] [PubMed] [Google Scholar]
- Costenaro L., Kaczmarska Z., Arnan C., Janowski R., Coutard B., Solà M., et al. (2011). Structural basis for antiviral inhibition of the main protease, 3C, from human enterovirus 93. J. Virol. 85, 10764–10773. 10.1128/JVI.05062-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin S. R., Camerer E. (2003). Participation in inflammation. J. Clin. Invest. 111, 25–27. 10.1172/JCI17564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer J. D., van't Veer C., Stroo I., van der Meer A. J., de Vos A. F., van der Zee J. S., et al. (2014). Protease-activated receptor-2 deficient mice have reduced house dust mite-evoked allergic lung inflammation. Innate Immun. 20, 618–625. 10.1177/1753425913503387 [DOI] [PubMed] [Google Scholar]
- DeConde A. S., Mace J. C., Levy J. M., Rudmik L., Alt J. A., Smith T. L. (2017). Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Laryngoscope 127, 550–555. 10.1002/lary.26391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers M. (2004). Refractory chronic rhinosinusitis: pathophysiology and management of chronic rhinosinusitis persisting after endoscopic sinus surgery. Curr. Allergy Asthma Rep. 4, 200–207. 10.1007/s11882-004-0027-z [DOI] [PubMed] [Google Scholar]
- Ebert C. S., McKinney K. A., Urrutia G., Wu M., Rose A. S., Fleischman G. M., et al. (2014). Expression of protease-activated receptors in allergic fungal rhinosinusitis. Int. Forum Allergy Rhinol. 4, 266–271. 10.1002/alr.21295 [DOI] [PubMed] [Google Scholar]
- Feldman R. E., Lam A. C., Sadow P. M., Bleier B. S. (2013). P-glycoprotein is a marker of tissue eosinophilia and radiographic inflammation in chronic rhinosinusitis without nasal polyps. Int. Forum Allergy Rhinol. 3, 684–687. 10.1002/alr.21176 [DOI] [PubMed] [Google Scholar]
- Fokkens W. J., Lund V. J., Mullol J., Bachert C., Alobid I., Baroody F., et al. (2012). EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 50, 1–12. 10.4193/Rhino50E2 [DOI] [PubMed] [Google Scholar]
- Fokkens W., Lund V., Bachert C., Clement P., Helllings P., Holmstrom M., et al. (2005). EAACI position paper on rhinosinusitis and nasal polyps executive summary. Allergy 60, 583–601. 10.1111/j.1398-9995.2005.00830.x [DOI] [PubMed] [Google Scholar]
- Gregory L. G., Lloyd C. M. (2011). Orchestrating house dust mite-associated allergy in the lung. Trends Immunol. 32, 402–411. 10.1016/j.it.2011.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad H., Chieppa M., Perros F., Willart M. A., Germain R. N., Lambrecht B. N. (2009). House dust mite allergen induces asthma via toll-like receptor 4 triggering of airway structural cells. Nat. Med. 15, 410–416. 10.1038/nm.1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad H., Lambrecht B. N. (2015). Barrier epithelial cells and the control of type 2 immunity. Immunity 43, 29–40. 10.1016/j.immuni.2015.07.007 [DOI] [PubMed] [Google Scholar]
- Hara K., Iijima K., Elias M. K., Seno S., Tojima I., Kobayashi T., et al. (2014). Airway uric acid is a sensor of inhaled protease allergens and initiates type 2 immune responses in respiratory mucosa. J. Immunol. 192, 4032–4042. 10.4049/jimmunol.1400110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershenson M. B. (2007). Proteases and protease-activated receptors signalling: at the crossroads of acquired and innate immunity. Clin. Exp. Allergy 37, 963–966. 10.1111/j.1365-2222.2007.02738.x [DOI] [PubMed] [Google Scholar]
- Hirasawa Y. (2010). Staphylococcus aureus extracellular protease causes epidermal barrier dysfunction. J. Invest. Dermat. 130, 614–617. 10.1038/jid.2009.257 [DOI] [PubMed] [Google Scholar]
- Huang L., Wring S. A., Woolley J. L., Brouwer K. R., Serabjit-Singh C., Polli J. W. (2001). Induction of P-glycoprotein and cytochrome P450 3A by HIV protease inhibitors. Drug Metabol. Disposition 29, 754–760. [PubMed] [Google Scholar]
- Ikeda K., Shiozawa A., Ono N., Kusunoki T., Hirotsu M., Homma H., et al. (2013). Subclassification of chronic rhinosinusitis with nasal polyp based on eosinophil and neutrophil. Laryngoscope 123, E1–E9. 10.1002/lary.24154 [DOI] [PubMed] [Google Scholar]
- Jacquet A. (2011). Interactions of airway epithelium with protease allergens in the allergic response. Clin. Exp. Allergy 41, 305–311. 10.1111/j.1365-2222.2010.03661.x [DOI] [PubMed] [Google Scholar]
- Kale S. L., Agrawal K., Gaur S. N., Arora N. (2017). Cockroach protease allergen induces allergic airway inflammation via epithelial cell activation. Sci Rep. 7:42341. 10.1038/srep42341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo S., Takeda H., Tokura T., Suzuki M., Inui K., Hara M., et al. (2013). IL-33–mediated innate response and adaptive immune cells contribute to maximum responses of protease allergen–induced allergic airway inflammation. J. Immunol. 190, 4489–4499. 10.4049/jimmunol.1201212 [DOI] [PubMed] [Google Scholar]
- Kesic M. J., Hernandez M., Jaspers I. (2012). Airway protease/antiprotease imbalance in atopic asthmatics contributes to increased Influenza A virus cleavage and replication. Respir. Res. 13:82. 10.1186/1465-9921-13-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheradmand F., Kiss A., Xu J., Lee S. H., Kolattukudy P. E., Corry D. B. (2002). A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J. Immunol. 169, 5904–5911. 10.4049/jimmunol.169.10.5904 [DOI] [PubMed] [Google Scholar]
- Kim D. Y., Cho S. H., Takabayashi T., Schleimer R. P. (2015). Chronic rhinosinusitis and the coagulation system. Allergy Asthma Immunol. Res. 7, 421–430. 10.4168/aair.2015.7.5.421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.-W., Hong S. L., Kim Y. K., Lee C. H., Min Y. G., Rhee C. S. (2007). Histological and immunological features of non-eosinophilic nasal polyps. Otolaryngol. Head Neck Surg. 137, 925–930. 10.1016/j.otohns.2007.07.036 [DOI] [PubMed] [Google Scholar]
- Kouzaki H., Matsumoto K., Kato T., Tojima I., Shimizu S., Shimizu T. (2016). Epithelial cell-derived cytokines contribute to the pathophysiology of eosinophilic chronic rhinosinusitis. J. Interferon. Cytokine Res. 36, 169–179. 10.1089/jir.2015.0058 [DOI] [PubMed] [Google Scholar]
- Kouzaki H., Matsumoto K., Kikuoka H., Kato T., Tojima I., Shimizu S., et al. (2017). Endogenous protease inhibitors in airway epithelial cells contribute to eosinophilic chronic rhinosinusitis. Am. J. Respir. Crit. Care Med. 195, 737–747. 10.1164/rccm.201603-0529OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht B. N., Hammad H. (2013). Asthma and coagulation. N. Engl. J. Med. 369, 1964–1966. 10.1056/NEJMcibr1311045 [DOI] [PubMed] [Google Scholar]
- Lan F., Zhang N., Gevaert E., Zhang L., Bachert C. (2016). Viruses and bacteria in Th2-biased allergic airway disease. Allergy 71, 1381–1392. 10.1111/all.12934 [DOI] [PubMed] [Google Scholar]
- Madeo J., Frieri M. (2013). Bacterial biofilms and chronic rhinosinusitis. Allergy Asthma Proc. 34, 335–341. 10.2500/aap.2013.34.3665 [DOI] [PubMed] [Google Scholar]
- Meaden E. R., Hoggard P. G., Newton P., Tjia J. F., Aldam D., Cornforth D., et al. (2002). P-glycoprotein and MRP1 expression and reduced ritonavir and saquinavir accumulation in HIV-infected individuals. J. Antimicrob. Chemother. 50, 583–588. 10.1093/jac/dkf161 [DOI] [PubMed] [Google Scholar]
- Mendelsohn D., Jeremic G., Wright E. D., Rotenberg B. W. (2011). Revision rates after endoscopic sinus surgery: a recurrence analysis. Ann. Otol. Rhinol. Laryngol. 120, 162–166. 10.1177/000348941112000304 [DOI] [PubMed] [Google Scholar]
- Millien V. O., Lu W., Shaw J., Yuan X., Mak G., Roberts L., et al. (2013). Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4. Science 341, 792–796. 10.1126/science.1240342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt M. (2004). SPINK5: a gene for atopic dermatitis and asthma. Clin. Exp. Allergy 34, 325–327. 10.1111/j.1365-2222.2004.01915.x [DOI] [PubMed] [Google Scholar]
- Mortuaire G., Leroy X., Gengler I., Chevalier D., Prin L., Picry A. (2015). Histopathological classification of refractory chronic rhinosinusitis with nasal polyps. Histol. Histopathol. 30, 1447–1454. 10.14670/HH-11-632 [DOI] [PubMed] [Google Scholar]
- Orlandi R. R., Kingdom T. T., Hwang P. H., Smith T. L., Alt J. A., Baroody F. M., et al. (2016). International consensus statement on allergy and rhinology: rhinosinusitis. Int. Forum Allergy Rhinol. 6(Suppl. 1), S22–S209. 10.1002/alr.21695 [DOI] [PubMed] [Google Scholar]
- Ou J., Wang J., Xu Y., Tao Z. Z., Kong Y. G., Chen S.-M., et al. (2014). Staphylococcus aureus superantigens are associated with chronic rhinosinusitis with nasal polyps: a meta-analysis. Eur. Arch. Otorhinolaryngol. 271, 2729–2736. 10.1007/s00405-014-2955-0 [DOI] [PubMed] [Google Scholar]
- Perloff M. D., von Moltke L. L., Fahey J. M., Daily J. P., Greenblatt D. J. (2000). Induction of P-glycoprotein expression by HIV protease inhibitors in cell culture. Aids 14, 1287–1289. 10.1097/00002030-200006160-00034 [DOI] [PubMed] [Google Scholar]
- Pfeffer P. E., Corrigan C. J. (2017). An imbalance between proteases and endogenous protease inhibitors in eosinophilic airway disease. Am. J. Respir. Crit. Care Med. 195, 707–708. 10.1164/rccm.201610-2020ED [DOI] [PubMed] [Google Scholar]
- Reed C. E., Kita H. (2004). The role of protease activation of inflammation in allergic respiratory diseases. J. Allergy Clin. Immunol. 114, 997–1008. 10.1016/j.jaci.2004.07.060 [DOI] [PubMed] [Google Scholar]
- Runswick S., Mitchell T., Davies P., Robinson C., Garrod D. R. (2007). Pollen proteolytic enzymes degrade tight junctions. Respirology 12, 834–842. 10.1111/j.1440-1843.2007.01175.x [DOI] [PubMed] [Google Scholar]
- Sachse F., Becker K., Von Eiff C., Metze D., Rudack C. (2010). Staphylococcus aureus invades the epithelium in nasal polyposis and induces IL-6 in nasal epithelial cells in vitro. Allergy 65, 1430–1437. 10.1111/j.1398-9995.2010.02381.x [DOI] [PubMed] [Google Scholar]
- Schleimer R. P. (2017). Immunopathogenesis of chronic rhinosinusitis and nasal polyposis. Annu. Rev. Pathol. 12, 331–357. 10.1146/annurev-pathol-052016-100401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleimer R. P., Berdnikovs S. (2017). Etiology of epithelial barrier dysfunction in patients with type 2 inflammatory diseases. J. Allergy Clin. Immunol. 139, 1752–1761. 10.1016/j.jaci.2017.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S. A., Ishinaga H., Takeuchi K. (2016). Pathogenesis of eosinophilic chronic rhinosinusitis. J. Immunol. 13:11. 10.1186/s12950-016-0121-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikani A. H., Sidhaye V. K., Basaraba R. J., Shikani H. J., Alqudah M. A., Kirk N., et al. (2014). Mucosal expression of aquaporin 5 and epithelial barrier proteins in chronic rhinosinusitis with and without nasal polyps. Am. J. Otolaryngol. 35, 377–383. 10.1016/j.amjoto.2013.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S., Gabazza E. C., Ogawa T., Tojima I., Hoshi E. H., Kouzaki, et al. (2011). Role of thrombin in chronic rhinosinusitis-associated tissue remodeling. Am. J. Rhinol. Allergy 25, 7–11. 10.2500/ajra.2011.25.3535 [DOI] [PubMed] [Google Scholar]
- Shin S.-H., Lee Y. H. (2010). Airborne fungi induce nasal polyp epithelial cell activation and toll-like receptor expression. Int. Arch. Allergy Immunol. 153, 46–52. 10.1159/000301578 [DOI] [PubMed] [Google Scholar]
- Shin S.-H., Lee Y. H., Jeon C. H. (2006). Protease-dependent activation of nasal polyp epithelial cells by airborne fungi leads to migration of eosinophils and neutrophils. Acta Otolaryngol. 126, 1286–1294. 10.1080/00016480500395179 [DOI] [PubMed] [Google Scholar]
- Snelgrove R. J., Gregory L. G., Peiró T., Akthar S., Campbell G. A., Walker S. A., et al. (2014). Alternaria-derived serine protease activity drives IL-33–mediated asthma exacerbations. J. Allergy Clin. Immunol. 134, 583–592.e6. 10.1016/j.jaci.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol C. L., Barton G. M., Farr A. G., Medzhitov R. (2008). A mechanism for the initiation of the Th2 response by an allergen. Nat. Immunol. 9, 310–318. 10.1038/ni1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stentzel S., Teufelberger A., Nordengrün M., Kolata J., Schmidt F., van Crombruggen K., et al. (2017). Staphylococcal serine protease–like proteins are pacemakers of allergic airway reactions to Staphylococcus aureus. J. Allergy Clin. Immunol. 139, 492–500.e8. 10.1016/j.jaci.2016.03.045 [DOI] [PubMed] [Google Scholar]
- Stevens W. W., Peters A. T., Hirsch A. G., Nordberg C. M., Schwartz B. S., Mercer D. G., et al. (2017). Clinical characteristics of patients with chronic rhinosinusitis with nasal polyps, asthma, and aspirin-exacerbated respiratory disease. J. Allergy Clin. Immunol. Pract. 5, 1061–1070.e3. 10.1016/j.jaip.2016.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta V., White A. A. (2015). Survey-defined patient experiences with aspirin-exacerbated respiratory disease. J. Allergy Clin. Immunol. Pract. 3, 711–718. 10.1016/j.jaip.2015.03.001 [DOI] [PubMed] [Google Scholar]
- Tai H. Y., Tam M., Chou H., Peng H. J., Su S. N., Perng D. W., et al. (2006). Pen ch 13 allergen induces secretion of mediators and degradation of occludin protein of human lung epithelial cells. Allergy 61, 382–388. 10.1111/j.1398-9995.2005.00958.x [DOI] [PubMed] [Google Scholar]
- Takai T., Ikeda S. (2011). Barrier dysfunction caused by environmental proteases in the pathogenesis of allergic diseases. Allergol Int. 60, 25–35. 10.2332/allergolint.10-RAI-0273 [DOI] [PubMed] [Google Scholar]
- Teufelberger A. R., Nordengrün M., Braun H., Maes T., De Grove K., Holtappels G., et al. (2017). The IL-33/ST2 axis is crucial in type 2 airway responses induced by the Staphylococcus aureus-derived serine protease-like protein D. J. Allergy Clin. Immunol. [Epub ahead of print]. 10.1016/j.jaci.2017.05.004 [DOI] [PubMed] [Google Scholar]
- Tieu D. D., Kern R. C., Schleimer R. P. (2009). Alterations in epithelial barrier function and host defense responses in chronic rhinosinusitis. J. Allergy Clin. Immunol. 124, 37–42. 10.1016/j.jaci.2009.04.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipirneni K. E., Woodworth B. A. (2017). Medical and surgical advancements in the management of cystic fibrosis chronic rhinosinusitis. Curr. Otorhinolaryngol. Rep. 5, 24–34. 10.1007/s40136-017-0139-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassen P., Vandeplas G., Van Zele T., Cardell L. O., Arebro J., Olze H., et al. (2016). Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J. Allergy Clin. Immunol. 137, 1449–1456.e4. 10.1016/j.jaci.2015.12.1324 [DOI] [PubMed] [Google Scholar]
- Tosun F., Arslan H. H., Karslioglu Y., Deveci M. S., Durmaz A. (2010). Relationship between postoperative recurrence rate and eosinophil density of nasal polyps. Ann. Otol. Rhinol. Laryngol. 119, 455–459. 10.1177/000348941011900705 [DOI] [PubMed] [Google Scholar]
- Van Zele T., Claeys S., Gevaert P., Van Maele G., Holtappels G., Van Cauwenberge P., et al. (2006). Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy 61, 1280–1289. 10.1111/j.1398-9995.2006.01225.x [DOI] [PubMed] [Google Scholar]
- Verma S., Dixit R., Pandey K. C. (2016). Cysteine proteases: modes of activation and future prospects as pharmacological targets. Front. Pharmacol. 7:107. 10.3389/fphar.2016.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H., Winton H. L., Soeller C., Tovey E. R., Gruenert D. C., Thompson P. J., et al. (1999). Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J. Clin. Invest. 104, 123–133. 10.1172/JCI5844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H., Winton H. L., Soeller C., Taylor G. W., Gruenert D. C., Thompson P. J., et al. (2001). The transmembrane protein occludin of epithelial tight junctions is a functional target for serine peptidases from faecal pellets of Dermatophagoides pteronyssinus. Clin. Exp. Allergy 31, 279–294. 10.1046/j.1365-2222.2001.00970.x [DOI] [PubMed] [Google Scholar]
- Wu D., Li L., Zhang M., Wang J., Wei Y. (2017). Two inflammatory phenotypes of nasal polyps and comorbid asthma. Ann. Allergy Asthma Immunol. 118, 318–325. 10.1016/j.anai.2016.12.018 [DOI] [PubMed] [Google Scholar]
- Wu D., Wang J., Zhang M. (2016). Altered Th17/Treg ratio in nasal polyps with distinct cytokine profile: association with patterns of inflammation and mucosal remodeling. Medicine 95:e2998. 10.1097/MD.000000000000299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn R., Har-El G. (2004). Recurrence rates after endoscopic sinus surgery for massive sinus polyposis. Laryngoscope 114, 811–813. 10.1097/00005537-200405000-00004 [DOI] [PubMed] [Google Scholar]
- Zastre J. A., Chan G. N., Ronaldson P. T., Ramaswamy M., Couraud P. O., Romero I. A., et al. (2009). Up-regulation of P-glycoprotein by HIV protease inhibitors in a human brain microvessel endothelial cell line. J. Neurosci. Res. 87, 1023–1036. 10.1002/jnr.21898 [DOI] [PubMed] [Google Scholar]
- Zhang N., Van Zele T., Perez-Novo C., Van Bruaene N., Holtappels G., DeRuyck N., et al. (2008). Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J. Allergy Clin. Immunol. 122, 961–968. 10.1016/j.jaci.2008.07.008 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Benet L. Z. (1998). Characterization of P-glycoprotein mediated transport of K02, a novel vinylsulfone peptidomimetic cysteine protease inhibitor, across MDR1-MDCK and Caco-2 cell monolayers. Pharm Res. 15, 1520–1524. 10.1023/A:1011990730230 [DOI] [PubMed] [Google Scholar]