Abstract
Plant viruses that are members of the Geminiviridae family have circular single-stranded DNA (ssDNA) genome and are responsible for major crop diseases worldwide. We have identified and characterized a novel monopartite geminivirus infecting tomato in Argentina. The full-length genome was cloned and sequenced. The genome-wide pairwise identity calculation that resulted in a maximum of 63% identity with all of other known geminiviruses indicated that it is a new geminivirus species. Biolistic infected plants presented interveinal yellowing, apical leaf curling and extreme root hypotrophy. Thus, the name proposed for this species is tomato apical leaf curl virus (ToALCV). The phylogenetic inferences suggested different evolutionary relationships for the replication-associated protein (Rep) and the coat protein (CP). Besides, the sequence similarity network (SSN) protein analyses showed that the complementary-sense gene products (RepA, Rep and C3) are similar to capulavirus while the viron-sense gene products (CP, MP and V3) are similar to topocuvirus, curtovirus and becurtovirus. Based on the data presented, ToALCV genome appears to have “modular organization” supported by its recombination origin. Analyses of the specificity-determining positions (SDPs) of the CP of geminiviruses defined nine subgroups that include geminiviruses that share the same type of insect vector. Our sequences were clustered with the sequences of topocuvirus, whose vector is the treehopper, Micrutalis malleifera. Also, a set of the highest scored amino acid residues was predicted for the CP, which could determine differences in virus transmission specificity. We predict that a treehopper could be the vector of ToALCV, but transmission assays need to be performed to confirm this. Given everything we demonstrate in this paper, ToALCV can be considered a type member of a new putative genus of the Geminiviridae family.
Keywords: ToALCV, coat protein, treehopper, recombination, specificity-determining positions, sequence similarity network, tomato, Argentina
Introduction
Argentina is South America’s third largest producer of fresh tomato after Brazil and Chile (Food and Agriculture Organization [FAO], 2014). Plant viruses that are members of the Geminiviridae family are responsible for major crop diseases worldwide (Varma and Malathi, 2003; Scholthof et al., 2011; Rybicki, 2015). Their circular ssDNA genome varies between 2.5 and 5.2 kb in length and is packed into twinned icosahedral particles (Zhang et al., 2001). Nowadays, this family comprises nine genera: Becurtovirus, Begomovirus, Capulavirus, Curtovirus, Eragrovirus, Grablovirus, Mastrevirus, Topocuvirus, and Turncurtovirus, classified according to the differences in their genome organization, their genome-wide pairwise sequence identities, their insect vector and host range. Known insect vectors are: whiteflies in the case of Begomovirus; leafhoppers for Becurtovirus, Curtovirus, Turncurtovirus and Mastrevirus; treehoppers for Topocuvirus and Grablovirus, and finally, aphids in the case of Capulavirus (Zerbini et al., 2017). Capulavirus and Grablovirus were the two last genera to be established and the most divergent viruses within the Geminiviridae family (Varsani et al., 2017). Capulaviruses include the species: Euphorbia caput medusae latent virus (EcmLV), French bean severe leaf curl virus (FbSLCV), Alfalfa leaf curl virus (ALCV) and Plantago lanceolata latent virus (PILV) (Bernardo et al., 2013, 2016; Roumagnac et al., 2015; Susi et al., 2017). ALCV was the only member that could be observed by electron microscopy and it is transmitted by the Aphis cracivora (Roumagnac et al., 2015). For the moment, there is only one Grablovirus species, Grapevine red blotch virus, and its proposed vector is the species Spissistilus festinus (GRBV) (Krenz et al., 2012; Poojari et al., 2013). In addition, there are other characterized divergent geminiviruses which are not yet assigned to a genus, like the citrus chlorotic dwarf-associated virus (CCDaV), the mulberry mosaic dwarf-associated virus (MMDaV), the apple geminivirus (AGV) and the grapevine geminivirus A (Loconsole et al., 2012; Liang et al., 2015; Ma et al., 2015; Al Rwahnih et al., 2017). All these viruses are putative members of the Geminiviridae family since they have a ssDNA genome, shared conserved protein domains and common replication motifs in their intergenic regions, like the hairpin-loop, with the characteristic geminivirus nonanucleotide (TAATATTAC).
Geminivirus-related tomato diseases in America are common, but generally, they are etiologically related with begomovirus and topocuvirus (Stanley et al., 1986; Briddon et al., 1996; Andrade et al., 2006; Castillo-Urquiza et al., 2008; Albuquerque et al., 2012; Vaghi Medina and López Lambertini, 2012; Melgarejo et al., 2013; Paz-Carrasco et al., 2014; Vaghi Medina et al., 2015). Here we identify, molecular characterize and demonstrate the infectivity of a new highly divergent species isolated from Solanum lycopersicum plants in Argentina. In addition, phylogenetic and recombination analyses were carried out. We use sequence similarity network (SSN) analysis to study the relationships of sequences and function among proteins codified by the different genus of the Geminiviridae family. Moreover, the specificity-determining positions (SDPs) of the coat protein (CP) analyses allow us to predict the putative vector of this virus. The name tomato apical leaf curl virus (ToALCV) is proposed and could be considered a type member of a new putative genus of the Geminiviridae family.
Materials and Methods
Plant Material and Molecular Characterization
Symptomatic tomato plants were collected in Yuto, Jujuy province, Argentina. Total DNA was purified with the Nucleospin® Plant II total DNA purification kit (Macherey-Nagel). Genomic DNA was amplified by rolling circle amplification (RCA) with phi29 polymerase (Templi-phiTM, GE Healthcare). RCA products were digested with PstI to obtain a complete monomeric genomic component, which was cloned into a pBluescriptII SK+, digested with PstI and dephosphorylated. The virus genome was sequenced by Primer Walking in Macrogen Inc. (Korea). Open reading frames (ORFs) were identified with the ORF finder in the Geneious R9 software1 (Kearse et al., 2012). BLASTX, BLASTN and BLASTP algorithms2 were used to identify which of the sequences were most related. After identifying the closest sequences with the BLAST algorithm, we used Muscle to align them with the query and calculate the pairwise identity without considering the gaps. Known protein domains were identified in the translated putative ORFs with SMART online software (Schultz et al., 1998; Letunic et al., 2015), the NCBI Conserved Domain tool (Marchler-Bauer et al., 2015) and the InterProScan tool in Geneious R9.
Sequence Similarity Network Analysis
We performed a SSN analysis to visualize the relationships of sequences and function among proteins from different genus of the Geminiviridae family. We retrieved sequences from the protein NCBI database, corresponding to the complementary-sense genes (C1, C2, C3, C4, and C5) of the 9 genera. As a large number of sequences were retrieved for mastrevirus and begomovirus (1785 and 18870, respectively), sequences of those groups were clustered at 95% of identity using the CD-hit (Li and Godzik, 2006). We also filtered out sequences with less than 50 residues in length, after which 2910 sequences remained, which belonged to the 5 proteins that were used to create a custom BLAST database. The pairwise relationships between sequences were calculated by an all-against-all BLAST in the custom database and the resulting E-value was taken as a measure of similarity between sequences (Atkinson et al., 2009). The network was visualized using Cytoscape (Shannon et al., 2003), where each sequence was represented as a node and edges were defined between any pair of nodes with an E-value of less than a threshold, using the Cytoscape force-directed layout. Similarly, a network was constructed to analyze protein sequences codified in the virion-sense strand. For this analysis, we retrieved all translated geminivirus sense genes (V1, V2, V3, and V4) and added the two movement protein genes from bipartite begomovirus (BC1-MP and BV1-NSP). The sequences of mastrevirus and begomovirus (2168 and 14136, respectively) were clustered to reduce the number of sequences and redundancy at 95 and 90% of identity, respectively. In total, the custom database comprises 1541 sequences belonging to the six proteins, including our query sequences.
Phylogenetic and Recombination Analysis
To establish the phylogenetic relationships, we assembled three datasets. The first dataset included 66 nucleotide sequences of the full-length genome or DNA-A (in the case of begomoviruses) of representative species of all the genera of the Geminiviridae family obtained from a public database (NCBI). The two other datasets were amino acid sequences of the coat protein (CP) and the replication-associated protein (Rep) of the sequence of each species and the geminivirus representative species. All sequences in the datasets were aligned with MUSCLE (Edgar, 2004). For phylogenetic analysis, the nucleotide substitution model was chosen as the best-fitting model by using jModeltest v2.1.6 (Guindon and Gascuel, 2003; Darriba et al., 2012). The models of protein evolution were inferred with Prottest v3.4 (Guindon and Gascuel, 2003; Darriba et al., 2011). In both cases, we used the Akaike Information Criterion (AIC) to select the best-fitting evolution model. The phylogenetic reconstructions were performed by Maximun likelihood criterion using FastTree software for full-length sequences (Price et al., 2010) and PHYML 3.0 software for the CP and Rep datasets (Guindon et al., 2010) with 1000 bootstrap replicates. Tree topologies were observed with FigTree v1.4.1. Recombination analyses of full-length genomes (DNA-A for begomovirus included) were performed using RDP, GENECONV, MaxChi, Bootscan, 3Seq, Chimera and SiScan statistic methods implemented in the RDP4.67 software (Martin et al., 2015).
CP Analyses for Prediction of the Tentative Type of Insect Vector
The same dataset of the CP amino acid sequences which was used for the phylogenetic tree was also used for the specificity-determining positions analyses (SDPs). SDPs are sites that show specific conservation patterns within subsets of proteins in a protein family alignment. Those sites are likely to be involved in the development of some kind of functional specificity. The goal of these analyses was the identification of amino acid residues that differ between groups of sequences, and to relate those amino acid residues to the viruses that have the same kind of insect vector. We hypothesize that those sites may be involved in the specificity of transmission; therefore, it is possible to predict the putative insect vector of this virus. SDPs were calculated with SPEER SERVER (Chakraborty et al., 2012) using MAFFT for sequence alignment (Katoh and Standley, 2013) and SCI-PHY for automated sub-grouping (Brown et al., 2007) setting the relative entropy term and the amino acids properties term weigh to one.
Infectivity Assay in Tomato and Virus Detection
Infectivity assays were performed with RCA products of virus DNA genome by biolistic inoculation using a PDS-1000/He particle delivery system (Bio-Rad) in tomato plants. Plasmids containing cloned full-length DNA genome were digested with PstI agarose-gel purification to obtain a monomeric component and then re-circularized by a ligation reaction (Knierim and Maiss, 2007; Lapidot et al., 2007). The RCA amplification product (5 μl) was precipitated on tungsten micro-particles and then inoculated into 10 Santa Clara tomato plants with 4–5 leaves. Plants were grown in greenhouse conditions with a 16/8 photoperiod.
In order to evaluate the virus infection, total DNA from apical leaves was purified 21 days after the inoculation and the viral genome was amplified by RCA and analyzed by Sau3AI restriction fragment length polymorphism (RFLP). Finally, we designed primers (NG_FW: 5′-ACTTCCAAAACTGGCTACAA-3′; NG_RV: 5′-AGAGCACATACCATCCAAAC-3′) to develop a PCR assay for specific virus identification. The reaction was as follows: 2 μl of total DNA, 0.25 mM each dNTPs, 2.5 mM of each primer and 1U of GoTaq DNA Polymerase (5 U/ml) (Promega, Madison, WI, United States). The temperature program used was: 94°C for 2 min, followed by 30 cycles of 94°C for 1 min, 51°C for 30 s, and 72°C for 2 min, with a final elongation step of 5 min at 72°C. The PCR product was evaluated by agarose gel electrophoresis and the presence of a 1375 bp DNA band confirmed the identity of the virus.
Results
Molecular Characterization
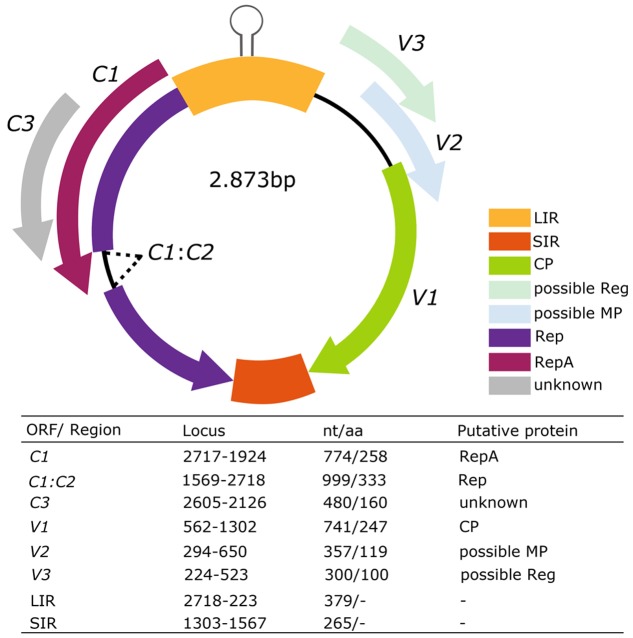
Tomato leaves with yellowing, reduction and wrinkling were collected in Yuto, Jujuy. We obtained a linear DNA fragment of about 2.8 kb generated from the digestion with PstI of RCA product amplified from three symptomatic plants. These fragments were isolated, cloned and sequenced by primer walking. The full-length virus insert was about 2874 pb for the isolation [AR:Yuto:Tom419:2008]-MG491195, 2873 pb for the isolation [AR:Yuto:Tom420:2008]-MG491196 and [AR:Yuto:Tom424:2008]-MG491197. The BLAST algorithm identified Alfalfa leaf curl virus (ALCV) as the most related sequence in the GenBank database. There was 63% of full-length pairwise identity with ALCV, which is below the 78% of the species demarcation threshold proposed by ICTV for Capulavirus classification (Varsani et al., 2017). We found the conserved geminivirus nonanucleotide (TAATATTAC) in all three genomic sequences obtained. Therefore, we identified a new geminivirus and proposed to name it tomato apical leaf curl virus (ToALCV). Six ORFs that might codify to putative proteins were identified. The inferred genome organization has three virion-sense genes (V1, V2, and V3) and three complementary-sense genes (C1, C1:C2 and C3) with two intergenic regions (LIR and SIR). The Replication-associated protein A (RepA) was codified by C1 as it shares a conserved domain with the RepA of geminiviruses. C1:C2 expressed for a spliced mRNA transcript and contained intron sequences. This protein shared sequence similarity with motifs conserved across the replication-associated protein (Rep) in geminiviruses, including the conserved motif I, II, and III, and the slightly different GRS motif (Supplementary Figure S1) (Nash et al., 2011). C3 predicted protein presented Rep-like motifs (E = 9.31e-42) in its sequence. On the other hand, the putative protein V1 was highly related with the geminivirus CP (E = 1.49e-19). V2 has a transmembrane domain which was detected by the SMART algorithm. V3 did not share any known conserved motif or domain with the database protein. In summary, the genome of ToALCV has six putative proteins and two intergenic regions (Figure 1).
FIGURE 1.
Genomic organization and ORFs sizes of the putative species tomato apical leaf curl virus (ToALCV). The table characterize of the open reading frames of ToALCV. Length (nt/aa), pairwise identity by the BLAST algorithm, protein motif identification by the Conserved Domain Tool (NCBI) and the SMART algorithm.
Sequence Similarity Network Analysis
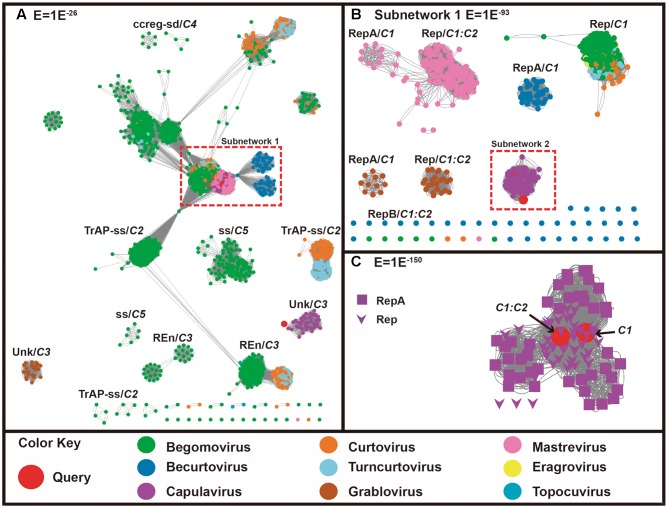
The complementary sense translated protein similarity network analysis indicates that the proteins coded by C1 and C1:C2 of our query genes clustered with all geminiviral RepA, RepB and Rep proteins (Figures 2A,B). Figure 2B shows further decomposition of a subnet (subnetwork 1) in the more stringent threshold value of 1E-93. It can be observed that, using this threshold, the Rep and RepA of mastrevirus, becurtovirus, grablovirus are clearly separated into different similarity groups, while the Rep and RepA of capulavirus lay in one group. The putative RepA-C1 and Rep-C1:C2 of ToALCV grouped with capulavirus. In subnetwork 2, with a more restrictive threshold of 1E-150, the RepA-C1 and the Rep-C1:C2 of ToALCV remained attached to the capulavirus Rep/RepA group, although both proteins seem to be closely related to the capulavirus Rep (Figure 2C). Interestingly, all the rest of the geminivirus genera that codified only a Rep stayed in the same group. Meanwhile, C3 protein of ToALCV grouped with C3 proteins of capulaviruses forming a detached group from the core with unknown function (Figure 2B).
FIGURE 2.
Sequence similarity networks (SSNs) including 2910 geminiviral complementary-sense coded protein sequences (codified by C1, C1:C2, C2, C3, C4, and C5 genes). Sequences are represented as nodes and the pairwise relationships, as edges (lines) between nodes. Nodes are colored according to the virus genera to which they belong, while the query sequences are colored in red. (A) SSNs using a permissive threshold (E-value = 1E-26). When sequences break out into clusters where the annotation is coincident, the name of the protein/function is indicated. One of the query sequences (putative C3) (red circle) clusters with the C3 sequences of capulavirus. Dotted red boxes enclose a group of sequences that was chosen for further analysis using more stringent thresholds (nodes are associated with more significant relationships). (B) Subnetwork 1, composed by 905 sequences, is shown at E-value threshold = 1E-93. The two query sequences cluster with capulavirus (enclosed in red dotted line). The subnetwork is further analyzed in (C) Subnetwork 2, which includes 91 sequences, and is shown at a more stringent threshold (E-value = 1E-150). Here the shape of the node indicates the protein annotation/function. The query sequences are closely related to the Rep associated protein of capulaviruses.
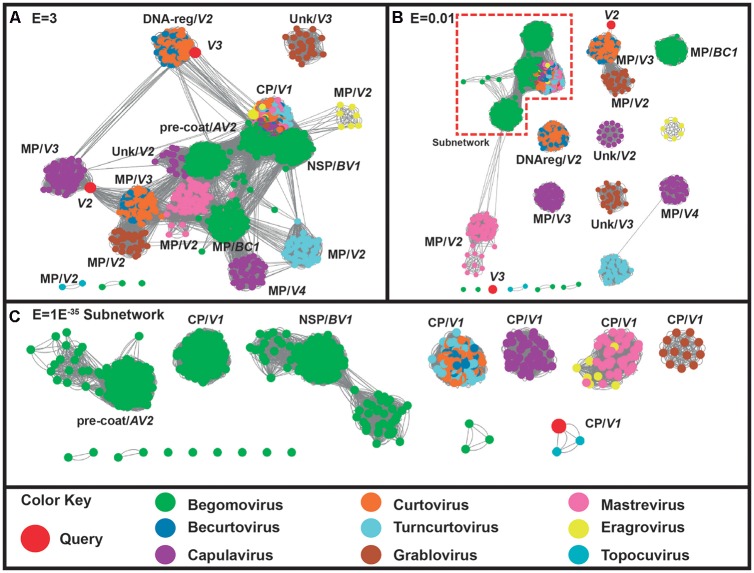
On the other hand, the proteins codified by virion-sense translated genes (V1, V2, and V3) of ToALCV clustered into a group that does not include the proteins of capulavirus. V1 is clearly related to the of all the geminiviruses at high threshold values (E = 3) (Figure 3A). Finally, the subnetwork in a threshold cut of E = 1E-35 presented different CP-V1 groups for capulaviruses, grabloviruses and begomoviruses (Figure 3C). The CPs of curtoviruses, becurtoviruses and turncurtoviruses are closely related and they formed another group. The same happened with the CPs of mastreviruses and eragroviruses. Contrary, the CP-V1 of ToALCV sequences clustered with the unique CP of topocuviruses and not with the CPs of capulaviruses. V2 grouped with movement proteins (MP) allowing the deduction of its function and suggesting a relationship with the MPs of curtoviruses and becurtoviruses (Figures 3A,B). V3 grouped with the proteins of curtoviruses and becurtoviruses involved in the regulation of the relative level of the ssDNA and dsDNA (Reg protein) (Figure 3A). However, V3 query protein was separated from all the other proteins at E = 0.03 threshold value making it difficult to confirm this proposed function (Figure 3B).
FIGURE 3.
Sequence similarity networks (SSNs) including 1541 sense coded protein geminiviral sequences (codified by V1, V2, V3, V4, BC1-MP and BV1-NSP genes). Sequences are represented as different colored nodes and the pairwise relationships, as edges (lines) between nodes. The nodes are colored according to the virus genera to which the sequence belongs, while the query sequences are colored in red. (A) SSNs using a permissive threshold (E-value = 3). When sequences break out into clusters where the annotation is coincident, the name of the coded protein is indicated. (B) The network is shown at E-value threshold = 0.03. (C) The subnetwork that comprises 898 sequences at an E-value threshold = E-35.
According to the protein similarity of the sequences and the order of codification, ToALCV presents modular organization. The complementary-sense gene products (RepA, Rep and C3) are similar to those of capulaviruses while the viron-sense gene products (CP, MP and V3) are similar to those belonging to topocuviruses, curtoviruses and becurtoviruses.
Phylogenetic Relationships
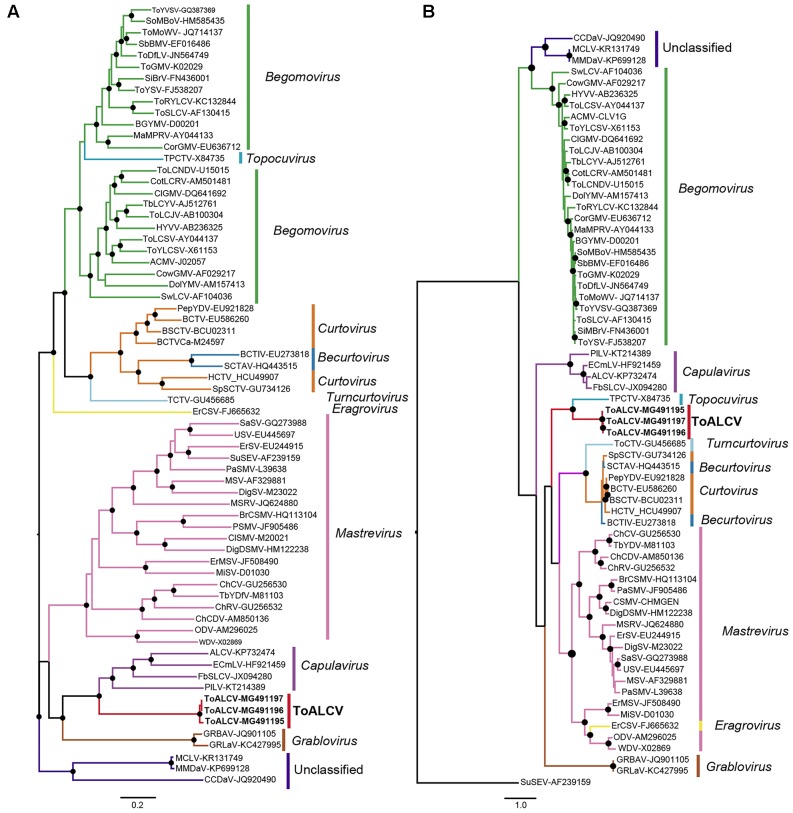
The evolutionary relationship between ToALCV and other known geminiviruses was inferred using full-length genome nucleotide sequences and amino acid sequences of the CP and the Rep proteins. The molecular evolutionary models were LG+I+G+F and VT+I+G for the CP and the Rep alignments, respectively. The phylogenetic tree for the full-length genome revealed that Capulavirus was the closest related genera. The three isolates ([AR:Yuto:Tom419:2008]-MG491195, [AR:Yuto:Tom420:2008]-MG491196 and [AR:Yuto:Tom424:2008]-MG491197) formed a monophyletic group with ALCV, EcmLV, FbSLCV and PLLV (Figure 4A). Nevertheless, ToALCV shaped a monophyletic group with the topocuvirus Tomato pseudo-curly top virus (ToPCTV) in the CP- phylogenetic tree (Figure 4B), while it grouped with the sequences of the capulavirus in the Rep-tree (Supplementary Figure S2), which corresponds with the results showed in the full-genome tree (Figure 4A).
FIGURE 4.
(A) Maximum likelihood phylogenetic tree of 66 full length genomes or DNA-A (bipartite begomovirus). (B) Maximum likelihood phylogenetic tree obtained with amino acid sequence alignments of the CP of the same 66 geminiviruses. Filled circles indicate 70 or more bootstrap percentages.
Recombination Analysis
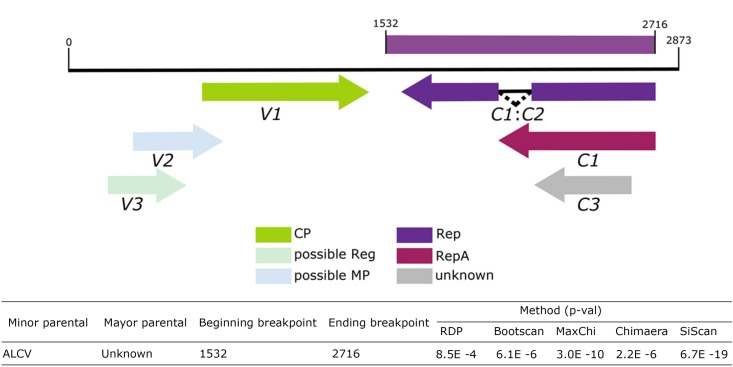
Five statistical methods, RDP (p-value = 8.5E-4), Bootscan (p-value = 6.1E-6), MaxChi (p-value = 3.0E-10), Chimera (p-value = 2.2E-6) and SiScan (p-value = 6.7E-19) in RDP v4.67 identified evidence of recombination for ToALCV. These analyses showed that the sequences of ALCV in the analyzed dataset are the sequences that most closely resemble the parental sequence; however, the major parental sequence remains unknown. The beginning breakpoint is at nucleotide 1532 and the ending breakpoint is at nucleotide 2716 involving a segment of the putative Rep sequences of ToALCV (Figure 5).
FIGURE 5.
Schematic representation of the recombination event detected by RDP4.67 in the ToALCV genome and table with corresponding the p-values.
Vector Specificity Prediction Based on Capsid Protein Amino Acidic Sequences
The automatic subgrouping defined nine subgroups that include geminiviruses that share the same type of insect vector. In that way, the subgroups 1, 2, and 3 include geminiviruses transmitted by the leafhopper (Cicadellidae). The subgroups 1 and 4, geminiviruses transmitted by the treehopper (Membracidae). The subgroup 8 contains those transmitted by the aphid (Aphididae) and subgroup 9 includes begomoviruses transmitted by the whitefly (Aleyrodidae). Interestingly, the subgroup 7 contains geminiviruses that have not been assigned to a family because their insect vector has not been identified. The geminiviruses transmitted by the leafhopper are divided into three subgroups and the geminiviruses transmitted by the treehopper, in two, according to the species of the insect-vector. SDP analyses clustered the three sequences of ToALCV with ToPCTV_X84735 for which the transmission vector is Micrutalis malleifera. Therefore, we propose that a treehopper (Membracidae) could be the vector of this virus. Also, a set of nine amino acid residues (L68, E86, A103, W112, Y203, N206, I212, I215 and P226) with the highest scoring positions was predicted for the CP that could determine differences in virus transmission specificity (Supplementary Figure S3). The predicted amino acid residues with functional specificity were concentrated in two positions in the CP; one, in the last part of the amino-terminal region, and the other, in the carboxy-terminal part.
Infectivity and Symptoms Development by Genomic Biolistic Inoculation
A total of 10 plants were biolistic inoculated with the RCA products of previously cloned full-length [AR:Yuto:Tom419:2008] and [AR:Yuto:Tom424:2008] genomes. Three plants showed interveinal yellowing, apical leaf curling and extreme root hypotrophy (Figure 6). The evaluation of the presence of ToALCV in the symptomatic plants by PCR with the specific primers resulted in the amplification of a 1400 bp fragment which confirmed the infection. Moreover, the Sau3AI RCA-RFLP (1027, 923, 735, and 188 bp) patterns obtained from the infected plants were the same as the predicted in silico patterns for ToALCV.
FIGURE 6.
Biolistic infected plants symptoms. (A,B) Leaf interveinal yellowing and curling symptoms exhibited by ToALCV of inoculated plants with [AR: Yuto: Tom424:2008] RCA product by the biolistic method. (C) Root hypotrophy in plants infected with [AR: Yuto: Tom424:2008].
Discussion
We identified and characterized a novel monopartite geminivirus infecting tomato in Argentina. The name tomato apical leaf curl virus (ToALCV) is proposed. A BLAST comparison shows that the nucleotide sequence only aligns in a small portion of this geminivirus genome, which matches the Rep portion of ALCV and that there is only 63% of nucleotide identity in the full-length genome. This indicates that it is highly divergent from all the Geminiviridae family. The full-length genome phylogenetic tree of the three isolates of ToALCV revealed that they cluster within capulavirus clades and share a common ancestor (Figure 4A). The same relationship was obtained with the Rep protein phylogenetic analyses (Supplementary Figure S1), but not with the CP protein (Figure 2B). In this case, the three isolate amino acid sequences formed a monophyletic group with ToPCTV, a topocuvirus that has not been identified in South America so far (Briddon et al., 1996). This lack of phylogenic relationship agreement of the Rep and the CP of ToALCV contrasts with the coincident results obtained for capulaviruses, where all proposed species clustered together in the capulavirus group in both trees (Varsani et al., 2017). The low percentage of nucleotide identity of the full-length genome, the different evolutionary relationships of the Rep and CP proteins, in addition to the recombination evidence, support a modular organization and a different evolutionary history of the virion-sense and complementary-sense frame of the ToALCV genome. A strong signal of recombination was found in the Rep segment of this virus. Beginning and ending recombination breakpoints are in nucleotides 1532 (within the small intergenic region, SIR) and 2716 (within the long intergenic region, LIR), respectively. The sequences in the recombination assay dataset that most resemble the recombination parental for the Rep segment belong to ALCV, agreeing with the results in the phylogenetic analysis. No other parental could be determined, which may be because the origin of this new species is ancient or its parental sequences have not been described yet. Topocuviruses and curtoviruses are examples of modular genome arrangement that is thought to have arisen through the recombination of fragments belonging to different Geminivirus genera (Stanley et al., 1986; Briddon et al., 1996; Klute et al., 1996; Varsani et al., 2009). The nucleotide sequence identity of virion-sense genes of becurtoviruses like Beet curly top Iran virus (BCTIV) are mostly related to those of curtoviruses, whereas the complementary-sense genes are distally related to mastreviruses (Yazdi et al., 2008; Bozorgi et al., 2017). Today, it is a challenge to determine with certainty, which genera are recombinant and which are parental; as with BCTIV, which was proposed to be involved as parent in the inter-genus recombination that originated curtoviruses (Varsani et al., 2009). The three replication modes postulated for geminiviruses; complementary strand replication (CSR), rolling circle replication (RCR) and recombination dependent recombination (RDR) have the potential to induce recombination in different parts of the genome. This plays a crucial role in geminivirus evolution as a motor for the switching of the host and the emergence of viruses (Saunders et al., 1991; Jeske et al., 2001; Preiss and Jeske, 2003; Lefeuvre and Moriones, 2015; Richter et al., 2016). When genetically distinct ssDNA virus genomes co-replicate within the same nucleus, there are a number of different mechanistic and selective processes that could determine the patterns of recombination that are conserved (Martin et al., 2011). The v-ori recombination hot spot was mapped for several geminiviruses (Lefeuvre et al., 2009). Complementary-sense genes in the geminivirus experience increased their recombination rate in relation to virion-sense genes, possibly due to mechanistic interferences between the transcription and replication complexes during RCR (Lefeuvre et al., 2009).
Genome organization of ToALCV showed the arrangement of six predicted ORFs encoding three viral-sense genes (C1, C1:C2, C3), three complementary-sense genes (V1, V2, V3) and two intergenic regions, LIR and SIR (Figure 1). Other geminivirus genera with two intergenic regions in their genome are becurtovirus, capulavirus, grablovirus, eragrovirus, mastevirus and CCDaV (Varsani et al., 2014, 2017). ToALCV has two putative genes involved in the replication by RCA: C1, which codified for a RepA protein, and C1:C2, which codified for a Rep fusion protein in which a splicing processing step was identified. Similar splicing of C1:C2 was described for mastreviruses, becurtoviruses, capulaviruses, grabloviruses and one begomovirus (Mungbean yellow mosaic virus) (Mullineaux et al., 1990; Dekker et al., 1991; Boulton, 2002; Shivaprasad et al., 2005; Bozorgi et al., 2017). The complementary-sense codified proteins are clearly similar to capulavirus (Figure 2). It is important to point out that the predicted expression products of C1 (RepA) and C1:C2 (Rep) presented more similarities with C1:C2 (Rep) of the capulavirus species (Figure 2C). As with capulavirus and grablovirus, they have a large C3 that overlaps with the Rep sequences (Figure 1). The C3 of ToALCV also has some degree of protein sequence similarity with capulaviruses, although no function is postulated yet for any of these proteins (Figures 2B,C). Of the virion-sense coding region, the main protein amongst all geminiviruses is the CP, due to its features to be conserved. The V1 gene codified for a putative CP that shares significant amino acid sequence similarity with the CP of ToPCTV. In addition, the evolutionary relationships with topocuviruses were displayed in the CP amino acid phylogenetic tree (Figures 1, 3C, 4B). Although the CP of ToALCV is clearly very similar to topocuvirus, it is not obviously derived from topocuvirus through recombination because no recombinant event was identified in these fragments in the analyses. The localization of the gene V2 that codified for a putative MP is immediately upstream of the CP like the other members of the Geminiviridae family and has sequence similarity with the MP proteins of curtoviruses and becurtoviruses (Figures 1, 3). V3 gene of ToALCV codified for the most divergent protein, but in the SSN analyses, (E = 3) V3 grouped with the Reg protein of curtoviruses and becurtoviruses (Figures 3A,B).
It is known that changes in the amino acidic sequence of the CP in geminiviruses can modify the vector specificity (Briddon et al., 1990). Based on that, we performed an SDP analysis to see if the CP amino acidic sequences of geminiviruses with similar insect vector exhibited features more akin amongst them. We found that a clustering based only on the amino acidic sequences (with no other information added) rendered 8 subgroups of proteins. Proteins in those subgroups surprisingly belong to viruses that use the same vector for its transmission. The nine highest scoring SDP amino acid positions are enough to separate the geminiviruses according to their specific transmission vector (Supplementary Figure S3). Our sequences were clustered with topocuviruses whose vector is the treehopper, Micrutalis malleifera (Briddon et al., 1996). This result sustains the prediction that the insect vector of ToALCV is probably the treehopper. Transmission assays need to be made in order to confirm this prediction. In spite of this, we have no record of any increase in the treehopper population or any new treehopper species threatening tomato cultivated in the northwest region of Argentina when these viruses were isolated. There is a report of Micrutalis malleifera Fowler in our country (Christensen, 1943), but its presence does not necessarily make it its vector and, besides, there are other reported treehoppers in Argentina (de Remes-Lenicov, 2014).
The SDP analysis of the CP was carried out, apart from the phylogenetic analyses of the CP here presented, to contribute with the prediction of the type of insect that could transmit ToALCV. Thus, the whitefly, the aphid, the treehopper and the leafhopper subgroups were defined and ToALCV was clustered within the treehopper subgroup (Supplementary Figure S3). The SDP analysis was useful to track down the crucial amino acid residue that could be involved in insect transmission specificity of geminiviruses (Supplementary Figure S2). The predicted amino acid residues with functional specificity were concentrated in two positions in the CP: one, in the last part of the amino-terminal region, and the other, in the carboxy-terminal part (Supplementary Figure S3). The CP is a multifunctional protein, so it was associated with virus genome packaging, movement and viral DNA replication, in addition to insect transmission (Fondong, 2013). There are reports that define amino acid residues and protein domain involved in whitefly transmission which have been mapped to the central part of the CP (Noris et al., 1998; Kheyr-Pour et al., 2000; Höhnle et al., 2001; Liu et al., 2001; Unseld et al., 2001). In addition, an N-terminal nuclear localization signal was found in the CP that interacts with the GroEL produced by the whitefly Bemisia tabaci endosymbiotic bacteria, probably to protect the virions in the haemolymph of the insect vector (Kunik et al., 1998; Morin et al., 2000; Yaakov et al., 2011; Rana et al., 2012). The amino acid residue predicted by SDP analysis could guide the design of experiments to define motifs of the CP involved in vector transmission of geminiviruses.
The biolistic inoculation with ToALCV infected tomato plants and developed characteristic viral symptoms including internerval yellowing and apical leaf curling (Figure 6). Furthermore, the three infected plants presented a marked root hypotrophy. This root symptom is rare in viral infections, but some authors describe similar behaviors within the Geminiviridae family, specifically in begomoviruses that infect sweet potato and cassava (Owor et al., 2004; Villordon and Clark, 2014).
ToALCV could be assigned to a new Geminivirus genus, with the tentative name of tomapivirus, due to its unique genome organization, its recombination origin and the evolutionary relationships of the nucleotide of the full-length and CP sequences. The unique modular genome arrangement of ToALCV has a capulavirus-like Rep, RepA and C3, a topocuvirus-like CP and a curtovirus and becurtovirus-like V2-MP and V3 proteins. Using SSN to analyze the relationships of sequences and function among proteins codified by the different genus of the Geminiviridae family supported the taxonomy proposal for ToALCV. Moreover, the identification of amino acid residues of the CP was employed to predict that a treehopper could be the vector of this virus but transmission assays need to be performed to confirm this.
Author Contributions
CVM, VB, CF, and PLL conducted the experiment. CVM, ET, CM-B and PLL analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to the excellent technical assistance of Verónica Ranieri. They are also thankful to Lilia Isabel Puch (EEA Yuto-INTA) for the information on treehoppers.
Funding. This work was supported by Instituto Nacional de Tecnología Agropecuaria (INTA, PE- PNBIO-1131044) and Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, PFDT-PRH N°75-13 FONCyT-INTA).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2017.02665/full#supplementary-material
FIGURE S1 | Alignment of the Rep 5′ ends of ALCV and the sequences of the three ToALCV isolations. Motif I, Motif II, Motif III and the GRS region are indicated with boxes.
FIGURE S2 | Maximum likelihood phylogenetic tree obtained with amino acid sequence alignments of the Rep of 66 geminiviruses. Filled circles indicate 70 or more bootstrap percentages.
FIGURE S3 | Specificity-determining positions alignment for the CP amino acid sequences of the Geminiviridae family. (Top) CP multiple sequence alignment colored by clustalX code. The sequences were automatically divided into 9 groups by SCI-PHY and SDPs calculated by the SPEER server. Colored boxes on the left indicate the different genus of the Geminiviridae family. The names of the sequences indicate the acronym, accession number, known insect vector and subgroup clustering. The numbered boxes on the center indicate the different clusters formed in the analysis. Subgroup 5 (where the interest sequences are) is pushed on top for clarity. The arrows show the highest scored amino acids that are informative to the clustering. (Bottom) Histograms of the conservation and quality of the alignment colored from brown to yellow (from lower to higher conservation and quality, respectively). The last histogram shows the sequence logo.
References
- Al Rwahnih M., Alabi O. J., Westrick N. M., Golino D., Rowhani A. (2017). Description of a novel monopartite geminivirus and its defective subviral genome in grapevine. Phytopathology 107 240–251. 10.1094/PHYTO-07-16-0282-R [DOI] [PubMed] [Google Scholar]
- Albuquerque L. C., Varsani A., Fernandes F. R., Pinheiro B., Martin D. P., de Tarso Oliveira, et al. (2012). Further characterization of tomato-infecting begomoviruses in Brazil. Arch. Virol. 157 747–752. 10.1007/s00705-011-1213-7 [DOI] [PubMed] [Google Scholar]
- Andrade E. C., Manhani G. G., Alfenas P. F., Calegario R. F., Fontes E. P., Zerbini F. M. (2006). Tomato yellow spot virus, a tomato-infecting begomovirus from Brazil with a closer relationship to viruses from Sida sp., forms pseudorecombinants with begomoviruses from tomato but not from Sida. J. Gen. Virol. 87 3687–3696. 10.1099/vir.0.82279-0 [DOI] [PubMed] [Google Scholar]
- Atkinson H. J., Morris J. H., Ferrin T. E., Babbitt P. C. (2009). Using sequence similarity networks for visualization of relationships across diverse protein superfamilies. PLOS ONE 4:e4345. 10.1371/journal.pone.0004345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo P., Golden M., Akram M., Naimuddin Nadarajan N., Fernandez E., et al. (2013). Identification and characterisation of a highly divergent geminivirus: evolutionary and taxonomic implications. Virus Res. 177 35–45. 10.1016/j.virusres.2013.07.006 [DOI] [PubMed] [Google Scholar]
- Bernardo P., Muhire B., François S., Deshoux M., Hartnady P., Farka S. K., et al. (2016). Molecular characterization and prevalence of two capulaviruses: Alfalfa leaf curl virus from France and Euphorbia caput-medusae latent virus from South Africa. Virology 493 142–153. 10.1016/j.virol.2016.03.016 [DOI] [PubMed] [Google Scholar]
- Boulton M. (2002). Functions and interactions of mastrevirus gene products. Physiol. Mol. Plant Pathol. 60 243–255. 10.1006/pmpp.2002.0403 21192819 [DOI] [Google Scholar]
- Bozorgi N., Heydarnejad J., Kamali M., Massumi H. (2017). Splicing features in the expression of the complementary-sense genes of Beet curly top Iran virus. Virus Genes 53 323–327. 10.1007/s11262-016-1422-y [DOI] [PubMed] [Google Scholar]
- Briddon R. W., Bedford I. D., Tsai J. H., Markham P. G. (1996). Analysis of the nucleotide sequence of the treehopper-transmitted geminivirus, tomato pseudo-curly top virus, suggests a recombinant origin. Virology 219 387–394. 10.1006/viro.1996.0264 [DOI] [PubMed] [Google Scholar]
- Briddon R. W., Pinner M. S., Stanley J., Markham P. G. (1990). Geminivirus coat protein gene replacement alters insect specificity. Virology 177 85–94. 10.1016/0042-6822(90)90462-Z [DOI] [PubMed] [Google Scholar]
- Brown D. P., Krishnamurthy N., Sjölander K. (2007). Automated protein subfamily identification and classification. PLOS Comput. Biol. 3:e160. 10.1371/journal.pcbi.0030160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Urquiza G. P., Beserra J. E., Jr., Bruckner F. P., Lima A. T., Varsani A., Alfenas-Zerbini P., et al. (2008). Six novel begomoviruses infecting tomato and associated weeds in Southeastern Brazil. Arch. Virol. 153 1985–1989. 10.1007/s00705-008-0172-0 [DOI] [PubMed] [Google Scholar]
- Chakraborty A., Mandloi S., Lanczycki C. J., Panchenko A. R., Chakrabarti S. (2012). SPEER-SERVER: a web server for prediction of protein specificity determining sites. Nucleic Acids. Res. 40 W242–W248. 10.1093/nar/gks559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J. R. (1943). Lista de Membracidae encontrados en la Argentina y algunos países limítrofes. Rev. Soc. Entomol. Argent. 11 440–445. [Google Scholar]
- Darriba D., Taboada G. L., Doallo R., Posada D. (2011). ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics 27 1164–1165. 10.1093/bioinformatics/btr088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darriba D., Taboada G. L., Doallo R., Posada D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9:772. 10.1038/nmeth.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Remes-Lenicov A. M. (2014). “Superfamilia membracoidea: fam. membracidae, aetalionidae y melizoderidae (Hemiptera:Auchenorrhyncha),” in Diversidad de Artrópodos Argentinos, eds Roig S., Claps L., Morrone O. (San Miguel de Tucumán: Editorial INSUE - UNT; ). [Google Scholar]
- Dekker E. L., Woolston C. J., Xue Y. B., Cox B., Mullineaux P. M. (1991). Transcript mapping reveals different expression strategies for the bicistronic RNAs of the geminivirus wheat dwarf virus. Nucleic Acids Res. 19 4075–4081. 10.1093/nar/19.15.4075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32 1792–1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondong V. N. (2013). Geminivirus protein structure and function. Mol. Plant Pathol. 14 635–649. 10.1111/mpp.12032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agriculture Organization [FAO] (2014). Available at: http://faostat3.fao.org/browse/Q/QC/E [accessed August 7, 2017]. [Google Scholar]
- Guindon S., Dufayard J. F., Lefort V., Anisimova M., Hordijk W., Gascuel O. (2010). New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59 307–321. 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- Guindon S., Gascuel O. (2003). A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Syst. Biol. 52 696–704. 10.1080/10635150390235520 [DOI] [PubMed] [Google Scholar]
- Höhnle M., Höfer P., Bedford I. D., Briddon R. W., Markham P. G., Frischmuth T. (2001). Exchange of three amino acids in the coat protein results in efficient whitefly transmission of a nontransmissible Abutilon mosaic virus isolate. Virology 290 164–171. 10.1006/viro.2001.1140 [DOI] [PubMed] [Google Scholar]
- Jeske H., Lütgemeier M., Preiss W. (2001). DNA forms indicate rolling circle and recombination-dependent replication of Abutilon mosaic virus. EMBO J. 20 6158–6167. 10.1093/emboj/20.21.6158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Standley D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30 772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., et al. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28 1647–1649. 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheyr-Pour A., Bananej K., Dafalla G. A., Caciagli P., Noris E., Ahoonmanesh A., et al. (2000). Watermelon chlorotic stunt virus from the Sudan and Iran: sequence comparisons and identification of a whitefly-transmission determinant. Phytopathology 90 629–635. 10.1094/PHYTO.2000.90.6.629 [DOI] [PubMed] [Google Scholar]
- Klute K. A., Nadler S. A., Stenger D. C. (1996). Horseradish curly top virus is a distinct subgroup II geminivirus species with rep and C4 genes derived from a subgroup III ancestor. J. Gen. Virol. 77 1369–1378. 10.1099/0022-1317-77-7-1369 [DOI] [PubMed] [Google Scholar]
- Knierim D., Maiss E. (2007). Application of Phi29 DNA polymerase in identification and full-length clone inoculation of tomato yellow leaf curl Thailand virus and tobacco leaf curl Thailand virus. Arch. Virol. 152 941–954. 10.1007/s00705-006-0914-9 [DOI] [PubMed] [Google Scholar]
- Krenz B., Thompson J. R., Fuchs M., Perry K. L. (2012). Complete genome sequence of a new circular DNA virus from grapevine. J. Virol. 86 7715. 10.1128/JVI.00943-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunik T., Palanichelvam K., Czosnek H., Citovsky V., Gafni Y. (1998). Nuclear import of the capsid protein of Tomato yellow leaf curl virus (TYLCV) in plant and insect cells. Plant J. 13 393–399. 10.1046/j.1365-313X.1998.00037.x [DOI] [PubMed] [Google Scholar]
- Lapidot M., Weil G., Cohen L., Segev L., Gaba V. (2007). Biolistic inoculation of plants with Tomato yellow leaf curl virus DNA. J. Virol. Methods 144 143–148. 10.1016/j.jviromet.2007.04.011 [DOI] [PubMed] [Google Scholar]
- Lefeuvre P., Lett J. M., Varsani A., Martin D. P. (2009). Widely conserved recombination patterns among single-stranded DNA viruses. J. Virol. 83 k2697–2707. 10.1128/JVI.02152-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefeuvre P., Moriones E. (2015). Recombination as a motor of host switches and virus emergence: geminiviruses as case studies. Curr. Opin. Virol. 10 14–19. 10.1016/j.coviro.2014.12.005 [DOI] [PubMed] [Google Scholar]
- Letunic I., Doerks T., Bork P. (2015). SMART: recent updates, new developments and status in 2015. Nucleic Acids. Res. 43 D257–D260. 10.1093/nar/gku949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Godzik A. (2006). Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22 1658–1659. 10.1093/bioinformatics/btl158 [DOI] [PubMed] [Google Scholar]
- Liang P., Navarro B., Zhang Z., Wang H., Lu M., Xiao H., et al. (2015). Identification and characterization of a novel geminivirus with a monopartite genome infecting apple trees. J. Gen. Virol. 96 2411–2420. 10.1099/vir.0.000173 [DOI] [PubMed] [Google Scholar]
- Liu H., Lucy A. P., Davies J. W., Boulton M. I. (2001). A single amino acid change in the coat protein of Maize streak virus abolishes systemic infection, but not interaction with viral DNA or movement protein. Mol. Plant Pathol. 2 223–228. 10.1046/j.1464-6722.2001.00068.x [DOI] [PubMed] [Google Scholar]
- Loconsole G., Saldarelli P., Doddapaneni H., Savino V., Martelli G. P., Saponari M. (2012). Identification of a single-stranded DNA virus associated with citrus chlorotic dwarf disease, a new member in the family Geminiviridae. Virology 432 162–172. 10.1016/j.virol.2012.06.005 [DOI] [PubMed] [Google Scholar]
- Ma Y., Navarro B., Zhang Z., Lu M., Zhou X., Chi S., et al. (2015). Identification and molecular characterization of a novel monopartite geminivirus associated with mulberry mosaic dwarf disease. J. Gen. Virol. 96 2421–2434. 10.1099/vir.0.000175 [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A., Derbyshire M. K., Gonzales N. R., Lu S., Chitsaz F., Geer L. Y., et al. (2015). CDD: NCBI’s conserved domain database. Nucleic Acids Res. 43 222–226. 10.1093/nar/gku1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. P., Biagini P., Lefeuvre P., Golden M., Roumagnac P., Varsani A. (2011). Recombination in eukaryotic single stranded DNA viruses. Viruses 3 1699–1738. 10.3390/v3091699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. P., Murrell B., Golden M., Khoosal A., Muhire B. (2015). RDP4: detection and analysis of recombination patterns in virus genomes. Virus Evol. 1:vev003. 10.1093/ve/vev003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melgarejo T. A., Kon T., Rojas M. R., Paz-Carrasco L., Zerbini F. M., Gilbertson R. L. (2013). Characterization of a new world monopartite begomovirus causing leaf curl disease of tomato in Ecuador and Peru reveals a new direction in geminivirus evolution. J. Virol. 87 5397–5413. 10.1128/JVI.00234-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin S., Ghanim M., Sobol I., Czosnek H. (2000). The GroEL protein of the whitefly Bemisia tabaci interacts with the coat protein of transmissible and nontransmissible begomoviruses in the yeast two-hybrid system. Virology 276 404–416. 10.1006/viro.2000.0549 [DOI] [PubMed] [Google Scholar]
- Mullineaux P. M., Guerineau F., Accotto G. P. (1990). Processing of complementary sense RNAs of Digitaria streak virus in its host and in transgenic tobacco. Nucleic Acids Res. 18 7259–7265. 10.1093/nar/18.24.7259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash T. E., Dallas M. B., Reyes M. I., Buhrman G. K., Ascencio-Ibañez J. T., Hanley-Bowdoin L. (2011). Functional analysis of a novel motif conserved across geminivirus Rep proteins. J. Virol. 85 1182–1192. 10.1128/JVI.02143-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noris E., Vaira A. M., Caciagli P., Masenga V., Gronenborn B., Accotto G. P. (1998). Amino acids in the capsid protein of Tomato yellow leaf curl virus that are crucial for systemic infection, particle formation, and insect transmission. J. Virol. 72 10050–10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owor B., Legg J. P., Okao-Okuja G., Obonyo R., Ogenga-Latigo M. W. (2004). The effect of cassava mosaic geminiviruses on symptom severity, growth and root yield of a cassava mosaic virus disease-susceptible cultivar in Uganda. Ann. Appl. Biol. 145 331–337. 10.1111/j.1744-7348.2004.tb00390.x [DOI] [Google Scholar]
- Paz-Carrasco L. C., Castillo-Urquiza G. P., Lima A. T., Xavier C. A., Vivas-Vivas L. M., Mizubuti E. S., et al. (2014). Begomovirus diversity in tomato crops and weeds Ecuador in tomato the detection of a recombinant isolate of rhynchosia golden mosaic Yucatan virus infecting. Arch. Virol. 159 2127–2132. 10.1007/s00705-014-2046-y [DOI] [PubMed] [Google Scholar]
- Poojari S., Alabi O. J., Fofanov V. Y., Naidu R. A. (2013). A leafhopper-transmissible DNA virus with novel evolutionary lineage in the family geminiviridae implicated in grapevine redleaf disease by next-generation sequencing. PLOS ONE 8:e6419. 10.1371/journal.pone.0064194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss W., Jeske H. (2003). Multitasking in replication is common among geminiviruses. J. Virol. 77 2972–2980. 10.1128/JVI.77.5.2972-2980.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M. N., Dehal P. S., Arkin A. P. (2010). FastTree 2 approximately maximum-likelihood trees for large alignments. PLOS ONE 5:e9490. 10.1371/journal.pone.0009490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana V. S., Singh S. T., Priya N. G., Kumar J., Rajagopal R. (2012). Arsenophonus GroEL interacts with CLCuV and is localized in midgut and salivary gland of whitefly B. tabaci. PLOS ONE 7:e42168. 10.1371/journal.pone.0042168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K. S., Götz M., Winter S., Jeske H. (2016). The contribution of translesion synthesis polymerases on geminiviral replication. Virology 15 137–148. 10.1016/j.virol.2015.10.027 [DOI] [PubMed] [Google Scholar]
- Roumagnac P., Granier M., Bernardo P., Deshoux M., Ferdinand R., Galzi S., et al. (2015). Alfalfa leaf curl virus: an aphid-transmitted geminivirus. J. Virol. 89 9683–9688. 10.1128/JVI.00453-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybicki E. P. (2015). A top ten list for economically important plant viruses. Arch. Virol. 160 17–20. 10.1007/s00705-014-2295-9 [DOI] [PubMed] [Google Scholar]
- Saunders K., Lucy A., Stanley J. (1991). DNA forms of the geminivirus African cassava mosaic virus consistent with a rolling circle mechanism of replication. Nucleic Acids Res. 19 2325–2330. 10.1093/nar/19.9.2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholthof K. B., Adkins S., Czosnek H., Palukaitis P., Jacquot E., Hohn T., et al. (2011). Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 12 938–954. 10.1111/j.1364-3703.2011.00752.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J., Milpetz F., Bork P., Ponting C. P. (1998). SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. U.S.A. 95 5857–5864. 10.1073/pnas.95.11.5857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P., Markiel A., Ozier O., Baliga N. S., Wang J. T., Ramage D., et al. (2003). Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13 2498–2504. 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivaprasad P. V., Akbergenov R., Trinks D., Rajeswaran R., Veluthambi K., Hohn T., et al. (2005). Promoters, transcripts, and regulatory proteins of Mungbean yellow mosaic geminivirus. J. Virol. 79 8149–8163. 10.1128/JVI.79.13.8149-8163.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J., Markham P. G., Callis R. J., Pinner M. S. (1986). The nucleotide sequence of an infectious clone of the geminivirus beet curly top virus. EMBO J. 5 1761–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susi H., Laine A. L., Filloux D., Kraberger S., Farkas K., Bernardo P., et al. (2017). Genome sequences of a capulavirus infecting Plantago lanceolata in the Åland archipelago of Finland. Arch. Virol. 162 2041–2045. 10.1007/s00705-017-3298-0 [DOI] [PubMed] [Google Scholar]
- Unseld S., Höhnle M., Ringel M., Frischmuth T. (2001). Subcellular targeting of the coat protein of African cassava mosaic geminivirus. Virology 286 373–383. 10.1006/viro.2001.1003 [DOI] [PubMed] [Google Scholar]
- Vaghi Medina C. G., López Lambertini P. M. (2012). Tomato dwarf leaf virus, a New World begomovirus infecting tomato in Argentina. Arch. Virol. 157 1975–1980. 10.1007/s00705-012-1355-2 [DOI] [PubMed] [Google Scholar]
- Vaghi Medina C. G., Martin D. P., López Lambertini P. M. (2015). Tomato mottle wrinkle virus, a recombinant begomovirus infecting tomato in Argentina. Arch. Virol. 160 581–585. 10.1007/s00705-014-2216-y [DOI] [PubMed] [Google Scholar]
- Varma A., Malathi V. G. (2003). Emerging geminivirus problems. A serious threat to crop production. Ann. Appl. Biol. 142 145–164. 10.1111/j.1744-7348.2003.tb00240.x [DOI] [Google Scholar]
- Varsani A., Navas-Castillo J., Moriones E., Hernandez-Zepeda C., Idris A., Brown J. K., et al. (2014). Establishment of three new genera in the family Geminiviridae: Becurtovirus, Eragrovirus and Turncurtovirus. Arch. Virol. 159 2193–2203. 10.1007/s00705-014-2050-2 [DOI] [PubMed] [Google Scholar]
- Varsani A., Roumagnac P., Fuchs M., Navas-Castillo J., Moriones E., Idris A., et al. (2017). Capulavirus and Grablovirus: two new genera in the family Geminiviridae. Arch. Virol. 162 1819–1831. 10.1007/s00705-017-3268-6 [DOI] [PubMed] [Google Scholar]
- Varsani A., Shepherd D. N., Dent K., Monjane A. L., Rybicki E. P., Martin D. P. (2009). A highly divergent South African geminivirus species illuminates the ancient evolutionary history of this family. Virol. J. 6:36. 10.1186/1743-422X-6-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villordon A. Q., Clark C. A. (2014). Variation in virus symptom development and root architecture attributes at the onset of storage root initiation in ‘beauregard’ sweetpotato plants grown with or without nitrogen. PLOS ONE 9:e107384. 10.1371/journal.pone.0107384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaakov N., Levy Y., Belausov E., Gaba V., Lapidot M., Gafni Y. (2011). Effect of a single amino acid substitution in the NLS domain of Tomato yellow leaf curl virus-Israel (TYLCV-IL) capsid protein (CP) on its activity and on the virus life cycle. Virus Res. 158 8–11. 10.1016/j.virusres.2011.02.016 [DOI] [PubMed] [Google Scholar]
- Yazdi H. R., Heydarnejad J., Massumi H. (2008). Genome characterization and genetic diversity of Beet curly top Iran virus: a geminivirus with a novel nonanucleotide. Virus Genes 36 539–545. 10.1007/s11262-008-0224-2 [DOI] [PubMed] [Google Scholar]
- Zerbini F. M., Briddon R. W., Idris A., Martin D. P., Moriones E., Navas-Castillo J., et al. (2017). ICTV virus taxonomy profile: geminiviridae. J. Gen. Virol. 98 131–133. 10.1099/jgv.0.000738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Olson N. H., Baker T. S., Faulkner L., Agbandje-McKenna M., Boulton M. I., et al. (2001). Structure of the Maize streak virus geminate particle. Virology 279 471–477. 10.1006/viro.2000.0739 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 | Alignment of the Rep 5′ ends of ALCV and the sequences of the three ToALCV isolations. Motif I, Motif II, Motif III and the GRS region are indicated with boxes.
FIGURE S2 | Maximum likelihood phylogenetic tree obtained with amino acid sequence alignments of the Rep of 66 geminiviruses. Filled circles indicate 70 or more bootstrap percentages.
FIGURE S3 | Specificity-determining positions alignment for the CP amino acid sequences of the Geminiviridae family. (Top) CP multiple sequence alignment colored by clustalX code. The sequences were automatically divided into 9 groups by SCI-PHY and SDPs calculated by the SPEER server. Colored boxes on the left indicate the different genus of the Geminiviridae family. The names of the sequences indicate the acronym, accession number, known insect vector and subgroup clustering. The numbered boxes on the center indicate the different clusters formed in the analysis. Subgroup 5 (where the interest sequences are) is pushed on top for clarity. The arrows show the highest scored amino acids that are informative to the clustering. (Bottom) Histograms of the conservation and quality of the alignment colored from brown to yellow (from lower to higher conservation and quality, respectively). The last histogram shows the sequence logo.