Abstract
Zika virus (ZIKV) is a mosquito-borne pathogen, and Aedes aegypti has been identified as the main vector of the disease. Other mosquito species in the Aedes and Culex genera have been suggested to have the potential for being competent vectors based on experimental exposition of mosquitoes to an infectious blood meal containing ZIKV. Here, we report the isolation in cell culture of ZIKV obtained from different body parts of wild-caught female mosquitoes (Ae. aegypti, Ae. vexans, Cx. quinquefasciatus, Cx. coronator, and Cx. tarsalis) and whole male mosquitoes (Ae. aegypti and Cx. quinquefasciatus) in Mexico. Importantly, this is the first report that shows the presence of the virus in the salivary glands of the wild-caught female mosquitoes species, Cx. coronator, Cx. tarsalis, and Ae. vexans. Our findings strongly suggest that all the species reported herein are potential vectors for ZIKV.
Introduction
In 2015, Brazil was the first country in the Western Hemisphere to report Zika virus (ZIKV)1, but currently the transmission has spread to more than 50 countries and territories in the region2. As of September 2017, there were 9,987 confirmed cases of ZIKV infection, including 5,925 pregnant women, in 26 out of 32 states of Mexico3. ZIKV is a member of the Flaviviridae family and the genus Flavivirus; the presumptive primary vector of the virus is Aedes aegypti (L.), and laboratory studies have demonstrated its ability to acquire and potentially transmit the virus in mosquitoes experimentally fed with infected blood4,5. On the other hand, different laboratory studies have reported that although Ae. aegypti and Ae. albopictus (Skuse) are susceptible to acquire the infection, their level of competence and efficiency to transmit ZIKV ranges from 0 to 70 percent6–9. Furthermore, it has been recently shown that other mosquito species may also transmit the virus in laboratory conditions, including Ae. vexans (Meigen)10,11, and mosquitoes in the Culex genus, such as Cx. quiquefasciatus Say12,13.
To gain insight into the vector competence of different species in the metropolitan area of Guadalajara in the State of Jalisco, Mexico, we collected mosquitoes inside houses in neighborhoods where at least one confirmed or probable case of ZIKV in humans had been reported by the local health authorities. The ZIKV present in different body parts of the mosquitoes was propagated by cell culture and the viral RNA was detected by RT-qPCR.
Results
Mosquito collection
The mosquitoes were collected over five days, from September to November 2016, in 3 different municipalities (18 blocks in 4 neighborhoods) of the metropolitan area of Guadalajara (Figs 1 and 2). In this study 579 mosquitoes representing 2 genera (Aedes and Culex) and 6 species (Ae. aegypti, Ae. epactius Dyar and Knab, Ae. vexans, Cx. quinquefasciatus, Cx. coronator Dyar and Knab, and Cx. tarsalis Coquillett) were collected. The mosquitoes were then separated by the block they were collected from and by species and sex and divided into pools with a maximum of 25 insects. Female mosquitoes from these pools were dissected to separate salivary glands, midguts, and the rest of their bodies, and the dissected parts were distributed into individual tubes containing viral transport medium (Table 1).
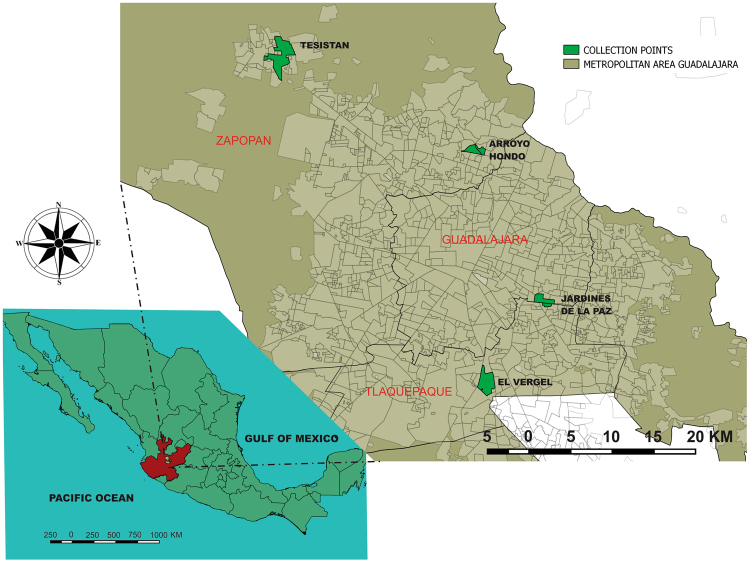
Figure 1.
Map of the collection points in the metropolitan area of Guadalajara, Jalisco, Mexico. Maps were generated using the free and open source software QGIS Chugiak version 2.4.0 (http://www.qgis.org/es/site/about/index.html).
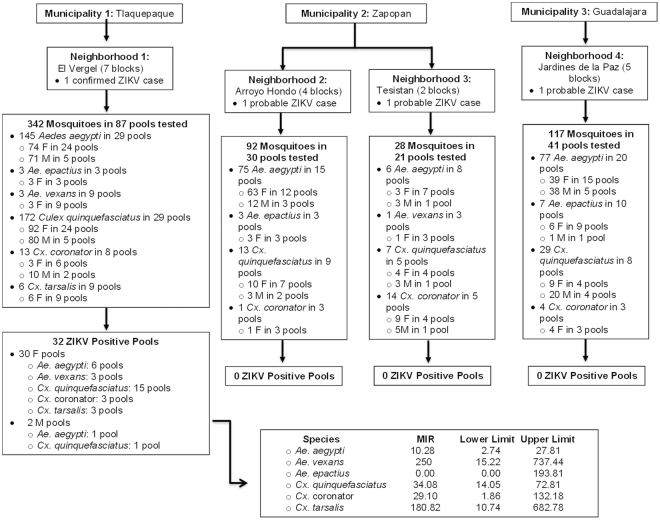
Figure 2.
Flow chart of mosquito collection in the metropolitan area of Guadalajara, Jalisco, Mexico. The figure outlines the collection municipalities and their neighborhoods, as well as the mosquitoes collected and the pools assembled for each area. The neighborhood El Vergel was the only area with mosquitoes positive for ZIKV. Also presented is the minimum infection rate (MIR) per species and the confidence intervals. F = Female; M = Male; ZIKV = Zika Virus.
Table 1.
Summary of mosquito species collected in neighborhoods from the metropolitan area of Guadalajara, Jalisco, Mexico.
| Number of mosquitoes of the indicated genus and species (579 total) | Female Mosquitoes | Male Mosquitoes | |||
|---|---|---|---|---|---|
| No. mosquitoes | No. assembled pools for dissection | No. pools of body parts | No. mosquitoes | No. assembled pools | |
| Aedes aegypti (n = 303) | 179 | 20 | 58 | 124 | 14 |
| Ae. epactius (n = 13) | 12 | 6 | 15 | 1 | 1 |
| Ae. vexans (n = 4) | 4 | 4 | 12 | 0 | 0 |
| Culex quinquefasciatus (n = 221) | 115 | 17 | 39 | 106 | 12 |
| Cx. coronator (n = 32) | 17 | 6 | 16 | 15 | 3 |
| Cx. tarsalis (n = 6) | 6 | 3 | 9 | 0 | 0 |
| Total | 333 | 56 | 149 | 246 | 30 |
Virus isolation
The separation and dissection of the 579 mosquitoes rendered 149 pools of female mosquito body parts and 30 pools of whole male mosquitoes (Table 1; Fig. 2), which were processed for virus isolation. A cytopathic effect (CPE) was observed in 30 of the 149 pools of female mosquito body parts; the observed CPE consisted in cell rounding, detachment, and culture degeneration in the first 1 to 5 days post inoculation (dpi) into C6/36 cell monolayers (Table 2, Fig. 3). These pools were from Ae. aegypti (2 pools), Ae. vexans (1 pool), Cx. quinquefasciatus (5 pools), Cx. coronator (1 pool), and Cx. tarsalis (1 pool). Two pools of male mosquitoes (Ae. aegypti and Cx. quinquefasciatus) also showed CPE. All the samples showing CPE represented 5 of the 6 species of mosquitoes collected.
Table 2.
ZIKV-positive pools for the different mosquito species. CPE appearance times and Ct values by RT-qPCR.
| Mosquitoes genera and species | Number of mosquitoes | Pools presenting CPE | CPE (dpi) | Ct mean values in RT-qPCR for ZIKV (SD) |
|---|---|---|---|---|
| Aedes aegypti | 25 | Male | 2 | 10.50 (0.06) |
| Ae. aegypti | 8 | SG | 3 | 14.14 (1.40) |
| MG | 3 | 13.87 (0.67) | ||
| B | 3 | 13.39 (0.35) | ||
| Ae. aegypti | 3 | SG | 3 | 12.97 (1.07) |
| MG | 3 | 14.19 (0.95) | ||
| B | 3 | 14.26 (0.47) | ||
| Ae. vexans | 1 | SG | 3 | 11.56 (0.91) |
| MG | 3 | 12.17 (0.56) | ||
| B | 3 | 13.74 (0.09) | ||
| Culex quinquefasciatus | 25 | Male | 2 | 11.91 (1.18) |
| Cx. quinquefasciatus | 16 | SG | 3 | 14.36 (0.33) |
| MG | 3 | 14.32 (0.35) | ||
| B | 3 | 14.01 (0.18) | ||
| Cx. quinquefasciatus | 20 | SG | 3 | 12.76 (0.43) |
| MG | 5 | 12.16 (0.51) | ||
| B | 3 | 14.49 (0.14) | ||
| Cx. quinquefasciatus | 25 | SG | 3 | 13.23 (0.55) |
| MG | 3 | 12.76 (0.22) | ||
| B | 3 | 12.59 (0.24) | ||
| Cx. quinquefasciatus | 9 | SG | 1 | 12.91 (1.79) |
| MG | 1 | 12.74 (0.22) | ||
| B | 2 | 13.74 (0.79) | ||
| Cx. quinquefasciatus | 3 | SG | 1 | 10.54 (0.71) |
| MG | 3 | 11.80 (1.19) | ||
| B | 2 | 14.74 (0.18) | ||
| Cx. coronator | 2 | SG | 3 | 12.65 (2.69) |
| MG | 3 | 13.26 (0.40) | ||
| B | 3 | 13.56 (0.09) | ||
| Cx. tarsalis | 3 | SG | 4 | 12.16 (0.58) |
| MG | 4 | 11.86 (0.58) | ||
| B | 1 | 13.89 (0.18) |
All RT-qPCR reactions were performed in triplicate. Ct mean and standard deviation were calculated. SG, salivary gland; MG, midgut; B, body; CPE, cytopathic effect; dpi, days post-inoculation; SD, standard deviation.
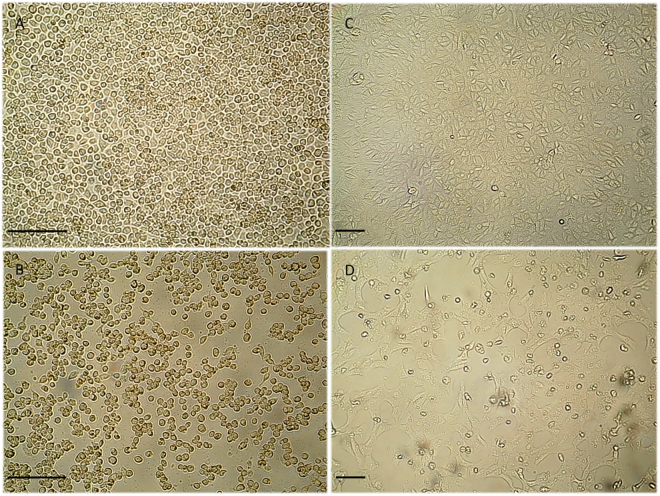
Figure 3.
Cytopathic effect in infected and uninfected cell cultures. Cytopathic effects consisting of cell rounding, detachment and culture degeneration were observed in C6/36 and Vero cell lines at day 4 post-ZIKV inoculation, under an inverted microscope (original magnification ×20 in C6/36 and ×10 in Vero cells. Scale bars, 100 μm). (A) Uninfected C6/36 cells, (B) C6/36 cells inoculated with salivary glands homogenates showing CPE, (C) Uninfected Vero cells, (D) Vero cells inoculated with salivary glands homogenates showing CPE.
All pools showing CPE were from the Vergel neighborhood of Tlaquepaque (Fig. 1). To confirm that the observed CPE was the result of virus infection and not a toxic component in the original samples, the cell media of inoculated cultures were re-inoculated into fresh C6/36 cells. All of the re-inoculated samples reproduced the initially observed CPE, suggesting virus growth. To discard the possibility that the isolated viruses were only entomopathogenic, the cell media from C6/36 cells were used to infect mammalian Vero cells (Fig. 3). All these inoculations produced CPE also presenting cell rounding, detachment, and culture degeneration between 4 to 6 dpi. These findings support the idea that the isolated viruses can infect mammals and discard the possibility that they are strictly entomopathogenic.
Virus identification
Since all the re-inoculations in Vero cells produced CPE, viral RNA was extracted from the collected supernatants of the C6/36 passage, and RT-qPCR was performed. All the supernatants were ZIKV-positive, with Ct values ranging from 10 to 15 (Table 2). Even though the isolates were positive for ZIKV, the presence of chikungunya and dengue viruses in these samples was also investigated by RT-qPCR; none of the samples resulted positive for these other viruses. These results indicate that ZIKV was isolated from the mosquito pools and no chikungunya or dengue viruses were present in our samples.
Minimum infection rates
The minimum infection rate (MIR) was calculated per 1,000 mosquitoes; MIR values of 250 for Ae. vexans, 180.82 for Cx. tarsalis, 34.08 for Cx. quinquefasciatus, 29.10 for Cx. coronator, and 10.28 for Ae. aegypti were found. The MIR values for the different mosquito species collected in this study showed high divergences, although the confidence intervals widely overlapped among them (Fig. 2).
Discussion
To the best of our knowledge this is the first report that shows the presence of ZIKV in the salivary glands of wild-caught female mosquitoes Cx. coronator, Cx. tarsalis, and Ae. vexans, as well as and in Ae. aegypti and Cx. quinquefasciatus, which have already been reported as potential vectors for ZIKV5–9,12,13. All virus isolates were from mosquitoes collected in a neighborhood of the city of Guadalajara, Jalisco, Mexico, where health authorities had recorded one confirmed human ZIKV infection. No viruses could be detected from mosquitoes collected in areas where probable cases had been reported.
Different MIRs were found among the collected mosquito species using the software PooledInfRate v.4.014; the highest were for Ae. vexans (250) and Cx. tarsalis (180.82) while the lowest was for Ae. aegypti (10.28) (Fig. 2). It cannot be disregarded, however, that these differences are due to the low number of collected mosquitoes for the first two species, which makes the confidence intervals overlap. Nevertheless, previous publications have experimentally evaluated the vector competence of Ae. aegypti using mosquitoes from other regions of the Americas6, as well as from Senegal7, and it was reported that these species had the lowest infection rate among those evaluated. To confirm the low MIR found in our study for Ae. aegypti, an equal number of mosquitoes per species would have to be analyzed. However, taking into consideration that Ae. aegypti mosquitoes have an endophilic and anthropophagic behavior15 in contrast with the other ZIKV-positive mosquito species found in this study, it could be assumed that despite the low infection rates reported by other researchers, Ae. aegypti is more likely the primary vector of the disease in the State of Jalisco, Mexico.
Most analyzed mosquito pools caused a CPE 3 dpi, but 2 pools of salivary glands of Cx. quinquefasciatus showed CPE at 1 dpi (Table 2). These findings support previously published results that suggest the Cx quinquefasciatus mosquito is a potential vector for ZIKV transmission12,13. On the other hand, the results presented in this work are discordant with previous publications reporting Culex spp. as poor ZIKV vectors. For instance, North American mosquito colonies maintained for decades in the laboratory were found to be non-efficient ZIKV vectors16. Similarly, in mosquitoes from Río de Janeiro, Brazil, Cx. quinquefasciatus were reported not to be competent to transmit the local strain of ZIKV17. These observations could be explained by the genetic variability of the mosquito populations, as previously suggested7,15. Hence, the implementation of vector competence surveillance programs should be mandatory for different geographic areas, even in the same country.
In those cases where ZIKV was found in salivary glands, a CPE was observed at a similar dpi in 5 wild-caught mosquito species, which can be then considered as potential ZIKV vectors. Nevertheless, further studies of a possible vector competence barrier to ZIKV in all mosquito species reported in this work are needed because many factors could be involved in the transmission of the virus, as has been suggested15. In the case of cells inoculated with salivary glands of Cx. tarsalis, a CPE was observed at 4 dpi, suggesting that this mosquito species may not be a competent vector, although it cannot be disregarded that the salivary glands in this pool had been recently infected, since the CPE in the rest of the body was observed at 1 dpi.
For the identification of the isolated viruses, RT-qPCR was performed. All the supernatants in the C6/36-infected cells were ZIKV-positive, with Ct values ranging from 10 to 15. Of particular interest, ZIKV was detected in a male pool of Ae. aegypti, supporting previous reports in mosquitoes from Brazil and in laboratory experimental infections18–20. In addition, we also found a ZIKV-positive male pool of Cx. quinquefasciatus, suggesting the occurrence of vertical transmission, a further cause of concern. If male mosquitoes are infected vertically, females from the same mother are probably also infected. Therefore, the number of mosquitoes with the potential to transmit the virus would increase; it will be important to address these possibilities through further studies analyzing the presence of the virus in the saliva of vertically infected female mosquitoes. Also, the finding of 3 ZIKV-infected Culex species (Cx. quinquefasciatus, Cx. coronator, and Cx. tarsalis) could be a major concern and potential complication for vector control programs since all these species have different breeding sites, and they could maintain viral populations during inter-epidemic periods, such as the dry season, and hibernate during colder temperatures21.
Recent studies indicate that ZIKV arrived in Mexico from northeast Brazil in the second half of 2014 or early 201522,23. It was introduced first to the state of Chiapas in the southeast of the country and then was dispersed to 26 (out of 32) additional states3,24. It would be very interesting to compare the genomic sequence of our ZIKV isolates with those determined from viruses infecting humans in different regions of Mexico.
In conclusion, we found the presence of ZIKV in the salivary glands of wild-caught female mosquitoes Cx. coronator, Cx. tarsalis, Ae. vexans, Ae. aegypti, and Cx. quinquefasciatus, suggesting that these species are potential vectors for the transmission of the disease. Also, the fact that pools of male mosquitoes were found to be positive for ZIKV, suggests vertical transmission in these species. Additional studies of female mosquitoes’ saliva from the different species reported in this work are needed to confirm the presence of ZIKV and determine if they have a vector competence barrier to the virus.
Methods
Mosquito collection
The collection was performed by mechanical aspiration using an InsectaZooka No. 2888 A (BioQuip Products, Rancho Dominguez, CA, USA) inside residences by Personnel of the Entomological Research Unit of the Public Health Department of the State of Jalisco and the mosquitoes were transported to this research unit. Neighborhoods and blocks were selected based on the reports of the Vector Borne and Zoonotic Diseases Department of Jalisco on ZIKV confirmed or probable human cases in the area.
Pools separation criteria
Collected mosquitoes from within each block were separated by species and sex into pools of 25 insects maximum. Female mosquitoes from each species-specific pool were dissected under a stereomicroscope. Their body parts (salivary glands, midguts, and the rest of the bodies) were distributed into individual 1.5 ml conical tubes containing 250 μL of viral transport medium (phosphate-buffered saline, pH 7.4, containing 30% fetal bovine serum and 2% of penicillin, streptomycin, and amphotericin B). Male mosquitoes were pooled without dissection, since they are not hematophagus, into 1.5 ml conical tubes containing 250 μL of viral transport medium. All pools were frozen at −20 °C to be transported to the Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco A.C. (CIATEJ).
Virus isolation
Mosquito pools were ground, and the resultant homogenates were centrifuged at 10,000 × g for 10 min. Next, 25 μL of each supernatant was placed into a single well of a 24-well plate containing Ae. albopictus cells C6/36 (ATCC® CRL-1660™). After the inoculum was adsorbed for 1 h at 28 °C, maintenance medium was added. The cell cultures were maintained at 28 °C and examined daily for evidence of viral CPE for 5 days. If no CPE was observed, the culture was freeze-thawed once and was re-inoculated in a blind passage in a fresh plate of C6/36 cells for another 5 days. If CPE was still not observed, the cultures were discarded. To confirm virus isolation, the cell media from cultures that showed CPE were re-inoculated in fresh C6/36 cells. To evaluate if these viruses could infect mammalian cells, viral passages were also performed in Vero cells (ATCC® CCL-81™), adding in both cases 25 μL of each cell media per well of a 24-well plate. After the inoculum was adsorbed for 1 h at 28 °C or 37 °C, maintenance medium was added. Cultures were maintained at 28 °C or 37 °C, for C6/36 and Vero cells, respectively, and examined daily for evidence of viral CPE. All cells observations were under an inverted microscope (Nikon Eclipse, TS100, Japan), and images were captured with a camera Optikam WiFi – 4083, and analyzed with Optika Vision Lite software 2.1 (Optika SRL, Ponteranica, Italy).
Virus identification
After virus re-infection in Vero cells was confirmed, the isolated viruses were detected by RT-qPCR. For this, viral RNA was extracted from the cell media of the C6/36 passage that yielded CPE using a QiAmp Viral RNA Mini Kit (Qiagen™, Hilden, Germany). RT-qPCRs were carried out in a Light Cycler 480 II PCR instrument (Roche Diagnostics, Penzberg, Germany) using Verso 1-step RT-qPCR Kit (Thermo Fisher™, MA, USA). Since ZIKV, dengue, and chikungunya viruses have been co-circulating in the same area, the presence of ZIKV was determined, first, using the primer pair and probe previously reported by Lanciotti et al. that can detect a minimum of 25 genomic copies of the virus25 (Table 3). As a positive control for the reaction, we used RNA extracted from a ZIKV strain kindly provided by A.A. Sall (Institut Pasteur, Dakar, Senegal). All RT-qPCR reaction per sample were performed by triplicate. If the cell cultures showing a CPE resulted negative to ZIKV, then RT-qPCRs for chikungunya and dengue, using QuantiFast SYBR Green RT-qPCR Kit (Qiagen™ Hilden Germany), were performed. In the case of chikungunya, we used a primer pair reported by Thavara et al.26 (Table 3). For a positive control, we used RNA extracted from an isolate obtained from a patient’s serum sample, previously collected in the Hospital Civil de Guadalajara Fray Antonio Alcalde during 2014–201627. In the case of dengue virus, we used the primer pair reported by Lai et al.28 (Table 3), and as a positive control, a PCR product previously cloned in our laboratory29. The negative controls for all the reactions were molecular biology grade water as a no template control.
Table 3.
Primers and probes used for the identification of Zika, chikungunya and dengue viruses.
| Primer name | Sequence 5′-3′ | Sensitivity, no. copies reported | References |
|---|---|---|---|
| ZIKV 1086 | CCGCTGCCCAACACAAG | Lanciotti, R.S., et al. (2007) | |
| ZIKV 1162c | CCACTAACGTTCTTTTGCAGACAT | 25 | |
| ZIKV 1107-FAM | AGCCTACCTTGACAAGCAGTCAGACACTCAA | ||
| CHIK-F3 | (ACGCAATTGAGCGAAGCAC) | 200 | Thavara, U., et al.26 |
| CHIK-B3 | (CTGAAGACATTGGCCCCAC) | ||
| DENV-For | TTGAGTAAACYRTGCTGCCTGTAGCTC | 35.30 | Lai Y.L., et al.28 and García-Ruíz, D., et al.29 |
| DENV-Rev | GAGACAGCAGGATCTCTGGTCTYTC |
Minimum infection rate analysis
We estimated the MIR per 1,000 mosquitoes, with the bias corrected by maximum likelihood estimator (MLE), with a skewness-corrected score confidence interval, using the program PooledInfRate v.4.014.
Acknowledgements
This project was partially supported by the National Council for Science and Technology (CONACyT)-Mexico through the Mexican Network for Virology. We are indebted to Drs. José Narro Robles, Pablo Kuri Morales, and Cuitláhuac Ruiz Matus from the Secretaría de Salud of México for their help and support to carry out this work. We also thank the personnel of the Unidad de Investigación Entomológica de Occidente for their assistance in the collection of mosquitoes and separation of species. We thank Rito Gerónimo Aguirre Cortés, for the elaboration of the collection maps. We also would like to thank Brian Walsh from CIATEJ/Peace Corps for his valuable remarks in editing the English of the manuscript.
Author Contributions
D.E.-Q., A.M.-S., J.M.S.-G., G.S.-T., F.C.-M, C.G.-A., C.F.A., S.L., R.M.d.A., V.P.-R., A.E.E.-Q. designed experiments, discussion of data and conceived the project. D.E.-Q., K.A.E., C.F.A., S.L., R.M.d.A., V.P.-R., A.E.E.-Q. drafted and reviewed the manuscript. D.E.-Q., E.V.-S. carried out virus experiments, isolation and molecular studies. A.M.-S., J.M.S.-G., A.R.N.-Z., A.E.E.-Q. performed mosquito collection and dissections. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
A correction to this article is available online at https://doi.org/10.1038/s41598-018-25807-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/15/2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has not been fixed in the paper.
References
- 1.Zanluca C, et al. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz. 2015;110:569–572. doi: 10.1590/0074-02760150192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC. Centers for Disease Control and Prevention, all countries and territories with active Zika virus transmission., (https://www.cdc.gov/zika/geo/active-countries.html) (2017).
- 3.Secretaría de Salud (Mexico). Avisos epidemiológicos Zika., (https://www.gob.mx/cms/uploads/attachment/file/258140/Cuadro_Casos_ZIKA_y_Emb_sem_37_2017.pdf) (2017).
- 4.Li MI, Wong PSJ, Ng LC, Tan CH. Oral susceptibility of Singapore Aedes (Stegomyia) aegypti (Linnaeus) to Zika virus. PLoS Negl Trop Dis. 2012;6:e1792. doi: 10.1371/journal.pntd.0001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa-da-Silva AL, et al. Laboratory strains of Aedes aegypti are competent to Brazilian Zika virus. PloS one. 2017;12:e0171951. doi: 10.1371/journal.pone.0171951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chouin-Carneiro T, et al. Differential susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika Virus. PLoS Negl Trop Dis. 2016;10:e0004543. doi: 10.1371/journal.pntd.0004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diagne CT, et al. Potential of selected Senegalese Aedes spp. mosquitoes (Diptera: Culicidae) to transmit Zika virus. BMC Infect Dis. 2015;15:492. doi: 10.1186/s12879-015-1231-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roundy CM, et al. Variation in Aedes aegypti Mosquito competence for Zika Virus transmission. Emerg Infect Dis. 2017;23:625–632. doi: 10.3201/eid2304.161484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciota AT, et al. Effects of Zika virus strain and Aedes mosquito species on vector competence. Emerg Infect Dis. 2017;23:1110–1117. doi: 10.3201/eid2307.161633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gendernalik A, et al. American Aedes vexans mosquitoes are competent vectors of Zika virus. Am J Trop Med Hyg. 2017;96:1338–1340. doi: 10.4269/ajtmh.16-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Donnell KL, Bixby MA, Morin KJ, Bradley DS, Vaughan JA. Potential of a northern population of Aedes vexans (Diptera: Culicidae) to transmit Zika Virus. J Med Entomol. 2017;54:1354–1359. doi: 10.1093/jme/tjx087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guedes DR, et al. Zika virus replication in the mosquito Culex quinquefasciatus in Brazil. Emerg Microbes Infect. 2017;6:e69. doi: 10.1038/emi.2017.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo XX, et al. Culex pipiens quinquefasciatus: a potential vector to transmit Zika virus. Emerg Microbes Infect. 2016;5:e102. doi: 10.1038/emi.2016.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biggerstaff, B. J. PooledInfRate, Version 4.0: a Microsoft® Office Excel© Add-In to compute prevalence estimates from pooled samples. Centers for Disease Control and Prevention, Fort Collins, CO, USA (2009).
- 15.Tabachnick WJ. Nature, nurture and evolution of intra-species variation in mosquito arbovirus transmission competence. Int J Environ Res Public Health. 2013;10:249–277. doi: 10.3390/ijerph10010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weger-Lucarelli J, et al. Vector competence of American mosquitoes for three strains of Zika virus. PLoS Negl Trop Dis. 2016;10:e0005101. doi: 10.1371/journal.pntd.0005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandes RS, et al. Culex quinquefasciatus from Rio de Janeiro is not competent to transmit the local Zika virus. PLoS Negl Trop Dis. 2016;10:e0004993. doi: 10.1371/journal.pntd.0004993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreira-de-Brito A, et al. First detection of natural infection of Aedes aegypti with Zika virus in Brazil and throughout South America. Mem Inst Oswaldo Cruz. 2016;111:655–658. doi: 10.1590/0074-02760160332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thangamani S, Huang J, Hart CE, Guzman H, Tesh RB. Vertical transmission of Zika virus in Aedes aegypti mosquitoes. Am J Trop Med Hyg. 2016;95:1169–1173. doi: 10.4269/ajtmh.16-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li CX, et al. Vector competence and transovarial transmission of two Aedes aegypti strains to Zika virus. Emerg Microbes Infect. 2017;6:e23. doi: 10.1038/emi.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hay SI, et al. Etiology of interepidemic periods of mosquito-borne disease. Proceedings of the National Academy of Sciences. 2000;97:9335–9339. doi: 10.1073/pnas.97.16.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faria NR, et al. Establishment and cryptic transmission of Zika virus in Brazil and the Americas. Nature. 2017;546:406–410. doi: 10.1038/nature22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Díaz-Quiñónez JA, et al. Evidence of the presence of the Zika virus in Mexico since early 2015. Virus Genes. 2016;52:855–857. doi: 10.1007/s11262-016-1384-0. [DOI] [PubMed] [Google Scholar]
- 24.Guerbois M, et al. Outbreak of Zika virus infection, Chiapas State, Mexico, 2015, and first confirmed transmission by Aedes aegypti mosquitoes in the Americas. The Journal of infectious diseases. 2016;214:1349–1356. doi: 10.1093/infdis/jiw302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanciotti RS, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia. Emerg Infect Dis. 2008;14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thavara U, et al. Outbreak of chikungunya fever in Thailand and virus detection in field population of vector mosquitoes, Aedes aegypti (L.) and Aedes albopictus Skuse (Diptera: Culicidae) Southeast Asian J Trop Med Public Health. 2009;40:951–962. [PubMed] [Google Scholar]
- 27.Resúmenes del XV concurso de trabajos libres en cartel, congreso internacional avances en medicina. Archivos de ciencia8, 37, https://www.academia.edu/31633121/Archivos_de_Ciencia (2016).
- 28.Lai YL, et al. Cost-effective real-time reverse transcriptase PCR (RT-PCR) to screen for dengue virus followed by rapid single-tube multiplex RT-PCR for serotyping of the virus. J Clin Microbiol. 2007;45:935–941. doi: 10.1128/JCM.01258-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.García-Ruíz D, et al. Detection of dengue, west Nile virus, rickettsiosis and leptospirosis by a new real-time PCR strategy. SpringerPlus. 2016;5:1–9. doi: 10.1186/s40064-016-2318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]