Abstract
Considering the intriguing relationship between immune system and behavior recently described in mammals, and the lack of information of this relationship in fish, here we describe for the first time the interaction between the immune system and social and exploratory behavior in zebrafish. Fish high responders to novelty (HRN) presented a proinflammatory profile, with increased IL-1β and reduced IL-10 expression compared to fish low responders to novelty (LRN). Likewise, fish less responsive to social stimuli have a reduced expression of INF-γ. We show that fish with different behavior patterns have differences in the immune response. Our findings indicate that the interplay between immune system and behavior in zebrafish is similar to that found in mammalian models and that zebrafish should be considered as a potential model organism to study the relationship between immune system and behavior.
Introduction
The immune system and behavior are deeply connected. Human behavioral disorders such as autism, schizophrenia and depression are associated with dysfunction of the immune system, with altered levels of cytokines, which in turn might directly affect neuronal function1–3. While the capacity of neurological diseases to interfere with immune system is widely studied4 but, in contrast, investigations on the role of the immune system in regulating neuronal physiology are only emerging recently5,6. Studies in rodents demonstranted that cells and molecules of the immune system are involved in neurological functions such as learning, memory, anxiety and social behavior5–9. Mast cells, which are involved in allergic responses, are present in meninges and brain and are key cells during neurogenesis: a deficiency in mast cells increases anxiety, and causes learning and memory deficits7. Learning and memory are also affected by Interleukin 4 (IL-4), a cytokine produced by mast cells during inflammation, and by T lymphocytes, which are found on meninges and central to adaptive immune responses8. Furthermore, Interferon gamma (INF-γ), a pleiotropic cytokine also produced by T lymphocytes is involved in controlling social behavior9.
Although the interaction of nervous and immune system has been investigated using mammals as animal models, there is a potential to exploit other species as model organisms. The zebrafish is one of the main animals species used in scientific research10, widely employed in behavioral studies11, because it presents behavior characteristics of other vertebrates12, clear individual differences of behavior13, besides there are several behavioral tests standardized for scientific use in the species14. Considering the intriguing relationship between immune system and behavior3,5–9,15, and the lack of information of this relationship in fish, here we describe the interaction between the immune system and social and exploratory behavior in zebrafish.
Results
Novel object test - immune system x exploratory behavior
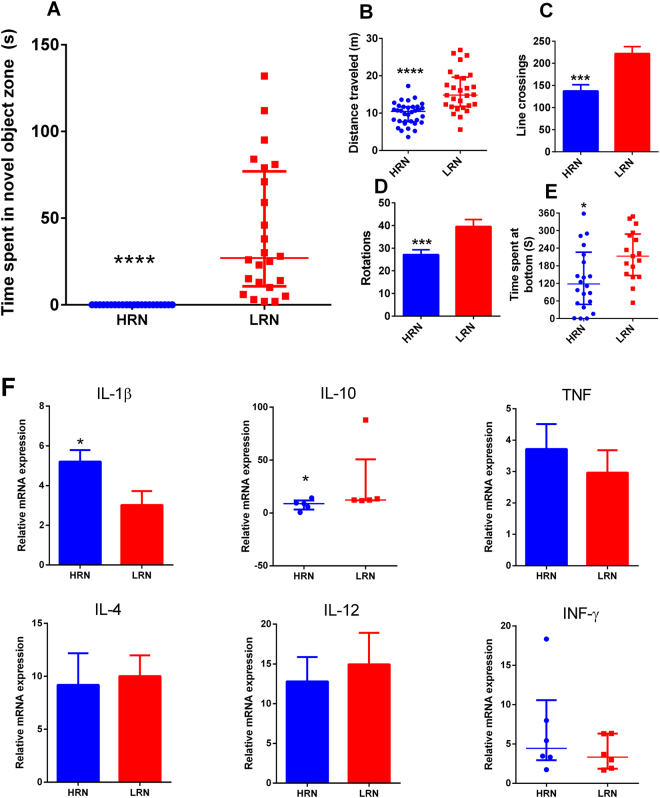
The time spent in the presence of a novel object was significantly lower in fish classified as high responders to novelty (HRN) (Wald-Wolfowitz rank test, P < 0.0001, Fig. 1A). These HRN fish also presented differences in locomotor parameters as decreased distance traveled (1B), line crossings (1C) and rotations (1D), and spent less time at the tank bottom (1E). In the HRN fish, the mRNA levels of IL-1β were upregulated while the levels of IL-10 mRNA were downregulated indicating a proinflammatory profile. No significant differences were found in the mRNA levels of the other cytokines tested (1F). The raw data and statistics are presented as Supplementary Information.
Figure 1.
Novel object test. Time spent in the novel object zone (A), distance traveled (B), line crossings (C), rotations (D), time spent at the bottom (E) and expression of cytokine genes (F). Each data represents the mean ± SEM or median ± interquartile range, depending on the data normality assessed by the Bartlett’s test. In panels A to E data represents the mean or median of 24 fish. In panel F, data represents the mean or median of 6 pooled samples. Significant differences are indicated by asterisk (*p < 0.05; ****p < 0.0001).
Social preference test - immune system x social behavior
Zebrafish exhibiting a “no preference” profile spent less time in the segment close to their conspecifics (p < 0.0001) (Fig. 2A). In these fish without social preference, the INF-γ gene expression was significantly reduced (p < 0.01). No differences in the mRNA levels were detected in the other genes evaluated (Fig. 2B). The raw data and statistics are provided as Supplementary Information.
Figure 2.
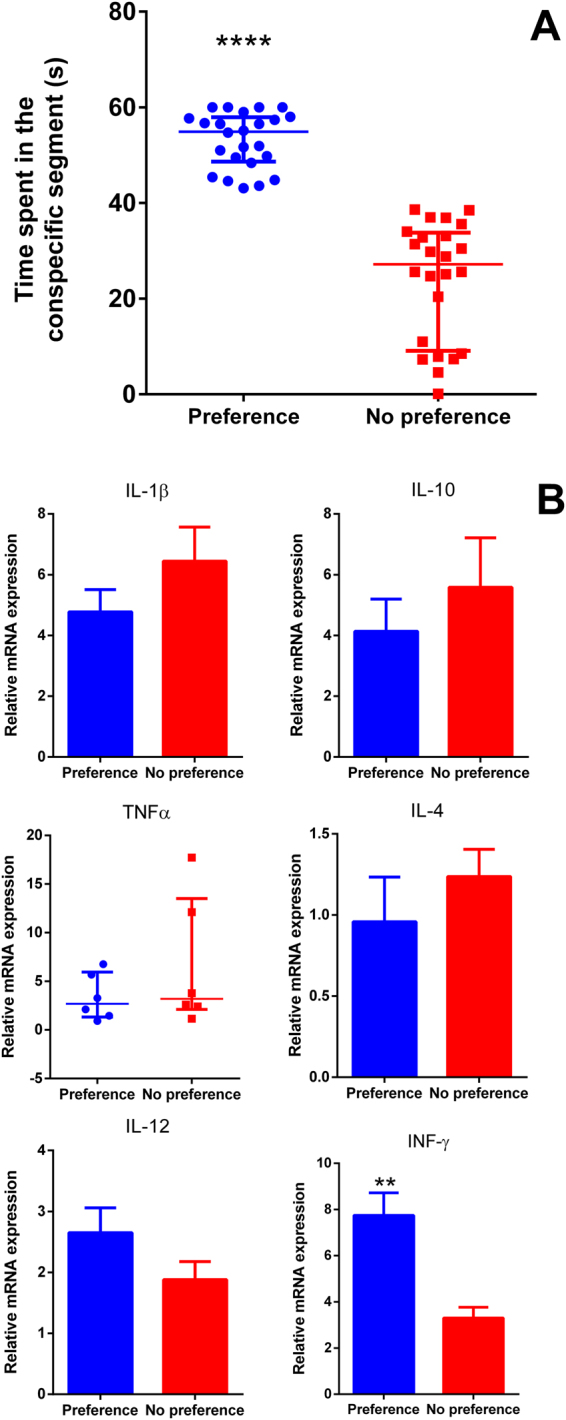
Social preference test- time spent in the conspecific segment. (A), and gene expression of cytokines (B). Each data represents the mean ± SEM or median ± interquartile range, depending on the data normality assessed by the Bartlett’s test. In panel A data represents the median of 24 fish and in panel B data represents the mean or median of 6 pooled samples. Significant differences are represented by asterisk (**p < 0.01; ****p < 0.0001).
Discussion
Here we show a clear relationship between the immune response of zebrafish and their exploratory and social behavior. Fish high responders to novelty (HRN) express cytokines with a proinflammatory profile, with increased IL-1β and reduced IL-10 expression compared to fish low responders to novelty (LRN). Likewise, fish less responsive to social stimuli have a reduced expression of INF-γ. Despite the clear relationship between the expression of selective immune-related genes and behavior, an intriguing question arises: is the immune system that leads to different behavioral patterns or is the behavioral pattern that alters cytokine profile and the outcome of the immune response?
Our main hypothesis is that the immune system modulates fish behavior. Challenging pathogens trigger a complex set of innate and adaptive immune mechanisms interconnected by cytokines secreted by several immune cells16. Besides promoting resistance to pathogens these cytokines modulate behavior to minimize the risk of new infections4,17. This cytokine-driven “sickness behavior”, that is a set of behavioral changes described in mammals, characterized by reduces social interaction, water and food intake and exploration of new environments and new objects2,18. This proinflammatory profile supports previous work suggesting that neophobia (HRN fish) may be a behavioural defense mechanism, lowering both risk of predation and exposure to infection through reduced exposure19–21.
HRN fish presented lower locomotor activity and remained less time at the bottom of the tank compared to LRN fish. At a simplistic view, LRN fish seems to exhibit an anxiety-like behavior14. However, we hypothesized that the increased locomotor activity observed with the LRN fish reflects their response to the new object. As they are exploring, they spend more time at the bottom of the tank where the new object is located. On the other hand, HRN fish may be avoiding the new object segment moving less and staying longer in the top of the tank, keeping a greater distance from the new object.
In the social behavior test, fish that spent less time close to their conspecific, had reduced levels of INF-γ mRNA. Interestingly, a recent study demonstrated that INF-γ knockout mice displayed reduced social behavior, indicating the central role of this cytokine on social interaction6. That study also demonstrated a relationship between the expression of IFN- γ and the social context in zebrafish6. Although social behaviour is crucial for survival and reproduction in many species19, it is also well documented that increasing exposure to conspecifics increases the spread of pathogens6.
Thus, it would be expected that increased social interactions would be indicative of a robust expression of immune related genes, mainly INF-γ, which appears as a key molecule in this interplay. Nonetheless, although we demonstrated the immune-behavioral relationship, we cannot ascertain weather the reduction of INF-γ leads the fish to respond less to social stimuli, or its lower interaction with the school reflects lower expression of INF-γ. Likewise, HRN behavior in fish results from the expression of proinflammatory cytokine genes or it is their fearful, stressing-type of behavior that leads to the expression IL-1B? There are evidences supporting both theories. Behavioral changes were observed in animals knockout for specific immune-related genes: for instance, lack of IL-4 expression might cause learning and memory deficits, and anxiolytic disorders5,8; IL-1β receptor knockouts have memory deficits9, and social dysfunction was observed in mice knockout for INF-γ6. Thus, at infection, the sudden rise on cytokine expression needed to mount an immune response to overcome threatening pathogens leads to behavioral changes known as sickness behavior17. These data support the hypothesis that cytokines, mastering communication between cells and tissues, act also upon the nervous system causing behavioral changes.
On the other hand, behavioral changes may also alter the immune response. It has been shown that social status (dominant vs. subordinate) leads to different patterns of immune response20,21. Subordinated animals have a profile of proinflammatory immune cells compared to their dominant cohorts. Furthermore, when the social hierarchy is changed, the immune system alters equally, so it appears that behaviour can also modulate immune response in some situations20. Thus, it is likely that subordinated animals are more stressed and, consequently, prone to have increased inflammatory response. Furthermore, several studies in humans showed an imbalance of cytokines concomitantly with pathologies such as depression and autism3,15,22–24.
Thus, neurological disturbances might alter functioning of the immune system, and immune dysfunctions can lead to behavioral changes. There is not a relationship of cause-effect, but an intimate interaction between the immune and nervous systems and little is known about the molecular pathways involved in this relationship.
Here, the relationship between behavior and immune system is described for the first time in fish. We show that the expression of specific cytokine genes in the brain of fishes varies according to behavior pattern. Our findings show that neuro-immunological interplay in fish is similar to current mammalian models highlighting the potential of zebrafish as model organism to study immune-behavioral relationship. In an ecological perspective, fish with an immune suppression cytokine profile would reduce interaction with conspecifics in several aspects of their social behavior like mating25, hierarchical contest26 and defense shoaling27. Immune suppressed fish might also have difficulties in tuning risk assessment of the environment during their exploratory activity in search for food and, as a consequence, become more susceptible to predators28. Aquatic contaminants like agrichemicals and drugs are known to alter the expression of specific cytokine genes29–32 which, in addition to increasing susceptibility to infections, might cause behavioral changes, letting the fish more vulnerable to predation. In this scenario, immune dysfunctions when associated with behavioral changes would have a greater ecological impact and could contribute to reduce fish population in the wilderness.
Materials and Methods
Study strategy
To evaluate the relationship between behavior and immune systems, we firstly discriminated zebrafish according to their exploratory behavior (high and low responders to novelty, HRN and LRN respectively) and according to their social behavior (preference and no preference for conspecifics). After, we evaluated the gene expression of selected cytokines in the brain to verify if there were differences in the immunological status in fish presenting different behaviors.
Animals and Maintenance
A stock population of 195 180-day old adult wild-type zebrafish (Danio rerio), mixed sex (50:50) from a single brood, were held in a tank equipped with biological filters, with a natural photoperiod (14 h light:10 h dark) and under constant aeration. The fish were fed twice a day with commercial flaked food provided ad libidum. Water temperature was maintained at 26 ± 2 C°, dissolved oxygen concentrations at 6.5 ± 0.4 mg/L, pH 7.0 ± 0.25, and the total ammonia concentration was less than 0.5 mg/L. Fish used in the study were in perfect health and were not subjected to any procedure or exposure to drugs prior to the experiments.
Behavioral evaluation
Two behavioral tests were performed in different zebrafish groups: the new object test, to classify fish into HRN and LRN in relationship to their exploratory behavior, and the social preference test, to discriminate fish according to their response to social stimuli. In both tests, fish behavior was recorded by a Logitech Quickcam PRO 9000 camera located in front of the tank, and the videos analyzed using ANY-maze® software (Stoelting CO, USA), which tracked animal behavior throughout testing. After testing, the water of the tank was completely changed prior to testing a new fish.
Novel object test
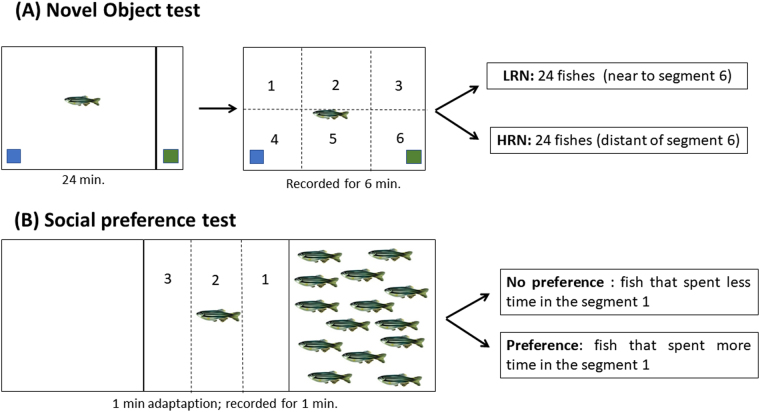
A total of 120 fish were tested to determine their responsiveness to novelty. The protocol was adapted from Braida et al.33 and May et al.34. Each fish was individually transferred to the test tank (24 × 8 × 20 cm; width × depth × height), which contained one object at each extremity (blue and green plastic squares measuring 4 × 4 cm). One of the object was covered by an opaque partition close to the extremity and could not be seen by the fish. The fish was introduced into the tank and for 24 minutes (based in the time to memory acquisition35) it was in contact with only one object. Then, the partition was removed and fish had access to the “new object”. After partition removal, the behavior was recorded for 6 minutes. The color of the new object (blue or green) varied in each test. For the analysis, the test tank was virtually divided into six segments and the fish were classified by the time spent in the segment of the new object. We considered as HRN the 24 fish that did not enter once in the segment of the new object and as LRN the 24 fish that stayed longer next to the new object (Fig. 3A). We analyzed also the total distance traveled (m), number of line crossings, number of rotations and time in the bottom part of the tank (s).
Figure 3.
Schematic representation of the methodology used to discriminate exploratory (A) and social (B) behavior of zebrafish. The drawings in the panels A and B were drawn by KK.
Social preference test
A total of 60 fish were tested to determine their preference for conspecifics. In this test, fish were transferred individually to the test tank (30 × 15 × 10 cm; width × depth × height). The test tank was positioned between two equal sized tanks, one without fish and the other containing 15 conspecifics36. After transferring to the test tank, fish were acclimated for 60 seconds and their behavior was recorded for 60 seconds. For the image analysis, the test tank was virtually divided into three vertical segments. The first segment was the one nearest to conspecifics, while the third segment was next to the empty tank. The relative time zebrafish spent in the first segment was calculated as response to social stimuli (Fig. 3B).
Euthanasia and brain extraction
Immediately after performing the behavioral tests, each fish was anesthetized with Eugenol (Sigma Aldrich, Brazil, 50 mg/L) and euthanized by sectioning the spinal cord. Then, each fish was separately packed in microtubes, frozen in liquid nitrogen for 30 seconds and stored at −80 °C. For brain extraction, the skull was opened, the cranial nerves severed, and the brain carefully removed37.
RNA extraction and cDNA synthesis
Due to the small size, the brains of four fish with the same behavioral profile were randomly pooled and used for total RNA extraction, totaling six RNA samples per group (24 fish) (6 samples per group). Tissue lysis was done using the Tissuelyser LT® (Qiagen, Brazil). RNA was extracted using RNeasy® Mini Kit (Qiagen, Brazil) and submitted to a DNAse I amplification grade treatment (Invitrogen, EUA) to eliminate genomic DNA. The RNA quality and concentration was measured by spectrophotometry (Nanophotometer Pearl®, IMPLEN, Germany), and stored at −80 °C. For cDNA synthesis, 500 ng of total RNA was used for the reverse transcription assay, using SuperScript® III Reverse Transcriptase (Invitrogen, EUA) and random primers.
Gene expression analysis by real-time quantitative PCR (qPCR)
Real time PCR (qPCR) was performed in 48-well plates (MicroAmp Fast optical 48 well plate reaction, 0.1 ml. Applied Biosystems, USA) in a final volume of 10 µl. The mix consisted of 400 nM of each primer, 5 µl of SYBR Select Master Mix (Applied Biosystems, USA) and 1 µl of cDNA (diluted 1:10). The reaction was carried out in a Step One equipment Applied Biosystems) with the following conditions: initial denaturing at 95 °C for 10 min followed by 40 cycles of 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s. At the end, a standard melting curve was included to confirm the specificity of the amplified product. Samples were performed in triplicate. The genes analyzed, their respective primers and the efficiency of each reaction are indicated in Table 1. Non template controls and the expression of a housekeeping gene (β-actin) were also analysed for comparison purposes. For the calibration curve, each gene was cloned into the pGEM-TEasy Vector System (Promega) and transformed into competent One Shot TOP10 E. coli (Promega) and cultured in LB supplemented with ampicillin. Cloning was confirmed by PCR and the resulting plasmid was extracted using the Wizard Plus SV minipreps DNA purification system (Promega). Then, the calibration curve consisted of decimal dilutions (1:10) of each cloned gene. To better compare the results from different groups the same threshold value (0.080) was used. The relative quantification of gene expression, was carried out by the 2−ΔΔct formula38.
Table 1.
Immune genes, primers nucleotide sequence and qPCR efficiency.
| Gene | Primer (5′-3′) | Efficiency | Accession number |
|---|---|---|---|
| TNF-α | F: GACCACAGCACTTCTACCG R: ACATTTTCCTCACTTTCGTTCAC | 98,3% | NM_212859 |
| IL-1β | F: GCTGGAGATGTGGACTTC R: ACTCTGTGGATTGGGGTTTG | 102% | NM_212844 |
| INF-γ | F: TGCCTCAAAATGGTGCTACTC R: AATCGGGTTCTCGCTCCTG | 97,1% | AB158361.1 |
| IL-4 | F: TCTCTGCCAAGCAGGAATG R: CAGTTTCCAGTCCCGGTATATG | 99,4% | AM403245.2 |
| IL-12 | F: CTGTAGGATCCATCCAAACATCT R: CACTGGCACTTCTACCCTATTT | 96,8% | AB183002.1 |
| IL-10 | F: CTCTGCTCACGCTTCTTCTT R: GCTCCCTCAGTCTTAAAGGAAA | 101,4% | BC163038.1 |
| β-Actin | F: GCAAAGGGAGGTAGTTGTCTAA R: GAGGAGGGCAAAGTGGTAAA | 97,7% | AF057040.1 |
Ethical Note
This study was approved by the Ethics Commission for Animal Use (CEUA) at Universidade de Passo Fundo, UPF, Passo Fundo, RS, Brazil (Protocol 008/2017) and met the guidelines of the National Council for the Control of Animal Experimentation (CONCEA).
Statistical analysis
Data of time spent in the new object segment exhibited unequal variance between treatment groups and was compared using a Wald-Wolfowits rank test. All other data of behavioral phenotypes were compared by Student’s t test or by Mann-Whitney test depending on the data normality (assessed by the Bartlett’s test). In all experiments, P was set at <0.05.
Electronic supplementary material
Acknowledgements
This study was funded by the Universidade de Passo Fundo and by the Secretaria de Desenvolvimento Econômico, Ciência e Tecnologia (SDECT). L.J.G.B. and L.C.K. hold CNPq research fellowships (301992/2014-2 and 307900/2016-9 respectively). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
K.K., L.C.K. and L.J.G.B. conceptualize the experiments, analyzed the results, wrote the manuscript and prepare the figures. K.K. and D.F. conducted experimental procedures. All authors have read and approved the manuscript for publication.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-19276-3.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Müller N, Schwarz MJ. Immune System and Schizophrenia. Curr. Immunol. Rev. 2010;6:213–220. doi: 10.2174/157339510791823673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dantzer RC. Sickness Behaviour, and Depression . Immunol. Allergy Clin. North Am. 2009;29:247–264. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibney SM, Drexhage HA. Evidence for a dysregulated immune system in the etiology of psychiatric disorders. J. Neuroimmune Pharmacol. 2013;8:900–920. doi: 10.1007/s11481-013-9462-8. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy DP, Adolphs R. The social brain in psychiatric and neurological disorders Daniel. Trends Cogn. Sci. 2012;16:559–572. doi: 10.1016/j.tics.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moon ML, et al. IL-4 Knock Out Mice Display Anxiety-Like Behavior. Behav. Genet. 2015;45:451–460. doi: 10.1007/s10519-015-9714-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filiano AJ, et al. Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature. 2016;535:425–429. doi: 10.1038/nature18626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nautiyal KM, Ribeiro AC, Pfaff DW, Silver R. Brain mast cells link the immune system to anxiety-like behavior. Proc. Natl. Acad. Sci. USA. 2008;105:18053–7. doi: 10.1073/pnas.0809479105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derecki NC, et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avital A, et al. Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus. 2003;13:826–834. doi: 10.1002/hipo.10135. [DOI] [PubMed] [Google Scholar]
- 10.Fetcho, J. R. Behaviors of Zebrafish. Encycl. Anim. Behav. 633–637 (2010).
- 11.Miklósi A, Andews RJ. The zebrafish as a model for behavioral studies. Zebrafish. 2006;3:227–234. doi: 10.1089/zeb.2006.3.227. [DOI] [PubMed] [Google Scholar]
- 12.Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. Integrating animal temperament within ecology and evolution. Biol. Rev. 2007;82:291–318. doi: 10.1111/j.1469-185X.2007.00010.x. [DOI] [PubMed] [Google Scholar]
- 13.Tran S, Gerlai R. Individual differences in activity levels in zebrafish (Danio rerio) Behav. Brain Res. 2013;257:224–229. doi: 10.1016/j.bbr.2013.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giacomini ACVV, et al. Fluoxetine and diazepam acutely modulate stress induced-behavior. Behav. Brain Res. 2016;296:301–310. doi: 10.1016/j.bbr.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 15.Dantzer R, et al. From inflammation to sickness and depression: when the immune system subjugates the brai. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinarello CA. Historical Review of Cytokines. Eur. J. Immunol. 2007;37:S34–S45. doi: 10.1002/eji.200737772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haba R, et al. Lipopolysaccharide affects exploratory behaviors toward novel objects by impairing cognition and/or motivation in mice: Possible role of activation of the central amygdala. Behav. Brain Res. 2012;228:423–431. doi: 10.1016/j.bbr.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 18.Johnson RW. The concept of sickness behavior: A brief chronological account of four key discoveries. Vet. Immunol. Immunopathol. 2002;87:443–450. doi: 10.1016/S0165-2427(02)00069-7. [DOI] [PubMed] [Google Scholar]
- 19.Bourke AFG. Hamilton’s rule and the causes of social evolution. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2014;369:20130362. doi: 10.1098/rstb.2013.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snyder-Mackler N, et al. Social status alters immune regulation and response to infection in macaques. Science (80-.). 2016;354:1041 LP–1045. doi: 10.1126/science.aah3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filby AL, Paull GC, Bartlett EJ, Van Look KJW, Tyler CR. Physiological and health consequences of social status in zebrafish (Danio rerio) Physiol. Behav. 2010;101:576–587. doi: 10.1016/j.physbeh.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Ross HE, Guo Y, Coleman K, Ousley O, Miller AH. Association of IL-12p70 and IL-6:IL-10 ratio with autism-related behaviors in 22q11.2 deletion syndrome: A preliminary report. Brain. Behav. Immun. 2013;31:76–81. doi: 10.1016/j.bbi.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Estes ML, McAllister AK. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat Rev Neurosci. 2015;16:469–486. doi: 10.1038/nrn3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogłodek E, Szota A, Just M, Moś D, Araszkiewicz A. The role of the neuroendocrine and immune systems in the pathogenesis of depression. Pharmacol. Reports. 2014;66:776–781. doi: 10.1016/j.pharep.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Griffiths SW, Magurran AE. Sex and schooling behaviour in the Trinidadian guppy. Anim. Behav. 1998;56:689–693. doi: 10.1006/anbe.1998.0767. [DOI] [PubMed] [Google Scholar]
- 26.Saverino C, Gerlai R. The social zebrafish: Behavioral responses to conspecific, heterospecific, and computer animated fish. Behav. Brain Res. 2008;191:77–87. doi: 10.1016/j.bbr.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller N, Gerlai R. Quantification of shoaling behaviour in zebrafish (Danio rerio) Behav. Brain Res. 2007;184:157–166. doi: 10.1016/j.bbr.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Gerlai R. Zebrafish antiproedatory responses: a future for translational research? Behav. Brain Res. 2011;207:223–231. doi: 10.1016/j.bbr.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Xing H, Li X, Xu S, Wang X. Effects of atrazine and chlorpyrifos on the mRNA levels of IL-1 and IFN-γ2b in immune organs of common carp. Fish Shellfish Immunol. 2011;31:126–33. doi: 10.1016/j.fsi.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Rihel, J. et al. NIH Public Access. 327, 348–351 (2011).
- 31.Devos S, et al. Inhibition of cytokine production by the herbicide atrazine: Search for nuclear receptor targets. Biochem. Pharmacol. 2003;65:303–308. doi: 10.1016/S0006-2952(02)01507-1. [DOI] [PubMed] [Google Scholar]
- 32.Kirsten KS, et al. Reduced expression of selective immune-related genes in silver catfish (Rhamdia quelen) monocytes exposed to atrazine. Fish Shellfish Immunol. 2017;64:78–83. doi: 10.1016/j.fsi.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Braida D, Ponzoni L, Martucci R, Sala M. A new model to study visual attention in zebrafish. Prog. Neuro-Psychopharmacology Biol. Psychiatry. 2014;55:80–86. doi: 10.1016/j.pnpbp.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 34.May Z, et al. Object recognition memory in zebrafish. Behav. Brain Res. 2016;296:199–210. doi: 10.1016/j.bbr.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 35.Lucon-Xiccato T, Dadda M. Assessing memory in zebrafish using the one-trial test. Behav. Processes. 2014;106:1–4. doi: 10.1016/j.beproc.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Gerlai R, Lahav M, Guo S, Rosenthal A. Drinks like a fish: Zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol. Biochem. Behav. 2000;67:773–782. doi: 10.1016/S0091-3057(00)00422-6. [DOI] [PubMed] [Google Scholar]
- 37.Vargas R, Jóhannesdóttir IT, Sigurgeirsson B, Thornorsteinsson H, Karlsson KA. The zebrafish brain in research and teaching: a simple in vivo and in vitro model for the study of spontaneous neural activity. Adv. Physiol. Educ. 2011;35:188–196. doi: 10.1152/advan.00099.2010. [DOI] [PubMed] [Google Scholar]
- 38.Rao X, Huang X, Zhou Z, Lin X. An improvement of the 2ˆ(−delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinforma. Biomath. 2013;3:71–85. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.