Abstract
A phosphoiminoBINOL ligand was designed to form a dinuclear metal complex that could hold a malononitrile molecule. The dinuclear bis(phosphoimino)binaphthoxy-Pd2(OAc)2 complex catalyzed a double Mannich reaction of N-Boc-imines with malononitrile to give chiral 1,3-diamines with high enantioselectivity. The rational asymmetric catalyst, which smoothly introduces the first coupling product to the second coupling reaction while avoiding the reverse reaction, facilitates the over-reaction into a productive reaction process.
Introduction
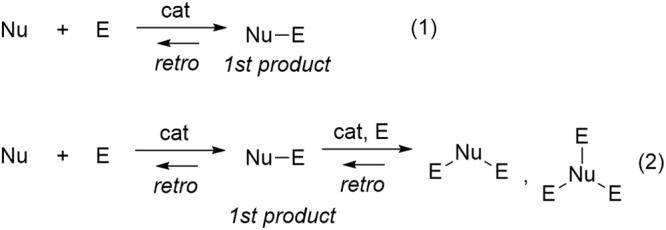
The importance of catalysts with high catalytic activity in achieving “green” or sustainable chemistry has been well documented1. The benefit of high catalytic activity is not limited to reducing the amount of catalyst used. Catalysts with superior activity have the potential to promote unprecedented chemical transformations. As shown in Fig. 1, in the reaction of a nucleophile (Nu) with an electrophile (E), conventional catalysts are used for the synthesis of the 1:1 coupling adduct Nu-E (eq. 1).
Figure 1.
Reaction of a nucleophile (Nu) with an electrophile (E).
If the first coupling product (Nu − E) can be made to react subsequently with further electrophiles through catalysis, 1:2 and/or 1:3 adducts (i.e. Nu-E2, Nu-E3) can be obtained. Although reactions of this type can be seen as over-reactions (eq. 2), such multicomponent coupling reactions are fascinating due to their potential as a direct approach toward highly functionalized advanced materials. List et al. reported an outstanding example: the condensation of acetaldehyde with two molecules of N-Boc imine using 20 mol% proline as a catalyst, which was originally developed for a single condensation reaction2. In order to utilize the double condensation reaction as a rational asymmetric catalytic reaction, new concepts for the design of highly active catalysts are required, and the first coupling product must be smoothly introduced to the second coupling reaction while avoiding the reverse reaction. Here, we report the design and development of a dinuclear palladium catalyst that enables a novel double Mannich reaction.
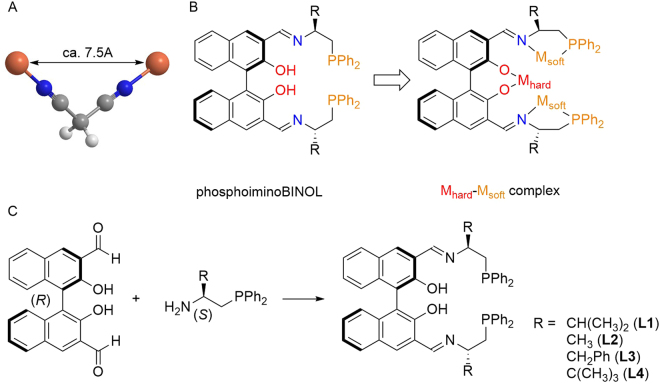
In this study, malononitrile was selected as a nucleophile with two acidic protons to be directed toward a double Mannich reaction3–6. In the interactions of nitriles with late transition metal salts, malononitrile can bind to two metal atoms7. For example, for the 5th-period elements, the two metal centers should be around 7.5 Å from each side of the malononitrile unit (Fig. 2A). In order to achieve a dinuclear reaction in one asymmetric reaction sphere while retaining the same geometry, a novel phosphoiminoBINOL ligand was designed, as shown in Fig. 2B. The phosphoimino moiety is designed to capture soft metals such as Pd, Rh, Au, Ag, and Cu, and the soft dinuclear complex binds strongly to malononitrile (or an anion of malononitrile generated during the reaction). The phenol functions in the ligand contribute by stabilizing the intermediate through hydrogen bonding. If the phenols incorporate a hard metal such as Li, Mg, or Zn, the resulting metal phenoxide acts as a Brønsted base, enhancing the catalytic activity. The hard Lewis acidity of the metal phenoxide is also characteristic.
Figure 2.
Design of Malononitrile Container. (A) Geometry of malononitrile bound to two metals, (B) Design of the multifunctional dinuclear phosphoiminoBINOL-metal complex for binding malononitrile. (C) Preparation of phosphoiminoBINOL ligands.
The synthesis of phosphoiminoBINOLs L1-L4 can be readily achieved through imine formation between 3,3′-formyl BINOL and the corresponding aminophosphine (Fig. 2C). The isopropyl-substituted phosphoiminoBINOL (L1) was stable to handling under air, and showed a high affinity with various soft metal salts. When 2 mol equiv. of Pd(OAc)2 was added to L1, the formation of a dinuclear palladium complex took place, as demonstrated by ESI-MS: an ion peak was detected at m/z = 1117.1705 in CH2Cl2, and this was attributed to [L1(−2H) + Pd2(OAc)]+. Fine crystals were obtained from a CH2Cl2 solution, and the structure of phosphoiminobinaphthoxy (L1)-Pd2(OAc)2 was determined by X-ray crystallographic analysis (Fig. 3).
Figure 3.
X-ray structure of L1-Pd2 (CCDC 1543349).
Based on this structure, one of the acetoxy anions of Pd(OAc)2 was replaced with L1 to make a palladium acetoxy phenoxide. The distance between two palladium cations is 6.907 Å, which is suitable for binding malononitrile (or a generated anion of malononitrile), and there is one H2O molecule positioned at the center of the asymmetric sphere. L1-Pd2 reacted smoothly with malononitrile. The 1H NMR spectrum of free malononitrile in CDCl3 shows a single methylene peak at 3.60 ppm. When malononitrile is added to 1 equiv. of L1-Pd2, the peak was shifted to 2.43 ppm 1H NMR (see details in Supplementary Information). ESI-MS analysis of a 1:1 mixture of malononitrile with L1-Pd2 showed a clear new peak at m/z = 1123.1764, attributed to [L1(−2H) + Pd2 + NCCHCN]+.
With this fascinating container for malononitrile (L1-Pd2) in hand, the optimum catalyst for the conventional single Mannich reaction was examined before we attempted the challenge of the double Mannich reaction (Table 1)8–23.
Table 1.
Development of dinuclear phosphoiminoBINOL-metal catalyst for Mannich reaction using malononitrile.

| |||||
|---|---|---|---|---|---|

| |||||
| Entry | Ligand | Additive | Yield (%)a | 2a/3a | ee of 2a (%) |
| 1 | L1 | — | 99 | 83/17 | 8 |
| 2 | L1 | LiOAc | 86 | 84/16 | 10 |
| 3 | L1 | NaOAc | 99 | 72/28 | 26 |
| 4 | L1 | Mg(OAc)2 | 91 | 57/43 | 74 |
| 5 | L1 | Ca(OAc)2 | 94 | 74/26 | 30 |
| 6 | L1 | Zn(OAc)2 | 93 | 60/40 | 68 |
| 7 | L1 | ZnCl2 | 88 | 92/8 | 7 |
| 8 | L1 | Zn(OTf)2 | 66 | 93/7 | 18 |
| 9 | L2 | Zn(OAc)2 | 46 | 96/4 | 11 |
| 10 | L3 | Zn(OAc)2 | 72 | 85/15 | 37 |
| 11 | L4 | Zn(OAc)2 | 78 | 68/32 | 77 |
| 12 | L5 | Zn(OAc)2 | 73 | 78/22 | 6 |
| 13b) | L6 | Zn(OAc)2 | 75 | 90/10 | 10 |
| 14b) | L7 | Zn(OAc)2 | 65 | 92/8 | rac |
| 15c) | L1 | Zn(OAc)2 | 70 | 73/27 | 91 |
a)Conversion yield (see details for the determination in SI). b)10 mol % of Pd(OAc)2 were used. c)5 mol % of PhosphoiminoBINOL, 10 mol % of Pd(OAc)2, and 5 mol % of Zn(OAc)2 were used. Imine 1a was slowly added over 4 h.
Although the Mannich reaction was smoothly catalyzed by the simple use of L1-Pd2, the Mannich product 2a was obtained with only 8% ee. As we expected cooperative effects due to the phenoxy unit of L1-Pd2 (Fig. 2B), the addition of several hard metal salts was examined (Entries 2–8). Several metal acetates were effective in improving asymmetric induction. With assistance from Zn(OAc)2 or Mg(OAc)2, L1-Pd2 smoothly catalyzed the Mannich reaction to give 2a with 68% ee and 74% ee, respectively. The structure-activity relationship of the ligands is also shown in Table 1. The equivalent catalyst using the methyl analog of L1 ((L2)-Pd2) gave 2a with only 11% ee (entry 9). Although the t-Butyl analog L4-Pd2 gave 2a with 77% ee, the catalyst activity was reduced (entry 11). The diastereomeric ligand L5 synthesized from (S)-BINOL, and L6 and L7 for constructing mono-nuclear palladium complex resulted in low levels of asymmetric induction. For L1-Pd2 with a Zn(OAc)2 catalyst system, when the N-Boc imine 1a was added slowly, the Mannich adduct 2a was obtained with 91% ee in 5 mol % catalyst use (entry 15).
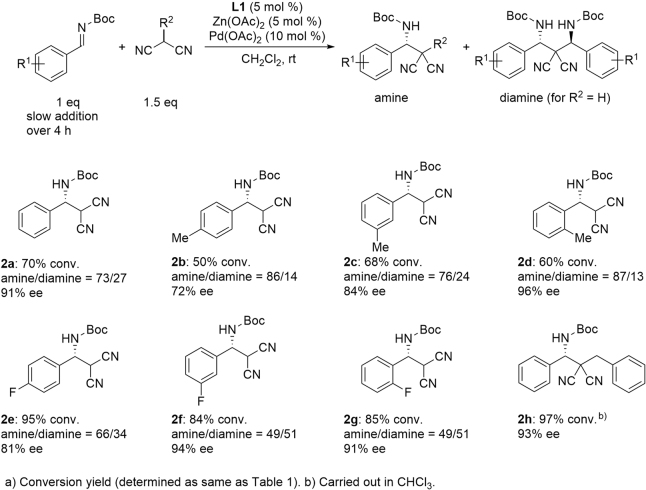
The results of the L1-Pd2-catalyzed asymmetric Mannich reaction under the optimized conditions are summarized in Fig. 4. Aromatic imines with various substituents were smoothly converted to the Mannich products with high enantioselectivity.
Figure 4.
Dinuclear phosphoiminoBINOL-Pd catalyzed asymmetric Mannich reaction using malononitrile.
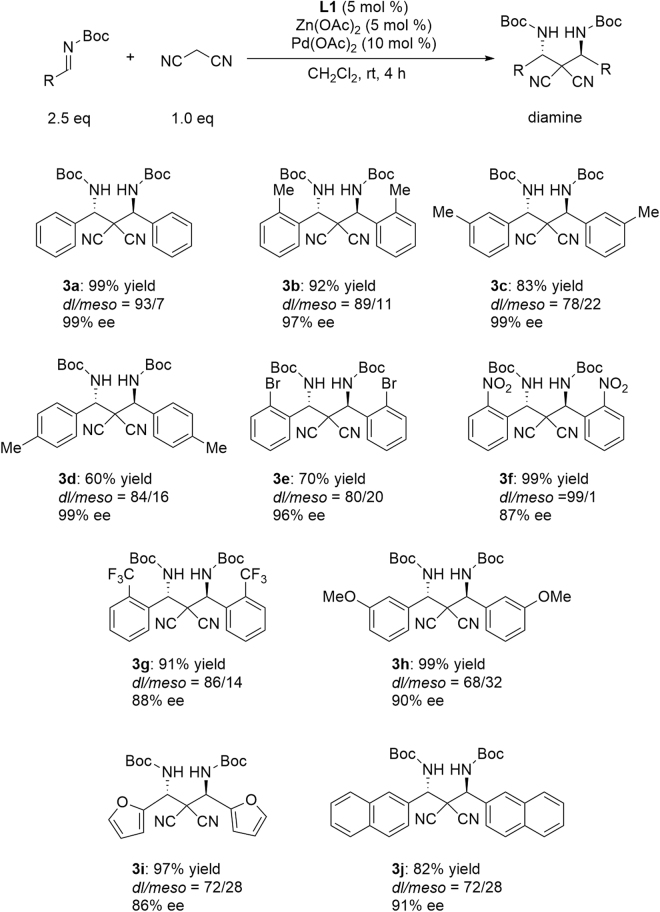
Using monobenzylated malononitrile, the chiral amine 2 h having adjacent quaternary carbon center was obtained in 97% yield with 93% ee. This suggests the strong catalyst activity of the dinuclear L1-Pd2 complex. We assumed that the highly active dinuclear L1-Pd2 catalyst would facilitate the conversion of the Mannich adducts (2a–d) to the second Mannich reaction for giving the 1,3-diamines 3. Actually, the use of L1-Pd2 with a Zn(OAc)2 catalyst system resulted in the production of a significant amount of the 1:2 adduct 3, as desired. For the synthesis of 2f and 2g, co-production of the same amount of diamines was observed even when 1.5 equiv. of malononitrile was applied to the imine substrate24,25. For the double Mannich reaction, the reaction conditions were modified so that 2.5 equiv. of N-Boc imine were used with respect to the malononitrile. L1-Pd2 with Zn(OAc)2 catalyzed the double Mannich reaction quite smoothly to give the 1,3-diamine 3a in quantitative yield with high diastereoselectivity (dl/meso = 93/7)26–43. The major dl-isomer was obtained in 99% ee. The results of 1,3-diamine synthesis by the double Mannich reaction are summarized in Fig. 5.
Figure 5.
Catalytic asymmetric double Mannich reaction.
For N-Boc-imines derived from both electron-donating and electron-deficient benzaldehydes, chiral 1,3-diamines were obtained in a highly enantioselective manner. A chiral bisfuryl-1,3-diamine(3i) was obtained with 86% ee, and a bisnaphthyl-1,3-diamine(3j) was also obtained successfully with 91% ee. When the second Mannich reaction was examined using rac-2a with N-Boc-imine, 3a was obtained in 73% ee with increasing co-production of the meso-form in dl/meso = 1:1. This result suggests that the second Mannich reaction is catalyzed independently from the first Mannich reaction, and the enantiomeric excess of 3a is improved due to the meso-trick44.
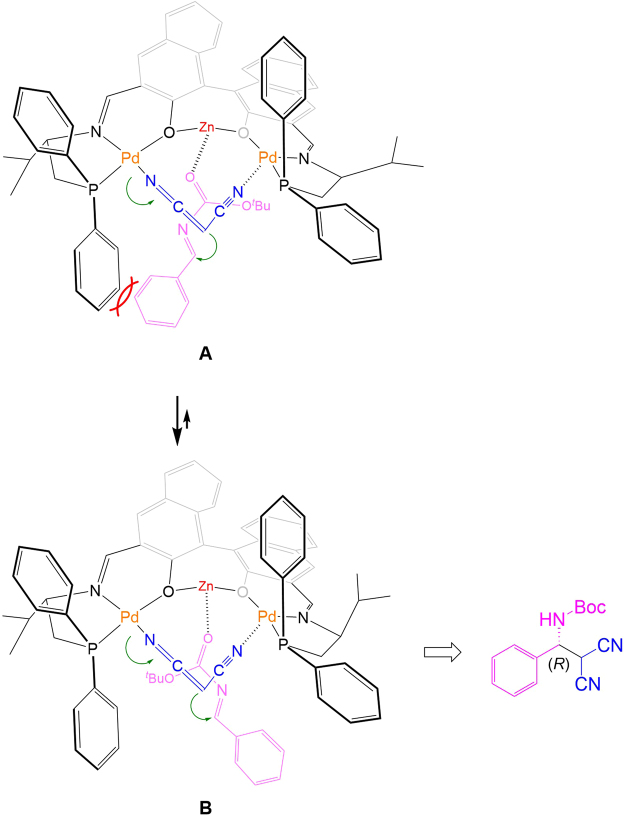
The formation of the (R)-enriched Mannich adduct using L1 can be explained using the working model described in Fig. 6.
Figure 6.
Plausible reaction model for asymmetric Mannich reaction using L1-Pd2.
The dinuclear L1-Pd2 complex binds to malononitrile at both nitrogen atoms of the nitrile moieties. Although the formation of palladium enolate by simple mixing of malononitrile with L1-Pd2 is suggested by the detection of an ion peak for [L1(−2H) + Pd2 + NCCHCN]+ at m/z = 1123.1764, the addition of Zn(OAc)2 also assists the smooth formation of the palladium enolate. The low asymmetric induction and catalyst activity of the mono-nuclear palladium complex using L6 and L7 suggest the effective role of the dinuclear palladium complex for converting 2a to 3a (Table 1, entries 13, 14) Moreover, because asymmetric induction of the Mannich adducts 2a and 3a is strongly influenced by the selection of hard metal salts, as shown in Table 1, a Zn(OAc)2-driven reactant is incorporated in the asymmetric reaction sphere45–53. When the hard zinc atom is captured by the two hard phenoxy oxygens of L1-Pd2, the zinc atom can act as a Lewis acidic site for activating Boc-imines. During nucleophilic attack by the Pd-enolate malononitrile of the zinc-activated N-Boc imine, the approach shown in model A (Fig. 6) involves serious steric repulsion between a benzene ring from the phosphine in L1 and the N-Boc imine. By flipping the face of the N-Boc imine, as shown in Model B, the steric repulsion can be released, and nucleophilic attack of the Re-face of the N-Boc imine gives the (R)-enriched Mannich product.
In conclusion, the dinuclear bis(phosphoimino)binaphthoxy-Pd2(OAc)2 complex described herein facilitated a double Mannich reaction of N-Boc-imine with malononitrile to give chiral 1,3-diamines in a highly enantioselective manner. This demonstrates that over-reaction need not always be useless and undesired: well managed over-reaction can open up novel synthetic processes.
Electronic supplementary material
Acknowledgements
This research is supported by JSPS KAKENHI Grant Number JP16H01004 in Precisely Designed Catalysts with Customized Scaffolding, and Workshop on Chirality at Chiba University (WCCU).
Author Contributions
T.A. wrote the main manuscript text, K.S., A.N. and H.M. did experiments, and H.M. operated X-ray crystallographic analysis.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-19178-4.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Eds. Sheldon, R. A., Arends, I. & Hanefeld, U. Green Chemistry and Catalysis (Wiley-VCH, 2007).
- 2.Chandler C, Galzerano P, Michrowska A, List B. The Proline-Catalyzed Double Mannich Reaction of Acetaldehyde with N-Boc Imines. Angew. Chem. Int. Ed. 2009;48:1978–1980. doi: 10.1002/anie.200806049. [DOI] [PubMed] [Google Scholar]
- 3.Taylor MS, Jacobsen EN. Enantioselective Michael Additions to α,β-Unsaturated Imides Catalyzed by a Salen−Al Complex. J. Am. Chem. Soc. 2003;125:11204–11205. doi: 10.1021/ja037177o. [DOI] [PubMed] [Google Scholar]
- 4.Hoashi Y, Okino T, Takemoto Y. Enantioselective Michael Addition to α,β-Unsaturated Imides Catalyzed by a Bifunctional Organocatalyst. Angew. Chem., Int. Ed. 2005;44:4032–4035. doi: 10.1002/anie.200500459. [DOI] [PubMed] [Google Scholar]
- 5.Molleti N, Rana NK, Singh VK. Highly Enantioselective Conjugate Addition of Malononitrile to 2-Enoylpyridines with Bifunctional Organocatalyst. Org. Lett. 2012;14:4322–4325. doi: 10.1021/ol3015607. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Wang B, Zhang J, Yan M. Asymmetric Organocatalytic Double-Conjugate Addition of Malononitrile to Dienones: Efficient Synthesis of Optically Active Cyclohexanones. Org. Lett. 2011;13:374–377. doi: 10.1021/ol102570b. [DOI] [PubMed] [Google Scholar]
- 7.Naota K, Tannna A, Murahashi S-I. Synthesis and Characterization of C- and N-Bound Isomers of Transition Metal α-Cyanocarbanions. J. Am. Chem. Soc. 2000;122:2960–2961. doi: 10.1021/ja994387l. [DOI] [Google Scholar]
- 8.For a review of catalytic asymmetric Mannich reaction, Kobayashi, S. & Ueno, M. Mannich Reaction, Comprehensive Asymmetric Catalysis, Supplement, 1, 143–150 (2004).
- 9.Lu Z, et al. Cinchona Alkaloid-catalyzed Asymmetric Direct Mannich Reaction of Malononitrile to Imine for Synthesis of β-Amino Malononitrile. Chinese J. Chem. 2012;30:2333–2337. doi: 10.1002/cjoc.201200586. [DOI] [Google Scholar]
- 10.Poulsen TB, Alemparte C, Saaby S, Bella M, Jørgensen KA. Direct Organocatalytic and Highly Enantio- and Diastereoselective Mannich Reactions of α-Substituted α-Cyanoacetates. Angew. Chem. Int. Ed. 2005;44:2896–2899. doi: 10.1002/anie.200500144. [DOI] [PubMed] [Google Scholar]
- 11.Nojiri A, Kumagai N, Shibasaki M. Asymmetric Catalysis via Dynamic Substrate/Ligand/Rare Earth Metal Conglomerate. J. Am. Chem. Soc. 2008;130:5630–5631. doi: 10.1021/ja800326d. [DOI] [PubMed] [Google Scholar]
- 12.Nojiri A, Kumagai N, Shibasaki M. Linking Structural Dynamics and Functional Diversity in Asymmetric Catalysis. J. Am. Chem. Soc. 2009;131:3779–3784. doi: 10.1021/ja900084k. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Kim DY. Enantio- and Diastereoselective Mannich-Type Reactions of α-Cyano Ketones with N-Boc Aldimines Catalyzed by Chiral Bifunctional Urea. Adv. Synth. Catal. 2009;351:1779–1782. doi: 10.1002/adsc.200900268. [DOI] [Google Scholar]
- 14.González PB, Lopez R, Palomo C. Catalytic Enantioselective Mannich-Type Reaction with β-Phenyl Sulfonyl Acetonitrile. J. Org. Chem. 2010;75:3920–3922. doi: 10.1021/jo1005872. [DOI] [PubMed] [Google Scholar]
- 15.Matsuzawa A, Nojiri A, Kumagai N, Shibasaki M. Solvent-Dependent Self-Discrimination of Bis(2-hydroxyphenyl)diamides. Chem. Eur. J. 2010;16:5036–5042. doi: 10.1002/chem.201000321. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita Y, Matsumoto M, Chen Y-J, Kobayashi S. Catalytic Mannich-type reactions of α-aminoacetonitrile using fluorenylidene as a protecting and activating group. Tetrahedron. 2012;68:7558–7563. doi: 10.1016/j.tet.2012.06.044. [DOI] [Google Scholar]
- 17.Ohmatsu K, Goto A, Ooi T. Catalytic asymmetric Mannich-type reactions of α-cyano α-sulfonyl carbanions. Chem. Commun. 2012;48:7913–7915. doi: 10.1039/c2cc32398b. [DOI] [PubMed] [Google Scholar]
- 18.Hyodo K, Nakamura S, Shibata N. Enantioselective Aza-Morita–Baylis–Hillman Reactions of Acrylonitrile Catalyzed by Palladium(II) Pincer Complexes having C2-Symmetric Chiral Bis(imidazoline) Ligands. Angew. Chem., Int. Ed. 2012;51:10337–10341. doi: 10.1002/anie.201204891. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, et al. Asymmetric One-Pot Sequential Mannich/Hydroamination Reaction by Organo- and Gold Catalysts: Synthesis of Spiro[pyrrolidin-3,2′-oxindole] Derivatives. Org. Lett. 2013;15:1846–1849. doi: 10.1021/ol4004542. [DOI] [PubMed] [Google Scholar]
- 20.Zhao J, et al. Asymmetric Synthesis of β-Amino Nitriles through a ScIII-Catalyzed Three-Component Mannich Reaction of Silyl Ketene Imines. Angew. Chem. Int. Ed. 2013;52:3473–3477. doi: 10.1002/anie.201209093. [DOI] [PubMed] [Google Scholar]
- 21.Zhao J, et al. Enantioselective Construction of Vicinal Tetrasubstituted Stereocenters by the Mannich Reaction of Silyl Ketene Imines with Isatin-Derived Ketimines. Angew. Chem. Int. Ed. 2015;54:241–244. doi: 10.1002/anie.201408730. [DOI] [PubMed] [Google Scholar]
- 22.Lin S, Kawato Y, Kumagai N, Shibasaki M. Catalytic Asymmetric Mannich-Type Reaction of N-Alkylidene-α-Aminoacetonitrile with Ketimines. Angew. Chem. Int. Ed. 2015;54:5183–5186. doi: 10.1002/anie.201412377. [DOI] [PubMed] [Google Scholar]
- 23.Lin S, Kumagai N, Shibasaki M. Enantioselective synthesis of α,α-disubstituted α-amino acids via direct catalytic asymmetric addition of acetonitrile to α-iminoesters. Org. Biomol. Chem. 2016;14:9725–9730. doi: 10.1039/C6OB01874B. [DOI] [PubMed] [Google Scholar]
- 24.Shimoda Y, Kubo T, Sugiura M, Kotani S, Nakajima M. Stereoselective Synthesis of Multiple Stereocenters by Using a Double Aldol Reaction. Angew. Chem. Int. Ed. 2013;52:3461–3464. doi: 10.1002/anie.201209848. [DOI] [PubMed] [Google Scholar]
- 25.Kotani S, Kai K, Sugiura M, Nakajima M. Sequential catalysis of phosphine oxide for stereoselective synthesis of stereopentads. Org. Lett. 2017;19:3672–3675. doi: 10.1021/acs.orglett.7b01719. [DOI] [PubMed] [Google Scholar]
- 26.Enders D, Jegelka U, Dücker B. Diastereo- and Enantioselective Synthesis of C2-Symmetrical HIV-1 Protease Inhibitors. Angew. Chem. Int. Ed. 1993;32:423–425. doi: 10.1002/anie.199304231. [DOI] [Google Scholar]
- 27.Wang J, Li H, Zu L, Jiang W, Wang W. Organocatalytic, Enantioselective Conjugate Addition of Nitroalkanes to Nitroolefins. Adv. Synth. Catal. 2006;348:2047–2050. doi: 10.1002/adsc.200600247. [DOI] [Google Scholar]
- 28.Zhao, C. H., Wangand, L. L. & Chen, Y. J. Asymmetric Mannich-Type Reaction of a Chiral N-(tert-Butylsulfinyl) Ketimine with Imines: Application to the Synthesis of Chiral 1,3-Diamines, Eur. J. Org. Chem., 2977–2986 (2006).
- 29.Couty, F. et al. Nucleophilic Ring-Opening of Azetidinium Ions: Insights into Regioselectivity. Eur. J. Org. Chem., 3479–3490 (2006).
- 30.Lu S-F, Du D-M, Xu J, Zhang S-W. Asymmetric Michael Addition of Nitroalkanes to Nitroalkenes Catalyzed by C2-Symmetric Tridentate Bis(oxazoline) and Bis(thiazoline) Zinc Complexes. J. Am. Chem. Soc. 2006;128:7418–7419. doi: 10.1021/ja0604008. [DOI] [PubMed] [Google Scholar]
- 31.Anderson JC, Blake AJ, Mills M, Ratcliffe PD. A General One-Step Synthesis of β-Nitronitriles. Org. Lett. 2008;10:4141–4143. doi: 10.1021/ol801691c. [DOI] [PubMed] [Google Scholar]
- 32.Yang X, et al. Highly Enantioselective Direct Michael Addition of Nitroalkanes to Nitroolefins Catalyzed by La(OTf)3/N,N′-Dioxide Complexes. Angew. Chem., Int. Ed. 2008;47:7079–7081. doi: 10.1002/anie.200802285. [DOI] [PubMed] [Google Scholar]
- 33.Giampietro NC, Wolfe JP. Stereoselective Synthesis of cis- or trans-3,5-Disubstituted Pyrazolidines via Pd-Catalyzed Carboamination Reactions: Use of Allylic Strain to Control Product Stereochemistry Through N-Substituent Manipulation. J. Am. Chem. Soc. 2008;130:12907–12911. doi: 10.1021/ja8050487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rabalakos C, Wulff WD. Enantioselective Organocatalytic Direct Michael Addition of Nitroalkanes to Nitroalkenes Promoted by a Unique Bifunctional DMAP-Thiourea. J. Am. Chem. Soc. 2008;130:13524–13525. doi: 10.1021/ja805390k. [DOI] [PubMed] [Google Scholar]
- 35.Kurokawa T, Kim M. & J. Du Bois, Synthesis of 1,3-Diamines Through Rhodium-Catalyzed C=H Insertion. Angew. Chem., Int. Ed. 2009;48:2777–2779. doi: 10.1002/anie.200806192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han Z-Y, Xiao H, Gong L-Z. Dynamic kinetic asymmetric transfer hydrogenation of racemic 2,4-diaryl-2,3-dihydrobenzo[b][1,4]diazepines catalyzed by chiral phosphoric acids. Bioorg. Med. Chem. Lett. 2009;19:3729–3732. doi: 10.1016/j.bmcl.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 37.Trost BM, Malhotra S, Olson DE, Maruniak A, Bois JD. Asymmetric Synthesis of Diamine Derivatives via Sequential Palladium and Rhodium Catalysis. J. Am. Chem. Soc. 2009;131:4190–4191. doi: 10.1021/ja809697p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dagousset G, Drouet F, Masson G, Zhu J. Chiral Brønsted Acid-Catalyzed Enantioselective Multicomponent Mannich Reaction: Synthesis of anti-1,3-Diamines Using Enecarbamates as Nucleophiles. Org. Lett. 2009;11:5546–5549. doi: 10.1021/ol9023985. [DOI] [PubMed] [Google Scholar]
- 39.Martjuga M, Shabashov D, Belyakov S, Liepinsh E, Suna E. Asymmetric Synthesis of 1,3-Diamines by Diastereoselective Reduction of Enantiopure N-tert-Butanesulfinylketimines: Unusual Directing Effects of the ortho-Substituent. J. Org. Chem. 2010;75:2357–2368. doi: 10.1021/jo100173f. [DOI] [PubMed] [Google Scholar]
- 40.Kodama K, Sugawara K, Hirose T. Synthesis of Chiral 1,3-Diamines Derived from cis-2-Benzamidocyclohexanecarboxylic Acid and Their Application in the Cu-Catalyzed Enantioselective Henry Reaction. Chem. - A Euro. J. 2011;17:13584–13592. doi: 10.1002/chem.201102136. [DOI] [PubMed] [Google Scholar]
- 41.Kumar P, Jha V, Gonnade VR. Proline-Catalyzed Asymmetric Synthesis of syn- and anti-1,3-Diamines. J. Org. Chem. 2013;78:11756–11764. doi: 10.1021/jo401722e. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Xie Y, Wang H, Huang H. Enantioselective Aminomethylamination of Conjugated Dienes with Aminals Enabled by Chiral Palladium Complex-Catalyzed C–N Bond Activation. J. Am. Chem. Soc. 2016;138:4314–4317. doi: 10.1021/jacs.6b00976. [DOI] [PubMed] [Google Scholar]
- 43.Ji X, Huang H. Synthetic methods for 1,3-diamines. Org. Biomol. Chem. 2016;14:10557–10566. doi: 10.1039/C6OB01976E. [DOI] [PubMed] [Google Scholar]
- 44.Fischli A, Klaus M, Mayer H, Schönholzer P, Ruegg R. Eine chiral ökonomische Totalsynthese von natürlichen und unnatürlichen Prostaglandinen. Helv. Chim. Acta. 1975;58:564–584. doi: 10.1002/hlca.19750580228. [DOI] [PubMed] [Google Scholar]
- 45.Eds. Shibasaki, M. & Yamamoto, Y. Multimetallic Catalysts in Organic Synthesis (Wiley-VCH, 2004).
- 46.Annamalai, V., DiMauro, E. F., Carroll, P. J. & Kozlowski, M. C. Catalysis of the Michael Addition Reaction by Late Transition Metal Complexes of BINOL-Derived Salens, J. Org. Chem., 68, 1973–1981 (2003), and references cited therein. [DOI] [PubMed]
- 47.Yang M, Zhu C, Yuan F, Huang Y, Pan Y. Enantioselective Ring-Opening Reaction of meso-Epoxides with ArSeH Catalyzed by Heterometallic Ti−Ga−Salen System. Org. Lett. 2005;7:1927–1930. doi: 10.1021/ol0503034. [DOI] [PubMed] [Google Scholar]
- 48.Li W, et al. Synthesis of optically active 2-hydroxy monoesters via-kinetic resolution and asymmetric cyclization catalyzed by heterometallic chiral (salen) Co complex. Tetrahedron Lett. 2006;47:3453–3457. doi: 10.1016/j.tetlet.2006.03.042. [DOI] [Google Scholar]
- 49.Mazet C, Jacobsen EN. Dinuclear {(salen)Al} Complexes Display Expanded Scope in the Conjugate Cyanation of α,β-Unsaturated Imides. Angew. Chem., Int. Ed. 2008;47:1762–1765. doi: 10.1002/anie.200704461. [DOI] [PubMed] [Google Scholar]
- 50.Hirahata W, Thomas RM, Lobkovsky EB, Coates GW. Enantioselective Polymerization of Epoxides: A Highly Active and Selective Catalyst for the Preparation of Stereoregular Polyethers and Enantiopure Epoxides. J. Am. Chem. Soc. 2008;130:17658–17659. doi: 10.1021/ja807709j. [DOI] [PubMed] [Google Scholar]
- 51.Mihara H, Xu Y, Shepherd NE, Matsunaga S, Shibasaki M. A Heterobimetallic Ga/Yb-Schiff Base Complex for Catalytic Asymmetric α-Addition of Isocyanides to Aldehydes. J. Am. Chem. Soc. 2009;131:8384–8385. doi: 10.1021/ja903158x. [DOI] [PubMed] [Google Scholar]
- 52.Arai T, et al. Trinuclear Zn3(OAc)4-3,3′-bis(aminoimino)binaphthoxide Complex for Highly Efficient Catalytic Asymmetric Iodolactonization. Chem. Comm. 2014;42:8287–8290. doi: 10.1039/C4CC02415J. [DOI] [PubMed] [Google Scholar]
- 53.Xu Y, Kaneko K, Kanai M, Shibasaki M, Matsunaga S. Regiodivergent Kinetic Resolution of Terminal and Internal rac-Aziridines with Malonates under Dinuclear Schiff Base Catalysis. J. Am. Chem. Soc. 2014;136:9190–9194. doi: 10.1021/ja5039165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.