Abstract
Purpose
Based on improvements of progression-free survival (PFS), new agents for metastatic renal cell carcinoma (mRCC) have been approved. It is assumed that one of the benefits is a delay in health-related quality of life (HRQoL) deterioration as a result of a delay in progression of disease. However, little data are available supporting this relationship. This study aims to provide insight into the most important determinants of HRQoL (including progression of disease) of patients with mRCC.
Methods
A patient registry (PERCEPTION) was created to evaluate treatment of patients with (m)RCC in the Netherlands. HRQoL was measured, using the EORTC QLQ-C30 and EQ-5D-5L, every 3 months in the first year of participation in the study, and every 6 months in the second year. Participation started as soon as possible following a diagnosis of (m)RCC. Random effects models were used to study associations between HRQoL and patient and disease characteristics, symptoms and treatment.
Results
Eighty-seven patients with mRCC completed 304 questionnaires. The average EORTC QLQ-C30 global health status was 69 (SD, 19) before progression and 61 (SD, 22) after progression of disease. Similarly, the average EQ-5D utility was 0.75 (SD, 0.19) before progression and 0.66 (SD, 0.30) after progression of disease. The presence of fatigue, pain, dyspnoea, and the application of radiotherapy were associated with significantly lower EQ-5D utilities.
Conclusions
Key drivers for reduced HRQoL in mRCC are disease symptoms. Since symptoms increase with progression of disease, targeted therapies that increase PFS are expected to postpone reductions in HRQoL in mRCC.
Electronic supplementary material
The online version of this article (doi:10.1007/s11136-017-1704-4) contains supplementary material, which is available to authorized users.
Keywords: Health-related quality of life, EQ-5D, EORTC QLQ-C30, Cost-effectiveness analysis, Metastatic renal cell carcinoma, Targeted therapy
Introduction
Renal cell carcinoma (RCC) accounts for 90% of all kidney cancers [1]. While the prognosis of patients with localised disease treated with surgery is relatively good, the prognosis of patients with advanced or metastatic disease is poor. Median overall survival (OS) ranges from 7.8 months for patients with a poor risk to 43.2 months for patients with a favourable risk according to the Heng criteria [2]. Besides the impact of metastatic renal cell carcinoma (mRCC) on survival, mRCC can be associated with severe symptoms, such as cachexia and/or anorexia, asthenia and/or fatigue, pain, anaemia, and venous thromboembolism [3].
Since 2006, several new targeted therapies have been approved for the treatment of mRCC such as sunitinib, sorafenib, pazopanib and everolimus. In phase III studies, these therapies improved progression-free survival (PFS) of patients with mRCC over the diverse comparators [4–11], but the effect on OS was less pronounced, likely (partly) due to treatment crossover. It is assumed that one of the benefits of the new therapies is a delay in HRQoL deterioration as a result of a delay in progression of disease. Clinicians feel that a better PFS translates into a better HRQoL [12], but little data are available supporting this relationship. In the context of the high prices of targeted therapies which form a strain on health care budgets, it is important to establish whether indeed a delay in progression delays HRQoL deterioration.
This study is the first to provide insight into the most important determinants of HRQoL (including progression of disease) of patients with mRCC using data from a patient registry in the Netherlands [13]. Additionally, this study aims to assess if the association between progression and HRQoL, if one exists, is also captured by measures used in economic evaluations to assess benefit (i.e. EQ-5D).
Patients and methods
Study population
A patient registry (i.e. PERCEPTION) was created to evaluate treatment of patients with (m)RCC in the Netherlands. Patients with RCC (all stages) of any histological subtype diagnosed from 2011 until June 30th 2013 in 25 of 32 hospitals (both general and academic) in three regions in the Netherlands were invited to participate, and fill out HRQoL questionnaires. Eligible patients were identified through the hospitals’ registration systems. Additionally, the Netherlands Cancer Registry (NCR), which maintains a cancer registration database of all cancer patients in the Netherlands, was used to ensure that no patients were missed.
The research protocol was approved by the medical ethics committee of Radboud university medical center in Nijmegen (CMO Region Arnhem-Nijmegen) in May 2010. Informed consent was obtained from all patients participating in the HRQoL study.
Data collection
Cancer-specific HRQoL was measured using the EORTC (European Organisation for Research and Treatment of Cancer) QLQ-C30 questionnaire (v3.0) [14]. This measure includes five functional scales (physical, role, emotional, social and cognitive), three symptom scales (fatigue, nausea & vomiting and pain), a global health status/QOL scale and six single items (dyspnoea, insomnia, appetite loss, constipation, diarrhoea and financial difficulties). In addition to the EORTC QLQ-C30, the EQ-5D-5L was used to measure HRQoL. The EQ-5D-5L is a preference-based generic measure, and measures HRQoL on five dimensions, i.e. mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each dimension includes five severity levels [15]. Patients were sent a HRQoL questionnaire every 3 months in the first year of participation in the study, and every 6 months in the second year. Participation started as soon as possible following a diagnosis of (m)RCC.
In addition to data on HRQoL, data on demographics, clinical and laboratory factors (to determine the patient’s risk group [16]) were collected retrospectively from individual patient records using uniform case report forms. Furthermore, data on treatment schemes and treatment endpoints (e.g. survival) were derived from patient records. Data collection stopped at the end of 2013.
Statistical analyses
For each scale of the EORTC QLQ-C30, the average of the items that contributed to that scale was calculated. They were then linearly transformed in line with the EORTC QLQ-C30 scoring manual [17]. EQ-5D utilities were derived by combining the answers to the EQ-5D-5L with the Dutch EQ-5D- 5L tariff [18]. Mean EQ-5D utilities and HRQoL based on the EORTC QLQ-C30 were calculated by taking the average of the observations for each patient. The proportion of reported problems for each EQ-5D dimension were presented by taking the modus (i.e. the level reported most frequently) across observations for each patient. If two or more modes exist, the highest level was taken.
HRQoL was evaluated separately for the periods before and after progression of disease. In the period before progression of disease, a further distinction was made between wait-and-see and treatment with (first-line) targeted therapy. Treatment was assumed to last until progression of disease. Response including progression of disease was defined based on RECIST (as mentioned in the radiology report). As a substitute (if unavailable) data managers were instructed to register the response as indicated by the physician in the medical record. Patients who did not start therapy within the follow-up period were assumed to wait for therapy during the entire follow-up.
Since data on HRQoL were clustered, random effects models [19] were used to study associations between HRQoL (i.e. EORTC QLQ-C30 global health status and EQ-5D utility) and patient and disease characteristics, symptoms and treatment. Use of random effects models ensured that multiple measurements from the same patient were analysed appropriately and made it possible to distinguish between intraindividual and interindividual variation. Backward selection was used to select the covariates for the models; any non-significant covariates were excluded from the models one at a time (significance level of 0.20 for entering and 0.10 for removing the explanatory variables). To control for heteroscedasticity, random effects models with robust standard errors were estimated.
Additionally, random effects logit models [19] were used to study associations between the individual EQ-5D dimensions and patient and disease characteristics, symptoms and treatment. EQ-5D levels were dichotomised into ‘no problems/(i.e. level 1) and ‘problems’ (i.e. levels 2–5).
Missing data regarding patient and disease characteristics were handled using multiple imputations by chained equations. This method generated imputations based on a set of imputation models, one for each variable with missing values [20].
The significance level was set at α = 0.10. Data analyses were conducted using STATA statistical analysis software (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP).
Results
Four hundred eleven (m)RCC patients participating in the study completed 1630 questionnaires. The median number of questionnaires per patient was four (range 1–7). The number of questionnaires collected at each time point is provided in the Supplementary material (Fig. S1), as are the number of questionnaires per patient (Fig. S2). The average EORTC QLQ-C30 global health status of patients diagnosed with localised disease (336 patients, 1326 questionnaires) was 76 (SD, 15), and the average EQ-5D utility was 0.82 (SD, 0.17).
Eighty-seven patients had mRCC (i.e. metastatic disease at initial presentation or after an initial diagnosis with localised disease). Of these patients, eighty-two percent were male, and the median age at diagnosis was 63 years (Table 1). Twenty-six percent of the population did not receive any systemic therapy during follow-up. Of the patients receiving systemic therapy, the majority (80%) was treated with first-line sunitinib. Twenty-three patients also received a second-line therapy within the follow-up period; the majority of these patients was treated with everolimus (13/23). Thirty-one patients received radiotherapy during follow-up.
Table 1.
Baseline characteristics at diagnosis
| Variable | Patients (n = 87) |
|---|---|
| Male sex, n (%) | 71 (82) |
| Age, median (range) | 63 (40–79) |
| Non-clear cell pathology, n (%) | 17 (20) |
| WHO performance status, n (%) | |
| 0–1 | 82 (94) |
| 2–4 | 5 (6) |
| More than one metastatic site, n (%) | 48 (55) |
| Liver metastasis, n (%) | 15 (17) |
| Lung metastasis, n (%) | 48 (56) |
| Bone metastasis, n (%) | 21 (24) |
| Brain metastasis, n (%) | 3 (3) |
| Haemoglobin < LLN, n (%) | 46 (52) |
| Neutrophil count > ULN, n (%) | 18 (21) |
| Platelet count > ULN, n (%) | 19 (22) |
| Corrected serum calcium > ULN, n (%) | 26 (30) |
| Lactate dehydrogenase >1.5 times ULN, n (%) | 11 (12) |
| Time since RCC diagnosis <1 year | 78 (90) |
| MSKCC risk score, n (%) | |
| Favourable | 6 (7) |
| Intermediate | 54 (62) |
| Poor | 27 (31) |
LLN lower limit of normal, ULN upper limit of normal, RCC renal cell carcinoma, MSKCC Memorial Sloan Kettering Cancer Center
In total, 304 questionnaires were completed by patients with mRCC and the median number of questionnaires per patient was three (range 1–7).
Table 2 shows HRQoL during the different stages of the disease. The mean EORTC QLQ-C30 global health status was 67 (SD, 19). Patients primarily experienced problems with role functioning (i.e. doing daily activities and pursuing leisure time activities). Problems with emotional (i.e. feeling tense, irritable, depressed or worrying) and cognitive functioning (i.e. concentrating and remembering) were experienced less often. Symptoms most commonly reported were fatigue, pain, insomnia and dyspnoea. A statistically significant difference was found between the EORTC QLQ-C30 global health status before and after progression of disease, i.e. 69 (SD, 19) and 61 (SD, 22) (p = 0.022). All functioning scales significantly decreased, except for emotional and cognitive functioning. Two symptom scales significantly increased; patients reported more problems regarding dyspnoea (p = 0.031) and diarrhoea (p = 0.057) after progression than before progression of disease.
Table 2.
Health-related quality of life based on the EQ-5D and QLQ-C30
| Total n = 87 patients (304 obs.) |
Before progression n = 81 patients (246 obs.) |
After progression n = 27 patients (58 obs.) |
|||
|---|---|---|---|---|---|
| Mean (SD) | Total mean (SD) | No systemic therapy n = 47 (125 obs.*) Mean (SD) |
First-line therapy n = 50 (119 obs.) Mean (SD) |
Total mean (SD) | |
| EQ-5D | |||||
| Utility | 0.74 (0.19) | 0.75 (0.19) | 0.76 (0.21) | 0.76 (0.18) | 0.66 (0.30)** |
| EORTC QLQ-C30 | |||||
| Global health status | 67 (19) | 69 (19) | 69 (22) | 70 (17) | 61 (22)*** |
| Functioning scales | |||||
| Physical functioning | 69 (23) | 71 (23) | 73 (22) | 69 (23) | 62 (29) |
| Role functioning | 59 (28) | 61 (29) | 61 (30) | 62 (29) | 52 (33) |
| Emotional functioning | 79 (16) | 80 (18) | 77 (19) | 82 (19) | 73 (19) |
| Cognitive functioning | 80 (20) | 80 (22) | 81 (21) | 79 (25) | 76 (22) |
| Social functioning | 76 (22) | 78 (22) | 77 (20) | 78 (22) | 67 (28) |
| Symptom scales | |||||
| Fatigue | 41 (25) | 39 (27) | 36 (27) | 41 (27) | 48 (30) |
| Nausea and vomiting | 12 (17) | 13 (20) | 8 (13) | 17 (24) | 10 (12) |
| Pain | 29 (24) | 27 (24) | 24 (25) | 29 (26) | 34 (30) |
| Single items | |||||
| Dyspnoea | 24 (24) | 23 (24) | 23 (25) | 26 (28) | 29 (34) |
| Sleeping | 28 (26) | 26 (27) | 24 (27) | 27 (30) | 35 (31) |
| Appetite loss | 19 (26) | 18 (25) | 15 (26) | 21 (26) | 22 (32) |
| Constipation | 10 (17) | 9 (17) | 12 (24) | 5 (10) | 12 (21) |
| Diarrhoea | 20 (26) | 19 (27) | 13 (27) | 23 (28) | 22 (26) |
| Financial difficulties | 10 (18) | 9 (18) | 9 (21) | 11 (19) | 8 (21) |
Obs observations
*Observations of patients who died within 90 days after being diagnosed with mRCC were excluded from this subgroup (n = 2), since these measurements would not contribute to the estimation of the HRQoL of a patient awaiting therapy
**Mean EQ-5D utility of these patients before progression of disease (n = 21) was 0.76 (0.23)
***Mean EORTC QLQ-C30 global health status of these patients before progression of disease (n = 21) was 69 (20)
In the period before progression of disease, a similar HRQoL was found for a period without therapy (i.e. wait-and-see) and a period with therapy; mean EORTC QLQ-C30 global health statuses were 69 (SD, 22) and 70 (SD, 17), respectively. However, in the period before progression of disease, patients experienced fewer problems with emotional functioning during a period with therapy compared to a period without therapy (p = 0.067). Additionally, patients reported fewer problems regarding constipation (p = 0.072), but more problems regarding diarrhoea during a period with therapy compared to a period without therapy (p = 0.005).
The average EQ-5D utility was 0.74 (SD, 0.19). As with the EORTC QLQ-C30 global health status, a significant difference was found in EQ-5D utility before progression of disease and after progression of disease; the average EQ-5D utility before progression of disease was 0.75 (SD, 0.19), whereas the average EQ-5D utility after progression of disease was 0.66 (SD, 0.30) (p = 0.032). In the period before progression of disease, no significant difference was found between a period without therapy (i.e. wait-and-see) and a period with therapy; mean utilities were 0.76 (SD, 0.21) and 0.76 (SD, 0.18), respectively. In the Supplementary material, Figs. S3 and S4 provide a summary of mean EORTC QLQ-C30 global health statuses and mean EQ-5D utilities by time.
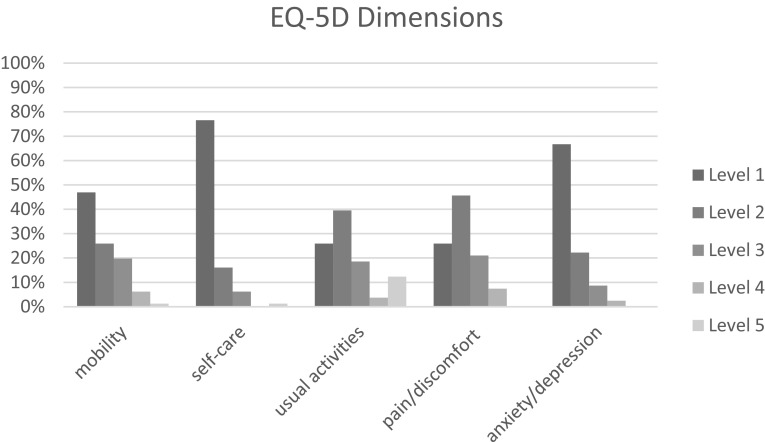
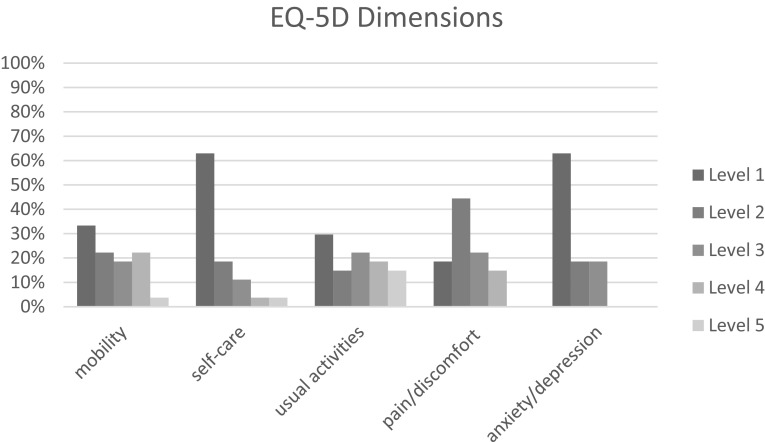
Figures 1 and 2 show the proportions of patients reporting levels 1–5 by EQ-5D dimension, before progression of disease and after progression of disease. Both before and after progression of disease, most problems were reported on the mobility, usual activities and pain/discomfort dimensions.
Fig. 1.
Proportion of patients reporting levels 1–5 by dimension, before progression of disease
Fig. 2.
Proportion of patients reporting levels 1–5 by dimension, after progression of disease
Univariable analyses show several relationships between disease characteristics, symptoms and treatment, and HRQoL (Table 3). Patients with brain metastases and patients with progression of disease reported a lower HRQoL than the other patients. Patients with more than one metastatic site or bone metastases reported a lower EQ-5D utility, a relationship that was not seen in the EORTC QLQ-C30 global health status. Additionally, symptoms (i.e. fatigue, nausea and vomiting, pain, dyspnoea, insomnia, appetite loss, constipation and diarrhoea) were associated with a lower HRQoL. Lastly, patients treated with radiotherapy reported a worse HRQoL than patients not treated with radiotherapy.
Table 3.
Associations between HRQoL and patient and disease characteristics, symptoms and treatment
| EQ-5D utility | EORTC QLQ-C30 global health status | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | |||||
| Coefficient | SE | Coefficient | SE | Coefficient | SE | Coefficient | SE | |
| Patient characteristics | ||||||||
| Male sex | 0.077 | 0.069 | NS | 2.748 | 5.198 | NS | ||
| Age (per year) | −0.001 | 0.002 | NS | −0.257 | 0.223 | NS | ||
| WHO performance score | ||||||||
| 0–1 | ||||||||
| 2–4 | −0.08 | 0.072 | NS | −5.919 | 7.304 | NS | ||
| Disease characteristics | ||||||||
| More than one metastatic site | −0.068* | 0.035 | NS | −5.048 | 3.342 | 4.048* | 2.276 | |
| Presence of liver metastases | −0.027 | 0.05 | NS | −3.992 | 4.779 | NS | ||
| Presence of lung metastases | −0.021 | 0.041 | NS | 0.465 | 4.074 | NS | ||
| Presence of bone metastases | −0.085** | 0.04 | NS | −3.39 | 3.915 | NS | ||
| Presence of brain metastases | −0.285* | 0.17 | NS | −21.143* | 10.239 | −13.586*** | 2.438 | |
| MSKCC risk score | ||||||||
| Favourable | ||||||||
| Intermediate | 0.015 | 0.062 | NS | −0.431 | 8.924 | NS | ||
| Poor | 0.054 | 0.063 | NS | 2.485 | 9.22 | NS | ||
| Progression of disease | −0.082** | 0.036 | NS | −6.897* | 3 | −3.859* | 2.249 | |
| Disease duration (in months) | −0.002 | 0.001 | NS | −0.081 | 0.117 | NS | ||
| Symptoms | ||||||||
| Fatigue | −0.004*** | 0.001 | −0.003*** | 0.001 | −0.451*** | 0.035 | −0.316*** | 0.042 |
| Nausea and vomiting | −0.001* | 0.001 | 0.001** | 0.001 | −0.360*** | 0.05 | NS | |
| Pain | −0.004*** | 0 | −0.002*** | 0 | −0.324*** | 0.036 | −0.143*** | 0.035 |
| Dyspnoea | −0.003*** | 0 | −0.001*** | 0 | −0.222*** | 0.04 | NS | |
| Sleeping | −0.002*** | 0 | NS | −0.219*** | 0.035 | NS | ||
| Appetite loss | −0.002*** | 0 | NS | −0.274*** | 0.034 | −0.111*** | 0.035 | |
| Constipation | −0.002*** | 0.001 | NS | −0.186*** | 0.054 | NS | ||
| Diarrhoea | −0.001* | 0 | NS | −0.089** | 0.04 | NS | ||
| Treatment | ||||||||
| Systemic therapy versus no systemic therapy | 0.026 | 0.027 | NS | −0.487 | 2.408 | NS | ||
| Radiotherapy | −0.150*** | 0.042 | −0.115*** | 0.036 | −10.017*** | 3.306 | NS | |
| Model intercept | 0.943*** | 0.016 | 85.380*** | 1.903 | ||||
| R2 (overall) | 0.559 | 0.534 | ||||||
| Wald test (p value) | <0.001 | <0.001 | ||||||
Several comorbidities at diagnosis were considered for inclusion in the multivariable analyses, but all appeared to be not significantly associated with HRQoL
SE standard error, NS not significant
*Significant at α = 0.1
**Significant at α = 0.05
***Significant at α = 0.01
Multivariable analysis showed that the EORTC QLQ-C30 global health status decreased with the presence of fatigue, pain and appetite loss. Furthermore, the presence of brain metastases and progression of disease were associated with a worse EORTC QLQ-C30 global health status. A similar association was found between fatigue and pain, and the EQ-5D utility. Furthermore, EQ-5D utility scores decreased with the presence of dyspnoea and treatment with radiotherapy.
Although the univariable analyses showed several relationships between disease characteristics (e.g. the presence of bone or brain metastases and progression of disease) and HRQoL, these characteristics were no longer associated with a deterioration of HRQoL in multivariable analyses after correction for symptoms (at a significance level of 0.05 and 0.01, except for the presence of brain metastases in the model with the EORTC QLQ-C30 global health status as the dependent variable). This seems to imply that symptoms might increase due to progression of disease (and/or due to the spread of the cancer to the bone or brain), which explains the reduced HRQoL.
Table 4 shows that fatigue was associated with all EQ-5D dimensions, except with the mobility dimension; fatigue was associated with a greater frequency of problems regarding self-care, usual activities, pain/discomfort and anxiety/depression. Patients having pain reported problems with all EQ-5D dimensions more often, with the exception of anxiety/depression.
Table 4.
Associations between the EQ-5D dimensions and patient and disease characteristics, symptoms and treatment
| Mobility | Self-care | Usual activities | Pain/discomfort | Anxiety/depression | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | SE | OR | SE | OR | SE | OR | SE | OR | SE | |
| Patient characteristics | ||||||||||
| Male sex | 0.149** | 0.112 | NS | 0.095** | 0.110 | NS | NS | |||
| Age (per year) | 1.078** | 0.032 | NS | NS | NS | NS | ||||
| Disease characteristics | ||||||||||
| Presence of liver metastases | 4.427* | 3.395 | NS | NS | NS | NS | ||||
| Presence of lung metastases | NS | NS | NS | NS | 0.300* | 0.191 | ||||
| Presence of bone metastases | 4.733** | 2.961 | NS | 15.054*** | 14.768 | NS | NS | |||
| MSKCC risk score | ||||||||||
| Favourable | ||||||||||
| Intermediate | NS | NS | NS | 0.041*** | 0.049 | NS | ||||
| Poor | NS | NS | NS | 0.143 | 0.176 | NS | ||||
| Disease duration | NS | 1.073** | 0.033 | NS | NS | NS | ||||
| Symptoms | ||||||||||
| Fatigue | NS | 1.044*** | 0.012 | 1.128*** | 0.028 | 1.034*** | 0.012 | 1.021** | 0.010 | |
| Nausea and vomiting | 0.967** | 0.015 | NS | NS | NS | NS | ||||
| Pain | 1.029*** | 0.009 | 1.030*** | 0.010 | 1.029* | 0.015 | 1.143*** | 0.023 | NS | |
| Dyspnoea | 1.025*** | 0.009 | NS | 1.024* | 0.014 | NS | NS | |||
| Sleeping | NS | NS | NS | NS | 1.016* | 0.009 | ||||
| Appetite loss | 1.031*** | 0.011 | NS | NS | NS | NS | ||||
| Treatment | ||||||||||
| Radiotherapy | NS | 6.062*** | 3.971 | NS | NS | NS | ||||
Odds ratios based on models created using multivariable logistic regression
OR odds ratio, SE standard error
*Significant at α = 0.1
**Significant at α = 0.05
***Significant at α = 0.01
Discussion
In this study differences were found between the health-related quality of life (HRQoL) of patients with metastatic renal cell carcinoma (mRCC) before and after progression of disease, with a reduced HRQoL after progression of disease. Progression of disease was no longer associated with a deterioration of HRQoL in multivariable analyses after correction for symptoms (at a significance level of 0.05 and 0.01). In line with Wilson and Cleary [21], a relationship between disease characteristics and symptoms was expected, which could explain why disease characteristics (such as progression) were no longer statistically significant in the multivariable analyses. Similarly, bone metastases were no longer associated with a deterioration of HRQoL in multivariable analyses. Since bone metastases can cause pain, then it is not surprising that bone metastases are not significantly associated with HRQoL once pain is included in the analysis. This seems to imply that symptoms increase due to progression of disease (and/or due to the spread of the cancer to the bone), which explains the reduced HRQoL.
Besides the relationship between symptoms and HRQoL, a significant association was found between radiotherapy and HRQoL (in the model with the EQ-5D utility as the dependent variable). It is possible that this observed association is not due to radiotherapy itself, but to the selection of which mRCC patients are to receive radiotherapy. That is, radiotherapy is mostly reserved for palliation of local and symptomatic disease or to prevent the progression of metastatic disease in critical sites (i.e. bones and brain) [22]. Either way, radiotherapy appears to be a significant determinant of HRQoL, even after correction for patient and disease characteristics (including bone and brain metastases) and symptoms.
The average EQ-5D utility of patients with mRCC was 0.74 compared to an average of 0.84 (SD, 0.18) in the Dutch population aged 60 to 69 [18]. Most patients (74%) in the study population were treated with a targeted therapy (the majority received sunitinib). The average EQ-5D utility of these patients was 0.76 before progression of disease. In a study by Cella et al., a similar EQ-5D utility was reported for patients treated with sunitinib (i.e. 0.75) [23]. In the economic evaluation of bevacizumab and sunitinib by Thompson-Coon and colleagues [24], a health state utility of 0.78 (95% CI 0.76–0.80) was used for progression-free survival and 0.70 (95% CI 0.66–0.74) for progressive disease. These utilities were derived from the data presented in the sunitinib submission to NICE and are somewhat higher than the utilities that we found in our study. The economic evaluation of sunitinib by Remák and colleagues [25] was based on the results of a phase II trial of sunitinib as second-line treatment in mRCC [26]; utilities of 0.72 and 0.76 were used for progression-free survival (i.e. during treatment or rest, respectively), whereas utilities of 0.63 and 0.55 were used for progressive disease (i.e. during second-line treatment or after termination of second-line treatment, respectively). The latter utilities are below the utilities found in our study, but this might be explained by differences in the study population, e.g. patients with progression on first-line cytokine therapy were enrolled in the phase II trial.
This study has several limitations that deserve mentioning. First, only 9% of the population (including those patients with RCC but not having metastatic disease) completed the 2-year follow-up period and filled in seven questionnaires. This is mainly because data collection stopped before many patients could be followed up for 2 years after diagnosis. That is, data collection stopped at the end of 2013, which meant that patients diagnosed after January 1st 2012 were not able to complete the full follow-up period. There are no reasons to expect important differences between the patients who did and did not complete the 2-year follow-up.
Second, a significant association between WHO performance status and HRQoL, and the MSKCC risk score and HRQoL was not found, although such a relationship would have been expected. The MSKCC risk score divides patients into three risk groups, and gives an indication of the life expectancy of patients with mRCC [16]. Whereas HRQoL was measured several times during the follow-up period, data on patient characteristics (e.g. WHO performance status) and disease characteristics (e.g. laboratory factors, which are part of the MSKCC risk score) were collected once before the start of each new treatment. As a consequence, too few observations on patient and disease characteristics might have been available to detect a significant association between WHO performance status and the MSKCC risk score, and HRQoL. Similarly, a significant association between comorbidities and HRQOL might have been expected, but data on comorbidities were only collected once (at diagnosis) which might explain why a significant association was not found. Nevertheless, the impact of comorbidities on HRQoL might be captured to some extent through age. Age appeared not to be significantly associated with HRQoL.
A third limitation is that our study sample was too small to find any difference in EQ-5D utilities between different types of targeted therapies, while these therapies differ in toxicity profiles [27]. Nevertheless, although adverse events have a high impact on HRQoL, an association between adverse events and HRQoL would not be found if the proportion of patients with grade 3 or 4 adverse events is relatively low. Hypertension and fatigue are the most commonly reported grade 3 or 4 adverse events in the randomised phase 3 trial of sunitinib [4], but these adverse events occurred in only 8 and 7% of the population. Therefore, a very large sample size is needed to find any difference in EQ-5D utilities between different types of targeted therapies. Additionally, it is unknown whether the improved HRQoL due to prolonged PFS outweighs reductions in HRQoL due to treatment-related adverse events. Importantly, this study did not find differences in HRQoL of patients treated with systemic therapy and patients not treated with systemic therapy, or between periods with or without systemic therapy. However, this study may have been underpowered to find such differences.
A fourth limitation is that no data were collected in the PERCEPTION-registry on assistance provided to patients who reported problems on one or more of the functioning scales of the EORTC QLQ-C30, while these patients could have received assistance to relieve their complaints. For example, patients could have received care at home to help with dressing and washing or emotional support by a psychologist or another healthcare professional. As a consequence, the impact of mRCC on HRQoL as presented in Table 2 might be underestimated.
Lastly, the total number of patients with mRCC was 233 in the 2011–2013 Cohort of the PERCEPTION-registry [13], while only 87 patients filled in one or more questionnaires about HRQoL. A comparison of the patient and disease characteristics and outcomes (in terms of overall survival) showed that the patients in the current study had a more favourable prognosis than the other patients in the PERCEPTION-registry. The impact on HRQoL as we estimated in this study is expected to be small, since we presented HRQoL associated with different stages of the disease.
To conclude, key drivers for reduced HRQoL in mRCC are symptoms of the disease. Since this study showed that symptoms increase with progression of disease, targeted therapies that increase PFS can help to delay loss in HRQoL. This study also showed that the EQ-5D is able to detect changes in HRQoL of patients with mRCC, as it found associations between well-known symptoms of mRCC and EQ-5D utilities. Similar associations were found between these symptoms and the disease-specific EORTC QLQ-C30.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the registration team of the Netherlands Comprehensive Cancer Organisation for data collection. The authors are also grateful to Dr. Jeannette Oosterwijk-Wakka for her help in the collection of data on HRQoL.
Funding
The PERCEPTION registry was financially supported by the Netherlands Organisation for Health Research and Development (Grant Number: 152001014); Pfizer BV (formerly Wyeth Pharmaceuticals BV); and Roche Nederland BV.
Compliance with ethical standards
Conflict of interest
MV is a member of the EuroQoL research foundation. SS received speaker honoraria from GlaxoSmithKline. LK received an unrestricted research grant from Pfizer to extend the data collection of the PERCEPTION registry. CU received unrestricted research grants from Pfizer (formerly Wyeth Pharmaceuticals BV) and Roche Nederland BV to support the PERCEPTION registry. All remaining authors declared no conflict of interest.
Ethical approval
The research protocol was approved by the medical ethics committee of Radboud university medical center in Nijmegen (CMO Region Arnhem-Nijmegen) in May 2010.
Informed consent
Informed consent was obtained from all patients participating in the HRQoL study.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s11136-017-1704-4) contains supplementary material, which is available to authorized users.
References
- 1.Ljungberg B, Campbell SC, Choi HY, Jacqmin D, Lee JE, Weikert S, et al. The epidemiology of renal cell carcinoma. European Urology. 2011;60(4):615–621. doi: 10.1016/j.eururo.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 2.Heng DY, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: A population-based study. The Lancet Oncology. 2013;14(2):141–148. doi: 10.1016/S1470-2045(12)70559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambea J, Hinojo C, Lainez N, Lazaro M, Leon L, Rodriguez A, et al. Quality of life and supportive care for patients with metastatic renal cell carcinoma. Cancer Metastasis Reviews. 2012;31(Suppl 1):S33–S39. doi: 10.1007/s10555-012-9357-9. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. The New England Journal of Medicine. 2007;356(2):115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 5.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. The New England Journal of Medicine. 2007;356(2):125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 6.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. The New England Journal of Medicine. 2007;356(22):2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 7.Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370(9605):2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 8.Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Ou SS, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2008;26(33):5422–5428. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 10.Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2010;28(6):1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 11.Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomised phase 3 trial. Lancet (London, England) 2011;378(9807):1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 12.Hotte SJ, Bjarnason GA, Heng DY, Jewett MA, Kapoor A, Kollmannsberger C, et al. Progression-free survival as a clinical trial endpoint in advanced renal cell carcinoma. Current Oncology (Toronto, Ont.) 2011;18(Suppl 2):S11–S19. doi: 10.3747/co.v18is2.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Groot S, Sleijfer S, Redekop WK, Oosterwijk E, Haanen JB, Kiemeney LA, et al. Variation in use of targeted therapies for metastatic renal cell carcinoma: Results from a Dutch population-based registry. BMC Cancer. 2016;16:364. doi: 10.1186/s12885-016-2395-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 15.Szende A, Oppe M, Devlin N, editors. EQ-5D value sets: Inventory, comparative review and user guide. 1. Dordrecht: Springer; 2007. [Google Scholar]
- 16.Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2002;20(1):289–296. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]
- 17.Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A, et al. The EORTC QLQ-C30 scoring manual. 3. New York: Wiley; 2001. [Google Scholar]
- 18.Versteegh MM, Vermeulen KM, Evers SM, de Wit GA, Prenger R, Stolk EA. Dutch tariff for the five-level version of EQ-5D. Value in Health. 2016;19(4):343–352. doi: 10.1016/j.jval.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Hedeker D, Gibbons RD. Longitudinal data analysis. New York: Wiley; 2006. [Google Scholar]
- 20.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Statistics in Medicine. 2011;30(4):377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 21.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273(1):59–65. doi: 10.1001/jama.1995.03520250075037. [DOI] [PubMed] [Google Scholar]
- 22.Escudier B, Porta C, Schmidinger M, Algaba F, Patard JJ, Khoo V, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology: Official Journal of the European Society for Medical Oncology/ESMO. 2014;25(Suppl 3):iii49–iii56. doi: 10.1093/annonc/mdu259. [DOI] [PubMed] [Google Scholar]
- 23.Cella D, Michaelson MD, Bushmakin AG, Cappelleri JC, Charbonneau C, Kim ST, et al. Health-related quality of life in patients with metastatic renal cell carcinoma treated with sunitinib vs interferon-alpha in a phase III trial: Final results and geographical analysis. British Journal of Cancer. 2010;102(4):658–664. doi: 10.1038/sj.bjc.6605552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson Coon J, Hoyle M, Green C, Liu Z, Welch K, Moxham T, et al. Bevacizumab, sorafenib tosylate, sunitinib and temsirolimus for renal cell carcinoma: A systematic review and economic evaluation. Health Technology Assessment. 2010;14(2):1–184. doi: 10.3310/hta14020. [DOI] [PubMed] [Google Scholar]
- 25.Remak E, Charbonneau C, Negrier S, Kim ST, Motzer RJ. Economic evaluation of sunitinib malate for the first-line treatment of metastatic renal cell carcinoma. Journal of Clinical Oncology: Official Journal Of The American Society of Clinical Oncology. 2008;26(24):3995–4000. doi: 10.1200/JCO.2007.13.2662. [DOI] [PubMed] [Google Scholar]
- 26.Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2006;24(1):16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 27.Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. The New England Journal of Medicine. 2013;369(8):722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.