Abstract
Purpose
Stress ulcer prophylaxis (SUP) is commonly prescribed in the intensive care unit. However, data from systematic reviews and conventional meta-analyses are limited by imprecision and restricted to direct comparisons. We conducted a network meta-analysis of randomized clinical trials (RCTs) to examine the safety and efficacy of drugs available for SUP in critically ill patients.
Methods
We searched MEDLINE, EMBASE, and the Cochrane Library Central Register of Controlled Trials through April 2017 for randomized controlled trials that examined the efficacy and safety of proton pump inhibitors (PPIs), histamine-2 receptor antagonists (H2RAs), and sucralfate for SUP in critically ill patients. No date or language restrictions were applied. Data on study characteristics, methods, outcomes, and risk of bias were abstracted by two reviewers.
Results
Of 96 potentially eligible studies, we included 57 trials enrolling 7293 patients. The results showed that PPIs are probably more effective for preventing clinically important gastrointestinal bleeding (CIB) than H2RAs [odds ratio (OR) 0.38; 95% confidence interval (95% CI) 0.20, 0.73], sucralfate (OR 0.30; 95% CI 0.13, 0.69), and placebo (OR 0.24; 95% CI 0.10, 0.60) (all moderate quality evidence). There were no convincing differences among H2RA, sucralfate, and placebo. PPIs probably increase the risk of developing pneumonia compared with H2RAs (OR 1.27; 95% CI 0.96, 1.68), sucralfate (OR 1.65; 95% CI 1.20, 2.27), and placebo (OR 1.52; 95% CI 0.95, 2.42) (all moderate quality). Mortality is probably similar across interventions (moderate quality). Estimates of baseline risks of bleeding varied significantly across studies, and only one study reported on Clostridium difficile infection. Definitions of pneumonia varied considerably. Most studies on sucralfate predate pneumonia prevention strategies.
Conclusions
Our results provide moderate quality evidence that PPIs are the most effective agents in preventing CIB, but they may increase the risk of pneumonia. The balance of benefits and harms leaves the routine use of SUP open to question.
Electronic supplementary material
The online version of this article (10.1007/s00134-017-5005-8) contains supplementary material, which is available to authorized users.
Keywords: Stress ulcers, Critical illness, Network meta-analysis, Proton pump inhibitors, Pneumonia, Histamine-2 receptor antagonists, Sucralfate
Introduction
Stress ulcer prophylaxis (SUP) is usual practice in intensive care units (ICUs) worldwide. Across North America, Europe, and Australia, most patients with risk factors receive SUP during their ICU stay [1, 2]. A survey of 58 ICUs in North America showed that proton pump inhibitors (PPIs) are the most commonly used agents, followed by histamine-2 receptor antagonists (H2RAs); none of the participating centers used sucralfate or anti-acids [1]. A recent survey of 97 centers in Europe, Australia, and Canada yielded similar findings [3]. These practices reflect the results of systematic reviews of randomized controlled trials (RCTs) suggesting that H2RAs, compared with sucralfate or no prophylaxis, reduce the risk of gastrointestinal (GI) bleeding without increasing the risk of pneumonia, and that PPIs in comparison with H2RAs further reduce the risk of clinically important GI bleeding (CIB) without increasing pneumonia risk. The recommendations of the Surviving Sepsis Campaign guidelines are consistent with these results [4–6].
Nevertheless, concerns have grown regarding the magnitude of benefit of SUP, as well as the safety of acid suppressive therapy with respect to pneumonia, Clostridium difficile infection, cardiovascular events, and mortality [7]. Conventional meta-analyses are restricted to head-to-head comparisons, and therefore cannot inform on the relative merit of candidate therapies that have not been compared directly. By including indirect comparisons, network meta-analyses can not only address this limitation but also—by combining direct and indirect estimates—improve precision [8].
We therefore conducted a network meta-analysis addressing the relative impact of SUP with PPI, H2RAs, sucralfate, and placebo (or no prophylaxis) on overt CIB, pneumonia, Clostridium difficile infection, and death.
Methods
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Extension statement for reporting network meta-analyses [Electronic Supplemental Material (ESM) Table 1] [9].
Data sources and searches
To identify RCTs comparing PPIs, H2RAs and sucralfate with one another and with placebo or no SUP in adult critically ill patients, we searched Cochrane CENTRAL, MEDLINE, and EMBASE from inception to April 2017 (ESM Table 2). We updated the search strategy for two systematic reviews of PPIs versus H2RA, and PPI versus placebo [6, 7], and conducted a complete search of the literature for other comparisons. We applied no restriction based on dose or route of drug administration or on language of publication. Eligible studies reported on at least one of the following: CIB, overt GI bleeding, pneumonia, mortality, and Clostridium difficile infection.
Study selection
Working in pairs, six reviewers screened citations and abstracts in duplicate and independently. The same pairs of reviewers evaluated all references judged potentially relevant for full-text eligibility.
Data extraction and quality assessment
Reviewers abstracted data in duplicate using piloted forms, and collected information on population demographics (age, sex, critical illness severity measure, ICU type, risk factors for bleeding), methodology and risk of bias, intervention and comparator (drug name, dose, route of administration, and duration of exposure), and outcomes. A third reviewer adjudicated disagreements not resolved by discussion.
We predefined CIB as evidence of upper GI bleeding with any of the following: significant hemodynamic changes not explained by other causes, need for transfusion of more than two units of blood, significant decrease in hemoglobin level, evidence of bleeding on GI endoscopy, or need for surgery to control the bleeding. Overt bleeding was defined as evidence of upper GI bleeding (hematemesis, melena, hematochezia, or coffee-grounds emesis or aspirate) regardless to other clinical findings. If an RCT only reported CIB, we considered all events as overt GI bleeding events. All studies used definitions consistent with those we prespecified.
We included pneumonia events in the ICU, whether or not they were associated with mechanical ventilation, accepting the definition used in each trial. We defined Clostridium difficile infection as a combination of clinical symptoms and a positive microbiologic test.
In duplicate, for each trial, reviewers assessed the risk of bias using the instrument recommended by the Cochrane Collaboration [10]. We provided a judgment of risk of bias as low (bias is not present or unlikely to alter the results seriously), unclear, or high (bias may seriously alter the results) for each of the following items: sequence generation, allocation sequence concealment, blinding of participants and clinicians, blinding of outcome assessment, incomplete outcome data assessment, and other bias. The overall risk of bias for each included trial was categorized as low if the risk of bias was low in all domains, unclear if the risk of bias was unclear in one or more domains and with no judgment of high risk of bias, or high if the risk of bias was high in one or more domains. We resolved disagreements by discussion.
Statistical analysis
Using a frequentist framework, we performed five random effects network meta-analyses, one for each outcome, calculating odds ratios (OR) and their corresponding 95% confidence intervals (CI). To evaluate inconsistency, we fit both a consistency and an inconsistency model for each outcome [11]. Using a node-splitting procedure [12], we calculated direct and indirect estimates for each pair of treatments. We calculated the frequentist analogue of the surface under the cumulative ranking curve (SUCRA) for each treatment (hereafter referred to as the SUCRA) [13]. All analyses were performed using the package mvmeta in Stata/IC 13.1 for Windows [14]. We assessed coherence (consistency) between direct and indirect estimates by performing node splitting and test for coherence [11]. We calculated the absolute treatment effect [risk difference (RD)] using the median event rate in the placebo arm in all trials for both clinically important GI bleeding and pneumonia outcomes. The median event rate in the placebo group was 2.1 and 6% for CIB and pneumonia outcomes; respectively.
Assessment of quality of evidence
For each outcome and each direct or indirect comparison, we applied the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess the quality of evidence [15, 16]. For rating quality in each indirect comparison, we focused on the first-order loop with the smallest variance which, therefore, contributed the most to the estimate of effect. We assigned quality of the indirect comparison according to the contributing direct comparison (of the two) within each first-order loop with the lowest of the quality rating. We considered rating down the quality of evidence further when intransitivity (clinical heterogeneity) was present, analogous to rating down the quality of evidence in direct comparison meta-analysis for indirectness [17]. For network estimates of any paired comparison, we chose the highest quality rating amongst the direct and indirect comparisons. We rated down quality in the network estimate if incoherence between direct and indirect estimates was present. In a network meta-analysis, incoherence represents the differences between the direct and indirect estimates of effect, while heterogeneity is the differences in estimates of effect across studies that assessed the same comparison [17].
Role of the funding source
The funders of this study did not contribute to its design or conduction. The authors were entirely responsible for data collection, analysis, interpretation and reporting. The corresponding author had access to all the data and final responsibility to submit for publication.
Results
Literature search
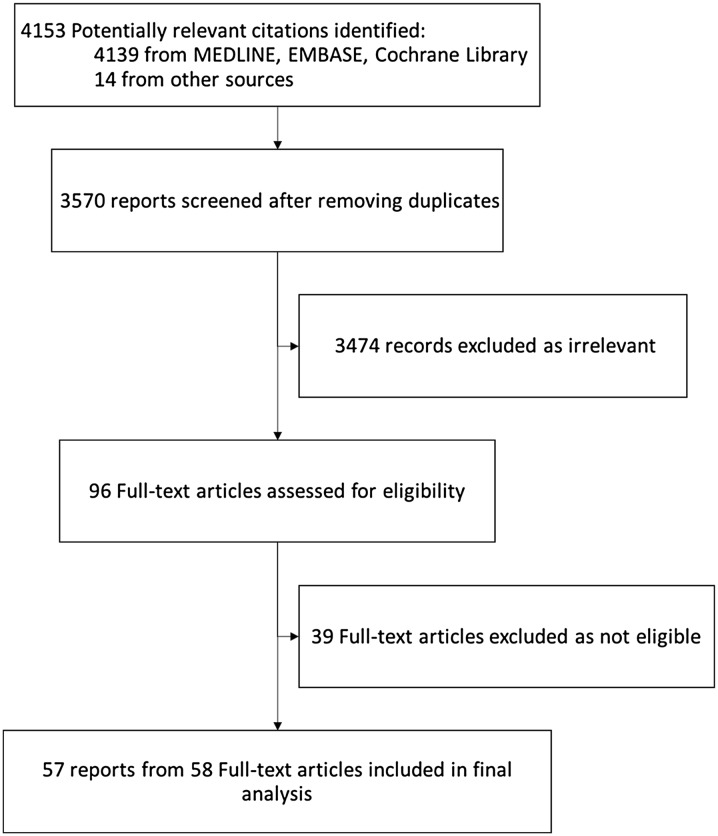
The initial search yielded 4139 citations; 93 proved potentially eligible after reviewing abstracts, of which 57 trials from 58 reports, representing 7293 patients, ultimately proved eligible (Fig. 1, ESM Table 2) [18–75].
Fig. 1.
Flow diagram of the search results. This figure shows the process of selecting eligible studies. Overall, we included 57 randomized clinical trials from 58 reports
Study characteristics
Of the 57 eligible trials, 18 compared PPIs with H2RAs; two, PPIs with sucralfate; four, PPIs with placebo; 18, H2RAs with sucralfate; 21, H2RAs with placebo; and six, sucralfate with placebo (ESM Figs. S1–S4). Trial sample size ranged from 28 to 1200 patients. Different doses, routes of administration, and durations of prophylaxis were used for PPIs and H2RAs across the trials (ESM Tables 3, 4).
Risk of bias
The risk of bias was high in 30 trials, low in 16 trials, and unclear in 11 trials (ESM Table 5).
Quality of evidence
Direct comparisons often suffered from limitations of risk of bias and imprecision. Regarding transitivity, although the intervention dosing and route varied across trials, the variation was not large enough to warrant concerns regarding intransitivity. Details can be found in Table 1 and ESM Table 5. For all comparisons across all outcomes, there was no statistical evidence of incoherence. Although no incoherence was detected by statistical testing, the test for incoherence could be underpowered; therefore, we further assessed incoherence by visually inspecting the direct and indirect estimates for any obvious differences. We considered problematic incoherence present for two comparisons based on visual inspection of direct and indirect estimates (Table 1).
Table 1.
Direct, indirect and network meta-analysis estimates of the odds ratios of the effects of different prophylaxis comparisons
| Comparison | RCTs | Direct estimate (95% CI) conventional MA | Direct estimate (95% CI) from node splitting | Quality | Indirect estimate (95% CI) | Qualityd | NMA estimate (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|
| Clinically important bleeding | ||||||||
| H2RA vs placebo | 7 | 0.52 (0.21, 1.33) | 0.53 (0.23, 1.19) | Moderatea | 1.36 (0.29, 6.51) | Lowe | 0.64 (0.32, 1.30) | Moderateh |
| PPI vs H2RA | 14 | 0.37 (0.20, 0.68) | 0.35 (0.18, 0.69) | Moderatec | 0.86 (0.11, 7.02) | Lowe | 0.38 (0.20, 0.73) | Moderateh |
| H2RA vs sucralfate | 12 | 0.79 (0.49, 1.27) | 0.86 (0.48, 1.55) | Moderatea | 0.32 (0.04, 2.67) | Lowe | 0.80 (0.46, 1.40) | Moderateh |
| PPI vs placebo | 4 | 0.67 (0.12, 3.59) | 0.66 (0.12, 3.74) | Lowb | 0.17 (0.06, 0.49) | Moderatef | 0.24 (0.10, 0.60) | Moderateh |
| Sucralfate vs placebo | 4 | 1.13 (0.44, 2.90) | 1.15 (0.41, 3.23) | Lowb | 0.48 (0.14, 1.64) | Moderatef | 0.80 (0.37, 1.73) | Lowh,i |
| PPI vs sucralfate | 1 | 0.31 (0.03, 3.05) | 0.23 (0.02, 2.30) | Lowb | 0.32 (0.13, 0.76) | Moderateg | 0.30 (0.13, 0.69) | Moderateh |
| Pneumonia | ||||||||
| H2RA vs placebo | 8 | 1.11 (0.61, 2.00) | 1.09 (0.70, 1.71) | Moderatea | 1.94 (0.73, 5.20) | Lowf,g | 1.19 (0.80, 1.78) | Moderateh |
| PPI vs H2RA | 13 | 1.15 (0.83, 1.59) | 1.15 (0.85, 1.57) | Moderatea | 2.10 (1.04, 4.21) | Moderateg | 1.27 (0.96, 1.68) | Moderateh |
| H2RA vs sucralfate | 16 | 1.36 (1.03, 1.79) | 1.32 (0.98, 1.77) | Moderatec | 1.35 (0.64, 2.86) | Lowf,g | 1.30 (1.08, 1.58) | Moderatej |
| PPI vs placebo | 3 | 1.53 (0.56, 4.16) | 1.48 (0.55, 3.99) | Lowa,c | 1.53 (0.90, 2.59) | Moderateg | 1.52 (0.95, 2.42) | Moderatej |
| Placebo vs sucralfate | 4 | 0.65 (0.34, 1.26) | 0.67 (0.34, 1.32) | Lowa,c | 1.54 (0.84, 2.80) | Moderateg | 1.09 (0.72, 1.66) | Lowh,i |
| PPI vs sucralfate | 4 | 2.37 (1.28, 4.42) | 2.16 (1.24, 3.77) | Moderatec | 1.44 (0.97, 2.14) | Moderateg | 1.65 (1.20, 2.27) | Moderatej |
| Mortality | ||||||||
| H2RA vs placebo | 17 | 0.95 (0.71, 1.26) | 0.95 (0.73, 1.25) | Moderatea | 1.04 (0.62, 1.73) | Moderatef | 0.97 (0.77, 1.23) | Moderateh |
| H2RA vs PPI | 11 | 0.86 (0.63, 1.17) | 0.86 (0.63, 1.17) | Moderatea | 0.75 (0.41, 1.37) | Moderatef | 0.83 (0.63, 1.10) | Moderateh |
| Sucralfate vs H2RA | 12 | 0.95 (0.79, 1.16) | 0.95 (0.78, 1.15) | Moderatea | 1.17 (0.53, 2.62) | Moderatef | 0.96 (0.79, 1.16) | Moderateh |
| Placebo vs PPI | 4 | 0.77 (0.47, 1.24) | 0.77 (0.47, 1.24) | Moderatea | 0.94 (0.61, 1.44) | Moderatef | 0.86 (0.62, 1.18) | Moderateh |
| Sucralfate vs placebo | 6 | 0.98 (0.66, 1.47) | 0.99 (0.66, 1.49) | Moderatea | 0.88 (0.60, 1.28) | Moderatef | 0.93 (0.71, 1.23) | Moderateh |
| Sucralfate vs PPI | 1 | 0.96 (0.42, 2.23) | 0.96 (0.41, 2.22) | Lowb | 0.77 (0.55, 1.10) | Moderatef | 0.80 (0.58, 1.10) | Moderateh |
CI confidence interval, H2RA histamine 2 receptor antagonists, MA meta-analysis, NMA network meta-analysis, PPI proton pump inhibitors, RCTs randomized controlled trials
aQuality of evidence for direct estimate rated down by one level for serious imprecision
bQuality of evidence for direct estimate rated down by two levels for very serious imprecision
cQuality of evidence for direct estimate rated down by one level for serious risk of bias
dWe did not downgrade for intransitivity in any of the indirect comparisons
eQuality of evidence for indirect estimate rated down by two level for very serious imprecision
fQuality of evidence for indirect estimate rated down by one level for serious imprecision
gQuality of evidence for indirect estimate was rated down by one level for risk of bias
hQuality of evidence for network estimate rated down by one level for serious imprecision
iQuality of evidence for network estimate rated down by one level for serious incoherence
jQuality of evidence for network estimate rated down by one level for serious risk of bias
The quality of evidence of network estimates for various comparisons across all outcomes ranged from low to moderate (Table 1).
Clinical outcomes
Clinically important GI bleeding
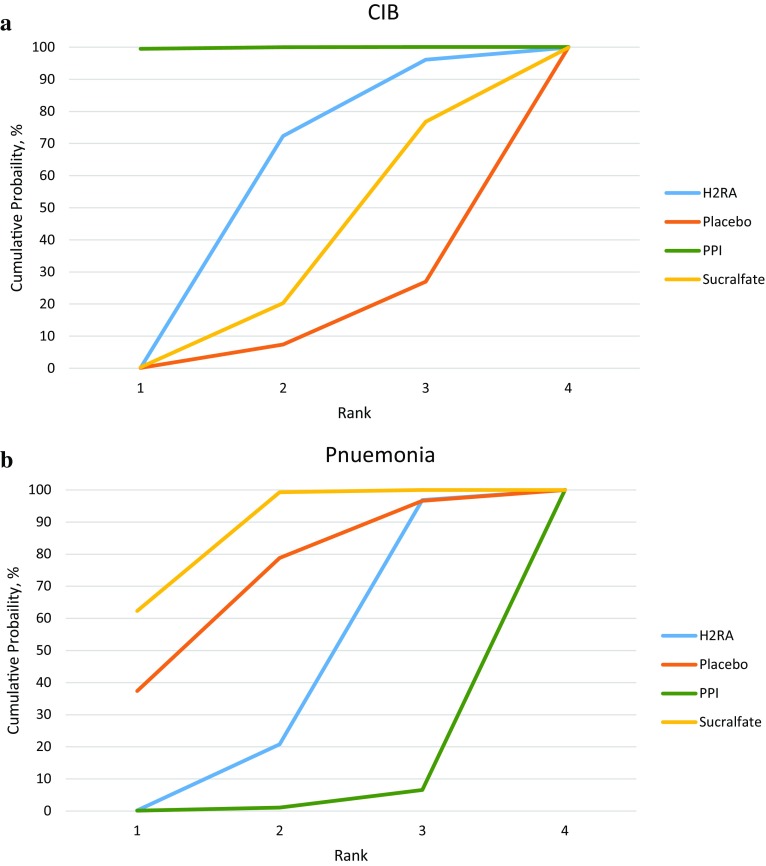
Thirty-one RCTs (5283 patients) reported on CIB [21, 25, 26, 30–32, 35, 36, 38–40, 42–44, 46–48, 57–59, 61, 63–67, 69–72, 74]. For three comparisons, the network estimate provided moderate-quality evidence with a CI excluding 1.0: PPIs versus H2RAs (OR 0.38; 95% CI 0.20, 0.73), PPIs versus no prophylaxis or placebo (OR 0.24; 95% CI 0.10, 0.60), and PPIs versus sucralfate (OR 0.30; 95% CI 0.13, 0.69). (Table 1, Fig. 2a). The SUCRA statistic showed that PPIs ranked first, followed by H2RAs, sucralfate, and placebo (ESM Table 6, Fig. 3a).
Fig. 2.
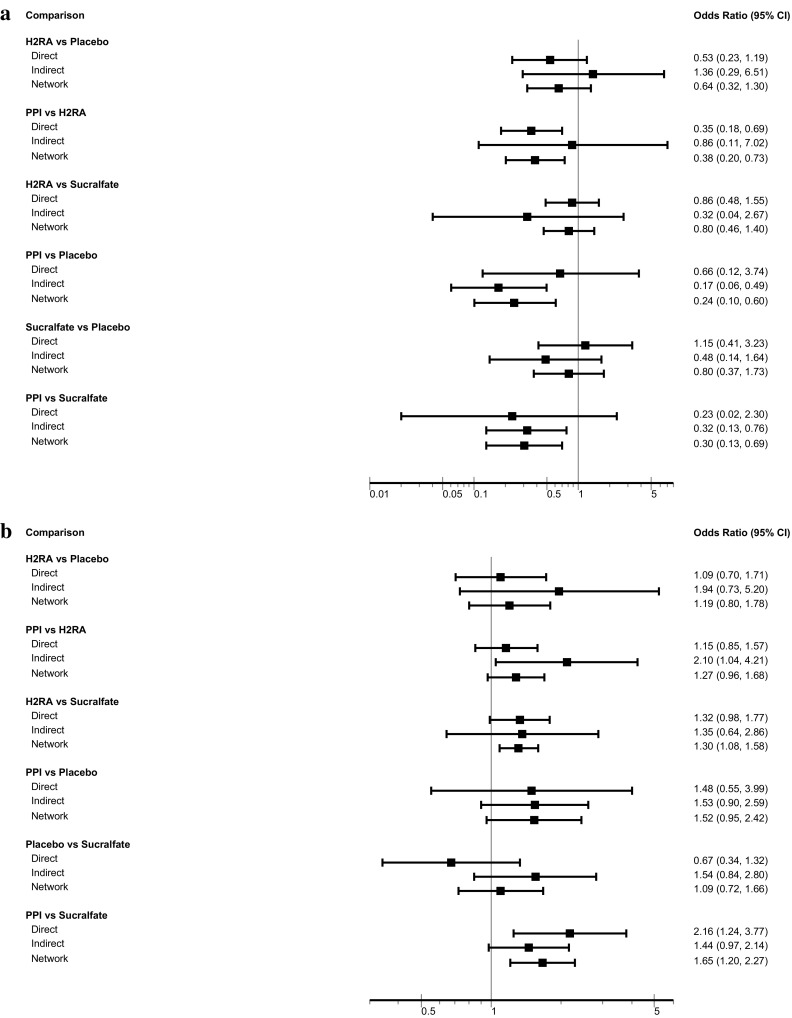
a Clinically important bleeding outcome. b Pneumonia outcome. a Test for inconsistency: p = 0.889 (indicating not inconsistent). b Test for inconsistency: p = 0.794 (indicating not inconsistent). CI confidence interval, H2RA histamine 2 receptor antagonists, PPI proton pump inhibitors
Fig. 3.
a Cumulative ranking curve for clinically important bleeding outcome. CIB clinically important bleeding, H2RA histamine 2 receptor antagonists, PPI proton pump inhibitors. b Cumulative ranking curve for pneumonia outcome. H2RA histamine 2 receptor antagonists, PPI proton pump inhibitors
Pneumonia
Thirty-five RCTs (5452 patients) reported on pneumonia [18, 19, 21, 22, 25, 26, 28–30, 32, 36, 37, 39–42, 44, 46–49, 51–54, 57, 58, 60, 61, 65–68, 70, 71]. PPIs ranked last compared with other interventions in terms of risk of pneumonia (ESM Table 7, Fig. 3b). Network meta-analysis results showed that PPIs and H2RAs probably increase the risk of pneumonia compared with sucralfate (OR 1.65; 95% CI 1.20, 2.27; moderate quality and OR 1.30; 95% CI 1.08, 1.58; moderate quality, respectively) but not compared with placebo or no prophylaxis (OR 1.09; 95% CI 0.72, 1.66; low quality). PPIs probably increase pneumonia compared with H2RAs (OR 1.27; 95% CI 0.96, 1.68; moderate quality), and no prophylaxis (OR 1.52; 95% CI 0.95, 2.42; moderate quality). H2RAs probably have no impact on pneumonia relative to placebo (Table 1, Fig. 2b).
Mortality
Thirty-six RCTs (5498 patients) reported on mortality [18, 19, 21, 23, 25, 26, 28, 29, 31, 34–40, 44, 46–51, 53, 56, 59–62, 64–66, 68–71, 75]. The results provide moderate-quality evidence that there is no difference between any of the management options in terms of all-cause mortality (Table 1, ESM Fig. S5). Given that all estimates were similar and approximated no effect, we did not calculate SUCRA values for the mortality outcome.
Clostridium difficile infection
Only one trial reported Clostridium difficile infection; therefore, we could not provide a network estimate for this outcome [66].
Overt GI bleeding
For overt GI bleeding, 49 studies (6662 patients) proved eligible [18, 20, 21, 23–36, 38–40, 42–75]. PPIs were superior to H2RAs (OR 0.34; 95% CI 0.19, 0.60, moderate quality), sucralfate (OR 0.35; 95% CI 0.18, 0.71, moderate quality), and placebo or no prophylaxis (OR 0.14; 95% CI 0.07, 0.28, moderate quality). H2RAs were superior to no prophylaxis in reducing risk of overt GI bleeding (OR 0.42; 95% CI 0.28, 0.63, moderate quality), but not sucralfate (OR 1.05; 95% CI 0.69, 1.59, moderate quality) (ESM Table 8 and Fig. S6). PPIs ranked first in reducing overt bleeding compared with other agents (ESM Table 9, Fig. S7).
Discussion
In this network meta-analysis, we included 57 RCTs enrolling 7293 patients comparing various SUP strategies. SUP with either PPIs or H2RAs likely reduces CIB (moderate quality evidence) (Table 1) relative to not using prophylaxis, and PPIs are likely superior to both H2RAs and sucralfate. Given the current estimates of the frequency of CIB, the magnitude of benefit is not large.
On the other hand, PPIs likely result in a higher risk of pneumonia than other prophylaxis regimens or no prophylaxis (moderate-quality evidence) (Table 1). These results are consistent with indirect evidence from multiple observational studies that have reported an increased risk of community- and hospital-acquired pneumonia in patients using PPIs [76–78]. Further direct supporting evidence comes from a retrospective observational study that reported a higher risk of pneumonia in critically ill patients on PPIs than for those on H2RAs in the ICU [79].
Low-quality evidence suggests that sucralfate may reduce pneumonia in comparison with placebo. This finding—together with the moderate-quality evidence of a lower pneumonia risk with sucralfate than PPIs—is consistent with prior physiologic and microbiologic data establishing the antibacterial effect of sucralfate, independently of the gastric pH level [80–82]. If the point estimate for pneumonia is accurate, PPIs relative to no SUP would result in a substantial increase in pneumonia.
The use of indirect comparisons within this network meta-analysis adds additional information beyond the multiple direct comparison meta-analyses that have compared PPIs, H2RAs, and sucralfate with placebo and with one another [6, 7, 83–87]. First, moderate-quality indirect evidence supports the previously observed effect of PPIs decreasing CIB relative to no SUP and relative to other agents (Table 1). Second, our results provide additional moderate quality evidence in support of the hypothesis that PPIs may increase the incidence of pneumonia relative to H2RAs, to sucralfate, and to no SUP. Prior systematic reviews were limited by imprecision and by the small number of studies for some direct comparisons, such as PPIs and placebo. Network meta-analytic techniques allowed us to generate more reliable estimates for these comparisons, particularly for the pneumonia outcome. Conventional meta-analyses are restricted to direct comparisons and do not consider indirect evidence in situations where evidence is sparse.
We have thus far focused on estimates of relative effects. In considering the implications of our results for use of SUP, consideration of absolute effects is crucial. The best estimates of the impact of PPIs on CIB suggest an absolute reduction of 1.6% relative to placebo, with a CI of 0.8–1.9% (Table 2). Similar estimates for pneumonia suggest an absolute increase of 3.1%, with a CI ranging from 0.3% fewer events to 8.5% more events. These estimates raise serious questions about the net benefit of PPIs for SUP.
Table 2.
Absolute treatment effect for clinically important bleeding and pneumonia outcomes
| Clinically important GI bleeding | ||
|---|---|---|
| Comparison | RD per 1000 patients (95% CI) for ACR 2.1 for placebo%a | Number needed to treat |
| H2RA vs placebo | 8 fewer per 1000 (6 more to 14 fewer) | 13 |
| PPI vs H2RA | 8 fewer per 1000 (from 4 fewer to 10 fewer) | 13 |
| H2RA vs sucralfate | 3 fewer per 1000 (from 7 more to 9 fewer) | 33 |
| PPI vs placebo | 16 fewer per 1000 (from 8 fewer to 19 fewer) | 6 |
| Sucralfate vs placebo | 4 fewer per 1000 (from 13 fewer to 15 more) | 25 |
| PPI vs sucralfate | 12 fewer per 1000 (from 6 fewer to 15 fewer) | 8 |
| Pneumonia outcome | ||
|---|---|---|
| Comparison | RD per 1000 patients (95% CI) for ACR 6% in placebob | Number needed to harm |
| H2RA vs placebo | 11 more per 1000 (from 12 fewer to 42 more) | 9 |
| PPI vs H2RA | 19 more per 1000 (from 3 fewer to 48 more) | 5 |
| H2RA vs sucralfate | 17 more per 1000 (from 4 more to 32 more) | 5 |
| PPI vs placebo | 31 more per 1000 (from 3 fewer to 85 more) | 3 |
| Placebo vs sucralfate | 5 more per 1000 (from 15 fewer to 36 more) | 20 |
| PPI vs sucralfate | 36 more per 1000 (from 11 more to 70 more) | 3 |
RD risk difference, ACR assumed control event rate, H2RA histamine-2 receptor antagonists, PPI proton pump inhibitor, GI gastrointestinal
aThe median event rate of clinically important bleeding across all trials in placebo arm was 2.1%
bThe median event rate of pneumonia across all trials in placebo arm was 6%
The strengths of this work include a comprehensive search, duplicate review of trial eligibility and risk of bias, and adherence to the PRISMA guidelines for reporting network meta-analyses (ESM Table 1) [9]. We adopted a frequentist (likelihood maximization) rather than a Bayesian approach to combine the results, avoiding issues relating to pre-specification of variance [88]. We used the node-splitting technique to provide estimates of indirect evidence, and applied the GRADE approach to assess the quality of evidence for each outcome, including specification of quality of direct, indirect, and network estimates [15]. To enhance the generalizability of our results, we included studies of patients with a wide spectrum of critical illness. We used best current estimates of effect for baseline risk to derive likely absolute effects of SUP on serious bleeding and pneumonia.
This study also has limitations. Although most RCTs used microbiological, radiological and clinical criteria to diagnose pneumonia, there was considerable variation in definitions [89]. Estimates of baseline risk vary appreciably across trials. In addition, most trials examining sucralfate predate the effective pneumonia prevention strategies, attenuating the applicability of these results to current practice. Only one trial addressed Clostridium difficile infection, which is important given a recent 30,000-patient retrospective observational study suggesting that PPIs may increase the risk of Clostridium difficile colitis [90]. Furthermore, the majority of trials did not report on nutritional management, which limited our ability to examine the effect of enteral nutrition as an effect modifier.
The universal use of SUP should be reconsidered in the light of uncertain net benefit. Two large multicenter RCTs are ongoing, the SUP-ICU in Europe (clinicaltrials.gov registration NCT02467621) and the REVISE Trial in North America, Australia, and Saudi Arabia. The results of these trials will provide further information about the safety of withholding stress ulcer prophylaxis in the ICU.
Conclusion
Our results provide moderate-quality evidence that PPIs are the most effective prophylactic strategy for SUP, with an absolute risk reduction in CIB relative to no prophylaxis of 1.6%. The benefit of PPIs must, however, be weighed against the risk of pneumonia, and possibly of Clostridium difficile infection. Moderate-quality evidence provides a best estimate of the increase in pneumonia with PPIs relative to no prophylaxis of over 3%. These estimates raise serious questions regarding the net benefit of SUP and its widespread use. The findings of this network meta-analysis can inform clinicians who consider the local incidence of CIB, pneumonia, and Clostridium difficile infection when deciding on uniform, selective, or sparing use of SUP in critically ill patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with ethical standards
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Funding
This study was funded by the Hamilton Chapter of the Canadian Intensive Care Foundation, the Critical Care Medicine Residency Program, and the Critical Care Division Alternate Funding Plan at McMaster University. Waleed Alhazzani holds a McMaster University Department of Medicine Internal Career Research Award. Deborah Cook is a Canada Research Chair of the Canadian Institutes for Health Research.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s00134-017-5005-8) contains supplementary material, which is available to authorized users.
A correction to this article is available online at https://doi.org/10.1007/s00134-017-5023-6.
References
- 1.Barletta JF, Kanji S, MacLaren R, Lat I, Erstad BL, American–Canadian consortium for Intensive care Drug utilization I Pharmacoepidemiology of stress ulcer prophylaxis in the United States and Canada. J Crit Care. 2014;29:955–960. doi: 10.1016/j.jcrc.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 2.Krag M, Perner A, Wetterslev J, Wise MP, Borthwick M, Bendel S, McArthur C, Cook D, Nielsen N, Pelosi P, Keus F, Guttormsen AB, Moller AD, Moller MH, Co-authors S-I. Prevalence and outcome of gastrointestinal bleeding and use of acid suppressants in acutely ill adult intensive care patients. Intensive Care Med. 2015;41:833–845. doi: 10.1007/s00134-015-3725-1. [DOI] [PubMed] [Google Scholar]
- 3.Krag M, Perner A, Wetterslev J, Wise MP, Borthwick M, Bendel S, McArthur C, Cook D, Nielsen N, Pelosi P, Keus F, Guttormsen AB, Moller AD, Moller MH, Collaborators S-I. Stress ulcer prophylaxis in the intensive care unit: an international survey of 97 units in 11 countries. Acta Anaesthesiol Scand. 2015;59:576–585. doi: 10.1111/aas.12508. [DOI] [PubMed] [Google Scholar]
- 4.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including The Pediatric S Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche J-D, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent J-L, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: International Guidelines for Management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 6.Alhazzani W, Alenezi F, Jaeschke RZ, Moayyedi P, Cook DJ. Proton pump inhibitors versus histamine 2 receptor antagonists for stress ulcer prophylaxis in critically ill patients: a systematic review and meta-analysis. Crit Care Med. 2013;41:693–705. doi: 10.1097/CCM.0b013e3182758734. [DOI] [PubMed] [Google Scholar]
- 7.Krag M, Perner A, Wetterslev J, Wise MP, Hylander Moller M. Stress ulcer prophylaxis versus placebo or no prophylaxis in critically ill patients. A systematic review of randomised clinical trials with meta-analysis and trial sequential analysis. Intensive Care Med. 2014;40:11–22. doi: 10.1007/s00134-013-3125-3. [DOI] [PubMed] [Google Scholar]
- 8.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 9.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catala-Lopez F, Gotzsche PC, Dickersin K, Boutron I, Altman DG, Moher D. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods G, Cochrane Statistical Methods G. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods. 2012;3:111–125. doi: 10.1002/jrsm.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29:932–944. doi: 10.1002/sim.3767. [DOI] [PubMed] [Google Scholar]
- 13.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 14.White IR. Multivariate random-effects meta-analysis. Stata J. 2009;9:40–56. [Google Scholar]
- 15.Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Puhan MA, Schunemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, Kessels AG, Guyatt GH, Group GW A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. doi: 10.1136/bmj.g5630. [DOI] [PubMed] [Google Scholar]
- 17.Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Falck-Ytter Y, Jaeschke R, Vist G, Akl EA, Post PN, Norris S, Meerpohl J, Shukla VK, Nasser M, Schunemann HJ, Group GW GRADE guidelines: 8. Rating the quality of evidence–indirectness. J Clin Epidemiol. 2011;64:1303–1310. doi: 10.1016/j.jclinepi.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Apte NM, Karnad DR, Medhekar TP, Tilve GH, Morye S, Bhave GG. Gastric colonization and pneumonia in intubated critically ill patients receiving stress ulcer prophylaxis: a randomized, controlled trial. Crit Care Med. 1992;20:590–593. doi: 10.1097/00003246-199205000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Bashar FR, Manuchehrian N, Mahmoudabadi M, Hajiesmaeili MR, Torabian S. Effects of ranitidine and pantoprazole on ventilator-associated pneumonia: a randomized double-blind clinical trial. Tanaffos. 2013;12:16–21. [PMC free article] [PubMed] [Google Scholar]
- 20.Basso N, Bagarani M, Materia A, Fiorani S, Lunardi P, Speranza V. Cimetidine and antacid prophylaxis of acute upper gastrointestinal bleeding in high risk patients. Controlled, randomized trial. Am J Surg. 1981;141:339–341. doi: 10.1016/0002-9610(81)90191-4. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Menachem T, Fogel R, Patel RV, Touchette M, Zarowitz BJ, Hadzijahic N, Divine G, Verter J, Bresalier RS. Prophylaxis for stress-related gastric hemorrhage in the medical intensive care unit. A randomized, controlled, single-blind study. Ann Intern Med. 1994;121:568–575. doi: 10.7326/0003-4819-121-8-199410150-00003. [DOI] [PubMed] [Google Scholar]
- 22.Bhanot RD (2010) Nosocomial pneumonia in mechanically ventilated patients receiving ranitidine, omeprazole or sucralfate as stress ulcer prophylaxis. American Thoracic Society International Conference, ATS 2010 New Orleans 181 (1 Meeting Abstracts)
- 23.Burgess P, Larson GM, Davidson P, Brown J, Metz CA. Effect of ranitidine on intragastric pH and stress-related upper gastrointestinal bleeding in patients with severe head injury. Dig Dis Sci. 1995;40:645–650. doi: 10.1007/BF02064385. [DOI] [PubMed] [Google Scholar]
- 24.Cannon LA, Heiselman D, Gardner W, Jones J. Prophylaxis of upper gastrointestinal tract bleeding in mechanically ventilated patients. A randomized study comparing the efficacy of sucralfate, cimetidine, and antacids. Arch Intern Med. 1987;147:2101–2106. doi: 10.1001/archinte.1987.00370120037009. [DOI] [PubMed] [Google Scholar]
- 25.Conrad SA, Gabrielli A, Margolis B, Quartin A, Hata JS, Frank WO, Bagin RG, Rock JA, Hepburn B, Laine L. Randomized, double-blind comparison of immediate-release omeprazole oral suspension versus intravenous cimetidine for the prevention of upper gastrointestinal bleeding in critically ill patients. Crit Care Med. 2005;33:760–765. doi: 10.1097/01.CCM.0000157751.92249.32. [DOI] [PubMed] [Google Scholar]
- 26.Cook D, Guyatt G, Marshall J, Leasa D, Fuller H, Hall R, Peters S, Rutledge F, Griffith L, McLellan A, Wood G, Kirby A. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. N Engl J Med. 1998;338:791–797. doi: 10.1056/NEJM199803193381203. [DOI] [PubMed] [Google Scholar]
- 27.Darlong V, Jayalakhsmi TS, Kaul HL, Tandon R. Stress ulcer prophylaxis in patients on ventilator. Trop Gastroenterol. 2003;24:124–128. [PubMed] [Google Scholar]
- 28.De Azevedo JRA, Soares MDGA, Silva GAE, De Lima Palacio G. Prevention of stress ulcer bleeding in high risk patients. Comparison of three drugs. GED Gastrenterol Endosco Dig. 2000;19:239–244. [Google Scholar]
- 29.Eddleston JM, Pearson RC, Holland J, Tooth JA, Vohra A, Doran BH. Prospective endoscopic study of stress erosions and ulcers in critically ill adult patients treated with either sucralfate or placebo. Crit Care Med. 1994;22:1949–1954. doi: 10.1097/00003246-199422120-00010. [DOI] [PubMed] [Google Scholar]
- 30.Fabian TC, Boucher BA, Croce MA, Kuhl DA, Janning SW, Coffey BC, Kudsk KA. Pneumonia and stress ulceration in severely injured patients. A prospective evaluation of the effects of stress ulcer prophylaxis. Arch Surg. 1993;128:185–191. doi: 10.1001/archsurg.1993.01420140062010. [DOI] [PubMed] [Google Scholar]
- 31.Fink M, Karlstadt RG, Maroko RT, Field B. Intravenous pantoprazole (IVP) and continuous infusion cimetidine (C) prevent upper gastrointestinal bleeding (UGIB) regardless of APSII score (APACHE II) in high risk intensive care unit (ICU) patients. Gastroenterology. 2003;124:A625–A626. doi: 10.1016/S0016-5085(03)83170-0. [DOI] [Google Scholar]
- 32.Fogas JF, Kiss KK, Gyura FG, Tobias ZT, Molnar ZM, (2013) Effects of proton pump inhibitor versus H2-receptor antagonist stress ulcer prophylaxis on ventilator-associated pneumonia: a pilot study. 33rd International Symposium on Intensive Care and Emergency Medicine Brussels Belgium 17:S150–S151
- 33.Friedman CJ, Oblinger MJ, Suratt PM, Bowers J, Goldberg SK, Sperling MH, Blitzer AH. Prophylaxis of upper gastrointestinal hemorrhage in patients requiring mechanical ventilation. Crit Care Med. 1982;10:316–319. [PubMed] [Google Scholar]
- 34.Groll A, Simon JB, Wigle RD, Taguchi K, Todd RJ, Depew WT. Cimetidine prophylaxis for gastrointestinal bleeding in an intensive care unit. Gut. 1986;27:135–140. doi: 10.1136/gut.27.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halloran LG, Zfass AM, Gayle WE, Wheeler CB, Miller JD. Prevention of acute gastrointestinal complications after severe head injury: a controlled trial of cimetidine prophylaxis. Am J Surg. 1980;139:44–48. doi: 10.1016/0002-9610(80)90228-7. [DOI] [PubMed] [Google Scholar]
- 36.Hanisch EW, Encke A, Naujoks F, Windolf J. A randomized, double-blind trial for stress ulcer prophylaxis shows no evidence of increased pneumonia. Am J Surg. 1998;176:453–457. doi: 10.1016/S0002-9610(98)00239-6. [DOI] [PubMed] [Google Scholar]
- 37.Harlaftis N, Basdanis G, Papapolychroniadis C, Prousalidis J, Apostolidis S, Kosmidis C, Avramidou E, Katsohis C. Nosocomial pneumonia in mechanically ventilated patients during stress ulcer prophylaxis with sucralfate and ranitidine. Hell J Gastroenterol. 1997;10:230–235. [Google Scholar]
- 38.Hata M, Shiono M, Sekino H, Furukawa H, Sezai A, Iida M, Yoshitake I, Hattori T, Wakui S, Soeda M, Taoka M, Negishi N, Sezai Y. Prospective randomized trial for optimal prophylactic treatment of the upper gastrointestinal complications after open heart surgery. Circ J. 2005;69:331–334. doi: 10.1253/circj.69.331. [DOI] [PubMed] [Google Scholar]
- 39.Kantorova I, Svoboda P, Scheer P, Doubek J, Rehorkova D, Bosakova H, Ochmann J. Stress ulcer prophylaxis in critically ill patients: a randomized controlled trial. Hepatogastroenterology. 2004;51:757–761. [PubMed] [Google Scholar]
- 40.Karlstadt RG, Iberti TJ, Silverstein J, Lindenberg L, Rright-Asare P, Rockhold F, Young MD. Comparison of cimetidine and placebo for the prophylaxis of upper gastrointestinal bleeding due to stress-related gastric mucosal damage in the intensive care unit. J Intensive Care Med. 1990;5:26–32. doi: 10.1177/088506669000500106. [DOI] [Google Scholar]
- 41.Khorvash F, Abbasi S, Meidani M, Dehdashti F, Ataei B. The comparison between proton pump inhibitors and sucralfate in incidence of ventilator associated pneumonia in critically ill patients. Adv Biomed Res. 2014;3:52. doi: 10.4103/2277-9175.125789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kotlyanskaya A, Luka B, Mukherji R. A comparison of lansoprazole disintegrating tablet, lansoprazole suspension or ranitidine for stress ulcer prophylaxis in critically ill patients. Crit Care Med. 2008;7:A194. [Google Scholar]
- 43.Labattut AG, Santolalla PM, De Andres AP, Ortigosa AM, Del Mar M, Serrano G, Gimeno OL. Efficacy of sucralfate in the prevention of upper gastrointestinal stress bleeding in intensive care patients: comparison vs a control group. Clin Intensive Care. 1992;3:19–25. [Google Scholar]
- 44.Laggner AN, Lenz K, Base W, Druml W, Schneeweiss B, Grimm G. Prevention of upper gastrointestinal bleeding in long-term ventilated patients. Sucralfate versus ranitidine. Am J Med. 1989;86:81–84. doi: 10.1016/0002-9343(89)90164-2. [DOI] [PubMed] [Google Scholar]
- 45.Laggner AN, Lenz K, Graninger W, Gremmel F, Grimm G, Base W, Schneeweiss B, Sertl K. Stress ulcer prophylaxis in a general intensive care unit: sucralfate versus ranitidine. Anaesthesist. 1988;37:704–710. [PubMed] [Google Scholar]
- 46.Lee T-H, Hung F-M, Yang L-H. Comparison of the efficacy of esomeprazole and famotidine against stress ulcers in a neurosurgical intensive care unit. Adv Dig Med. 2014;1:50–53. doi: 10.1016/j.aidm.2013.06.001. [DOI] [Google Scholar]
- 47.Levy MJ, Seelig CB, Robinson NJ, Ranney JE. Comparison of omeprazole and ranitidine for stress ulcer prophylaxis. Dig Dis Sci. 1997;42:1255–1259. doi: 10.1023/A:1018810325370. [DOI] [PubMed] [Google Scholar]
- 48.Lin CC, Hsu YL, Chung CS, Lee TH. Stress ulcer prophylaxis in patients being weaned from the ventilator in a respiratory care center: a randomized control trial. J Formos Med Assoc. 2016;115:19–24. doi: 10.1016/j.jfma.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Liu BL, Li B, Zhang X, Fei Z, Hu SJ, Lin W, Gao DK, Zhang L. A randomized controlled study comparing omeprazole and cimetidine for the prophylaxis of stress-related upper gastrointestinal bleeding in patients with intracerebral hemorrhage. J Neurosurg. 2013;118:115–120. doi: 10.3171/2012.9.JNS12170. [DOI] [PubMed] [Google Scholar]
- 50.Macdougall BR, Bailey RJ, Williams R. H2-receptor antagonists and antacids in the prevention of acute gastrointestinal haemorrhage in fulminant hepatic failure. Two controlled trials. Lancet. 1977;1:617–619. doi: 10.1016/S0140-6736(77)92055-4. [DOI] [PubMed] [Google Scholar]
- 51.Martin LF, Booth FV, Karlstadt RG, Silverstein JH, Jacobs DM, Hampsey J, Bowman SC, D’Ambrosio CA, Rockhold FW. Continuous intravenous cimetidine decreases stress-related upper gastrointestinal hemorrhage without promoting pneumonia. Crit Care Med. 1993;21:19–30. doi: 10.1097/00003246-199301000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Metz CA, Livingston DH, Smith JS, Larson GM, Wilson TH. Impact of multiple risk factors and ranitidine prophylaxis on the development of stress-related upper gastrointestinal bleeding: a prospective, multicenter, double-blind, randomized trial. The Ranitidine Head Injury Study Group. Crit Care Med. 1993;21:1844–1849. doi: 10.1097/00003246-199312000-00010. [DOI] [PubMed] [Google Scholar]
- 53.Misra UK, Kalita J, Pandey S, Mandal SK, Srivastava M. A randomized placebo controlled trial of ranitidine versus sucralfate in patients with spontaneous intracerebral hemorrhage for prevention of gastric hemorrhage. J Neurol Sci. 2005;239:5–10. doi: 10.1016/j.jns.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 54.Mustafa NA, Akturk G, Ozen I, Koksal I, Erciyes N, Solak M. Acute stress bleeding prophylaxis with sucralfate versus ranitidine and incidence of secondary pneumonia in intensive care unit patients. Intensive Care Med. 1995;21:287. doi: 10.1007/BF01701491. [DOI] [PubMed] [Google Scholar]
- 55.Pan X, Zhang W, Li Z, Xu G, Yin N, Wang D, Shi X. The preventive effects of rabeprazole on upper gastrointestinal tract hemorrhage in patients with severe acute pancreatitis. Chin J Gastroenterol. 2004;9:30–32. [Google Scholar]
- 56.Peura DA, Johnson LF. Cimetidine for prevention and treatment of gastroduodenal mucosal lesions in patients in an intensive care unit. Ann Intern Med. 1985;103:173–177. doi: 10.7326/0003-4819-103-2-173. [DOI] [PubMed] [Google Scholar]
- 57.Phillips JO, Metzler MH, Huckfeldt RE, Olsen K. A multicenter, prospective, randomized clinical trial of continuous infusion IV ranitidine vs. omeprazole suspension in the prophylaxis of stress ulcers. Crit Care Med. 1998;26:101A. [Google Scholar]
- 58.Pickworth KK, Falcone RE, Hoogeboom JE, Santanello SA. Occurrence of nosocomial pneumonia in mechanically ventilated trauma patients: a comparison of sucralfate and ranitidine. Crit Care Med. 1993;21:1856–1862. doi: 10.1097/00003246-199312000-00012. [DOI] [PubMed] [Google Scholar]
- 59.Powell H, Morgan M, Li SK, Baron JH. Inhibition of gastric acid secretion in the intensive care unit after coronary artery bypass graft. A pilot control study of intravenous omeprazole by bolus and infusion, ranitidine and placebo. Theor Surg. 1993;8:125–130. [Google Scholar]
- 60.Prakash S, Rai A, Gogia AR, Prakash S. Nosocomial pneumonia in mechanically ventilated patients receiving ranitidine or sucralfate as stress ulcer prophylaxis. Indian J Anaesth. 2008;52:179–184. [Google Scholar]
- 61.Prod’hom G, Leuenberger P, Koerfer J, Blum A, Chiolero R, Schaller MD, Perret C, Spinnler O, Blondel J, Siegrist H, Saghafi L, Blanc D, Francioli P. Nosocomial pneumonia in mechanically ventilated patients receiving antacid, ranitidine, or sucralfate as prophylaxis for stress ulcer. A randomized controlled trial. Ann Intern Med. 1994;120:653–662. doi: 10.7326/0003-4819-120-8-199404150-00005. [DOI] [PubMed] [Google Scholar]
- 62.Reusser P, Gyr K, Scheidegger D, Buchmann B, Buser M, Zimmerli W. Prospective endoscopic study of stress erosions and ulcers in critically ill neurosurgical patients: current incidence and effect of acid-reducing prophylaxis. Crit Care Med. 1990;18:270–274. doi: 10.1097/00003246-199003000-00004. [DOI] [PubMed] [Google Scholar]
- 63.Risaliti A, Terrosu G, Uzzau A, Petri R, Intini S, Carcoforo P, Noce L, Anania G, Cedolini C, Soro P. Intravenous omeprazole vs ranitidine in the prophylaxis of stress ulcers. Acta Chirurgica Italica. 1993;49:397–401. [Google Scholar]
- 64.Ruiz-Santana S, Ortiz E, Gonzalez B, Bolanos J, Ruiz-Santana AJ, Manzano JL. Stress-induced gastroduodenal lesions and total parenteral nutrition in critically ill patients: frequency, complications, and the value of prophylactic treatment. A prospective, randomized study. Crit Care Med. 1991;19:887–891. doi: 10.1097/00003246-199107000-00011. [DOI] [PubMed] [Google Scholar]
- 65.Ryan P, Dawson J, Teres D, Celoria G, Navab F. Nosocomial pneumonia during stress ulcer prophylaxis with cimetidine and sucralfate. Arch Surg. 1993;128:1353–1357. doi: 10.1001/archsurg.1993.01420240061011. [DOI] [PubMed] [Google Scholar]
- 66.Selvanderan SP, Summers MJ, Finnis ME, Plummer MP, Ali Abdelhamid Y, Anderson MB, Chapman MJ, Rayner CK, Deane AM. Pantoprazole or placebo for stress ulcer prophylaxis (POP-UP): randomized double-blind exploratory study. Crit Care Med. 2016;44:1842–1850. doi: 10.1097/CCM.0000000000001819. [DOI] [PubMed] [Google Scholar]
- 67.Simms HH, De Maria E, McDonald L, Peterson D, Robinson A, Burchard KW. Role of gastric colonization in the development of pneumonia in critically ill trauma patients: results of a prospective randomized trial. J Trauma. 1991;31:531–536. doi: 10.1097/00005373-199104000-00013. [DOI] [PubMed] [Google Scholar]
- 68.Solouki M, Mar’ashian SM, Koochak M, Nasiri A, Mokhtari M, Amirpour A. Ventilator-associated pneumonia among ICU patients receiving mechanical ventilation and prophylaxis of gastrointestinal bleeding. Iran J Clinical Infect Dis. 2009;4:177–180. [Google Scholar]
- 69.Solouki M, Marashian SM, Kouchak M, Mokhtari M, Nasiri E. Comparison between the preventive effects of ranitidine and omeprazole on upper gastrointestinal bleeding among ICU patients. Tanaffos. 2009;8:37–42. [Google Scholar]
- 70.Somberg L, Morris J, Jr, Fantus R, Graepel J, Field BG, Lynn R, Karlstadt R. Intermittent intravenous pantoprazole and continuous cimetidine infusion: effect on gastric pH control in critically ill patients at risk of developing stress-related mucosal disease. J Trauma. 2008;64:1202–1210. doi: 10.1097/TA.0b013e31815e40b5. [DOI] [PubMed] [Google Scholar]
- 71.Thomason MH, Payseur ES, Hakenewerth AM, Norton HJ, Mehta B, Reeves TR, Moore-Swartz MW, Robbins PI. Nosocomial pneumonia in ventilated trauma patients during stress ulcer prophylaxis with sucralfate, antacid, and ranitidine. J Trauma. 1996;41:503–508. doi: 10.1097/00005373-199609000-00020. [DOI] [PubMed] [Google Scholar]
- 72.Tryba M, Zevounou F, Grabhoefer P, Seifert V, Torok M. Prevention of acute stress hemorrhages of the upper gastrointestinal tract with pirenzepine and antacids. A controlled comparison between pirenzepine and cimetidine. Anasth Intensivther Notfallmed. 1984;19:240–244. doi: 10.1055/s-2007-1003446. [DOI] [PubMed] [Google Scholar]
- 73.van den Berg B, van Blankenstein M. Prevention of stress-induced upper gastrointestinal bleeding by cimetidine in patients on assisted ventilation. Digestion. 1985;31:1–8. doi: 10.1159/000199170. [DOI] [PubMed] [Google Scholar]
- 74.Wee B, Liu CH, Cohen H, Kravchuk S, Reddy K, Mukherji R. 731: IV Famotidine vs. IV Pantoprazole for stress ulcer prevention in the ICU: a prospective Study. Crit Care Med. 2013;41:A181. doi: 10.1097/01.ccm.0000439969.36301.c9. [DOI] [Google Scholar]
- 75.Zinner MJ, Zuidema GD, Smith P, Mignosa M. The prevention of upper gastrointestinal tract bleeding in patients in an intensive care unit. Surg Gynecol Obstet. 1981;153:214–220. [PubMed] [Google Scholar]
- 76.Giuliano C, Wilhelm SM, Kale-Pradhan PB. Are proton pump inhibitors associated with the development of community-acquired pneumonia? A meta-analysis. Expert Rev Clin Pharmacol. 2012;5:337–344. doi: 10.1586/ecp.12.20. [DOI] [PubMed] [Google Scholar]
- 77.Johnstone J, Nerenberg K, Loeb M. Meta-analysis: proton pump inhibitor use and the risk of community-acquired pneumonia. Aliment Pharmacol Ther. 2010;31:1165–1177. doi: 10.1111/j.1365-2036.2010.04284.x. [DOI] [PubMed] [Google Scholar]
- 78.Herzig SJ, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for hospital-acquired pneumonia. JAMA. 2009;301:2120–2128. doi: 10.1001/jama.2009.722. [DOI] [PubMed] [Google Scholar]
- 79.MacLaren R, Kassel LE, Kiser TH, Fish DN. Proton pump inhibitors and histamine-2 receptor antagonists in the intensive care setting: focus on therapeutic and adverse events. Expert Opin Drug Saf. 2015;14:269–280. doi: 10.1517/14740338.2015.986456. [DOI] [PubMed] [Google Scholar]
- 80.Tryba M, Mantey-Stiers F. Antibacterial activity of sucralfate in human gastric juice. Am J Med. 1987;83:125–127. doi: 10.1016/0002-9343(87)90841-2. [DOI] [PubMed] [Google Scholar]
- 81.Welage L, Carver P, Welch K. Antibacterial activity of sucralfate versus aluminum chloride in simulated gastric fluid. Eur J Clin Microbiol Infect Dis. 1994;13:1046–1052. doi: 10.1007/BF02111825. [DOI] [PubMed] [Google Scholar]
- 82.West AP, Abdul S, Sherratt MJ, Inglis TJ. Antibacterial activity of sucralfate against Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa in batch and continuous culture. Eur J Clin Microbiol Infect Dis. 1993;12:869–871. doi: 10.1007/BF02000412. [DOI] [PubMed] [Google Scholar]
- 83.Barkun AN, Adam V, Martel M, Bardou M. Cost-effectiveness analysis: stress ulcer bleeding prophylaxis with proton pump inhibitors, H2 receptor antagonists. Value Health. 2013;16:14–22. doi: 10.1016/j.jval.2012.08.2213. [DOI] [PubMed] [Google Scholar]
- 84.Marik PE, Vasu T, Hirani A, Pachinburavan M. Stress ulcer prophylaxis in the new millennium: a systematic review and meta-analysis. Crit Care Med. 2010;38:2222–2228. doi: 10.1097/CCM.0b013e3181f17adf. [DOI] [PubMed] [Google Scholar]
- 85.Huang J, Cao Y, Liao C, Wu L, Gao F. Effect of histamine-2-receptor antagonists versus sucralfate on stress ulcer prophylaxis in mechanically ventilated patients: a meta-analysis of 10 randomized controlled trials. Crit Care. 2010;14:R194. doi: 10.1186/cc9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alquraini M, Alshamsi F, Moller MH, Belley-Cote E, Almenawer S, Jaeschke R, MacLaren R, Alhazzani W. Sucralfate versus histamine 2 receptor antagonists for stress ulcer prophylaxis in adult critically ill patients: a meta-analysis and trial sequential analysis of randomized trials. J Crit Care. 2017;40:21–30. doi: 10.1016/j.jcrc.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 87.Alshamsi F, Belley-Cote E, Cook D, Almenawer SA, Alqahtani Z, Perri D, Thabane L, Al-Omari A, Lewis K, Guyatt G, Alhazzani W. Efficacy and safety of proton pump inhibitors for stress ulcer prophylaxis in critically ill patients: a systematic review and meta-analysis of randomized trials. Crit Care. 2016;20:120. doi: 10.1186/s13054-016-1305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lambert PC, Sutton AJ, Burton PR, Abrams KR, Jones DR. How vague is vague? A simulation study of the impact of the use of vague prior distributions in MCMC using WinBUGS. Stat Med. 2005;24:2401–2428. doi: 10.1002/sim.2112. [DOI] [PubMed] [Google Scholar]
- 89.Nair GB, Niederman MS. Ventilator-associated pneumonia: present understanding and ongoing debates. Intensive Care Med. 2015;41:34–48. doi: 10.1007/s00134-014-3564-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.MacLaren R, Reynolds PM, Allen RR. Histamine-2 receptor antagonists vs proton pump inhibitors on gastrointestinal tract hemorrhage and infectious complications in the intensive care unit. JAMA Intern Med. 2014;174:564–574. doi: 10.1001/jamainternmed.2013.14673. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.