Abstract
Contraction of skeletal muscle cells is initiated by a well-known signaling pathway. An action potential in a motor nerve triggers an action potential in a muscle cell membrane, a transient increase of intracellular calcium concentration, binding of calcium to troponin in the actin-containing thin filaments, and a structural change in the thin filaments that allows myosin motors from the thick filaments to bind to actin and generate force. This calcium/thin filament mediated pathway provides the “START” signal for contraction, but it is argued that the functional response of the muscle cell, including the speed of its contraction and relaxation, adaptation to the external load, and the metabolic cost of contraction is largely determined by additional mechanisms. This review considers the role of the thick filaments in those mechanisms, and puts forward a paradigm for the control of contraction in skeletal muscle in which both the thick and thin filaments have a regulatory function. The OFF state of the thick filament is characterized by helical packing of most of the myosin head or motor domains on the thick filament surface in a conformation that makes them unavailable for actin binding or ATP hydrolysis, although a small fraction of the myosin heads are constitutively ON. The availability of the majority fraction of the myosin heads for contraction is controlled in part by the external load on the muscle, so that these heads only attach to actin and hydrolyze ATP when they are required. This phenomenon seems to be the major determinant of the well-known force-velocity relationship of muscle, and controls the metabolic cost of contraction. The regulatory state of the thick filament also seems to control the dynamics of both muscle activation and relaxation.
Main Text
Functional requirements for the control of skeletal muscle
Skeletal muscles are the motors of the animal body, and are responsible for a wide range of its functions including posture, locomotion, breathing, and eye movements. The central nervous system (CNS) controls skeletal muscles through motor neurons that innervate a functional group of muscle cells—a motor unit. CNS control is essentially digital; each action potential in a motor neuron produces an action potential in its target muscle cells, acting as a “START” signal for contraction and eliciting a unitary mechanical response called a twitch. The CNS controls the strength of muscle contraction by changing the frequency of START pulses sent to a motor unit and by recruiting additional units. Although the motor neurons have inhibitory as well as excitatory inputs, there is no inhibitory innervation of the muscle cells themselves in vertebrates; the muscle cells do not receive “STOP” signals. The mechanical response to a START signal, including the speed of contraction and relaxation and the adaptation to external load, is determined by mechanisms intrinsic to the muscle cell.
Muscle contraction is powered by the hydrolysis of ATP and thus by cellular and whole-body metabolism. Skeletal muscle has high thermodynamic efficiency, converting about half the energy available from ATP hydrolysis into mechanical work in optimal conditions (1). Muscle strength can, therefore, only be increased by increasing muscle mass, with a corresponding increase in metabolic cost. In man, skeletal muscle mass is ∼40% of body mass, with a large contribution to whole body metabolism at rest, and a very high metabolic cost when activated. Control systems in skeletal muscle are likely to have evolved to optimize the ratio of performance to metabolic cost across the broad dynamic range of in vivo requirements from its OFF or resting state, in which the primary driver would be inhibition of ATP utilization, to its various ON states including steady muscle tone, rapid movements of the skeleton, and intense exercise. In addition to their fundamental role in the normal physiological performance of skeletal muscle, those control systems offer opportunities for therapeutic intervention in muscle weakness or disease and, through their dominant contribution to whole-body metabolism, in diabetes and obesity (2).
Molecular components of muscle contraction and its control
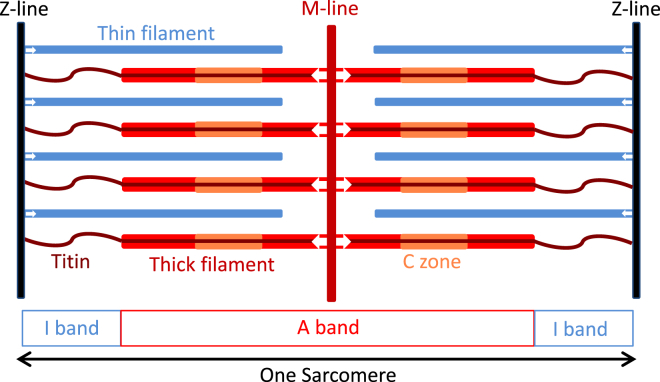
At the molecular level, muscle contraction is due to the relative sliding of two overlapping arrays of filaments, called the “thick” and “thin” filaments (Fig. 1). The thick filaments have the same length, close to 1.6 μm, in all vertebrate skeletal muscles and in the heart, and contain the same number of myosin monomers (3). Regular arrays of thick and thin filaments assemble during development into contractile units called “sarcomeres”, which are ∼2 μm long at the normal muscle length in vivo. Thick filaments are symmetrical about their midpoint at the M-line of the sarcomere, and thin filaments reverse polarity at the Z-line, so the fundamental functional unit of skeletal muscle is the half-sarcomere. The principal protein component of the thick filaments is the motor protein myosin. The thin filaments, of which the principal protein is actin, provide the track along which the myosin motors move.
Figure 1.
Arrangement of the thick (red, orange) and thin (blue) filaments in the muscle sarcomere. The midpoints of the thick filaments are connected by the M-line (dark red) and the ends of the thin filaments by the Z-line (black), which is considered to define the ends of the sarcomere. The central third of each half thick filament is the myosin binding protein C-containing C zone (orange). Titin (brown) runs from the M-line to the Z-line. The white arrows indicate the forces imposed on the Z and M lines during contraction, and the reversal of filament polarity at those lines. To see this figure in color, go online.
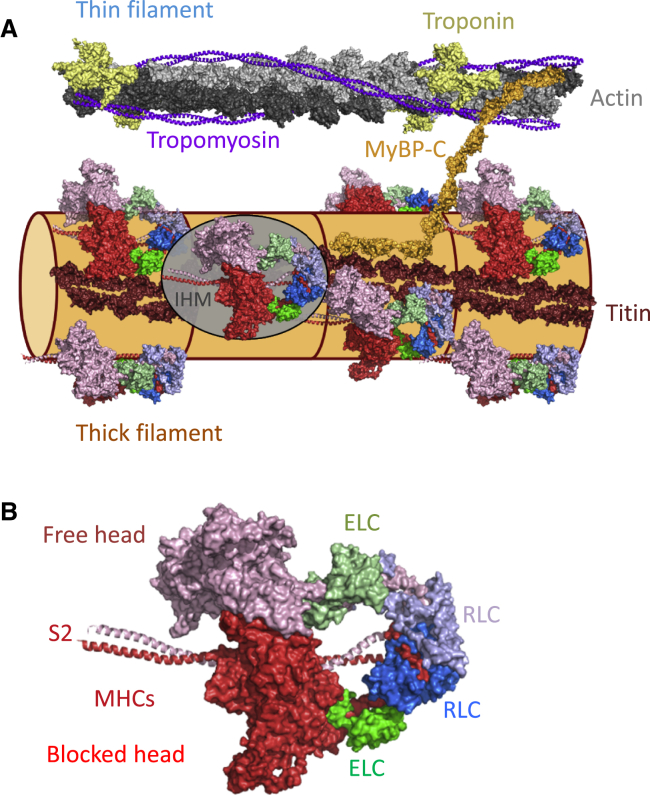
The thin and thick filaments have roughly helical structures (Fig. 2 A). The thin filament contains two strands of actin monomers, and the structure effectively repeats every 38 nm along the filament. The thick filament has three pairs of myosin head or motor domains around its circumference at each axial level, in a quasi-helical organization that repeats every 43 nm along the filament. Each myosin molecule has two head domains, which are ∼20 nm long, linked to a 150-nm-long tail that packs into the thick filament backbone. The molecular structure of the backbone of the thick filaments of vertebrate muscles is unknown.
Figure 2.
(A) Schematic model of possible structures of the thin and thick filaments in the sarcomeric C zone in the OFF state. The thin filaments (drawn from coordinates in PDB: 2W4U) have a helical periodicity of ∼38 nm and contain actin (light and dark gray), tropomyosin (purple) and troponin (yellow). The thick filaments have a helical periodicity of ∼43 nm, with three myosin molecules, each with a pair of myosin heads in the interacting heads motif (IHM), every 14.3 nm. The single IHM outlined in the gray ellipse is shown in greater detail in (B). Titin (brown; based on PDB: 3LCY) and the C terminus of myosin binding protein-C (orange; based on PDB: 5K6P and 2EDN) are shown in the approximate conformations suggested by electron microscopy of isolated filaments (46, 47); the N terminus of MyBP-C is shown bound to actin. The detailed structural relationships between the thick filament components, and the organization of the long C-terminal regions of the myosin tails in the filament backbone are unknown; the latter is shown as a simple cylinder. (B). The IHM thought to correspond to the OFF conformation of the myosin heads on the surface of the thick filament. Myosin heavy chain (MHC), red; essential light chain (ELC), green; regulatory light chain (RLC), blue; with lighter and darker shades corresponding to the two myosin heads in each myosin molecule (free and blocked heads. respectively), folding back onto the coiled-coil S) tail. Coordinates from PDB: 3DTP. To see this figure in color, go online.
Muscle contraction is driven by relative sliding between the thick and thin filaments, which in turn is driven by a cyclical interaction of the myosin heads with actin coupled to the hydrolysis of ATP. In each cycle, the myosin head binds to an actin monomer and changes shape while bound; this working stroke drives filament sliding or, if the filaments cannot slide because the muscle is prevented from shortening, generates force in an “isometric contraction” (4, 5, 6).
Thin filament regulation: the calcium START signal
The organization of myosin and actin in the thick and thin filaments of the muscle sarcomere suggests a general paradigm for muscle regulation in which the “OFF” state of resting muscle is achieved by blocking the interaction between myosin and actin. Such blocking must be highly efficient, to minimize ATP utilization by resting muscle, but unblocking must be fast, to allow physiological activation of mammalian muscles on timescales as short as 10 ms. The thin filaments contain a blocking protein, tropomyosin, a long coiled-coil molecule that can cover the myosin binding sites on seven actin monomers (Fig. 2 A). Each tropomyosin is bound to a second regulatory protein, troponin, that sensitizes the thin filaments to calcium (7, 8). At the low intracellular calcium concentration ([Ca2+]i) typical of resting muscle, <100 nM, the thin filament is OFF and tropomyosin blocks myosin binding to actin (9, 10, 11). In actively contracting muscle, [Ca2+]i increases to ∼20 μM, and calcium ions bind to troponin. This leads to a change in thin filament structure linked to a movement of tropomyosin that exposes binding sites on actin for the myosin head domains, permitting the myosin-actin interaction that drives contraction (12). According to this standard model of muscle regulation, the OFF (resting) and ON (actively contracting) states of skeletal muscle correspond to OFF and ON states of the thin filament, and the switch between these two states is controlled by [Ca2+]i.
This calcium/thin filament model of muscle regulation is consistent with the first major functional requirement for the control of skeletal muscle summarized above—rapid transient activation of muscle in response to an action potential in a motor nerve. Each nerve action potential triggers an action potential in the surface membrane of the target muscle cell, which in turn triggers release of calcium ions from their intracellular store—the sarcoplasmic reticulum—on the millisecond timescale. The released calcium ions bind rapidly to troponin, activating the thin filament as described above. They are then pumped back into the sarcoplasmic reticulum, reducing the intracellular calcium concentration and inducing dissociation of calcium from troponin, dissociation of myosin from actin, and relaxation of the muscle. The intracellular calcium transient links the action potential to the muscle twitch in an overall process referred to as excitation-contraction coupling.
This calcium/thin filament paradigm has dominated ideas about muscle regulation since its original exposition around 1970. However, it has become clear that other pathways are involved, and that the calcium/thin filament mechanism alone cannot fulfill the second functional requirement of muscle control highlighted above, optimization of the performance: metabolic cost ratio across the full dynamic range of muscle action. This review presents the evidence supporting an extended paradigm for muscle regulation, in which the thick filament, like the thin filament, is considered to have structural and functional OFF and ON states. It will be argued that, whereas the rapid increase in [Ca2+]i triggered by the action potential is the intracellular START signal for contraction, the regulatory state of the thick filament is the fundamental determinant of muscle performance, dynamics, and efficiency.
Proposed paradigm for thick filament regulation
The thick filament does not contain a blocking protein analogous to tropomyosin that can cover all the myosin head domains and prevent their binding to actin. Instead, in the paradigm for thick filament regulation considered here, the OFF state of the thick filament is achieved through a distinct conformation of myosin—the conformation diagrammed in Fig. 2, A and B in which its head domains cannot bind to actin or hydrolyze ATP. Structurally, the regulatory transition in the thick filament seems to involve changes in the organization of both the myosin head domains on the filament surface and their tail domains in the filament backbone, including the N-terminal parts of the tails that have unknown packing and are not shown in Fig. 2. The OFF state of the thick filament depends on multiple protein-protein interactions, including intramolecular head-head and head-tail interactions in myosin and intermolecular interactions between neighboring myosin molecules and probably between myosin and two other thick filament proteins, titin and myosin binding protein-C (MyBP-C). Individually, these interactions are relatively weak compared with the binding of myosin to actin, but together they stabilize a lattice of myosin heads on the thick filament surface that effectively competes with and prevents myosin-actin interaction.
According to this paradigm, the regulatory state of the thick filament is defined functionally in terms of the availability of myosin heads for actin binding, just as that of the thin filament is defined by the availability of actin sites for myosin head binding. In the simplest form of this concept, there are two permissive steps for muscle activation; muscle is ON when both the thin and thick filaments are ON. However, just as muscle has a range of ON states for different mechanical conditions, the concept will subsequently be extended to a range of regulatory states of the thick filament, and the presence of multiple populations of myosin heads with distinct regulatory properties in each thick filament.
This review examines the experimental support for the above paradigm, emphasizing cell-based studies in which the lattice of thin and thick filaments necessary for physiological regulation is preserved. It makes briefer reference to in vitro studies of isolated thick and thin filaments and their protein components to provide the necessary molecular context. It does not discuss the possible role of thick-filament-based regulation in heart muscle, although the close similarity between the protein isoforms and filament structures in the two muscle types strongly suggests that thick filament-based regulation is also important in the heart, albeit modified for the distinct functional requirements of that tissue. A few studies of the cardiac isoforms of thick filament proteins are included when they are more extensive than those of their skeletal muscle counterparts.
Potential regulatory proteins of the thick filament
Before discussing potential regulatory mechanisms in the thick filament, it is necessary to describe the structure and function of its protein components in more detail. The three major components are myosin, MyBP-C, and titin. A thick filament from fast twitch skeletal muscles of vertebrates contains 294, 42 (or possibly up to 54), and 12 copies of these proteins, respectively (3, 13, 14, 15). Additional proteins are bound to the thick filaments near the M line, and there are less well-characterized homologs of MyBP-C.
MyBP-C is mainly composed of a string of globular immunoglobulin (Ig)-like and fibronectin type-3 (Fn) domains, each ∼4 nm in diameter (Fig. 2 A). There are 10 Ig/Fn domains in the skeletal muscle isoforms. MyBP-C is confined to the central one-third of each half-thick filament, called the “C-zone” (Fig. 1; (13, 14)), and antibodies to MyBP-C label stripes in the C-zone with the same 43-nm axial periodicity as myosin (13, 16). The constitutive interactions of MyBP-C with myosin and titin in the thick filament backbone are mediated by its C-terminal domains. The cardiac isoform of MyBP-C (cMyBP-C; encoded by the MYBPC3 gene) has been studied more extensively than its skeletal muscle counterparts, which will be referred to collectively here as sMyBP-C, although they are the products of two genes, MYBPC1 and MYBPC2. All these isoforms are similar in much of their sequence, but cMyBP-C has an extra Ig domain at its N-terminus. The N-terminus of cMyBP-C can bind to actin, myosin heads, or the part of the myosin tail next to the heads with micromolar affinity (17, 18).
MyBP-C is widely regarded as a regulatory protein in both skeletal and cardiac muscle (18, 19, 20), and these regulatory functions are modulated by phosphorylation. sMyBP-C expression is strongly dependent on muscle fiber type; slow skeletal muscles have multiple splice variants of MYBPC1, and mutations in this gene are linked to skeletal muscle myopathies (21). Mutations in MYBPC3 are frequently associated with cardiomyopathy, and cMyBP-C knockout mice have a hypertrophic phenotype (22). In isolated muscle cells, sMyBP-C and cMyBP-C inhibit muscle shortening and work production (23, 24). cMyBP-C reduces unloaded sliding velocity in a purified filament assay (25). N-terminal fragments of cMyBP-C inhibit active force in heart and skeletal muscle cells at high [Ca2+], but in the absence of Ca2+ they can activate force in muscle cells (26, 27, 28) and displace tropomyosin to the ON position in isolated thin filaments (29). Thus, MyBP-C seems to have both activating and inhibitory effects on contractility, but the mechanisms underlying these effects are poorly understood.
Titin is a giant (3-MDa) protein that extends from the M to the Z line of the sarcomere (Fig. 1) and has multiple functions. Like MyBP-C, much of its sequence is composed of a string of Ig and Fn domains (Fig. 2 A). The domains in the thick filament or A-band region of titin are bound to myosin; this region probably acts as an assembly template for the thick filament and determines its length, but may also have a regulatory role. Mutations in this segment of titin, which has the same sequence in skeletal and cardiac muscle, are associated with cardiomyopathies (30). In the C-zone of the thick filament, the Ig/Fn domains have an 11-domain super-repeat corresponding to the 43 nm periodicity of myosin and MyBP-C (Fig. 2 A; (31)), suggesting a stoichiometric complex of myosin, MyBP-C, and titin domains in this region, although the detailed molecular interactions between the three components have not been characterized. The I-band region of titin, between the tip of the thick filament and the Z line (Fig. 1), provides an elastic element that contributes to the resting elasticity of muscle at long sarcomere length (32, 33).
Finally, myosin itself is recognized as a regulatory component of the thick filament in some muscle types and species, in addition to the structural and contractile roles described in previous sections. The regulatory function of myosin is normally associated with its essential and regulatory light chains (ELC and RLC), which are bound to a long α-helix of the myosin heavy chain (MHC) in the myosin head, near the junction between the heads and the tail (Fig. 2 B). This region of the myosin head is called the light chain domain (LCD) or lever arm, the latter term describing its role in the working stroke in the myosin head that drives filament sliding. In invertebrate myosins, the LCD is sometimes called the regulatory domain, reflecting the fact that such myosins are activated directly by calcium binding to the ELC or by phosphorylation of the RLC (34). RLC phosphorylation is the primary mechanism for the control of contraction in vertebrate smooth muscle (35, 36). Vertebrate skeletal muscle myosins retain the phosphorylatable serine residue in the RLC, and RLC phosphorylation by myosin light chain kinase increases active force in skeletal and cardiac muscle at submaximal [Ca2+] (37). This phosphorylation is too slow to have a significant impact on the time course of activation by a single action potential, but mediates force potentiation of skeletal muscle on repeated stimulation (38).
Structural and biochemical studies of thick filaments in myosin-regulated muscles
Ideas about thick-filament regulation in vertebrate skeletal muscle have been strongly influenced by studies of myosin-regulated muscles, including vertebrate smooth muscle and some invertebrate skeletal muscles. Although contraction of these muscles is regulated through myosin, either by phosphorylation of the RLC or by calcium binding to the ELC as described above, the structures of the myosins and thick filaments from these myosin-regulated muscles are similar to those of their vertebrate skeletal muscle counterparts. These similarities, described in more detail below, suggest that the myosin-based regulatory mechanisms in these muscle types and species may have been retained and adapted to work in parallel with thin filament-based mechanisms that appeared later in evolution.
Biochemical studies of smooth muscle myosins established that regulation by RLC phosphorylation is dependent on an interaction between the two head domains of each myosin molecule that is lost in the ON- or phosphorylated state (39). A key insight into the structural basis of myosin inhibition in the OFF state of smooth muscle myosin came from electron microscopy of a dephosphorylated smooth muscle myosin fragment containing both head domains and the subfragment-2 (S2) region of the myosin tail adjoining the heads (Fig. 2 B; (40)). This OFF structure, together with later structural studies of thick filaments from skeletal muscles described below, suggested that the OFF state of myosin has the heads folded back against S2, with the actin-binding site of one head, the so-called “blocked” head, binding to the side of the other head—the “free” head. As a result of this head-head interaction, the blocked head is prevented from binding to actin, and the free head is prevented from hydrolyzing ATP.
This OFF structure of smooth muscle myosin, also called the “J motif” or “interacting heads motif” (IHM), was subsequently identified in isolated thick filaments from skeletal muscles of tarantula, which are regulated by myosin phosphorylation (41). In these filaments the IHM seems to be stabilized by inter- and intramolecular interactions between the blocked head and S2, and between the blocked and free heads. The result is to lock the myosin heads into a helical lattice on the surface of the thick filaments in a conformation in which they can neither bind actin nor hydrolyze ATP: a structural and functional OFF state. These interactions are destabilized by RLC phosphorylation, releasing the heads for actin interaction and contraction (42). A similar myosin conformation, albeit stabilized by somewhat different molecular interactions, has been observed in isolated thick filaments from an invertebrate muscle regulated by calcium binding to the ELC, suggesting that it is a conserved feature of myosin-regulated muscles (43, 44, 45). More surprisingly perhaps, a similar myosin conformation is observed in the C-zone of isolated thick filaments from vertebrate cardiac muscle (46, 47), suggesting that the intramolecular interaction between the blocked and free myosin heads is preserved across muscle types and species. The intermolecular interactions are not completely preserved in thick filaments from cardiac muscle, however, and are not the same for the three layers of myosin heads in each 43-nm repeat (Fig. 2 A), reflecting the fact that the arrangement of myosin heads has systematic perturbations from a perfect helix. X-ray studies of vertebrate skeletal muscle show that such systematic perturbations from a helical organization are also present in the thick filaments of that muscle type (48).
The level of structural detail of the myosin head organization described above has not yet been attained in structural studies of thick filaments from vertebrate skeletal muscle. However, biochemical evidence, together with lower resolution structural data, described in the next two sections, suggests that the OFF state of myosin in vertebrate skeletal muscle is closely related to the IHM described above.
Biochemical evidence for an OFF state of the thick filament in vertebrate skeletal muscle
Resting skeletal muscles of vertebrates hydrolyze ATP at a very low rate. In amphibian muscles at 0°C, this rate corresponds to ∼0.002/s/myosin head, estimated from resting heat production (49) or oxygen consumption (50). Although these measurements include the contributions of cellular ATPases other than myosin, this muscle ATPase rate is five times lower than that of isolated head fragments of the same myosin isoform in the same conditions (51). There is a similar discrepancy for mammalian skeletal muscle and its isolated myosin fragments at physiological temperature (52).
These comparisons suggest that a control factor that is responsible for maintaining the very low ATP utilization and associated metabolic cost in intact skeletal muscles at rest has been lost in the isolated myosin head preparations that are normally used for biochemical studies, and which cannot form thick filaments. Cooke and co-workers (53) overcame some of the limitations of conventional ATPase measurements on thick filaments in muscle cells using displacement of fluorescent nucleotide analogs by ATP to isolate slow steps in the ATPase mechanism. Applying this method to demembranated muscle cells in relaxing conditions, they showed that, in contrast to myosin head fragments in solution, most of the myosin molecules in thick filaments have the same very low ATP turnover as resting intact muscle, but this very slow kinetic component is lost in actively contracting muscle. They also provided evidence that the low ATPase state of myosin in resting muscle, which they called the “superrelaxed” state, corresponds to myosin heads that are ordered and immobilized on the surface of the thick filaments, i.e., to the IHM state described in the previous section (53, 54, 55).
Structural evidence for an OFF state of the thick filament in vertebrate skeletal muscle
Early x-ray studies of vertebrate skeletal muscles showed that the quasi-helical arrangement of the myosin head domains on the surface of the thick filaments in resting muscle is lost during contraction (48, 56). With hindsight, those early observations may be considered to be the first evidence for OFF and ON structural states of the thick filament in vertebrate skeletal muscle, although they were not interpreted in that way at the time. The calcium/thin filament paradigm of muscle regulation was emerging in the same period, and contemporary biochemical studies focused on the strong ATP-dependent interaction between the myosin head domain and actin, best studied with soluble proteolytic fragments of myosin that do not form filaments. Much less was (and still is) known about the interaction between the head domain and the filament backbone, and the latter was widely regarded as a simple scaffold to present the head domains to actin and to transmit the contractile force.
Structural characterization of the thick filament has continued to lag behind that of the thin filament. At the time of writing, the structure of myosin head domains strongly bound to actin/tropomyosin has been described at almost atomic resolution (57), but the interaction of the myosin heads with their tails in the filament have only been described at much lower resolution. Very little is known about the structural organization of the myosin tails in the backbone of the thick filament, with the recent exception of a study of thick filaments from insect flight muscle (58), which have a substantially different protein composition from their vertebrate counterparts. The longstanding technical limitations of structural and biochemical measurements on native thick filaments have probably helped to maintain an actin-centric focus in models of the regulation of contraction in vertebrate skeletal muscle.
There was one notable early exception to that actin-centric perspective. Haselgrove (59) argued that the loss of the helical order of the myosin heads on the surface of the thick filaments during muscle contraction was a consequence of muscle activation per se rather than the interaction of the myosin heads with actin. This proposal was based on his finding that the part of the x-ray diffraction pattern from resting muscle that signals the helical order of the myosin heads, called the “first myosin layer line” (ML1), still decreases on muscle activation after the sarcomeres have been extended to reduce the region of overlap between the thick and thin filaments, and consequently the fraction of myosins that are able to interact with actin. Haselgrove also followed up earlier evidence that the axial periodicity of the thick filament, measured very precisely from the spacings of the x-ray reflections, increases by ∼1% on muscle activation. He proposed that this periodicity change signals a change in the packing of the myosin tails in the thick filament backbone linked to the loss of the helical order of the heads on the filament surface, and that both these structural changes are associated with myosin-linked regulation of muscle contraction.
Recent structural studies have confirmed and extended Haselgrove’s results, and shown that the helically ordered OFF state of the thick filaments in resting vertebrate skeletal muscle described above is closely related to the IHMs of myosin-regulated muscles. Synchrotron x-ray diffraction and interference studies of single intact muscle cells, in which sarcomere length can be precisely measured and controlled, showed that myosin heads in the regions of the thick filaments that do not overlap with thin filaments in stretched muscle lose their helical order during isometric contraction (60). Thick filament structures determined by cryo-electron tomography of resting skeletal muscles of vertebrates are consistent with the IHM-like motif, albeit at relatively low resolution (14). X-ray studies on intact single cells from vertebrate skeletal muscle used interference between the diffracted x-rays from the two arrays of myosin heads in each thick filament to show that the center of mass of the myosin heads moves by ∼10 nm away from the M-line in the transition from the resting state to isometric contraction (61). Because the axial center of mass of the heads with respect to the head-rod junction in isometric contraction was known from previous studies (62), this allowed the center of mass in the resting state to be calculated, and the result matched that of the IHM conformation. The orientation of bifunctional probes on the RLC in single demembranated cells from mammalian skeletal muscle in near-physiological relaxing conditions suggest that most but not all of the myosin heads are in the IHM conformation (63). Finally, the longer periodicity of the myosin tails in the filament backbone during isometric contraction than in resting muscle was shown to be due to a structural transition in the thick filament backbone distinct from the much smaller compliance of the filament (64, 65, 66), consistent with Haselgrove’s interpretation.
The structural and biochemical studies described in this and the previous section provide strong support for the hypothesis of an OFF state of the thick filaments in vertebrate skeletal muscle that is similar to the IHM in myosin-regulated muscles, and show that this OFF state is lost when vertebrate skeletal muscle is activated. Because vertebrate skeletal muscles have a calcium/thin filament regulatory mechanism, these findings raise a fundamental question. How do OFF myosin heads that are parked in helical tracks on the surface of the thick filament sense that the neighboring thin filament has been activated in response to a calcium transient? Before considering possible answers to this question, the results of two types of study that indicate coupling between the regulatory states of the thin and thick filaments during partial activation will be summarized. The next section describes the relationship between contractile force, thin and thick filament structure, and free calcium concentration in the steady state, and the subsequent one describes the relative kinetics of the changes in these parameters during muscle activation after action potential stimulation.
Calcium regulation of contraction: cooperativity, sensitivity, and the influence of the thick filament
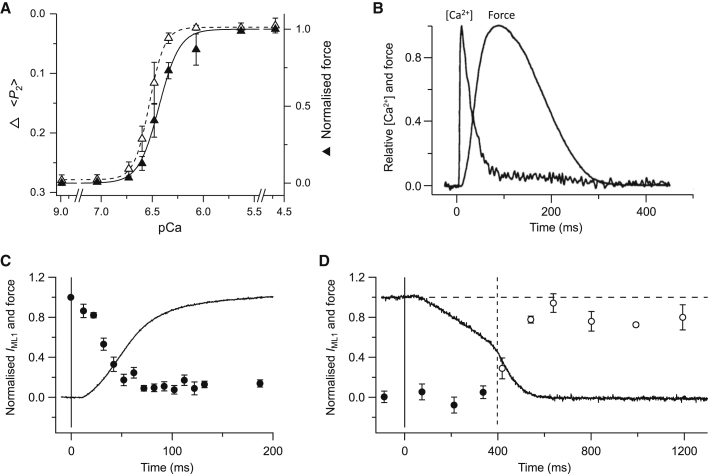
Calcium control of muscle contraction can be studied in demembranated or skinned muscle cells or myofibrils in which the normal membrane-based systems responsible for the [Ca2+]i transient have been removed, allowing steady [Ca2+]i to be controlled directly using calcium buffers. The dependence of isometric force on [Ca2+]i in a steady-state experiment of this type (Fig. 3 A) is usually plotted against pCa (–log10 [Ca2+]i) and described by the Hill equation with two fitted parameters. pCa50, the calcium sensitivity, is the pCa value corresponding to one-half the maximum force and nH, the Hill coefficient, describes the steepness of the calcium dependence. For a simple first-order binding reaction, nH would be 1. In skinned fibers from vertebrate skeletal muscle, pCa50 is ∼6.0 and nH varies from ∼2 to 6 depending on the experimental conditions and protocol, indicating that calcium activation is cooperative.
Figure 3.
(A) Dependence on steady [Ca2+], plotted as pCa= −log10 [Ca2+], of isometric force (solid triangles) and the orientation of a fluorescent probe attached to the RLC region of the myosin heads (〈P2〉, open triangles), replotted from (68). (B) Time course of [Ca2+] and isometric force after single action potential stimulation, from (82). (C) Time course of the intensity of the first myosin-based x-ray layer line (IML1; solid circles) signaling the helical order of the myosin heads on the surface of the thick filaments and force (solid line) during the rising phase of an isometric tetanus, replotted from (61). Time zero and the vertical line mark the first electrical stimulus. (D). Time course of IML1 (circles) and force (solid line) during isometric relaxation (solid circles) and chaotic relaxation (open circles), replotted from (111). The full vertical line and time zero mark the last electrical stimulus; the dashed vertical line the transition between isometric and chaotic relaxation; the dashed horizontal line is IML1 in resting muscle. Data in (A) from skinned fibers of rabbit psoas muscle, 25°C; those in (B–D) from intact fibers of frog fast twitch muscle, 4°C.
Some of this cooperativity is intrinsic to the thin filament (12), and has been attributed in part to coupling between adjacent tropomyosin molecules along the thin filaments. However, probes on troponin in the thin filaments report calcium-dependent structural changes with an nH lower than that for force (67), suggesting that some of the cooperativity of the force-pCa relation is not due to that of the thin filament. Moreover, probes on the RLC of myosin heads that report the regulatory state of thick filament have both an nH and pCa50 that are higher than those of force (Fig. 3 A; (68)). These results suggest that thick filament structure does not simply follow the regulatory state of the thin filament or force production, but has an independent role in muscle regulation and in the cooperativity of its calcium dependence in the steady state.
Consistent with that interpretation, interventions that promote the ON state of the thick filaments in mammalian skeletal muscle increase the calcium sensitivity (pCa50) of both isometric force and the regulatory state of the thin filament, and conversely those that promote the OFF state decrease the calcium sensitivity. Lowering the temperature from the physiological value promotes the ON state of the thick filament (63, 69), and pCa50 for isometric force is increased (70). Disrupting the OFF state of the thick filament by phosphorylating the RLC also increases pCa50 for force (38, 71). Conversely, the small molecule myosin inhibitors butanedione monoxime, N-benzyl-p-toluene sulphonamide, and blebbistatin that stabilize the ADP.Pi state of myosin and the OFF state of the thick filament (55, 72, 73) decrease pCa50 for both force (74, 75) and the regulatory state of the thin filament as measured by a probe on troponin (67). Similar effects of RLC phosphorylation and blebbistatin on the calcium sensitivity of force and thin filament structure have been observed in cardiac muscle (76, 77), although the effect of temperature on pCa50 for isometric force in cardiac muscle is opposite to that in skeletal muscle (78), perhaps reflecting the different temperature dependence of other contractile and regulatory parameters.
Structural dynamics of muscle activation
The studies summarized in the previous section demonstrate a coupling between the regulatory states of the thin and thick filaments when the level of thin filament activation is controlled by varying the steady level of [Ca2+]i. That approach gives information about the calcium control of muscle activation, including its calcium sensitivity (pCa50) and cooperativity (nH) in equilibrium conditions of calcium binding. Moreover, the widely used extension of that approach to the time domain through measurement of the rate of force redevelopment (kTR) after rapid shortening at different [Ca2+] (reviewed in (12)) has the same limitation. During physiological activation of skeletal muscle, however, the calcium signaling pathways are far from equilibrium. The intracellular calcium transient after action potential stimulation rises in a few milliseconds from its resting value, which is at least an order of magnitude lower than the calcium dissociation constant of troponin, to a peak value that is at least an order of magnitude higher (Fig. 3 B). Moreover, there is negligible force development during the upstroke of the calcium transient—the so-called “latent period”—and the calcium transient is largely over before force has reached its peak. These relative time courses underline the limitation of extrapolating from the equilibrium calcium titrations described in the previous section to physiological mechanisms of muscle activation. Those mechanisms are more reliably deduced from direct measurements of the kinetics of the molecular structural transitions in muscle cells during activation.
Structural dynamics experiments of this type on intact muscle cells in physiological conditions, using time-resolved synchrotron x-ray diffraction, were pioneered by Huxley and co-workers (79, 80). To compare kinetic data from different studies, the results are presented here for the example of isometric activation of amphibian muscle at 4°C, as used for most studies of this type. In contrast with the skinned fibers from mammalian skeletal muscle used for the studies described in the previous section, the thick filaments in resting amphibian muscles are OFF at 4°C, which is a physiological temperature for these species. In these conditions, the intracellular calcium transient triggered by a single action potential peaks ∼10 ms after the stimulus, then declines rapidly over the next few tens of milliseconds (Fig. 3 B; (81, 82)).
The half-time of both myosin binding to actin and isometric force generation is ∼50 ms under these conditions (83), much slower than the upstroke of the calcium transient. The rate of force development is not limited by that of calcium binding to troponin; the x-ray reflections associated with troponin change substantially during the latent period (61, 84, 85). The increase in the intensity of the so-called “second actin layer line” associated with azimuthal motion of tropomyosin is also very fast, with a half-time of ∼20 ms (80). Changes in the intensities of x-ray reflections associated with the OFF structure of the thick filament, including the 43 nm reflection associated with MyBP-C, and with the axial periodicity of the thick filament backbone, are also fast, with half-times of 20–30 ms (Fig. 3 C; (61)). All these processes are significantly faster than attachment of myosin heads to actin and force generation by the attached myosin heads, which have a half-time of ∼50 ms (61).
These time-resolved x-ray studies of intact muscle cells suggest that fast structural changes in both the thick and thin filaments on muscle activation are required for contraction. Activation of vertebrate skeletal muscle by the calcium transient depends on two fast permissive switches, one in the thin and one in the thick filaments. Attachment of myosin heads to actin is slower than the structural switches in both filaments.
Mechanisms of thick filament activation and interfilament communication
The results described in the previous two sections suggest that the regulatory states of the thin and thick filaments are positively coupled, both in the steady state in response to a change in calcium concentration, and kinetically after action potential stimulation. Four mechanisms that could potentially couple the regulatory states of the thin and thick filaments are considered below, although these are neither mutually exclusive nor comprehensive.
Calcium signaling to thick filaments. After the discovery that some invertebrate muscles are regulated by direct calcium binding to the myosin light chains, early studies investigated the possibility that a related mechanism might operate in vertebrate skeletal muscle. The RLC of vertebrate skeletal muscle myosin binds Ca2+ with an affinity of ∼10 μM at physiological [Mg2+] (86, 87), so it is expected to bind Ca2+ during steady-state activation. However, these binding sites would be predominantly occupied by Mg2+ in resting muscle, and the rate of displacement of Mg2+ by Ca2+ after the Ca2+ transient is much too slow for this to be a significant signaling pathway in normal muscle activation (86). In general, studies of isolated myosin from vertebrate skeletal muscle have provided no support for the hypothesis of direct calcium signaling to myosin. In addition, electron microscopy of isolated thick filaments from vertebrate skeletal muscle showed that Ca2+ does not disrupt the helically ordered OFF structure at concentrations up to 100 μM (71). It remains possible that Ca2+ signaling to thick filaments might be mediated by other protein components in the intact sarcomere, for example, via a change in the stiffness of the PEVK region of titin (88), which connects the tip of the thick filament to the sarcomeric Z-line (Fig. 1), or by Ca2+ binding to MyBP-C (25). However, x-ray studies from intact skeletal muscle fibers that will be described below seem to provide compelling evidence that the regulatory state of the thick filament is not controlled directly by the intracellular Ca2+ transient.
Activation of thin filaments by myosin heads. The hypothesis that binding of myosin heads activates the thin filaments has a long history (89). It is well established that binding of myosin heads to actin in rigor (i.e., in the absence of ATP) can activate thin filaments by displacing tropomyosin toward its ON position; in these conditions there are effectively three regulatory states of the thin filament, referred to as “blocked”, “closed”, and “open” (11, 90). In this model, Ca2+ binding to troponin induces the blocked-to-closed transition and myosin heads binding to actin promote the closed-to-open transition, with the latter considered to be the fully ON state of the thin filament. However, the rigor state would be occupied for a very small fraction of the ATPase cycle at physiological ATP concentrations, so activation of the thin filaments by rigor myosin heads does not imply that a mechanism of this type is significant during normal contraction.
In principle, the influence of the myosin heads on the level of thin filament activation at physiological [ATP] could be determined from the effects of interventions that reduce the number of myosin heads interacting with actin, and three such interventions have been used for this purpose: stretching muscle to a sarcomere length of ∼4.0 μm to abolish the overlap between thick and thin filaments; myosin-specific inhibitors of active force; and the imposition of rapid muscle shortening. In general, these interventions produce either no change or a small decrease in the activation state of the thin filament at maximal [Ca2+], as judged by the intensity of the second actin x-ray layer line associated with the azimuthal position of tropomyosin (80, 91), or orientation-sensitive fluorescent probes on troponin (92, 93). In one study (93) that used all three types of intervention, a probe on the E-helix of troponin C reported a reduction of the level of thin filament activation by ∼20% on removal of filament overlap, 30% on inhibition of active force with the myosin inhibitor N-benzyl-p-toluene sulphonamide, and ∼25% on imposition of rapid shortening. Small-molecule inhibitors of active force also decrease pCa50 for force and thin filament activation during partial calcium activation (67, 74, 75), as noted in a previous section. All these effects might be interpreted as a contribution of actin-bound myosin heads to thin filament activation. However, there is an alternative interpretation. All three interventions used to decrease the number of force-generating myosin heads in the above studies have also been reported to decrease the activation level of the thick filament (60, 72, 94). Therefore, the effects on thin filament activation observed in those protocols might be an indirect effect of positive coupling between the regulatory states of the thin and thick filaments mediated by another mechanism, such as those described below, rather than a direct effect of actin-bound myosin heads.
Nevertheless, the results described above do not exclude the possibility that actin-bound myosin heads contribute to activation of the thin filament during steady-state calcium activation. Indeed, such an effect is expected to be a fundamental property of calcium-induced thin filament regulation in the steady state. Calcium-induced motion of tropomyosin permits myosin head binding to actin, but the bound myosin heads inhibit the reverse tropomyosin movement associated with switching OFF the thin filament. Thus actin-bound myosin heads are expected to displace the equilibrium state of the thin filament in the ON direction. However, it seems unlikely that this mechanism has a significant effect on the timescale of physiological muscle activation in response to an action potential, because thin filament activation is much faster than myosin head binding to actin, as described in Structural Dynamics of Muscle Activation. Moreover, as also discussed in that section, the structural changes in the thick filament associated with switching ON the thick filament on activation are almost as fast as those in the thin filament, and significantly faster than myosin head binding to actin (Fig. 3 C). It therefore seems likely that some other interfilament signaling mechanism is responsible for transmitting the calcium activation signal to the thick filament after action potential stimulation.
Interfilament signaling by MyBP-C. MyBP-C is an attractive candidate for an interfilament signaling protein, because although its C-terminus is a constitutive component of the thick filament, its N-terminus can bind to the thin filament, as noted in an earlier section and diagrammed in Fig. 2 A. MyBP-C is associated with axial x-ray reflections from the thick filament related to its ∼43-nm axial periodicity in resting muscle. Electron tomography of resting muscles shows densities with the same periodicity extending from the thick to the thin filaments in the C-zone (14). The dependence on sarcomere length of the x-ray reflections associated with MyBP-C in resting muscle provides further evidence for such C-links between thick and thin filaments. When resting muscle is stretched, the intensities of these reflections are reduced only when the region of the thick filament that contains MyBP-C—the C-zone (Fig. 1)—is withdrawn from the thin filament lattice, i.e., when binding of MyBP-C to actin is prevented (60). The x-ray reflections associated with the helical arrangement of the myosin heads on the surface of the thick filament also become weaker when overlap of the C-zone with thin filaments is removed, and the periodicity of the thick filament backbone increases toward the value associated with its ON state in the same sarcomere length range (60).
These structural studies suggest that MyBP-C cross links the thick and thin filaments in resting skeletal muscle, and that these C-links might contribute to stabilizing the OFF state of the thick filament in resting muscle. Such C-links might also be responsible for the so-called “short-range” elastic component detected by mechanical studies of resting muscle, and its resting viscoelasticity (95). The decrease in the intensity of the MyBP-C-related x-ray reflections on muscle activation is much faster than myosin binding to actin and force development (94). The loss of the short-range elasticity and viscoelasticity characteristic of resting muscle is similarly rapid (96, 97). These observations suggest that loss of the C-links after calcium activation might constitute a fast interfilament signaling pathway that switches ON the thick filaments.
However, that hypothesis is likely to be an oversimplification. In contrast with the complete loss of the x-ray reflections associated with the helical surface lattice of the myosin heads during full isometric activation of isolated muscle cells, the reflections associated with MyBP-C are still present, although they are weaker than in resting muscle (61). Functional studies also suggest that C-links are still present in calcium-activated muscle, and can reduce the velocity of filament sliding (23, 24, 25). Moreover, N-terminal fragments of MyBP-C can activate the thin filament (26, 27, 28, 29). Finally, the regulatory function of MyBP-C is likely to involve direct interactions with myosin heads and S2 region in addition to those with actin described above (18). C-links may be retained but modified during calcium activation, and there may be OFF and ON states of the C-link with distinct structures and mechanical properties.
Many questions remain about the regulatory function of MyBP-C and its mechanistic basis, and there are additional mechanisms of interfilament communication as discussed below. Nevertheless, MyBP-C remains the most likely protein mediator of interfilament signaling in vertebrate skeletal and cardiac muscle. MyBP-C may act as a regulatory hub between the thick and thin filaments, integrating the effects of direct signaling to MyBP-C itself with sensitivity to the regulatory states of both the thin and thick filaments and to filament sliding.
Thick filament mechanosensing. Mechanosensing is a fundamental property of myosin as a motor protein, and is necessary for efficient coupling of its mechanical and ATPase cycles (98, 99, 100). According to the concept introduced by Huxley (98), strain in the internal elasticity of the myosin head controls the rate constants of its attachment to and detachment from actin, matching its motor function to the external load. The key strain-dependent step is release of ADP by the actin-bound myosin head after ATP hydrolysis. In muscle myosin this rate is low at high load, reducing the rate of ATP utilization in isometrically contracting muscle, but increases at low load, as in rapidly shortening muscle. In these conditions, ADP release from the myosin head at a critical strain allows it to rapidly bind ATP and detach from actin before filament sliding carries it into the negative strain or drag region (99, 101).
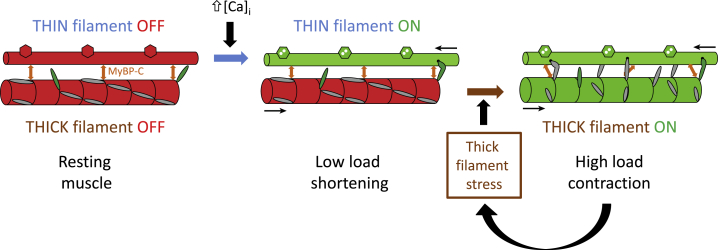
It has recently become clear that the thick filaments of vertebrate skeletal muscle have a second mechanosensing mechanism that is distinct from that of the individual myosin heads: thick filaments are switched ON by mechanical stress (68, 94). This concept emerged from the observation that the x-ray signatures of the OFF state of the thick filament—its helical order and short axial periodicity—show no measurable changes if rapid shortening is imposed at the start of electrical stimulation to prevent active force development (94). In these conditions, calcium activation of the thin filament is maximal, and the muscle shortens at the maximum velocity (vmax) characteristic of zero load, but the thick filament remains OFF. Unloaded shortening in muscle therefore requires only an ON thin filament, not an ON thick filament (Fig. 4). This observation shows that the regulatory state of the thick filament is not solely determined by that of the thin filament, and provides clear evidence that calcium does not directly activate thick filaments in vertebrate skeletal muscle. Although it may seem surprising that filament sliding can be driven at maximum unloaded velocity by thick filaments that appear to be structurally OFF, filament sliding against very low load requires only a small number of myosin heads (101), and recent evidence from sarcomere mechanics (102) suggests that this number may be as low as one head per filament. The small number of myosin heads that drive unloaded shortening seem to be outside thick filament control, or “constitutively ON” (94).
Figure 4.
Dual filament regulation in skeletal muscle. The thin filament (upper) is shown as a simple cylinder with hexagons symbolizing troponin; the thin filament is switched ON by calcium binding (blue arrow). Part of the C zone of the thick filament (lower) is shown with a surface array of myosin head domains (gray or green); the thick filament can be switched ON by mechanical stress (brown arrow). OFF and ON states of both filaments are red and green respectively. Constitutively ON myosin heads are green; myosin heads under thick filament control are gray. The double-headed orange arrows symbolize coupling of the regulatory states of the thin and thick filaments by myosin binding protein-C, which may have a different conformation in the OFF and ON states of the thick filament. To see this figure in color, go online.
A more general implication of the above result is that the thick filaments are only switched ON when the load is high; the switch operates by direct mechanosensing in the thick filaments (94). At high load, as for example, in an isometric or fixed-end contraction, the constitutively ON myosin heads generate stress in the backbone of the thick filament. This stress directly triggers the transition to the ON state of the thick filament (Fig. 4), which would mobilize additional myosin heads, generating additional force and filament stress in a positive feedback loop that culminates in a fully ON thick filament for loads larger than about half the isometric force (94, 103). According to this thick filament mechanosensing hypothesis, the regulatory state of the thick filament is determined by the external load on the muscle, independently of the canonical calcium/thin filament regulation pathway.
The thick filament mechanosensing hypothesis predicts that it should be possible to switch the thick filament OFF by reducing the stress in the thick filament when [Ca2+]i remains high and the thin filament remains ON. This prediction was tested by imposing rapid shortening from the isometric steady state (94), showing that the x-ray signatures of the OFF state of the thick filament reappear when its stress is held at a low level, as expected. Moreover, force redevelopment at the end of such a period of unloaded shortening was fast if the shortening period was brief, so that the thick filament remained almost fully ON, but slower and preceded by a lag phase if the shortening period was long enough to allow the thick filament to switch OFF. These results provide further support for the thick filament mechanosensing hypothesis, and show that the time course of force development in physiological conditions is determined by the level of activation of the thick filament. Although the rate of force development in skinned fibers from mammalian muscle is lower at lower steady state [Ca2+] (12), in physiological conditions the rising phase of the calcium transient and thin filament activation are so fast that they have a negligible impact on the rate of force generation.
An independent test of the thick filament mechanosensing hypothesis is enabled by the existence of titin links between the tips of the thick filaments and the sarcomeric Z-line (Fig. 1). When resting muscle is stretched, these titin links transmit the resting force to the thick filament, so the mechanosensing hypothesis predicts that thick filaments should be switched on by stretching resting muscle. This prediction was tested in skinned muscle fibers using fluorescent probes on the RLC. The results confirmed that the orientation of the RLC probes is sensitive to thick filament stress in relaxing conditions as predicted (68). Moreover, by inhibiting active force with blebbistatin, it was possible to show that the mechanism was independent of [Ca2+] in the physiological range, clearly establishing thick filament mechanosensing as a regulatory mechanism that works independently of the calcium/thin filament pathway.
The concept of the thick filament as a regulatory mechanosensor provides, to my knowledge, a novel explanation for the well-known force-velocity relationship of muscle (104). Contraction against a high load needs both an ON thick filament and an ON thin filament, but contraction against a low load requires only an ON thin filament (Fig. 4). Thus, thick filament mechanosensing performs the role of an automatic gearbox in muscle, matching the number of myosin heads engaged, and the associated ATP utilization, to the mechanical load against which the muscle has to work. In this respect, thick filament mechanosensing provides a necessary functional complement to motor mechanosensing, which as noted above couples motor detachment from actin to ADP release from the active site of the motor, maximizing the efficiency of the working stroke of the motor at moderate loads and velocities. Motor mechanosensing could not explain why the rate of ATP utilization becomes low at very low loads (105); this requires an additional mechanism to inhibit attachment of the motors to actin so that they do not become committed to ATP hydrolysis (101). In terms of the cross-bridge cycle of Huxley (98), motor mechanosensing would be regarded as controlling the detachment rate constant g, with thick filament mechanosensing controlling the attachment rate constant f. This two-step cycle is clearly an oversimplification, however, and recent mechanical studies show that more sophisticated models are required to describe the energetics of shortening and mechanical-chemical coupling in muscle (102, 105, 106).
The mechanism of thick filament mechanosensing is not known at the molecular level, and a detailed explanation will probably require a high-resolution structure of the thick filament and its backbone. Because the OFF state of the thick filament, with its slightly shorter axial periodicity, seems to be stabilized by a network of intermolecular interactions involving the helically ordered myosin heads on the surface of the thick filaments with the myosin tails, MyBP-C and titin, one possibility is that the shorter backbone periodicity provides the optimal axial spacing for those periodic intermolecular surface interactions. However, given the likely complexity of the packing arrangement of myosin tails in the filament backbone (58), more complicated structural changes may be involved. Finally, the existence of thick filament mechanosensing does not exclude a role for MyBP-C in interfilament signaling; the regulatory state of the thick filament may be influenced by both thick filament stress and by the regulatory state of MyBP-C, although the interaction between these two signaling pathways remains to be worked out.
Mechanisms of muscle relaxation
So far, this review has focused on muscle activation, as is common in most studies and discussion of muscle regulation, in which there is often an implicit assumption that relaxation is simply the reversal of activation. However, control of the rate of muscle relaxation in response to external factors is critical for its physiological function, both for control of body movements and to control mechanical efficiency and metabolic cost. Thin-filament-mediated regulation has a kinetic asymmetry between activation and relaxation, arising from the possibility that actin-bound myosin heads prevent the return of tropomyosin to its OFF position, so that the thin filament might remain ON after calcium has dissociated from troponin. This section explores the possible roles of thick filament regulation in the relaxation of skeletal muscle, and relates them to the higher-level asymmetry in the control of skeletal muscle by the CNS noted at the start of this review, the fact that motor nerves switch muscle ON, but the subsequent switching OFF is outside CNS control.
After its peak, ∼10 ms after the action potential in amphibian skeletal muscle at 4°C, intracellular free calcium concentration [Ca2+]i declines to ∼10% of its peak value in the next few tens of milliseconds, although final recovery of [Ca2+]i to its steady-state resting value is much slower (Fig. 3 B; (81, 85, 107)). Dissociation of calcium from troponin is likely to be significantly slower than the decline of [Ca2+]i (108), but has not been measured directly in muscle fibers. The best available estimate of the time course of thin filament activation in these conditions is probably the intensity of the second actin x-ray layer line associated with the azimuthal position of tropomyosin in the thin filaments, which in whole muscles recovers with a time course slightly faster than that of force (80).
In single muscle cell preparations with low series compliance activated by repetitive electrical stimulation in isometric conditions, mechanical relaxation consists of a series of distinct kinetic phases (109, 110, 111). Force stays constant for ∼50 ms after the last action potential, then decreases at a constant slow rate, to typically one-half the maximum value, for several hundred milliseconds (Fig. 3 D). This period is called “isometric relaxation” because sarcomere lengths remain constant, and is terminated by a sudden redistribution of sarcomere lengths along the fiber, linked to acceleration of the force decline, called “chaotic relaxation”. Slow mechanical relaxation during the isometric phase is not related to the slow component of [Ca2+]i recovery, because it is also present in isolated myofibrils in which [Ca2+] can be reduced from a level producing full activation to ∼10 nM within 10 ms (112, 113). Experiments of this type established that the rate of isometric relaxation is determined primarily by that of the dissociation of myosin heads from actin, which is very much slower than Ca2+ dissociation from troponin.
There is no recovery of the x-ray signals and periodicities associated with the OFF state of the thick filament during isometric relaxation; the thick filament remains fully ON (Fig. 3 D). This observation provides further evidence that the regulatory state of the thick filament is not directly sensitive to [Ca2+]i. Moreover, the x-ray signal associated with the ON state of MyBP-C is also maintained during isometric relaxation (111). The maintained ON state of the thick filament during isometric relaxation is consistent with the positive feedback loop of thick filament mechanosensing (Fig. 4); thick filament stress is sufficiently high to hold the thick filaments ON. In this respect, thick filament mechanosensing is antagonistic to fast relaxation, as indeed is the thin filament-based mechanism in which actin-bound myosin heads prevent the movement of tropomyosin to the thin filament OFF position after [Ca2+]i has fallen to its resting level.
Isometric relaxation is terminated by mechanical yielding of a population of sarcomeres accompanied by shortening of the other sarcomeres in series, and by collapse of the isometric state—chaotic relaxation (109, 110, 111, 112). All the x-ray signatures of the OFF state of the thick filament recover quickly during chaotic relaxation (Fig. 3 D). Chaotic relaxation may be triggered by the cumulative increase in the strain of the attached myosin heads during isometric relaxation, eventually leading to a markedly increased rate of detachment of heads from actin through myosin head mechanosensing (111, 114). Consistent with this idea, isometric relaxation is terminated prematurely when a small stretch is imposed during this phase (81, 115, 116).
Functionally, the very slow relaxation of skeletal muscle in isometric conditions allows force to be maintained very efficiently when no change in muscle length is required, as in static postural muscles. In these conditions the energy bill for isometric contraction has already been paid during force development, and very slow relaxation allows isometric force to be maintained at very low metabolic cost. Moreover, detachment of myosin heads from actin during isometric relaxation is likely to be at least partly achieved by reversal of the force-generating step in the myosin head, potentially further reducing the overall ATP utilization in a single muscle activation (117, 118). Isometric relaxation of skeletal muscle appears similar in some respects to the catch or latch states of smooth muscle (119, 120).
From the perspective of maximizing the ratio of muscle performance to metabolic cost highlighted at the start of this review, the very slow rate of muscle relaxation in sarcomere-isometric conditions is a positive functional adaptation. Exit from this latchlike state during the transition to chaotic relaxation is rapid, as the positive feedback loop that sustains the ON state of the thick filaments breaks down, and the transition to chaotic relaxation is controlled mechanically rather than by [Ca2+]i, as shown by the experiments described above in which muscles were stretched during isometric relaxation. In vivo, this implies that relaxation of a postural muscle would be very slow if there were no movement of the skeleton, but even a small (∼1%) change in muscle length, produced for example by a change in external load, would trigger chaotic relaxation. This phenomenon might provide a signaling pathway by which the CNS could control muscle relaxation indirectly, by activating an antagonist muscle to produce a small stretch of the agonist. In muscle fibers with high tendon compliance, the initial force decrease at the start of isometric relaxation would shorten the tendon sufficiently to extend the active sarcomeres by the small amount required to trigger chaotic relaxation (114), allowing such muscle fibers and motor units to have characteristically fast relaxation, in contrast with the sustained activation of isometric postural muscles.
These considerations emphasize the general principle of mechanical control of the rate of relaxation of skeletal muscle, and the idea that the falling phase of the [Ca2+]i transient is a necessary but not sufficient condition for mechanical relaxation. In contrast with activation, in which high cooperativity and positive feedback contribute to very rapid switching ON of contraction, relaxation is delayed by these properties of thin and thick filament regulation in sarcomere-isometric conditions, and the trigger for exit from this latchlike state seems to be mechanical.
Conclusions
It has been argued that the thick filaments of skeletal muscle play a fundamental role in the regulation of contraction that extends the well-established paradigm of thin filament regulation in several respects. Whereas thin filament pathways mediate the START signal for contraction, thick filament regulation matches the response of the muscle to external mechanical conditions. It optimizes the ratio of muscle performance to metabolic cost across the wide range of muscle function from rest, through low-load shortening to high-load force bearing, acting as an automatic gearbox. It controls the kinetics of muscle activation and mediates the kinetics of relaxation in response to external mechanical conditions. The molecular details of thick filament regulation remain to be elucidated, although it is clear that the OFF state of the thick filament is associated with a helical surface lattice of myosin head domains and that activation of the thick filaments is associated with the loss of this lattice and a slight extension of the filament backbone. This structural transition is controlled by stress in the thick filament itself, although it seems likely that myosin binding protein C plays an additional role in signaling between the thin and thick filaments. Finally, these general principles of thick filament regulation in skeletal muscle probably operate in a modified form in heart muscle, and a better understanding of these thick filament-mediated regulatory mechanisms could underpin the development of novel therapies for myopathies affecting both types of muscle.
Acknowledgments
I am grateful to P. Bennett, E. Brunello, L. Fusi, T. Kampourakis, and D.R. Trentham for helpful comments on an earlier version of this review, and to E. Brunello, L. Fusi, and T. Kampourakis for help with preparation of the figures.
Editor: Brian Salzberg.
References
- 1.Woledge R.C., Curtin N.A., Homsher E. Academic Press; London, UK: 1985. Energetic Aspects of Muscle Contraction. [PubMed] [Google Scholar]
- 2.Cooke R. The role of the myosin ATPase activity in adaptive thermogenesis by skeletal muscle. Biophys. Rev. 2011;3:33–45. doi: 10.1007/s12551-011-0044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Squire J.M. Plenum Press; New York: 1981. The Structural Basis of Muscle Contraction. [Google Scholar]
- 4.Huxley H.E. The mechanism of muscular contraction. Science. 1969;164:1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- 5.Huxley A.F. Muscular contraction. J. Physiol. 1974;243:1–43. [PMC free article] [PubMed] [Google Scholar]
- 6.Geeves M.A., Holmes K.C. The molecular mechanism of muscle contraction. Adv. Protein Chem. 2005;71:161–193. doi: 10.1016/S0065-3233(04)71005-0. [DOI] [PubMed] [Google Scholar]
- 7.Ebashi S., Endo M., Otsuki I. Control of muscle contraction. Q. Rev. Biophys. 1969;2:351–384. doi: 10.1017/s0033583500001190. [DOI] [PubMed] [Google Scholar]
- 8.Greaser M.L., Gergely J. Reconstitution of troponin activity from three protein components. J. Biol. Chem. 1971;246:4226–4233. [PubMed] [Google Scholar]
- 9.Huxley H.E. Structural changes in the actin- and myosin-containing filaments during contraction. Cold Spring Harb. Symp. Quant. Biol. 1973;37:361–376. [Google Scholar]
- 10.Parry D.A.D., Squire J.M. Structural role of tropomyosin in muscle regulation: analysis of the x-ray diffraction patterns from relaxed and contracting muscles. J. Mol. Biol. 1973;75:33–55. doi: 10.1016/0022-2836(73)90527-5. [DOI] [PubMed] [Google Scholar]
- 11.Vibert P., Craig R., Lehman W. Steric-model for activation of muscle thin filaments. J. Mol. Biol. 1997;266:8–14. doi: 10.1006/jmbi.1996.0800. [DOI] [PubMed] [Google Scholar]
- 12.Gordon A.M., Homsher E., Regnier M. Regulation of contraction in striated muscle. Physiol. Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 13.Bennett P., Craig R., Offer G. The ultrastructural location of C-protein, X-protein and H-protein in rabbit muscle. J. Muscle Res. Cell Motil. 1986;7:550–567. doi: 10.1007/BF01753571. [DOI] [PubMed] [Google Scholar]
- 14.Luther P.K., Winkler H., Liu J. Direct visualization of myosin-binding protein C bridging myosin and actin filaments in intact muscle. Proc. Natl. Acad. Sci. USA. 2011;108:11423–11428. doi: 10.1073/pnas.1103216108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granzier H.L., Irving T.C. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys. J. 1995;68:1027–1044. doi: 10.1016/S0006-3495(95)80278-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rome E., Offer G., Pepe F.A. X-ray diffraction of muscle labelled with antibody to C-protein. Nat. New Biol. 1973;244:152–154. doi: 10.1038/newbio244152a0. [DOI] [PubMed] [Google Scholar]
- 17.Shaffer J.F., Kensler R.W., Harris S.P. The myosin-binding protein C motif binds to F-actin in a phosphorylation-sensitive manner. J. Biol. Chem. 2009;284:12318–12327. doi: 10.1074/jbc.M808850200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfuhl M., Gautel M. Structure, interactions and function of the N-terminus of cardiac myosin binding protein C (MyBP-C): who does what, with what, and to whom? J. Muscle Res. Cell Motil. 2012;33:83–94. doi: 10.1007/s10974-012-9291-z. [DOI] [PubMed] [Google Scholar]
- 19.Flashman E., Redwood C., Watkins H. Cardiac myosin binding protein C: its role in physiology and disease. Circ. Res. 2004;94:1279–1289. doi: 10.1161/01.RES.0000127175.21818.C2. [DOI] [PubMed] [Google Scholar]
- 20.Sadayappan S., de Tombe P.P. Cardiac myosin binding protein-C: redefining its structure and function. Biophys. Rev. 2012;4:93–106. doi: 10.1007/s12551-012-0067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geist J., Kontrogianni-Konstantopoulos A. MYBPC1, an emerging myopathic gene: what we know and what we need to learn. Front. Physiol. 2016;7:410. doi: 10.3389/fphys.2016.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris S.P., Bartley C.R., Moss R.L. Hypertrophic cardiomyopathy in cardiac myosin binding protein-C knockout mice. Circ. Res. 2002;90:594–601. doi: 10.1161/01.res.0000012222.70819.64. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann P.A., Greaser M.L., Moss R.L. C-protein limits shortening velocity of rabbit skeletal muscle fibres at low levels of Ca2+ activation. J. Physiol. 1991;439:701–715. doi: 10.1113/jphysiol.1991.sp018689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korte F.S., McDonald K.S., Moss R.L. Loaded shortening, power output, and rate of force redevelopment are increased with knockout of cardiac myosin binding protein-C. Circ. Res. 2003;93:752–758. doi: 10.1161/01.RES.0000096363.85588.9A. [DOI] [PubMed] [Google Scholar]
- 25.Previs M.J., Mun J.Y., Craig R. Phosphorylation and calcium antagonistically tune myosin-binding protein C’s structure and function. Proc. Natl. Acad. Sci. USA. 2016;113:3239–3244. doi: 10.1073/pnas.1522236113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunst G., Kress K.R., Fink R.H. Myosin binding protein C, a phosphorylation-dependent force regulator in muscle that controls the attachment of myosin heads by its interaction with myosin S2. Circ. Res. 2000;86:51–58. doi: 10.1161/01.res.86.1.51. [DOI] [PubMed] [Google Scholar]
- 27.Herron T.J., Rostkova E., Kentish J.C. Activation of myocardial contraction by the N-terminal domains of myosin binding protein-C. Circ. Res. 2006;98:1290–1298. doi: 10.1161/01.RES.0000222059.54917.ef. [DOI] [PubMed] [Google Scholar]
- 28.Kampourakis T., Yan Z., Irving M. Myosin binding protein-C activates thin filaments and inhibits thick filaments in heart muscle cells. Proc. Natl. Acad. Sci. USA. 2014;111:18763–18768. doi: 10.1073/pnas.1413922112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mun J.Y., Previs M.J., Craig R. Myosin-binding protein C displaces tropomyosin to activate cardiac thin filaments and governs their speed by an independent mechanism. Proc. Natl. Acad. Sci. USA. 2014;111:2170–2175. doi: 10.1073/pnas.1316001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gigli M., Begay R.L., Mestroni L. A review of the giant protein titin in clinical molecular diagnostics of cardiomyopathies. Front Cardiovasc. Med. 2016;3:21. doi: 10.3389/fcvm.2016.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labeit S., Gautel M., Trinick J. Towards a molecular understanding of titin. EMBO J. 1992;11:1711–1716. doi: 10.1002/j.1460-2075.1992.tb05222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukuda N., Granzier H.L., Kurihara S. Physiological functions of the giant elastic protein titin in mammalian striated muscle. J. Physiol. Sci. 2008;58:151–159. doi: 10.2170/physiolsci.RV005408. [DOI] [PubMed] [Google Scholar]
- 33.Tskhovrebova L., Trinick J. Roles of titin in the structure and elasticity of the sarcomere. J. Biomed. Biotechnol. 2010;2010:612482. doi: 10.1155/2010/612482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehman W., Szent-Györgyi A.G. Regulation of muscular contraction. Distribution of actin control and myosin control in the animal kingdom. J. Gen. Physiol. 1975;66:1–30. doi: 10.1085/jgp.66.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sellers J.R. Regulation of cytoplasmic and smooth muscle myosin. Curr. Opin. Cell Biol. 1991;3:98–104. doi: 10.1016/0955-0674(91)90171-t. [DOI] [PubMed] [Google Scholar]
- 36.Somlyo A.V., Khromov A.S., Somlyo A.P. Smooth muscle myosin: regulation and properties. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004;359:1921–1930. doi: 10.1098/rstb.2004.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamm K.E., Stull J.T. Signaling to myosin regulatory light chain in sarcomeres. J. Biol. Chem. 2011;286:9941–9947. doi: 10.1074/jbc.R110.198697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sweeney H.L., Bowman B.F., Stull J.T. Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function. Am. J. Physiol. 1993;264:C1085–C1095. doi: 10.1152/ajpcell.1993.264.5.C1085. [DOI] [PubMed] [Google Scholar]
- 39.Ikebe M., Hartshorne D.J. Phosphorylation of smooth muscle myosin at two distinct sites by myosin light chain kinase. J. Biol. Chem. 1985;260:10027–10031. [PubMed] [Google Scholar]
- 40.Wendt T., Taylor D., Taylor K. Three-dimensional image reconstruction of dephosphorylated smooth muscle heavy meromyosin reveals asymmetry in the interaction between myosin heads and placement of subfragment 2. Proc. Natl. Acad. Sci. USA. 2001;98:4361–4366. doi: 10.1073/pnas.071051098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woodhead J.L., Zhao F.Q., Padrón R. Atomic model of a myosin filament in the relaxed state. Nature. 2005;436:1195–1199. doi: 10.1038/nature03920. [DOI] [PubMed] [Google Scholar]
- 42.Alamo L., Wriggers W., Padrón R. Three-dimensional reconstruction of tarantula myosin filaments suggests how phosphorylation may regulate myosin activity. J. Mol. Biol. 2008;384:780–797. doi: 10.1016/j.jmb.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woodhead J.L., Zhao F.Q., Craig R. Structural basis of the relaxed state of a Ca2+-regulated myosin filament and its evolutionary implications. Proc. Natl. Acad. Sci. USA. 2013;110:8561–8566. doi: 10.1073/pnas.1218462110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jung H.S., Burgess S.A., Knight P.J. Conservation of the regulated structure of folded myosin 2 in species separated by at least 600 million years of independent evolution. Proc. Natl. Acad. Sci. USA. 2008;105:6022–6026. doi: 10.1073/pnas.0707846105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung H.S., Komatsu S., Craig R. Head-head and head-tail interaction: a general mechanism for switching off myosin II activity in cells. Mol. Biol. Cell. 2008;19:3234–3242. doi: 10.1091/mbc.E08-02-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zoghbi M.E., Woodhead J.L., Craig R. Three-dimensional structure of vertebrate cardiac muscle myosin filaments. Proc. Natl. Acad. Sci. USA. 2008;105:2386–2390. doi: 10.1073/pnas.0708912105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al-Khayat H.A., Kensler R.W., Morris E.P. Atomic model of the human cardiac muscle myosin filament. Proc. Natl. Acad. Sci. USA. 2013;110:318–323. doi: 10.1073/pnas.1212708110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huxley H.E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J. Mol. Biol. 1967;30:383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- 49.Clinch N.F. On the increase in rate of heat production caused by stretch in frog’s skeletal muscle. J. Physiol. 1968;196:397–414. doi: 10.1113/jphysiol.1968.sp008514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kushmerick M.J., Paul R.J. Aerobic recovery metabolism following a single isometric tetanus in frog sartorius muscle at 0°C. J. Physiol. 1976;254:693–709. doi: 10.1113/jphysiol.1976.sp011253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferenczi M.A., Homsher E., Trentham D.R. Reaction mechanism of the magnesium ion-dependent adenosine triphosphatase of frog muscle myosin and subfragment 1. Biochem. J. 1978;171:165–175. doi: 10.1042/bj1710165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Myburgh K.H., Franks-Skiba K., Cooke R. Nucleotide turnover rate measured in fully relaxed rabbit skeletal muscle myofibrils. J. Gen. Physiol. 1995;106:957–973. doi: 10.1085/jgp.106.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stewart M.A., Franks-Skiba K., Cooke R. Myosin ATP turnover rate is a mechanism involved in thermogenesis in resting skeletal muscle fibers. Proc. Natl. Acad. Sci. USA. 2010;107:430–435. doi: 10.1073/pnas.0909468107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naber N., Cooke R., Pate E. Slow myosin ATP turnover in the super-relaxed state in tarantula muscle. J. Mol. Biol. 2011;411:943–950. doi: 10.1016/j.jmb.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilson C., Naber N., Cooke R. The myosin inhibitor blebbistatin stabilizes the super-relaxed state in skeletal muscle. Biophys. J. 2014;107:1637–1646. doi: 10.1016/j.bpj.2014.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haselgrove J.C., Huxley H.E. X-ray evidence for radial cross-bridge movement and for the sliding filament model in actively contracting skeletal muscle. J. Mol. Biol. 1973;77:549–568. doi: 10.1016/0022-2836(73)90222-2. [DOI] [PubMed] [Google Scholar]
- 57.von der Ecken J., Heissler S.M., Raunser S. Cryo-EM structure of a human cytoplasmic actomyosin complex at near-atomic resolution. Nature. 2016;534:724–728. doi: 10.1038/nature18295. [DOI] [PubMed] [Google Scholar]
- 58.Hu Z., Taylor D.W., Taylor K.A. Structure of myosin filaments from relaxed Lethocerus flight muscle by cryo-EM at 6 Å resolution. Sci. Adv. 2016;2:e1600058. doi: 10.1126/sciadv.1600058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haselgrove J.C. X-ray evidence for conformational changes in the myosin filaments of vertebrate striated muscle. J. Mol. Biol. 1975;92:113–143. doi: 10.1016/0022-2836(75)90094-7. [DOI] [PubMed] [Google Scholar]
- 60.Reconditi M., Brunello E., Piazzesi G. Sarcomere-length dependence of myosin filament structure in skeletal muscle fibres of the frog. J. Physiol. 2014;592:1119–1137. doi: 10.1113/jphysiol.2013.267849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reconditi M., Brunello E., Irving M. Motion of myosin head domains during activation and force development in skeletal muscle. Proc. Natl. Acad. Sci. USA. 2011;108:7236–7240. doi: 10.1073/pnas.1018330108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Irving M., Piazzesi G., Lombardi V. Conformation of the myosin motor during force generation in skeletal muscle. Nat. Struct. Biol. 2000;7:482–485. doi: 10.1038/75890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fusi L., Huang Z., Irving M. The conformation of myosin heads in relaxed skeletal muscle: implications for myosin-based regulation. Biophys. J. 2015;109:783–792. doi: 10.1016/j.bpj.2015.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huxley H.E., Stewart A., Irving T. X-ray diffraction measurements of the extensibility of actin and myosin filaments in contracting muscle. Biophys. J. 1994;67:2411–2421. doi: 10.1016/S0006-3495(94)80728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wakabayashi K., Sugimoto Y., Amemiya Y. X-ray diffraction evidence for the extensibility of actin and myosin filaments during muscle contraction. Biophys. J. 1994;67:2422–2435. doi: 10.1016/S0006-3495(94)80729-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reconditi M., Linari M., Lombardi V. The myosin motor in muscle generates a smaller and slower working stroke at higher load. Nature. 2004;428:578–581. doi: 10.1038/nature02380. [DOI] [PubMed] [Google Scholar]
- 67.Sun Y.-B., Brandmeier B., Irving M. Structural changes in troponin in response to Ca2+ and myosin binding to thin filaments during activation of skeletal muscle. Proc. Natl. Acad. Sci. USA. 2006;103:17771–17776. doi: 10.1073/pnas.0605430103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fusi L., Brunello E., Irving M. Thick filament mechano-sensing is a calcium-independent regulatory mechanism in skeletal muscle. Nat. Commun. 2016;7:13281. doi: 10.1038/ncomms13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malinchik S., Xu S., Yu L.C. Temperature-induced structural changes in the myosin thick filament of skinned rabbit psoas muscle. Biophys. J. 1997;73:2304–2312. doi: 10.1016/S0006-3495(97)78262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goldman Y.E., McCray J.A., Ranatunga K.W. Transient tension changes initiated by laser temperature jumps in rabbit psoas muscle fibres. J. Physiol. 1987;392:71–95. doi: 10.1113/jphysiol.1987.sp016770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Levine R.J., Kensler R.W., Sweeney H.L. Myosin light chain phosphorylation affects the structure of rabbit skeletal muscle thick filaments. Biophys. J. 1996;71:898–907. doi: 10.1016/S0006-3495(96)79293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao F.Q., Padrón R., Craig R. Blebbistatin stabilizes the helical order of myosin filaments by promoting the switch 2 closed state. Biophys. J. 2008;95:3322–3329. doi: 10.1529/biophysj.108.137067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu S., White H.D., Yu L.C. Stabilization of helical order in the thick filaments by blebbistatin: further evidence of coexisting multiple conformations of myosin. Biophys. J. 2009;96:3673–3681. doi: 10.1016/j.bpj.2009.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martyn D.A., Freitag C.J., Gordon A.M. Ca2+ and cross-bridge-induced changes in troponin C in skinned skeletal muscle fibers: effects of force inhibition. Biophys. J. 1999;76:1480–1493. doi: 10.1016/S0006-3495(99)77308-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brandt P.W., Poggesi C. Clusters of bound Ca2+ initiate contraction in fast skeletal muscle. Arch. Biochem. Biophys. 2014;552-553:60–67. doi: 10.1016/j.abb.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 76.Sun Y.-B., Lou F., Irving M. Calcium- and myosin-dependent changes in troponin structure during activation of heart muscle. J. Physiol. 2009;587:155–163. doi: 10.1113/jphysiol.2008.164707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kampourakis T., Sun Y.-B., Irving M. Myosin light chain phosphorylation enhances contraction of heart muscle via structural changes in both thick and thin filaments. Proc. Natl. Acad. Sci. USA. 2016;113:E3039–E3047. doi: 10.1073/pnas.1602776113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Tombe P.P., Stienen G.J. Impact of temperature on cross-bridge cycling kinetics in rat myocardium. J. Physiol. 2007;584:591–600. doi: 10.1113/jphysiol.2007.138693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huxley H.E., Faruqi A.R., Koch M.H.J. Time-resolved x-ray diffraction studies of the myosin layer-line reflections during muscle contraction. J. Mol. Biol. 1982;158:637–684. doi: 10.1016/0022-2836(82)90253-4. [DOI] [PubMed] [Google Scholar]
- 80.Kress M., Huxley H.E., Hendrix J. Structural changes during activation of frog muscle studied by time-resolved x-ray diffraction. J. Mol. Biol. 1986;188:325–342. doi: 10.1016/0022-2836(86)90158-0. [DOI] [PubMed] [Google Scholar]
- 81.Caputo C., Edman K.A., Sun Y.-B. Variation in myoplasmic Ca2+ concentration during contraction and relaxation studied by the indicator fluo-3 in frog muscle fibres. J. Physiol. 1994;478:137–148. doi: 10.1113/jphysiol.1994.sp020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun Y.B., Lou F., Edman K.A. The relationship between the intracellular Ca2+ transient and the isometric twitch force in frog muscle fibres. Exp. Physiol. 1996;81:711–724. doi: 10.1113/expphysiol.1996.sp003971. [DOI] [PubMed] [Google Scholar]
- 83.Brunello E., Bianco P., Lombardi V. Structural changes in the myosin filament and cross-bridges during active force development in single intact frog muscle fibres: stiffness and x-ray diffraction measurements. J. Physiol. 2006;577:971–984. doi: 10.1113/jphysiol.2006.115394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matsuo T., Yagi N. Structural changes in the muscle thin filament during contractions caused by single and double electrical pulses. J. Mol. Biol. 2008;383:1019–1036. doi: 10.1016/j.jmb.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 85.Matsuo T., Iwamoto H., Yagi N. Monitoring the structural behavior of troponin and myoplasmic free Ca2+ concentration during twitch of frog skeletal muscle. Biophys. J. 2010;99:193–200. doi: 10.1016/j.bpj.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bagshaw C.R., Reed G.H. The significance of the slow dissociation of divalent metal ions from myosin ‘regulatory’ light chains. FEBS Lett. 1977;81:386–390. doi: 10.1016/0014-5793(77)80560-7. [DOI] [PubMed] [Google Scholar]
- 87.Holroyde M.J., Potter J.D., Solaro R.J. The calcium binding properties of phosphorylated and unphosphorylated cardiac and skeletal myosins. J. Biol. Chem. 1979;254:6478–6482. [PubMed] [Google Scholar]
- 88.Labeit D., Watanabe K., Granzier H. Calcium-dependent molecular spring elements in the giant protein titin. Proc. Natl. Acad. Sci. USA. 2003;100:13716–13721. doi: 10.1073/pnas.2235652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bremel R.D., Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat. New Biol. 1972;238:97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- 90.McKillop D.F., Geeves M.A. Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys. J. 1993;65:693–701. doi: 10.1016/S0006-3495(93)81110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tamura T., Wakayama J., Iwamoto H. Dynamics of thin-filament activation in rabbit skeletal muscle fibers examined by time-resolved x-ray diffraction. Biophys. J. 2009;96:1045–1055. doi: 10.1016/j.bpj.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ferguson R.E., Sun Y.-B., Irving M. In situ orientations of protein domains: troponin C in skeletal muscle fibers. Mol. Cell. 2003;11:865–874. doi: 10.1016/s1097-2765(03)00096-0. [DOI] [PubMed] [Google Scholar]
- 93.Fusi L., Brunello E., Irving M. Structural dynamics of troponin during activation of skeletal muscle. Proc. Natl. Acad. Sci. USA. 2014;111:4626–4631. doi: 10.1073/pnas.1321868111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Linari M., Brunello E., Irving M. Force generation by skeletal muscle is controlled by mechanosensing in myosin filaments. Nature. 2015;528:276–279. doi: 10.1038/nature15727. [DOI] [PubMed] [Google Scholar]
- 95.Hill D.K. Tension due to interaction between the sliding filaments in resting striated muscle. The effect of stimulation. J. Physiol. 1968;199:637–684. doi: 10.1113/jphysiol.1968.sp008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ford L.E., Huxley A.F., Simmons R.M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J. Physiol. 1977;269:441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lombardi V., Menchetti G. The maximum velocity of shortening during the early phases of the contraction in frog single muscle fibres. J. Muscle Res. Cell Motil. 1984;5:503–513. doi: 10.1007/BF00713257. [DOI] [PubMed] [Google Scholar]
- 98.Huxley A.F. Muscle structure and theories of contraction. Prog. Biophys. Biophys. Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- 99.Nyitrai M., Geeves M.A. Adenosine diphosphate and strain sensitivity in myosin motors. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004;359:1867–1877. doi: 10.1098/rstb.2004.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Greenberg M.J., Arpağ G., Ostap E.M. A perspective on the role of myosins as mechanosensors. Biophys. J. 2016;110:2568–2576. doi: 10.1016/j.bpj.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Piazzesi G., Reconditi M., Lombardi V. Skeletal muscle performance determined by modulation of number of myosin motors rather than motor force or stroke size. Cell. 2007;131:784–795. doi: 10.1016/j.cell.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 102.Fusi L., Percario V., Piazzesi G. Minimum number of myosin motors accounting for shortening velocity under zero load in skeletal muscle. J. Physiol. 2017;595:1127–1142. doi: 10.1113/JP273299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cecchi G., Colomo F., Lombardi V. Force-velocity relation in normal and nitrate-treated frog single muscle fibres during rise of tension in an isometric tetanus. J. Physiol. 1978;285:257–273. doi: 10.1113/jphysiol.1978.sp012570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marcucci L., Reggiani C. Mechanosensing in myosin filament solves a 60 years old conflict in skeletal muscle modeling between high power output and slow rise in tension. Front. Physiol. 2016;7:427. doi: 10.3389/fphys.2016.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Homsher E., Irving M., Wallner A. High-energy phosphate metabolism and energy liberation associated with rapid shortening in frog skeletal muscle. J. Physiol. 1981;321:423–436. doi: 10.1113/jphysiol.1981.sp013994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Caremani M., Melli L., Linari M. The working stroke of the myosin II motor in muscle is not tightly coupled to release of orthophosphate from its active site. J. Physiol. 2013;591:5187–5205. doi: 10.1113/jphysiol.2013.257410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Konishi M. Cytoplasmic free concentrations of Ca2+ and Mg2+ in skeletal muscle fibers at rest and during contraction. Jpn. J. Physiol. 1998;48:421–438. doi: 10.2170/jjphysiol.48.421. [DOI] [PubMed] [Google Scholar]
- 108.Rosenfeld S.S., Taylor E.W. Kinetic studies of calcium binding to regulatory complexes from skeletal muscle. J. Biol. Chem. 1985;260:252–261. [PubMed] [Google Scholar]
- 109.Huxley A.F., Simmons R.M. Rapid ‘give’ and the tension ‘shoulder’ in the relaxation of frog muscle fibres. J. Physiol. 1970;210:32P–33P. [PubMed] [Google Scholar]
- 110.Edman K.A., Flitney F.W. Laser diffraction studies of sarcomere dynamics during ‘isometric’ relaxation in isolated muscle fibres of the frog. J. Physiol. 1982;329:1–20. doi: 10.1113/jphysiol.1982.sp014287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brunello E., Fusi L., Irving M. Structural changes in myosin motors and filaments during relaxation of skeletal muscle. J. Physiol. 2009;587:4509–4521. doi: 10.1113/jphysiol.2009.176222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Poggesi C., Tesi C., Stehle R. Sarcomeric determinants of striated muscle relaxation kinetics. Pflugers Arch. 2005;449:505–517. doi: 10.1007/s00424-004-1363-5. [DOI] [PubMed] [Google Scholar]
- 113.Stehle R. Force responses and sarcomere dynamics of cardiac myofibrils induced by rapid changes in [Pi] Biophys. J. 2017;112:356–367. doi: 10.1016/j.bpj.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Campbell K.S. Compliance accelerates relaxation in muscle by allowing myosin heads to move relative to actin. Biophys. J. 2016;110:661–668. doi: 10.1016/j.bpj.2015.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lombardi V., Piazzesi G., Goldman Y.E. Following tetanic stimulation, relaxation is accelerated by quick stretches of frog single muscle fibers. J. Physiol. 1990;426:38. [Google Scholar]
- 116.Tesi C., Piroddi N., Poggesi C. Relaxation kinetics following sudden Ca2+ reduction in single myofibrils from skeletal muscle. Biophys. J. 2002;83:2142–2151. doi: 10.1016/S0006-3495(02)73974-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dantzig J.A., Goldman Y.E., Homsher E. Reversal of the cross-bridge force-generating transition by photogeneration of phosphate in rabbit psoas muscle fibres. J. Physiol. 1992;451:247–278. doi: 10.1113/jphysiol.1992.sp019163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Linari M., Woledge R.C., Curtin N.A. Energy storage during stretch of active single fibres from frog skeletal muscle. J. Physiol. 2003;548:461–474. doi: 10.1113/jphysiol.2002.032185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Siegman M.J., Funabara D., Butler T.M. Phosphorylation of a twitchin-related protein controls catch and calcium sensitivity of force production in invertebrate smooth muscle. Proc. Natl. Acad. Sci. USA. 1998;95:5383–5388. doi: 10.1073/pnas.95.9.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Murphy R.A., Rembold C.M. The latch-bridge hypothesis of smooth muscle contraction. Can. J. Physiol. Pharmacol. 2005;83:857–864. doi: 10.1139/y05-090. [DOI] [PMC free article] [PubMed] [Google Scholar]