Abstract
Angiogenesis is the process through which new blood vessels are formed, while therapeutic angiogenesis aims to promote and control the angiogenic response. Ischemia results from the lack of blood flow with oxygen and nutrients. Therapeutic angiogenesis is crucial in preserving brain tissue and bodily functions after ischemic stroke. Various approaches have been proposed to promote angiogenesis in ischemic diseases. Traditional protein/gene and subsequent stem/progenitor cell approaches have not shown consistent efficacy for ischemic diseases in clinical trials. Exosomes are microparticles secreted from cells and conduct cell-cell communication including stem cell or cancer cell induced pro-angiogenesis. Utilization of exogenous exosomes for the treatment of ischemic diseases is an emerging approach which may prevent certain disadvantages such as easy degradation and tumor formation happened in other strategies. This review highlights recent reports on the use of exosomes as a therapeutic agent to promote angiogenesis in ischemic stroke.
Keywords: Exosome, ischemic stroke, angiogenesis
Background
Ischemic diseases are the leading cause of disabilities and deaths due to the reduction in the blood supply that limits the transfer of oxygen, uptake of nutrients, and removal of metabolic waste. Oxygen and nutrients are vital for cellular metabolism and bodily functions. Ischemic diseases caused by insufficient blood delivery include coronary artery disease, ischemic stroke, peripheral artery disease, diabetic ulcers, chronic wounds, etc. Ischemic stroke is a common cerebrovascular disease accounting for more than 80% of all strokes [1]. Thrombotic stroke is a result of platelet coagulation that creates a blood clot blocking the blood flow to the brain. Embolic stroke occurs when a transient or permanent blockage forms in an artery from an emboli that travels to the brain and narrows the arterial lumen and therefore limits the blood supply [2]. Both forms are severe conditions that require immediate medical treatment.
In response to insufficient blood supply, the body undergoes angiogenesis as natural processes to restore blood flow. Inspired by this physiological or pathological occurrence, researchers have leaned towards stimulating and enhancing the efficiency of angiogenesis as a treatment strategy. Therapeutic angiogenesis, is a concept that introduces an agent to pharmacologically facilitate the stimulation of the growth of new blood vessels to restore circulation to the ischemic tissue. This therapeutic option is currently being used to combat all ischemic diseases including ischemic stroke. Protein/gene therapy, stem/progenitor cell therapy and exosome/microvesicle therapy are approaches employed to induce this therapeutic response.
Previous studies have revealed that exosomes may be a novel agent to promote angiogenesis [3]. Exosomes are cell-secreted, endosome-derived membrane microvesicles, approximately 30 to 100 nm in diameter that contain mRNA and microRNAs, lipids and proteins. In addition to various biological functions, exosomes can be used as biomarkers for diagnosis, evaluation of prognosis and efficacy, and directly as therapeutic agents [3].
Therapeutic angiogenesis using exosomes
Exosomes as a therapeutic agent gained much attention due to the clinically relevant outcomes of stem cell therapy. Stem cell therapy has been implied to promote angiogenesis and these findings are believed via paracrine/autocrine mechanisms. Exosomes, secreted from cells, have been investigated and acknowledged as key mediators of cell-to-cell communication [4]. Exosomes are smaller in diameter and are feasible for transport across membranes to deliver their cargo, and can circumvent the risk of tumorigenesis that have been observed with stem cells. Exosomes moderate signal transduction in both autocrine and paracrine styles by the transfer of proteins and RNA essential for angiogenesis to take place under an ischemic condition. Proteins and microRNAs are the major regulators found in exosomes that promote angiogenesis through intracellular communication that influences growth factors and gene expression. In an ischemic myocardial model, researchers have determined the rationale for angiogenesis via the expression of pro-angiogenic growth factors and upregulation of microRNAs [5]. Therefore, exosomes can promote angiogenesis by upregulation of pro-angiogenic molecules and down-regulation of anti-angiogenic molecules.
More importantly, studies have shown that exosomes can be secreted from mesenchymal stem cells (MSCs), cardiac progenitor cells, endometriotic stromal cells and human induced pluripotent stem cells (iPSCs) to promote angiogenesis [5-8]. In a mouse hindlimb ischemia model, exosomes derived from iPSC-derived MSCs increased the microvessel density thus inducing angiogenesis [8]. Similar exosomes secreted from human adipose-derived MSCs promotes angiogenesis determined by the increase of tube length and number of branches [9]. These studies support the use of exosomes as a pro-angiogenic therapeutic option in a variety of ischemic diseases.
Therapeutic angiogenesis for ischemic stroke
In addition to administration of thrombolytics, such as recombinant tissue plasminogen activator, for ischemic stroke, pro-angiogenesis has been proposed to protect the brain tissue by increasing blood supply. Angiogenesis together with neurogenesis and synaptic plasticity are the crucial processes in the recovery of ischemic stroke [10].
The use of protein or stem cell therapy to enhance therapeutic angiogenesis has been immensely studied. Vascular endothelial growth factor (VEGF) is a known potent angiopeptide. However, there has been controversial results on the use of VEGF as a protein and gene therapy in ischemic models. Navaratna et al., have shown in a rat stroke model that VEGF stimulated angiogenesis which was suggested based on the reduction of infarct volume [10]. Conversely, studies have also shown VEGF to be detrimental as it may lead to blood-brain barrier leakage, brain edema, vasodilation and aberrant systemic hemodynamics [11]. On the contrary, studies have indicated the use of stem cells, such as adipose stem cells (ACS) to induce angiogenesis in an ischemic model. In an ischemic model, xenogeneic ASCs and allogeneic ASCs have been proven to increase functional recovery, the prevention of ischemic brain damage, and the enhancement of angiogenesis [12].
Exosomes promote angiogenesis for ischemic stroke
The use of exosomes in ischemic stroke has acquired much attention recently. Xin H, et al. investigated the effects of multipotent MSC derived exosomes on neurovascular remodeling and brain function after a middle cerebral artery occlusion (MCAo) in rats. After 24 hours of the stroke, 100 μg protein of MSC-generated exosomes in 0.5 mL phosphate-buffered saline were injected through the tail vein. The rat’s forebrain tissues were analyzed 28 days after the stroke. Exosomes were observed to promote proliferation of cerebral endothelial cells by measuring bromodeoxyuridine (BrdU) and von Willebrand factor (vWF) positive cells compared to treatment with phosphate-buffered saline only. The number of vWF positive cells, as a measure of blood vessel formation, was increased, suggesting improved vascular network [13]. In another report, MSCs and MSC-derived exosomes were examined on the recovery of striatum brain tissue after focal cerebral ischemia in mice. The mice received normal saline, 1 × 106 of MSCs or MSC-exosomes released from 2 × 106 MSCs, 24 hours after stroke. The cells or exosomes were delivered through the right femoral vein. On the third and fourth day after the surgery, both MSCs and MSC-exosomes were shown to enhance CD31+ and BrdU+ merged cells indicating new vascular endothelial cells. In addition, the findings reveal that there is no significant difference between MSC-treated and MSC-exosomes treated groups. These findings support the idea that MSC-exosomes can be used in place of MSCs [14].
Further studies on the preconditioned exosome-donor MSCs have been performed. MSCs pre-treated with Buyang Huanwu decoction (BYHWD), a Chinese medicine, have been investigated on the promotion of angiogenesis in a rat stroke model. The results demonstrated that administration of 400 μg/kg exosomes from BYHWD-pretreated MSCs increased VEGF and Ki-67 expression levels and enhanced vascular density in rat brain after bilateral carotid artery ligation. Mechanistic studies further revealed that these exosomes promoted the expression of VEGF, pro-angiogenic microRNA-126, but reduced the expression of antiangiogenic microRNA, miRNA-221 and miRNA-222 in vascular endothelial cells, while the expression of VEGF were not significant induced in fibroblasts [15]. In another study, angiogenic effects of three different exosomes in an ischemic stroke rat model were examined: exosomes from untreated MSCs, exosomes from MSCs pretreated with normal rat brain extract (NBE-MSC-Ex), and exosomes from MSCs pretreated with stroke-injured brain extract (SBE-MSC-Ex). The rats were injected with a single dose of 0.2 mg/kg of the different exosomes via the right common carotid artery after 48 hours of a permanent MCAo. Angiogenesis was determined based on the proliferation of α-smooth muscle actin, a label of vascular smooth muscle cells. α-smooth muscle actin positive cells significantly increased in NBE-MSC-Ex treated compared to control groups. There was no significant difference in infarct lesion volume between groups treated with NBE-MSC-Ex and SBE-MSC-Ex [16].
Conclusion
Administrations of exosomes from MSCs have been reported to promote angiogenesis in ischemic brain tissue in rodent stroke models (Figure 1). The pro-angiogenic effects can be enhanced by pretreatment of exosome donor cells. VEGF and certain angiogenesis related miRNAs in exosomes may be involved in this effect. Engineered exosomes and exosomes from other donor cells may be examined in the future. Further studies in underlining molecular mechanisms of exosomal angiogenesis are required. Investigation of therapeutic angiogenesis of exosomes in large animal stroke models need to be performed before clinical trials.
Figure 1.
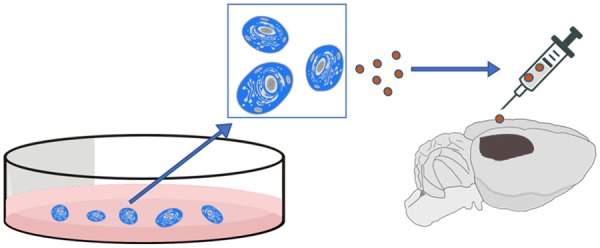
Schematic diagram for the treatment of ischemic stroke with exosomes.
Disclosure of conflict of interest
None.
References
- 1.Writing Group Members; Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart disease and stroke statistics-2016 update: a report from the American heart association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Zhao L, Johnson T, Liu D. Therapeutic angiogenesis of adipose-derived stem cells for ischemic diseases. Stem Cell Res Ther. 2017;8:125. doi: 10.1186/s13287-017-0578-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J, Sun X, Zhao J, Yang Y, Cai X, Xu J, Cao P. Exosomes: a novel strategy for treatment and prevention of diseases. Front Pharmacol. 2017;8:300. doi: 10.3389/fphar.2017.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gangoda L, Boukouris S, Liem M, Kalra H, Mathivanan S. Extracellular vesicles including exosomes are mediators of signal transduction: are they protective or pathogenic? Proteomics. 2015;15:260–271. doi: 10.1002/pmic.201400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray WD, French KM, Ghosh-Choudhary S, Maxwell JT, Brown ME, Platt MO, Searles CD, Davis ME. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ Res. 2015;116:255–263. doi: 10.1161/CIRCRESAHA.116.304360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, De Veirman K, Faict S, Frassanito MA, Ribatti D, Vacca A, Menu E. Multiple myeloma exosomes establish a favourable bone marrow microenvironment with enhanced angiogenesis and immunosuppression. J Pathol. 2016;239:162–173. doi: 10.1002/path.4712. [DOI] [PubMed] [Google Scholar]
- 7.Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Hu GW, Li Q, Niu X, Hu B, Liu J, Zhou SM, Guo SC, Lang HL, Zhang CQ, Wang Y, Deng ZF. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res Ther. 2015;6:10. doi: 10.1186/scrt546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang X, Zhang L, Wang S, Han Q, Zhao RC. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci. 2016;129:2182–2189. doi: 10.1242/jcs.170373. [DOI] [PubMed] [Google Scholar]
- 10.Navaratna D, Guo S, Arai K, Lo EH. Mechanisms and targets for angiogenic therapy after stroke. Cell Adh Migr. 2009;3:216–223. doi: 10.4161/cam.3.2.8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005;437:497–504. doi: 10.1038/nature03987. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez-Fernandez M, Rodriguez-Frutos B, Ramos-Cejudo J, Otero-Ortega L, Fuentes B, Vallejo-Cremades MT, Sanz-Cuesta BE, Diez-Tejedor E. Comparison between xenogeneic and allogeneic adipose mesenchymal stem cells in the treatment of acute cerebral infarct: proof of concept in rats. J Transl Med. 2015;13:46. doi: 10.1186/s12967-015-0406-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33:1711–1715. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doeppner TR, Herz J, Gorgens A, Schlechter J, Ludwig AK, Radtke S, de Miroschedji K, Horn PA, Giebel B, Hermann DM. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med. 2015;4:1131–1143. doi: 10.5966/sctm.2015-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J, Gao F, Zhang Y, Liu Y, Zhang D. Buyang huanwu decoction (BYHWD) enhances angiogenic effect of mesenchymal stem cell by upregulating VEGF expression after focal cerebral ischemia. J Mol Neurosci. 2015;56:898–906. doi: 10.1007/s12031-015-0539-0. [DOI] [PubMed] [Google Scholar]
- 16.Lee JY, Kim E, Choi SM, Kim DW, Kim KP, Lee I, Kim HS. Microvesicles from brain-extracttreated mesenchymal stem cells improve neurological functions in a rat model of ischemic stroke. Sci Rep. 2016;6:33038. doi: 10.1038/srep33038. [DOI] [PMC free article] [PubMed] [Google Scholar]


