Abstract
It is unclear how social drinking can contribute to the development of addiction in susceptible individuals. However, alcohol’s aversive properties are a well-known factor contributing to its abuse. The lateral habenula (LHb) is a key brain structure responding to various aversive stimuli, including those related to alcohol. We recently reported that ethanol at 10 mM or less that can be achieved by social drinking activates many LHb neurons and drives aversive conditioning. The current study sought to identify LHb circuits that are activated by a low-dose of ethanol using immunohistochemistry and anatomic tracing techniques on adult Sprague-Dawley rats. We showed here that an intraperitoneal injection of ethanol (0.25 g/kg), resulting in a blood ethanol concentration of 5.6 mM, significantly increased the number of cFos immunoreactive (IR) neurons in the LHb. Most of the ethanol-activated cFos-IR LHb neurons expressed vGluT2 (vesicular glutamate transporters 2, a marker of a glutamatergic phenotype). These LHb neurons projected to the ventral tegmental area (VTA), rostromedial tegmental nucleus (RMTg), and dorsal raphe. Moreover, injections of the anterograde tracer AAV-CaMKIIa-eGFP into the lateral hypothalamus produced a significant amount of labeled fibers with vGluT2 positive terminals on the ethanol-activated LHb cells. These results indicate that the LHb neurons stimulated by a low-dose of ethanol project to the VTA, RMTg, and dorsal raphe, and receive excitatory projections from the lateral hypothalamus. These neurocircuits may play a crucial role in mediating the initial aversive effects produced by a low-dose of ethanol.
Keywords: cFos, lateral hypothalamus, median raphe, dorsal raphe, RMTg, anterograde
Introduction
An estimated 4.9% of the world’s adult population suffers from alcohol use disorder (AUD) [1]. However, the mechanism underlying the transition from social drinking to AUD in susceptible individuals remains elusive [2-4]. Accumulating evidence suggests that alcohol’s aversive properties play important roles in relapse drinking and the development of AUD [5]. The lateral habenula (LHb), a relay station between the forebrain and hindbrain, has been found to encode aversive signals [6]. For example, LHb hyperactivity may represent a cellular mechanism underlying the depressive-like symptoms seen in AUD [7-9].
LHb activity has been linked to drugs of abuse and their associated aversive behaviors. About 95% of LHb neurons are glutamatergic [10-12]. The LHb receives inputs from the limbic areas and basal ganglia [13,14]. Neural signals from the LHb affect the ventral tegmental area (VTA) and the raphe nucleus directly, and indirectly through the rostromedial tegmental nucleus (RMTg) [15-18]. Stimulation of the LHb inhibits dopaminergic neurons, reducing dopamine-mediated reward signaling, which could account for the aversive effect of cocaine [19]. Also, the LHb is involved in ethanol-related aversive behaviors, such as conditional taste aversion (CTA), anxiety and depression [20-24]. We recently reported that low concentrations (10 mM or less) of ethanol could activate LHb neurons and contribute to conditioned place aversion [25]. Together, these studies suggest that alcohol’s aversive properties may involve the activation of LHb-related circuits.
In the current study, we examined cFos expression in the LHb and the related circuitries following the administration of a low-dose of ethanol, using a combination of anatomical tracing techniques and immunohistochemistry. We found that the LHb neurons stimulated by low-dose ethanol project to the VTA, RMTg, and raphe, and receive excitatory inputs from the lateral hypothalamus. These results offer new information regarding the targets of a low-dose of ethanol at the cellular and circuitry levels.
Material and method
Animals and housing
We conducted all experiments on adult male Sprague-Dawley rats (250-300 g at the start of the studies). Animals were housed two per cage under controlled temperature and illumination (12 h/12 h, light/dark cycle) with water and food ad libitum. All procedures were approved by the Animal Care and Utilization Committee of Rutgers, the State University of New Jersey, per National Institutes of Health guidelines, minimizing the number of animals used and their suffering.
Measurement of blood ethanol concentration
The blood ethanol concentrations (BECs) were measured as described [26]. Briefly, tail blood was collected 10 min after an intraperitoneal injection (i.p.) of 0.25 or 0.5 g/kg ethanol (12.5% w/v, prepared with normal saline) from an independent group of rats (n=7/group/time point). The samples were centrifuged at room temperature (21-22°C) for 15 minutes at 8000 rpm, and 10 μl serum from each blood sample was analyzed using NAD+-ADH enzyme spectrophotometry [27].
Induction of cFos expression by ethanol
To habituate the rats to the injection procedure, we gave them intraperitoneal injection (i.p.) of saline (1 ml/kg) per day) for two days. On the third day, we injected either saline or 12.5% (w/v) ethanol (0.25 or 0.5 g/kg) in isotonic saline to these rats. To examine the time course of cFos expression, at 30 min, 90 min, 4 h and 8 h post-injection, we sacrificed the rats under deep anesthesia with an overdose of sodium pentobarbital (50 mg/kg) and transcardially perfused with saline followed by 4% paraformaldehyde (PFA). We then harvested the brain tissues and post-fixed (overnight, at 4°C) in 4% PFA and cryoprotected. Serial 30-µm coronal sections were cut on a freezing microtome (Microm HM550, Walldorf) and collected in phosphate-buffered solution (PBS, pH 7.4). cFos expression was identified using immunohistochemistry (IHC) or immunofluorescence (IF) as described [28]. In brief, the tissue was incubated overnight with rabbit anti-cFos antibody (ABE457, EMD Millipore) diluted to 1:2000 in PBS with 0.25% Triton-X with 0.01% sodium azide at 4°C. For immunohistochemistry, after two hours of incubation with biotinylated anti-rabbit immunoglobulin G (1:200, Vector Laboratories), the brain sections were incubated with avidin-biotin-horseradish peroxidase complex (Vector Elite kit, Vector Laboratories) for 45 min and visualized using a diaminobenzidine (DAB) staining kit (Vector Laboratories). After that, sections were mounted, dehydrated, coverslipped and observed under a bright field microscope. For IF, the brain sections were first incubated with an anti-cFos antibody, mouse anti-NeuN (1:1000, ab104225, Abcam) or guinea pig anti-vGluT2 (1:1000, AB2251-I, EMD Millipore) antibody. These sections were then incubated in one or two of the following: (1) Goat anti-rabbit-Texas Red (1:200, Vector Laboratories), (2) Goat anti-mouse Texas Red (1:200, ab6787, Abcam), (3) Goat anti-rabbit- Alexa Flour 405 (1:200, Thermo Fisher Scientific) or (4) Goat anti-guinea pig Dylight 650 (1:200, Thermo Fisher Scientific) for two hours. Finally, we mounted and examined them under a Nikon Eclipse 90i fluorescence microscope or an A1R confocal microscope.
Stereotaxic surgeries and anatomical tracing
To identify the circuits of ethanol-activated LHb neurons, we injected 38 rats with retrobeads (Lumafluor, Naples, FL) into the VTA, the RMTg, and the dorsal raphe (DR), three major projection areas of the LHb [17,18,29,30]. We then examined cFos expression in the LHb in conjunction with the retrograde tracing. To determine whether the ethanol-sensitive LHb neurons receive projections from the lateral hypothalamus (LH), we injected adeno-associated virus type 5 (AAV5)-CaMKIIa-eGFP into the LH of 16 rats. The LH is known to send glutamatergic projections to the medial division of the LHb [31,32]. AAV vectors are well-accepted tools for anterograde tracing of axonal pathways in the CNS without causing gliosis or cellular inflammatory reactions [33].
We performed surgery using a stereotaxic apparatus (Kopf Instruments, Tujunga, CA) on rats under anesthesia, induced by ketamine/xylazine (80/20 mg/kg) and maintained with isoflurane, as described previously [34]. Tracers or viruses were loaded in glass pipettes (Science Products). For retrograde tracing, we injected either Red or Green Retrobeads IX (150~300 nl/side) to the stereotaxic coordinates relative to the bregma as follows (in mm): VTA: AP-6.0, DV-8.5, ML±2.1; RMTg: AP-7.1, DV-8.4, ML±0.8; DR: AP-8.5, ML 0, DV-6.6. We injected AAV5-eGFP (300 nl/side, 2.4×1010 gc/mL, Vector Core, UNC) into the LH: AP-2.6, DV-8.6, ML±1.5. All coordinates were in reference to the Paxinos and Watson rat brain atlas [35]. We delivered the tracers or viruses with the Nanoliter 2000 microinjection system (World Precision Instruments). To minimize tissue damage, we injected slowly at a speed of 50 nl/10 min. After each injection, we left the micropipette in place for an additional 10 min to prevent backflow. Then, we sealed the burr holes with sterile bone wax and sutured the scalp. The rats were then returned to their home cages. To enable optimal virus or tracer expression in the target region, the animals were kept under observation for two-to-three weeks following virus injection or for 9-11 days after tracer injections, respectively [33].
Imaging and cell counting
The ethanol-stimulated neurons were mapped using the cFos IHC approach in the whole habenula complex. For a neuron to be considered cFos-immunoreactive (IR), the nucleus must be stained with a characteristic dark brown dot in a round shape. We counted the numbers of IR cells per level in each rat for 5-6 sections with Nikon NIS software package under 10× magnification and averaged the results.
In all tracing experiments, we analyzed only data from animals with satisfactory tracing or with virus injection within the boundaries of the VTA, RMTg, DR, or LH. For each retrobeads-injected rat, we (two researchers who were blind to the rat’s treatment history) manually counted the LHb cells with retrobeads/cFos under 10× magnification in 8-9 sections and averaged. To avoid confounding from the difference in total number of retrogradely-labeled cells in the areas of interest among treatment groups, we used the number of double stained (retrobeads and NeuN) cells, as the total labeled neuron population. For the double-tracing experiments, we considered only those cells that showed a red (and blue) fluorescence restricted to the nucleus and distinct from the background as cFos fluorescence positive. Then two-dimensional overview pictures (tiles) or three-dimensional z stacks were acquired using a Nikon A1R confocal laser scanning microscope and three different laser lines (488, 543 and 633 nm). The contact sites between LHb cFos IR cells and LH axonal terminals were observed using a Nikon 60× (1.4 NA) oil immersion lens.
Statistical analysis
Analyses were performed using Sigmaplot 12.5 software (Systat Software). Statistical comparisons were carried out with paired or unpaired Student’s t-test or with a one-or two-way ANOVA followed by a post hoc Bonferroni or Tukey’s test as appropriate (two-tailed p < 0.05 was considered significant). All values shown in the text and figures are expressed as means ± SEM.
Results
Ethanol increases cFos expression in the LHb
To determine the effect of ethanol on LHb neurons and the time course, we conducted IHC staining and counted cFos IR (cFos+) cells in the LHb and its neighboring regions at 30 min, 90 min, 4 h and 8 h after ethanol administration. Compared to saline injection, a single ethanol administration (0.25 or 0.5 g/kg, i.p.) substantially increased cFos+ cell density in the LHb (Figure 1A-C), which was highest at 90 min, declined and then returned near baseline levels 4 to 8 h post-injection (Figure 1D). We observed no significant difference in the cFos+ cell density between 0.25 and 0.5 g/kg at any time points (all p > 0.05). Conversely, ethanol (0.25, and 0.5 g/kg, i.p.) did not cause a significant change in cFos+ cell density in the medial habenula (MHb, Figure 1E) and the posterior paraventricular thalamic nucleus (PVP, Figure 1F) at any time points (all p > 0.05). Moreover, ethanol (0.25 or 0.5 g/kg, i.p.) resulted in BEC of 5.6±0.3 and 13.0±1.2 mM, respectively at 10 min post injection, which decreased to 2.5±0.5 and 9.4±1.4 mM by 90 min. In the following experiments, we used ethanol at the dose of 0.25 g/kg.
Figure 1.
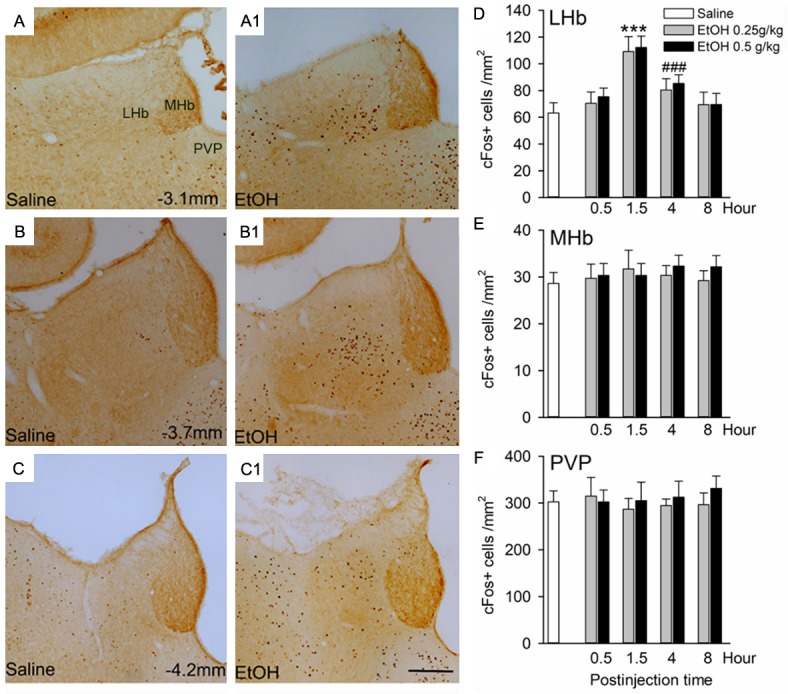
Ethanol causes a significant increase in cFos expression in the lateral habenula (LHb). Images of three rostrocaudal levels demonstrate cFos expression in the LHb, medial habenula (MHb) and paraventricular thalamic nucleus (PVP) at 90 min after an intraperitoneal injection of saline (A-C) or 0.25 g/kg ethanol (EtOH, A1-C1). (D-F)Summary graph of the increase of cFos immunoreactive cell density in the LHb, but not in the MHb, and PVP at 90 min, 4 h and 8 h after the injection of saline or ethanol (0.25 and 0.5 g/kg). Since there was no difference in cFos expression in the LHb of rats treated with saline across the various time points, the data across time points were collapsed for analysis. ***p < 0.001 vs. any other groups. ###p < 0.001 vs. saline. n=8-10 rats/group, unpaired t-test; Scale bar =200 μm.
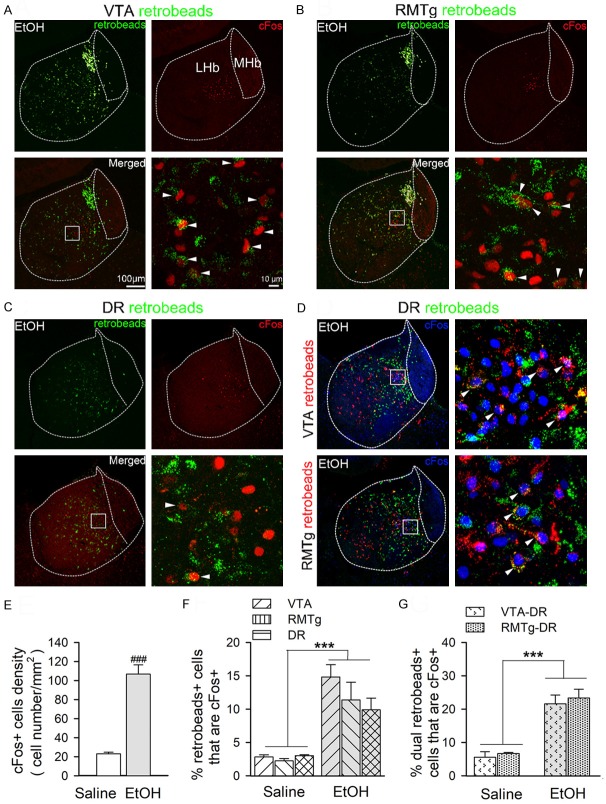
Ethanol excites LHb neurons with projections to the VTA, RMTg, and DR
To investigate the projection targets of ethanol-activated LHb neurons, we used a combination of cFos IR and retrograde tracing technologies. We injected green retrobeads alone or together with red retrobeads into the VTA, RMTg, and DR. We also injected green and red retrobeads respectively into the VTA and DR, or the RMTg and DR, to explore whether ethanol-activated LHb neurons project to both dopaminergic/GABAergic and serotonergic nuclei. Ten days after intra-nucleus microinjections, we injected ethanol (0.25 g/kg, i.p.) or saline to the rat. We performed c-Fos IHC 90 minutes after ethanol or saline injection. As illustrated in Figure 2, the VTA injection was centered at the paranigral or parabrachial pigmented nucleus of the VTA, almost throughout its entire rostrocaudal extent (Figure 2A left panel). The RMTg injection covered mainly the area lateral to the caudal linear nucleus (Cli), beneath the decussation of the superior cerebellar peduncle (XSCP), and above the trigeminothalamic tract and interpeduncular nucleus (Figure 2A middle panel). The DR injection mostly covered the dorsal raphe center (DRc), with minimal spread to the pericentral part of the dorsal tegmental nucleus (DTgP) and laterodorsal tegmental nucleus (LTDg) (Figure 2C right panel).
Figure 2.
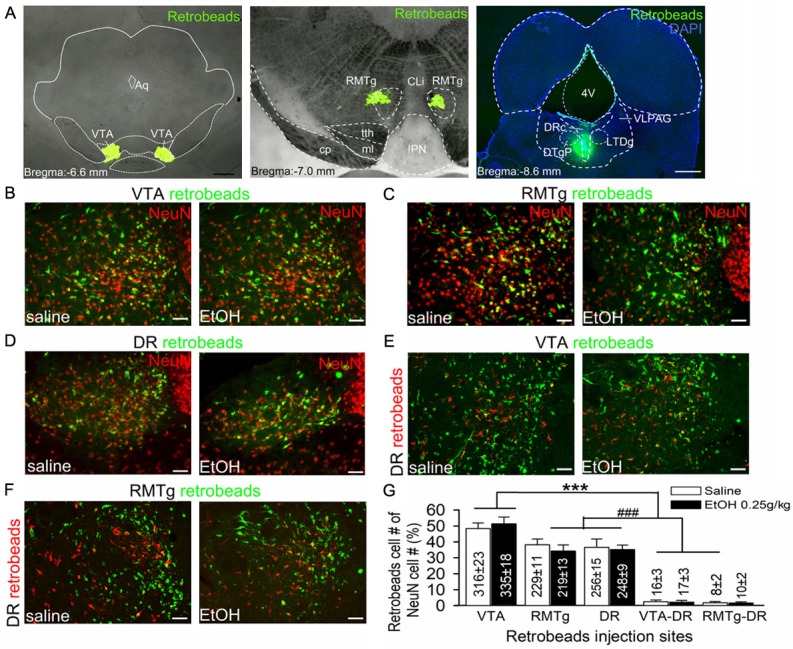
LHb neurons project to the ventral tegmental area (VTA), rostral medial tegmental nucleus (RMTg), and dorsal raphe (DR). Images showing green retrobeads injected into the VTA (A, left panel), RMTg (A, middle panel) or DR (A, right panel) of ethanol naive rats. Ten days after injection, rats were randomly divided into two groups; one group received an intraperitoneal injection (i.p.) of 0.25 g/kg ethanol (EtOH), the other of saline (1 ml/Kg). Then the brain tissue was harvested for double staining of retrobeads and NeuN (a neuronal marker) to assess the distribution of retrobeads in the LHb. Intra-VTA retrobead injection resulted in a strong retrograde labeling in the LHb (B). Intra-RMTg or DR injection of retrobeads resulted in a moderate labeling in the LHb (C and D). Intra-VTA and DR or RMTg and DR dual-retrobeads injections resulted in a minority of LHb neurons labeled with green and red retrobeads (E and F). Compared with saline group, acute ethanol (0.25 g/kg, i.p.) did not significantly alter the ratios of neurons projecting to these areas. (G) Summary graph of percentages of cell counting from 5 sections of each rat (n=5/group). The percentage was calculated by (retrobeads labeling cells/NeuN cells) ×100%. Cell numbers are indicated in the bars. ***p < 0.001 vs. RMTg, DR, VTA-DR or RMTg-DR; ###p < 0.001 vs. VTA-DR or RMTg-DR, Bonferroni t-test followed by two-way ANOVA. 4v, 4th ventricle; Aq, aqueduct; CLi, the caudal linear nucleus of the raphe; DRc, dorsal raphe center; DTgP dorsal tegmental nucleus, pericentral part; IPN, interpeduncular nucleus; ml, medial lemniscus; PMnR, Paramedian raphe nucleus; tth, trigeminothalamic tract; VLPAG, ventrolateral periaqueductal gray. Scale bar =1 mm (A left panel-C right panel) and 20 μm (B-F).
We then compared retrograde labeling in the LHb produced by injections in these three areas, by calculating the ratio of double stained (retrobeads+ and NeuN+ neurons) cells over the total number of NeuN+ cells. The results revealed that injection of retrobeads into the VTA resulted in an intense labeling in the LHb, which was stronger than those produced by RMTg or DR injection (Two-way ANOVA: F (4,59)=347.62, p < 0.001, Figure 2G). RMTg or DR injection resulted in a moderate retrograde labeling in the LHb. Also, we identified a small population of LHb neurons that contained retrobeads from both the DR and VTA or RMTg injections. Specifically, 2.2% and 1.4% of LHb neurons collateralized to innervate VTA and DR, and RMTg and DR respectively, indicating a minority of LHb neurons project to more than one monoaminergic nucleus. Compared to saline, ethanol (0.25 g/kg, i.p.) did not significantly alter the ratios of LHb neurons with single or dual projections to these areas (Two-way ANOVA: F (1,59)=0.41, p=0.53).
Next, to assess whether there is any projection specificity of LHb neurons in response to ethanol, we quantified the ethanol-activated LHb neurons by double staining of the retrobeads and cFos. Similar to what is shown in Figure 1, we repeatedly observed an increased number of c-fos+ LHb neurons in the ethanol-treated group (0.25 g/kg, i.p.) than in the saline group in rats with single or dual retrobeads injection (t (2,63)=65.182, p < 0.001, Figure 3E). We then assessed the double-staining of the retrobeads and cFos in the LHb. The percentages of double stained LHb cells in total retrobead-labeled neurons were significantly higher in the ethanol-treated groups (0.25 g/kg, i.p.) than in the saline-treated group (Two-way ANOVA: F (1,35)=236.3, p < 0.001, Figure 3F, F (1,23)=704.76, p < 0.001, Figure 3G). However, within ethanol groups, we observed no significant difference either in projection preference for ethanol-activated neurons among the three targets, VTA, RMTg, and DR (Two-way ANOVA: F (2,35)=1.68, p=0.24], or between LHb neurons that simultaneously project to VTA and DR and that project to RMTg and DR (Two-way ANOVA: F (1,23)=0.31, p=0.87]. Also, we observed no significant difference in the interaction between treatment and injection sites either in the single injection (Two-way ANOVA: F (2,35)=1.72, p=0.24) and dual injection groups (F (1,23)=1.03, p=0.36). Together, these results suggest that low-dose ethanol excites the LHb neurons projecting to the VTA, RMTg or DR, and only very few of these project to two areas simultaneously.
Figure 3.
Ethanol-activated LHb neurons project to VTA, RMTg, and DR. Compared to saline, ethanol (0.25 g/kg, i.p.) significantly elevated the number of double-labeled (retrobeads- and cFos-positive) LHb cells that project to VTA (A), RMTg (B), and DR (C). However, there was no significant difference in the percentage of double-labeled cells in retrobeads+ LHb cells among these three projection sites. Moreover, few LHb neurons activated by ethanol simultaneously project to both the VTA and DR or both the RMTg and DR (D). Right panels show an enlarged view of the boxed areas. Arrowheads indicate the cells with cFos and retrobead co-labeling. (E) Summary graph of cFos+ cells density in ethanol and saline treatment groups. Since no significant differences were found in total number of cFos+ neurons within saline or ethanol treatment group, the within-group data were collapsed for analysis. ###p < 0.001, vs. saline, by t-test. (F and G) Summary graphs of percentage of cFos+ and retrobeads colabeled cells in total retrobeads retrogradely labeled LHb neurons. ***p < 0.001, vs. saline group. Two-way ANOVA followed by Tukey’s post hoc analysis, n=6 rats/group, 8-9 sections/rat. Scale bars =100 μm or 10 μm.
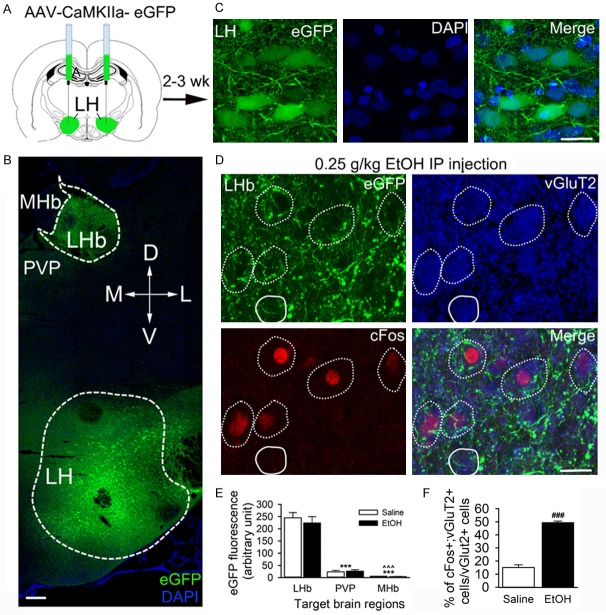
Ethanol-activated LHb neurons receive excitatory innervation from the lateral hypothalamus
We recently reported that acute ethanol enhances excitatory glutamatergic neurotransmission to LHb neurons [25]. The LHb receives inputs mainly from the limbic system, including the glutamatergic projections from the lateral hypothalamus (LH) [31,32]. To test whether the LH sends excitatory projections to ethanol-activated LHb neurons, two weeks before ethanol administration, we injected the LH with an AAV carrying eGFP under the Ca2+/calmodulin-dependent protein kinase-α (CaMKIIα) promoter (AAV5-CaMKIIa-eGFP), which is expressed mainly in excitatory neurons [36]. We observed robust eGFP fluorescent expression in the LH (Figure 4B, 4C), especially around the fornix, with only minimal eGFP expression spreading to the perifornical area. All 9 rats (3 injected with saline, and 6 injected with ethanol) shared a similar distribution pattern: eGFP florescence covered 300-500 μm of the rostrocaudal extent within the LH nucleus.
Figure 4.
Ethanol-activated LHb neurons receive inputs from the lateral hypothalamus (LH). AAV5-CaMKIIa-eGFP was infused into the LH of rats, 2-3 weeks before ethanol (0.25 g/kg, i.p.) or saline (1 ml/kg, i.p.). Brain slices containing the LH and the LHb were harvested 90 min post-i.p.-injections. (A) Schematic showed the AAV synaptic labeling tracing approach. (B) A confocal image of a coronal section illustrating the expression of AAV-eGFP in a brain slice containing both the LH and LHb (counterstained with DAPI). (C) High-resolution images of AAV-mediated expression of CaMKIIa-eGFP in LH neurons. (D) Confocal images demonstrated the overlap of ethanol-activated cFos+ (red) with vGluT2 staining (blue). Notably, a lot of eGFP+ (green) fibers were overlapped with the cell bodies (dotted white outlines), and a few vGluT2+ cells targeted by LH terminals were cFos- (white outlines). (E) Summary graph of LH-LHb eGFP fluorescence intensity in and around the LHb. MHb, medial habenula; PVP, posterior part of paraventricular thalamic nucleus; ***p < 0.001, vs. LHb; ^^^p < 0.001, vs. PVP. (F) Summary graph: ethanol treatment increased the percentage of cFos and vGluT2 co-localized cells/vGluT2 cells in LHb. ***p < 0.001, vs. Saline, n=5, unpaired t-test. Scale bar =200 μm (B), 20 μm (C & D).
The highest density of eGFP fluorescence-positive fibers was observed in the LHb, compared to other LH target brain regions, including the MHb and paraventricular thalamic nucleus (PVP, Two Way ANOVA: F (2,100)=69.4, p < 0.001, n=5 rats/group, 3 sections/rat, Figure 4E). The LHb eGFP fluorescence covered the region between Bregma -3.36 to -3.96 mm, consistent with previous findings [31]. Moreover, cFos IR neurons were overlapped with vGluT2, the marker of glutamatergic neurons (Figure 4D). Compared to saline, low-dose ethanol (0.25 g/kg, i.p.) significantly increased the percentage of LHb cells that were stained with both vGluT2 and cFos (t (1,8)=14.2, p < 0.001, n=5 rats/group, 3-4 sections/rats). Consistent with the immunohistochemistry result, the eGFP-labeled LH axon fibers and terminals seen around these cells looked dense and patchy. High- magnification confocal imaging found that eGFP+ terminals made contacts with LHb cFos IR cell bodies (confirmed by 32 deconvoluted stacks, Figure 5A, 5B). Remarkably, eGFP+ terminals containing vGluT2 also made contact with LHb cFos IR cell bodies (Figure 5C, 5D). These results suggest that ethanol-activated LHb neurons receive excitatory inputs from the LH.
Figure 5.
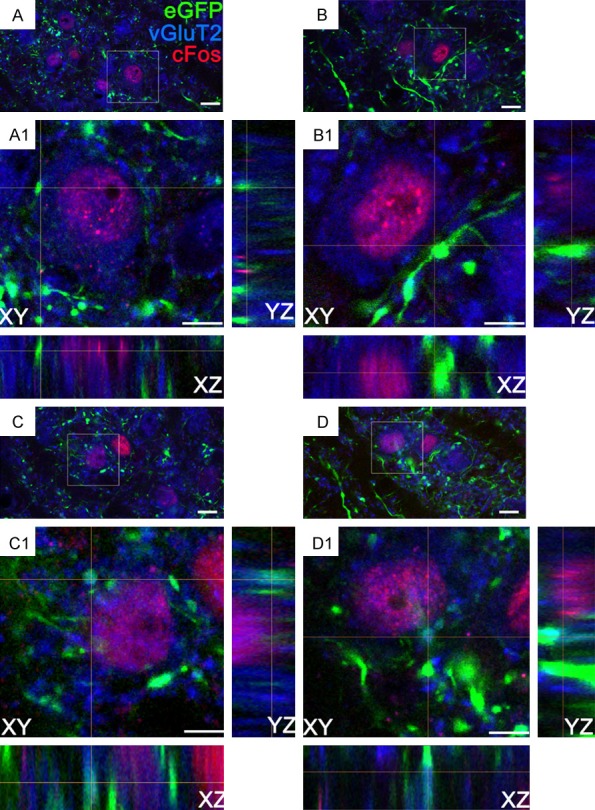
Three-dimensional confocal images of LH axonal terminations in LHb vGluT2 cells expressing ethanol-induced cFos from rats that received an intra-LH injection of AAV5-CaMKIIa-eGFP, and ethanol (0.25 g/kg, i.p.) 2-3 weeks later. (A-D), show the LHb vGluT2+ cells (blue) expressing cFos (red), were contacted by axonal buttons (green) from the LH. Square boxes indicate the locations of the contacts. (A1-D1) show enlarged xy-, yz-, and xz- orthogonal views. Notably, (C, D) show the LH axonal varicosities expressing vGluT2 and, forming contact on LHb cFos+ cells. Since the confocal laser-scanning microscope generates in-focus images of selected depth, projection phenomena, in the conventional fluorescence microscope can be ruled out. Scale bars =10 μm (A-D) and 5 μm (A1-D1).
Discussion
In this study, we identified circuits connecting to LHb neurons that were activated by a low-dose of ethanol. Ethanol (0.25 g/kg, i.p.), producing a BEC of 5.6 mM, significantly increased the number of cFos IR cells in the LHb. Most of these neurons expressed vGluT2, indicating that they were glutamatergic. These ethanol-activated LHb cells project to the VTA, RMTg, and DR, and receive excitatory projections from the LH. These results provide new insight into the targets of low-dose ethanol at the cellular and circuit levels.
cFos has been widely used as an anatomical marker of cell activity. After exposure to a stimulus, such as ethanol, a transient induction of cFos occurs [37,38], and the change in cFos expression between subjects exposed to the stimulus and those in basal conditions indicates a difference in neuronal activity. Here, we showed that low-dose ethanol (0.25 g/kg, i.p.) significantly increased cFos expression in the LHb, indicating that LHb neurons are sensitive to ethanol [25]. Additionally, we examined the time course of cFos expression in response to ethanol and found it peaked at 90 min and returned near control levels by 8 hours, consistent with a previous study examining different brain regions [39].
The cFos approach has its caveat. Specifically, cFos expression is particularly sensitive to stimuli that induce stress [40]. Additionally, LHb neurons have been shown to be excited [41] or inhibited by nociceptive stimuli [42]. Several studies indicated that different forms of stress, such as foot shock and water deprivation can activate LHb neurons [43,44]. To minimize potential false positive results caused by stress, we habituated all the rats to handling and the injection procedure before cFos experiments.
Given that cFos expression level was very low in the LHb of saline-treated animals, the elevated cFos expression in the ethanol group was likely a result of the pharmacological effects of ethanol. We also acknowledge the limitation regarding the passive administration of ethanol by intraperitoneal injection. Compared to voluntary drinking, however, injection may provide better control over the resulting BEC. Still, this route of administration cannot emulate the use of alcohol in humans. Therefore, future studies should test the response of LHb neurons in relation to voluntary consumption of alcohol in an animal model.
Functional significance of LHb output circuits in ethanol-related behaviors
Although recent evidence has associated alcohol’s aversive properties with its abuse, the underlying neuronal mechanisms remain unclear. The LHb has emerged as a critical brain region in aversion response, as it is activated by aversive signals such as depression, anxiety, and abused drugs including alcohol. We observed that the LHb neurons activated by a single injection of low-dose ethanol projected to the VTA, RMTg, and DR, confirming the efferent fibers from LHb to the midbrain [45,46]. Ethanol-activated LHb neurons are vGluT2 positive, consistent with the molecular characteristics of LHb neurons that were almost uniformly glutamatergic [12]. In this study, we did not identify the neuronal type of VTA and RMTg neurons that receive inputs from ethanol-excited LHb cells. However, previous studies have documented that LHb neurons form excitatory synapses onto VTA-GABAergic interneurons [47,48], and dopaminergic neurons [29,48,49], as well as RMTg-GABAergic neurons [30]. Functionally, VTA-dopaminergic neurons have been widely reported in motivation and reward behavior [50], as well as in aversion [51,52]. Moreover, VTA-GABAergic neurons drive aversion [53]. Furthermore, the RMTg encodes aversion [54] and responds to negative reward [55], as well as drugs of abuse [56]. Based on these findings, we propose that activation of LHb neurons or their midbrain output circuits (LHb to VTA/RMTg) may be associated with ethanol’s aversive properties. This hypothesis is supported by our recent report that ethanol drives aversive conditioning through the activation of LHb neurons [25]. Recent evidence has linked the RMTg to ethanol-related behaviors. For example, RMTg cFos expression was elevated in rats subjected to ethanol-induced condition taste aversion (CTA) [20]. Also, RMTg lesions accelerated the extinction of ethanol-induced CTA [57] and pharmacological inhibition or lesion of the RMTg enhanced ethanol consumption in rats [34,57,58]. Together, these results suggest that the RMTg contributes to the aversive properties of alcohol. However, more studies are needed to further investigate the function of the LHb-VTA or LHb-RMTg circuit in ethanol-related aversive behaviors.
Emerging evidence has demonstrated a role for the serotonin-rich DR in mediating alcohol reward, preference, dependence, and craving. Here we showed that acute ethanol activated the LHb-DR circuit. Acute ethanol has also been shown to inhibit DR serotoninergic neurons [59,61], but can elevate 5-HT levels in their target areas [61,62]. The possible interpretations of these conflicting findings could be that the elevated 5-HT may suppress DR neuronal firing through the activation of 5-HT1 autoreceptors [63], or that ethanol may enhance GABA transmissions to the DR [59]. Considering LHb glutamatergic neurons target both GABAergic and serotonergic neurons in the raphe nucleus [64], we propose that ethanol may, through the activation of LHb-DR GABAergic circuit, inhibit serotonergic neurons. Ultimately, this may contribute to the underlying mechanism connecting serotonin with ethanol reward and aversion.
The circuit linking the LHb and DR has also been of great interest in other neurobiological functions that are related to DR serotonin neurotransmission, including cognitive and emotional functions, pain sensitivity and sleep and circadian rhythm regulation [65]. It is noteworthy to investigate the function of LHb-DR in binge alcohol drinking and the associated symptoms such as anxiety, depression or hyperalgesia.
Also, there are reciprocal connections between the LHb and the raphe. The 5HT-2c receptor is also widely distributed in the LHb [12]. We recently reported that serotonin increased glutamate release in the LHb via the activation of 5-HT 2, 3 receptors. 5-HT modulates glutamatergic transmissions to LHb neurons or stimulates the LHb via activation of 5-HT 2/3 receptors [66,67]. These studies emphasize the regulatory role of 5-HT signaling on LHb neurons. However, the role of the LHb-raphe circuit in ethanol-related behaviors has not been well explored.
LH inputs on ethanol-activated neurons in the LHb
Ethanol could excite LHb neurons through enhanced glutamate transmission to LHb neurons [25]. The LHb receives inputs from the LH [31], which consists of heterogeneous cell populations, including glutamate and GABA, or neuropeptides, including orexin, melanin-concentrating hormone, neurotensin and galanin [68]. To label the LH excitatory neurons, we used AAV-CaMKIIa-eGFP with a CaMKIIα promoter as a presynaptic tracer, which exhibits expression primarily in excitatory neurons. We found that LH axonal varicosities expressed vGluT2, forming button-like structures on LHb neurons [69]. Also, vGluT2 was extensively expressed in the cell body of LHb neuron, indicating LHb neurons are glutamatergic. More importantly, ethanol significantly increased the percentage of the neurons with cFos and vGluT2 double staining in all vGluT2+ cells but did not significantly alter the density of vGluT2+ inputs around LHb cells, suggesting that the ethanol-induced excitation of LHb neurons occurs via enhanced glutamate transmission but not by increased number of the excitatory terminals. Ethanol-induced enhancement of glutamate transmission in the LHb neurons is mediated by the D1 receptor [25]. Also, the LH is sensitive to low-dose ethanol: acute oral administration of low (0.75 g/kg) but not higher (2.5 g/kg) doses of ethanol-enhanced orexin expression in the LH [70]. However, it remains unclear whether ethanol directly activates LH glutamatergic neurons. Functionally, optogenetic stimulation of the LH-LHb pathway produced real-time place avoidance [31], suggesting the LH-LHb circuit may produce aversive behavioral phenotypes. A lesion in the LH-LHb circuit increased voluntary ethanol consumption [71], suggesting a link between this pathway and alcohol’s aversive properties.
Conclusion
We report that a low-dose of ethanol activates LHb neurons that project to the VTA, RMTg, and raphe and that these LHb neurons receive excitatory projections from the LH. These results extend our knowledge on how a low-dose of alcohol specifically affects the LHb, at a cellular and circuit level.
Acknowledgements
The work was supported by NIH-NIAAA AA021657, AA022292. The authors thank Dr. Rose Paluose, Dr. Nimisha Shiwalkar, and Dr. Priscilla White for reading over the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Gowing LR, Ali RL, Allsop S, Marsden J, Turf EE, West R, Witton J. Global statistics on addictive behaviours: 2014 status report. Addiction. 2015;110:904–919. doi: 10.1111/add.12899. [DOI] [PubMed] [Google Scholar]
- 2.Cui C, Koob GF. Titrating tipsy targets: the neurobiology of low-dose alcohol. Trends Pharmacol Sci. 2017;38:556–568. doi: 10.1016/j.tips.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- 5.Gilpin NW, Koob GF. Neurobiology of alcohol dependence: focus on motivational mechanisms. Alcohol Res Health. 2008;31:185–195. [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–U1111. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- 7.Lecca S, Pelosi A, Tchenio A, Moutkine I, Lujan R, Herve D, Mameli M. Rescue of GABAB and GIRK function in the lateral habenula by protein phosphatase 2A inhibition ameliorates depression-like phenotypes in mice. Nat Med. 2016;22:254–261. doi: 10.1038/nm.4037. [DOI] [PubMed] [Google Scholar]
- 8.Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, Henn F, Malinow R. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shabel SJ, Proulx CD, Piriz J, Malinow R. Mood regulation. GABA/glutamate co-release controls habenula output and is modified by antidepressant treatment. Science. 2014;345:1494–1498. doi: 10.1126/science.1250469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meye FJ, Lecca S, Valentinova K, Mameli M. Synaptic and cellular profile of neurons in the lateral habenula. Front Hum Neurosci. 2013;7:860. doi: 10.3389/fnhum.2013.00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss T, Veh RW. Morphological and electrophysiological characteristics of neurons within identified subnuclei of the lateral habenula in rat brain slices. Neuroscience. 2011;172:74–93. doi: 10.1016/j.neuroscience.2010.10.047. [DOI] [PubMed] [Google Scholar]
- 12.Aizawa H, Kobayashi M, Tanaka S, Fukai T, Okamoto H. Molecular characterization of the subnuclei in rat habenula. J Comp Neurol. 2012;520:4051–4066. doi: 10.1002/cne.23167. [DOI] [PubMed] [Google Scholar]
- 13.Kim U. Topographic commissural and descending projections of the habenula in the rat. J Comp Neurol. 2009;513:173–187. doi: 10.1002/cne.21951. [DOI] [PubMed] [Google Scholar]
- 14.Shabel SJ, Proulx CD, Trias A, Murphy RT, Malinow R. Input to the lateral habenula from the basal ganglia is excitatory, aversive, and suppressed by serotonin. Neuron. 2012;74:475–481. doi: 10.1016/j.neuron.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klemm WR. Habenular and interpeduncularis nuclei: shared components in multiple-function networks. Med Sci Monit. 2004;10:RA261–273. [PubMed] [Google Scholar]
- 16.Balcita-Pedicino JJ, Omelchenko N, Bell R, Sesack SR. The inhibitory influence of the lateral habenula on midbrain dopamine cells: ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus. J Comp Neurol. 2011;519:1143–1164. doi: 10.1002/cne.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Proulx CD, Hikosaka O, Malinow R. Reward processing by the lateral habenula in normal and depressive behaviors. Nat Neurosci. 2014;17:1146–1152. doi: 10.1038/nn.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sego C, Goncalves L, Lima L, Furigo IC, Donato J Jr, Metzger M. Lateral habenula and the rostromedial tegmental nucleus innervate neurochemically distinct subdivisions of the dorsal raphe nucleus in the rat. J Comp Neurol. 2014;522:1454–1484. doi: 10.1002/cne.23533. [DOI] [PubMed] [Google Scholar]
- 19.Jhou TC, Good CH, Rowley CS, Xu SP, Wang H, Burnham NW, Hoffman AF, Lupica CR, Ikemoto S. Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. J Neurosci. 2013;33:7501–7512. doi: 10.1523/JNEUROSCI.3634-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glover EJ, McDougle MJ, Siegel GS, Jhou TC, Chandler LJ. Role for the rostromedial tegmental nucleus in signaling the aversive properties of alcohol. Alcohol Clin Exp Res. 2016;40:1651–61. doi: 10.1111/acer.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haack AK, Sheth C, Schwager AL, Sinclair MS, Tandon S, Taha SA. Lesions of the lateral habenula increase voluntary ethanol consumption and operant self-administration, block yohimbine-induced reinstatement of ethanol seeking, and attenuate ethanol-induced conditioned taste aversion. PLoS One. 2014;9:e92701. doi: 10.1371/journal.pone.0092701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tandon S, Keefe KA, Taha SA. Excitation of lateral habenula neurons as a neural mechanism underlying ethanol-induced conditioned taste aversion. J Physiol. 2017;595:1393–1412. doi: 10.1113/JP272994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang S, Li J, Zuo W, Fu R, Gregor D, Krnjevic K, Bekker A, Ye JH. Ethanol withdrawal drives anxiety-related behaviors by reducing M-type potassium channel activity in the lateral habenula. Neuropsychopharmacology. 2017;42:1813–1824. doi: 10.1038/npp.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang S, Li J, Bekker A, Ye JH. Rescue of glutamate transport in the lateral habenula alleviates depression- and anxiety-like behaviors in ethanol-withdrawn rats. Neuropharmacology. 2018;129:47–56. doi: 10.1016/j.neuropharm.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zuo W, Fu R, Hopf FW, Xie G, Krnjevic K, Li J, Ye JH. Ethanol drives aversive conditioning through dopamine 1 receptor and glutamate receptor-mediated activation of lateral habenula neurons. Addict Biol. 2017;22:103–116. doi: 10.1111/adb.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Bian W, Dave V, Ye JH. Blockade of GABA(A) receptors in the paraventricular nucleus of the hypothalamus attenuates voluntary ethanol intake and activates the hypothalamic-pituitary-adrenocortical axis. Addict Biol. 2011;16:600–614. doi: 10.1111/j.1369-1600.2011.00344.x. [DOI] [PubMed] [Google Scholar]
- 27.Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE, Koob GF. Ethanol selfadministration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J Neurosci. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Zuo W, Fu R, Xie G, Kaur A, Bekker A, Ye JH. High frequency electrical stimulation of lateral habenula reduces voluntary ethanol consumption in rats. Int J Neuropsychopharmacol. 2016 doi: 10.1093/ijnp/pyw050. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neurol. 2009;513:597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- 31.Stamatakis AM, Van Swieten M, Basiri ML, Blair GA, Kantak P, Stuber GD. Lateral hypothalamic area glutamatergic neurons and their projections to the lateral habenula regulate feeding and reward. J Neurosci. 2016;36:302–311. doi: 10.1523/JNEUROSCI.1202-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poller WC, Madai VI, Bernard R, Laube G, Veh RW. A glutamatergic projection from the lateral hypothalamus targets VTA-projecting neurons in the lateral habenula of the rat. Brain Research. 2013;1507:45–60. doi: 10.1016/j.brainres.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 33.Chamberlin NL, Du B, de Lacalle S, Saper CB. Recombinant adeno-associated virus vector: use for transgene expression and anterograde tract tracing in the CNS. Brain Res. 1998;793:169–175. doi: 10.1016/s0006-8993(98)00169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu R, Chen X, Zuo W, Li J, Kang S, Zhou LH, Siegel A, Bekker A, Ye JH. Ablation of mu opioid receptor-expressing GABA neurons in rostromedial tegmental nucleus increases ethanol consumption and regulates ethanol-related behaviors. Neuropharmacology. 2016;107:58–67. doi: 10.1016/j.neuropharm.2016.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. London: Academic Press; 2007. [DOI] [PubMed] [Google Scholar]
- 36.Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY, Adhikari A, Tye KM, Frank LM, Deisseroth K. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492:428–432. doi: 10.1038/nature11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgan JI, Cohen DR, Hempstead JL, Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987;237:192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- 38.Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- 39.Chang SL, Patel NA, Romero AA. Activation and desensitization of Fos immunoreactivity in the rat brain following ethanol administration. Brain Res. 1995;679:89–98. doi: 10.1016/0006-8993(95)00210-h. [DOI] [PubMed] [Google Scholar]
- 40.Ryabinin AE, Criado JR, Henriksen SJ, Bloom FE, Wilson MC. Differential sensitivity of c-Fos expression in hippocampus and other brain regions to moderate and low doses of alcohol. Mol Psychiatry. 1997;2:32–43. doi: 10.1038/sj.mp.4000206. [DOI] [PubMed] [Google Scholar]
- 41.Matsumoto N, Yahata F, Kawarada K, Kamata K, Suzuki TA. Tooth pulp stimulation induces c-fos expression in the lateral habenular nucleus of the cat. Neuroreport. 1994;5:2397–2400. doi: 10.1097/00001756-199411000-00046. [DOI] [PubMed] [Google Scholar]
- 42.Dafny N, Qiao JT. Habenular neuron responses to noxious input are modified by dorsal raphe stimulation. Neurol Res. 1990;12:117–121. doi: 10.1080/01616412.1990.11739929. [DOI] [PubMed] [Google Scholar]
- 43.Park H, Rhee J, Park K, Han JS, Malinow R, Chung C. Exposure to stressors facilitates longterm synaptic potentiation in the lateral habenula. J Neurosci. 2017;37:6021–6030. doi: 10.1523/JNEUROSCI.2281-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L, Hernandez VS, Vazquez-Juarez E, Chay FK, Barrio RA. Thirst is associated with suppression of habenula output and active stress coping: is there a role for a non-canonical vasopressin-glutamate pathway? Front Neural Circuits. 2016;10:13. doi: 10.3389/fncir.2016.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. J Comp Neurol. 1979;187:19–47. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- 46.Quina LA, Tempest L, Ng L, Harris JA, Ferguson S, Jhou TC, Turner EE. Efferent pathways of the mouse lateral habenula. J Comp Neurol. 2015;523:32–60. doi: 10.1002/cne.23662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brinschwitz K, Dittgen A, Madai VI, Lommel R, Geisler S, Veh RW. Glutamatergic axons from the lateral habenula mainly terminate on GABAergic neurons of the ventral midbrain. Neuroscience. 2010;168:463–476. doi: 10.1016/j.neuroscience.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 48.Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, Gao XJ, Kremer EJ, Malenka RC, Luo L. Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell. 2015;162:622–634. doi: 10.1016/j.cell.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Omelchenko N, Bell R, Sesack SR. Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. Eur J Neurosci. 2009;30:1239–1250. doi: 10.1111/j.1460-9568.2009.06924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 51.Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouebe G, Deisseroth K, Tye KM, Luscher C. GABA neurons of the VTA drive conditioned place aversion. Neuron. 2012;73:1173–1183. doi: 10.1016/j.neuron.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O. Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J Neurosci. 2011;31:11457–11471. doi: 10.1523/JNEUROSCI.1384-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lecca S, Melis M, Luchicchi A, Ennas MG, Castelli MP, Muntoni AL, Pistis M. Effects of drugs of abuse on putative rostromedial tegmental neurons, inhibitory afferents to midbrain dopamine cells. Neuropsychopharmacology. 2011;36:589–602. doi: 10.1038/npp.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sheth C, Furlong TM, Keefe KA, Taha SA. Lesion of the rostromedial tegmental nucleus increases voluntary ethanol consumption and accelerates extinction of ethanol-induced conditioned taste aversion. Psychopharmacology (Berl) 2016;233:3737–3749. doi: 10.1007/s00213-016-4406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fu R, Zuo W, Gregor D, Li J, Grech D, Ye JH. Pharmacological manipulation of the rostromedial tegmental nucleus changes voluntary and operant ethanol self-administration in rats. Alcohol Clin Exp Res. 2016;40:572–582. doi: 10.1111/acer.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lowery-Gionta EG, Marcinkiewcz CA, Kash TL. Functional alterations in the dorsal raphe nucleus following acute and chronic ethanol exposure. Neuropsychopharmacology. 2015;40:590–600. doi: 10.1038/npp.2014.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pistis M, Muntoni AL, Gessa G, Diana M. Effects of acute, chronic ethanol and withdrawal on dorsal raphe neurons: electrophysiological studies. Neuroscience. 1997;79:171–176. doi: 10.1016/s0306-4522(96)00643-4. [DOI] [PubMed] [Google Scholar]
- 61.Thielen RJ, Morzorati SL, McBride WJ. Effects of ethanol on the dorsal raphe nucleus and its projections to the caudate putamen. Alcohol. 2001;23:131–139. doi: 10.1016/s0741-8329(01)00126-4. [DOI] [PubMed] [Google Scholar]
- 62.Yoshimoto K, McBride WJ, Lumeng L, Li TK. Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol. 1992;9:17–22. doi: 10.1016/0741-8329(92)90004-t. [DOI] [PubMed] [Google Scholar]
- 63.Wang RY, Aghajanian GK. Antidromically identified serotonergic neurons in the rat midbrain raphe: evidence for collateral inhibition. Brain Res. 1977;132:186–193. doi: 10.1016/0006-8993(77)90719-3. [DOI] [PubMed] [Google Scholar]
- 64.Weissbourd B, Ren J, DeLoach KE, Guenthner CJ, Miyamichi K, Luo L. Presynaptic partners of dorsal raphe serotonergic and GABAergic neurons. Neuron. 2014;83:645–662. doi: 10.1016/j.neuron.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao H, Zhang BL, Yang SJ, Rusak B. The role of lateral habenula-dorsal raphe nucleus circuits in higher brain functions and psychiatric illness. Behav Brain Res. 2015;277:89–98. doi: 10.1016/j.bbr.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 66.Zuo W, Zhang Y, Xie G, Gregor D, Bekker A, Ye JH. Serotonin stimulates lateral habenula via activation of the post-synaptic serotonin 2/3 receptors and transient receptor potential channels. Neuropharmacology. 2016;101:449–459. doi: 10.1016/j.neuropharm.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xie G, Zuo W, Wu L, Li W, Wu W, Bekker A, Ye JH. Serotonin modulates glutamatergic transmission to neurons in the lateral habenula. Sci Rep. 2016;6:23798. doi: 10.1038/srep23798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stuber GD, Wise RA. Lateral hypothalamic circuits for feeding and reward. Nat Neurosci. 2016;19:198–205. doi: 10.1038/nn.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Poller WC, Madai VI, Bernard R, Laube G, Veh RW. A glutamatergic projection from the lateral hypothalamus targets VTA-projecting neurons in the lateral habenula of the rat. Brain Res. 2013;1507:45–60. doi: 10.1016/j.brainres.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 70.Morganstern I, Chang GQ, Barson JR, Ye Z, Karatayev O, Leibowitz SF. Differential effects of acute and chronic ethanol exposure on orexin expression in the perifornical lateral hypothalamus. Alcohol Clin Exp Res. 2010;34:886–896. doi: 10.1111/j.1530-0277.2010.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sheth C, Furlong TM, Keefe KA, Taha SA. The lateral hypothalamus to lateral habenula projection, but not the ventral pallidum to lateral habenula projection, regulates voluntary ethanol consumption. Behav Brain Res. 2017;328:195–208. doi: 10.1016/j.bbr.2017.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]