Abstract
Cola nitida (Kolanut) is conventionally used in tropical Africa for the treatment of all kinds of ailments such as migraine, morning sickness, metabolic disorders etc. However, this study was designed to investigate the diuretic, natriuretic and kaliuretic activities of methanolic extract of Cola nitida (MECN) in male Wistar rats. Adult male Wistar rats were randomly allotted into control (25 ml/kg b.w.), furosemide (20 mg/kg b.w; standard), MECN1 (100 mg/kg), MECN2 (200 mg/kg), MECN3 (300 mg/kg), MECN4 (400 mg/kg), MECN5 (500 mg/kg), MECN6 (600 mg/kg) groups with n=6. The extract was prepared as previously described and the treatment lasted for 14 days. Urine volume and diuretic indices were estimated. Urine electrolytes, plasma electrolytes, plasma/renal AST/ALT, plasma creatinine and urea were assayed using flame photometry and standard colorimetric method respectively.Administration of different doses of C. nitida significantly altered body weight gain and water intake but not food intake compared with control group. There were significant increases in urine volume and urine electrolytes (Na+, K+ and Cl-), a decrease in plasma/renal ALT and AST activities, a decrease in plasma creatinine and urea concentration and no alteration in plasma electrolytes when compared with control and furosemide-treated groups. Our study suggests that MECN elicits diuretic, natriuretic, and kaliuretic activities without causing electrolyte impairment, hepatotoxicity and nephrotoxicity. These effects are dose-dependent.
Keywords: Cola nitida, creatinine, diuretic, hepatotoxicity, urea
Introduction
Cola is an evergreen forest plant that is common in tropical region of Africa. It has different species such as Cola acuminata which is predominant in Togo, Angola, Liberia, Ivory Coast, Senegal and Nigeria [1]. Both species are now cultivated in tropical zones of the America and in Southeast Asia [2]. Cola tree grow up to 25 meters tall with pale yellow flowers, purple stripes, star-shaped fruits and woody hulls. It has a shiny and light green color leaves. The fruits can weigh up to 3 kilograms and contain large seeds with reddish brown nuts known as cola nut [2].
Cola nitida belongs to the family of sterculiaceae, specie of acuminata, nitida and to the Genus of cola. It is commonly called Kola nut in English language, Goro in Hausa language, Oji in Igbo language and Obi in Yoruba language [3]. Its usefulness for traditional purposes in West Africa span from social life, religious events to sealing business contracts [1,4]. It has also been used in folk medicine as an aphrodisiac, an appetite suppressant, to treat morning sickness, migraine headache, and indigestion [5]. C. nitida has been applied directly to the skin to treat wounds and inflammations [6]. The tree’s bitter twig has been used as well for cleaning the teeth and gums [5]. The medicinal uses of Cola nitida in Africa cannot be over emphasized; cola fruits are used as tonics, stimulants and concoction for the treatment of fever, dysentery and exhaustion [2].
In Europe, Kola nuts were once used to treat migraines, neuralgia, nausea, and diarrhea [2]. Kola preparations are used today to treat physical and mental exhaustion [7]. The medicinal importance of Cola nitida is based mainly on the chemical constituents of the plant from its roots to its seeds. The plant is known to contain several chemical constituents noted for their medicinal values among them are caffeine, theophylline and theobromine [7]. Cola nitida also contain traces of essential minerals like potassium, calcium, magnesium, sodium, iron, zinc, manganese, and phosphorus. Some of these minerals act as a source of macro and micro nutrient needed for growth, development and metabolic activity. It is used in the manufacturing of beverages such as Coca cola and Pepsi cola (Javies, 2002). It is also used in the manufacturing of dyes [8]. Studies have shown that Cola nitida acts as a stimulant that fight tiredness, reduces hunger and thirst, act as a bronchodilator and most people take it to stay alert [8].
Diuresis is increased in urination and the physiologic processes that produce such an increase in the renal system. It involves extra urine production in the kidneys as part of the body’s homeostatic maintenance of fluid balance. Drug-induced diuresis is beneficial in many life-threatening disease conditions such as congestive heart failure (CHF), nephritis, hypertension and pregnancy toxemia [9]. A number of diuretics like mannitol, thiazides, furosemide, and spironolactone are used in practice [10]. However, most diuretic drugs have been associated with numerous adverse effects such as electrolyte imbalance, metabolic alterations, development of new-onset diabetes, activation of the renin-angiotensin and neuroendocrine systems, and impairment of sexual function [1,12]. Studies have shown that higher loop diuretic dosages in patients with CHF are at a high risk of mortality [13]. In this scenario, the need for novel diuretics such as plant-based substances, which are considered to be relatively safe and possessing lower potential for adverse effects, is imperative. This forms the rationale of the current study to investigate the diuretic, natriuretic and kaliuretic activities of methanolic extract of Cola nitida (MECN) in male Wistar rats.
Materials and methods
Drugs and chemicals
Furosemide was obtained from Apro Pharmaceutical Ltd, Ado-Ekiti, Nigeria, and the chemicals used in the study were of AR grade, which were obtained from Sigma Chemical, St. Louis, MO, USA.
Preparation of the extract
Samples of C. nitida were commercially obtained locally in Nigeria, and authenticated by a botanist in the Department of Agricultural science, Afe Babalola University where a voucher specimen was documented. The plant material was washed with distilled water, and chopped into small pieces with a kitchen knife. Drying was achieved under room temperature for 7 days in an open space. Dried sample were pulverized and extraction was carried out by soaking the sample in 80% methanol (Sigma-Aldrich, Germany) for 24 hours in a glass bottle. The C. nitida was filtered with Whatman No. 1 filter paper. The filtrate was concentrated by evaporation in a rotary evaporator (Search Instrument). The concentrate (extract) was stored in a refrigerator.
Animals, grouping and protocol
Adult male Wistar rats weighing 180-200 g were obtained from the animal house, College of Medicine and Health Sciences, Afe Babalola University, Ado-Ekiti, Ekiti State, Nigeria. The rats were housed in wire mesh cages and maintained in a well ventilated room at 25±2°C, on a 12-h light/12-h dark cycle. Rats had unrestricted access to standard rat chow and tap water. After acclimatized for two weeks, the rats were randomly allotted into different groups (n=6 each); Control (25 ml/kg body weight; b.w.), Furosemide (20 mg/kg b.w; Standard), MECN1 (100 mg/kg b.w. of MECN), MECN2 (200 mg/kg b.w. of MECN), MECN3 (300 mg/kg b.w. of MECN), MECN4 (400 mg/kg b.w. of MECN), MECN5 (500 mg/kg b.w. of MECN) and MECN6 (600 mg/kg b.w. of MECN). The treatment lasted for 14 days and the administration was done daily by oral gavage. The investigation was conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Review Board of Afe Babalola University, Ado-Ekiti, Ekiti State and every effort was made to minimize both the number of animals used and their suffering. Initial and final body weights were monitored using animal weighing balance (Olympia SCL66110 model, Kent Scientific Corporation, Torrington, CT06790, USA) and the body weight change was estimated.
Investigation of diuretic, natriuretic and kaliuretic activities
Diuretic potential of different doses of the extract was determined following the previous method [3,14]. The rats were allowed to acclimatized to metabolic cages fabricated by Central Technological Laboratory and Workshops, Afe Babalola University, according to Ohasu R Model; Ohasu, Pine Brook, New Jersey, USA) for 3 days before the commencement of the study. The last 24 h of this study investigated the possible diuretic, natriuretic and kaliuretic activities of C. nitida. The animals were fasted for 12 hours with free access to water. There after the drug and extract were administered to the rats. Urine volume and electrolytes (Na+, K+ and Cl-) were estimated in the samples of urine collected after 6 h of the study. Diuretic indices were calculated as previously described [14].
Sample preparation and biochemical analysis
At the end of treatment, the rats were anesthetized with pentobarbital sodium (50 mg/kg, i.p). Blood was collected from the apex of the heart into heparinized bottle and centrifuged at 3000 rpm for 15 minutes using a bench centrifuge and the plasma was stored frozen until it was needed for biochemical assay. Biochemical analysis of plasma electrolytes concentration (Na+, K+ and Cl-) were performed using flame photometry and AST, ALT, creatinine and urea were performed by standard colorimetric method using assay kit obtained from Randox Laboratory Ltd. (Co. Antrim, UK).
Tissue homogenate
The kidneys were excised, blotted and weighed. After weighing, 500 mg of tissue was carefully removed and homogenized with a glass homogenizer. Following centrifugation at 3000 rpm for 10 minutes, supernatant was removedand used for the measurement of renal AST and ALT activities by standardized enzymatic colorimetric methods using assay kit obtained from Randox Laboratory Ltd. (Co. Antrim, UK).
Statistical analysis
All data were expressed as means ± SEM. Statistical group analysis was performed with SPSS, version 22 of statistical software. One-way analysis of variance (ANOVA) was used to compare the mean values of variables among the groups. Bonferroni’s test was used to identify the significance of pair wise comparison of mean values among the groups. Statistically significant differences were accepted at p<0.05.
Results
Effect of methanolic extract of Cola nitida on body weight, food intake and water intake in male Wistar rats
Sub-chronic administration of different doses of C. nitida significantly altered body weight gain and water intake but not food intakecompared with control group. In addition, 300 mg-treated group showed a significant increase in water intake compared with furosemide-treated group (Table 1).
Table 1.
Effect of methanolic extract of Cola nitida on body weight, food intake and water intake in male Wistar rats
| GROUPS | CONTROL | FRD | MECN1 | MECN2 | MECN3 | MECN4 | MECN5 | MECN6 |
|---|---|---|---|---|---|---|---|---|
| Weight change (g/week) | 10.2±1.5 | 3.5±1.2* | 4.8±0.5* | 2.0±1.4* | 4.0±0.7* | 4.5±0.8* | 4.6±1.0* | 4.3±0.8* |
| Food intake (g/day) | ||||||||
| W0 | 10.5±3.0 | 11.1±1.3 | 9.6±1.0 | 10.7±1.2 | 12.3±1.1 | 11.1±0.8 | 13.2±0.2 | 10.9±0.5 |
| ΔW1 | 0.4±0.9 | 2.0±1.1 | 0.5±0.9 | 3.0±1.2 | 0.7±0.8 | 1.1±0.6 | 1.4±1.1 | 0.9±0.5 |
| ΔW2 | 0.5±0.1 | 1.4±1.3 | 0.7±0.7 | 3.3±1.7 | 1.5±0.8 | 1.5±0.7 | 1.2±0.9 | 0.4±0.8 |
| Water intake (ml/day) | ||||||||
| W0 | 15.6±8.0 | 14.8±5.7 | 16.0±3.7 | 16.8±3.0 | 17.6±3.4 | 15.7±3.3 | 16.1±4.2 | 17.6±10.7 |
| ΔW1 | 5.0±0.8 | 6.8±0.8 | 0.5±0.6*,# | 2.8±0.7# | 3.8±0.7# | 2.0±0.6*,# | 7.2±0.9 | 4.0±1.1 |
| ΔW2 | 6.1±0.6 | 7.9±0.9 | 0.3±1.0*,# | 2.8±0.9*,# | 11.0±0.5*,# | 9.8±0.5* | 3.7±0.6# | 9.6±0.5* |
Data are expressed as mean ± S.E.M. n=6. Data were analyzed by one-way ANOVA followed by Bonferroni post hoc test.
p<0.05 vs Control;
p<0.05 vs FRD.
ΔW1 (change in week 1); ΔW2 (change in week 2).
Effect of methanolic extract of Cola nitida on urine volume in male Wistar rats
Treatment with different doses of C. nitida significantly increased urine volume except for the groups that received 100 mg of C. nitida when compared with control group (Table 2).
Table 2.
Effect of methanolic extract of Cola nitida on urine volume and its diuretic indices in male Wistarrats
| GROUPS | CONTROL | FRD | MECN1 | MECN2 | MECN3 | MECN4 | MECN5 | MECN6 |
|---|---|---|---|---|---|---|---|---|
| Urine Volume (ml) | 0.58±0.05 | 0.75±0.02* | 0.65±0.03 | 0.70±0.01* | 0.94+0.06*,# | 1.10±0.07*,# | 0.95±0.05*,# | 1.05±0.13*,# |
| Diuretic index | 0 | 1.29±0.08* | 1.12±0.05* | 1.2±0.09* | 1.6±0.10*,# | 1.9±0.07*,# | 1.6±0.08*,# | 1.8±0.12*,# |
Data are expressed as mean ± S.E.M. n=6. Data were analyzed by one-way ANOVA followed by Bonferroni post hoc test.
p<0.05 vs Control;
p<0.05 vs FRD.
Diuretic index of methanolic extract of Cola nitida in male Wistar rats
Diuretic index as previously described is the ratio of urine volume of the experimental group to that of the control group [14]. Indices of 1.0-1.49 and 1.5 and more are regarded as moderate and potent diuretics respectively [14,15]. As detailed in Table 2, the groups treated with 100 mg and 200 mg of C. nitida showed moderate diuretic effect (indices <1.5), while other experimental groups with high doses showed potent diuretic effect (indices >1.5).
Effect of methanolic extract of Cola nitida on urine electrolyte concentration (Na+, K+ and Cl-) in male Wistar rats
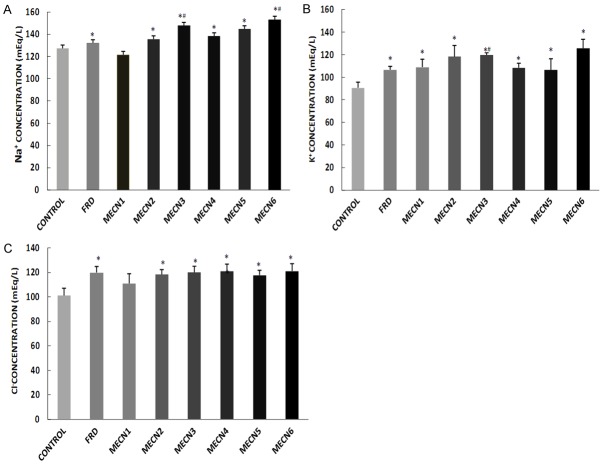
Sub-chronic treatment with different doses of C. nitida led to significant increases in urine electrolyte concentration (Na+, K+ and Cl-) when compared with control group except the group treated with 100 mg of C. nitida that did not significantly increased urine Na+ and Cl- compared with control (Figure 1). In addition, 300 mg-treated group of C. nitida significantly increased urine Na+ and K+ levels when compared with furosemide-treated group (Figure 1A, 1B).
Figure 1.
Effect of methanolic extract of Cola nitida on urine electrolyte concentration; Na+ (A), K+ (B) and Cl- (C) in male Wistar rats. Data are expressed as mean ± S.E.M. n=6. Data were analyzed by one-way ANOVA followed by Bonferroni post hoc test.(*p<0.05 vs Control; #p<0.05 vs FRD).
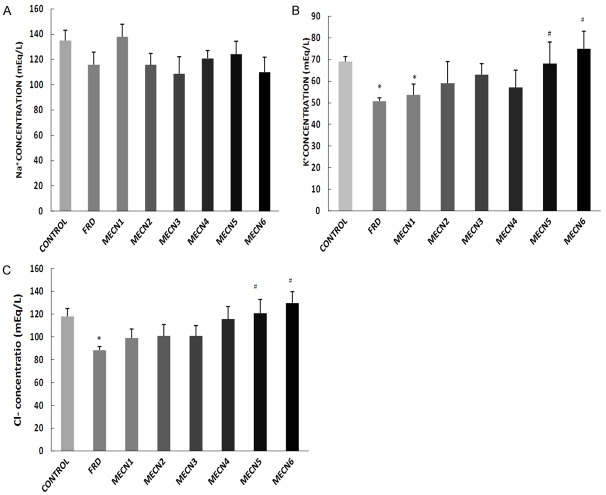
Effect of methanolic extract of Cola nitida on plasma electrolyte concentration (Na+, K+ and Cl-) in male Wistar rats
Sub-chronic treatment with different doses of C. nitida did not alter plasma Na+, K+ and Cl- concentration when compared with control group except the group treated with 100 mg/kg of C. nitida that significantly decreased plasma K+ concentration compared with control (Figure 2). In addition, 500 mg- and 600 mg-treated groups of C. nitida significantly increased plasma Cl- and K+ concentration compared with furosemide-treated group (Figure 2B, 2C).
Figure 2.
Effect of methanolic extract of Cola nitida on plasma electrolyte concentration; Na+ (A), K+ (B) and Cl- (C) in male Wistar rats. Data are expressed as mean ± S.E.M. n=6. Data were analyzed by one-way ANOVA followed by Bonferroni post hoc test. (*p<0.05 vs Control; #p<0.05 vs FRD).
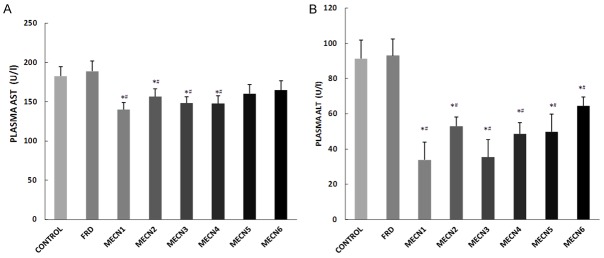
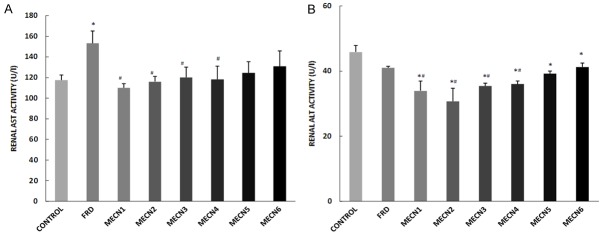
Effect of methanolic extract of Cola nitida on AST and ALT activities of male Wistar rats
Serum or plasma AST and ALT are commonly measured biomarkers of hepatic injury or toxicity (Wang et al., 2012), while renal AST and ALT are biomarkers of renal toxicity or nephrotoxicity. Administration of different doses of C. nitida significantly reduced plasma AST and ALT when compared with both the control and furosemide-treated groups, except the groups treated with 500 mg and 600 mg of C. nitida that showed no significant effect on plasma AST compared to control and furosemide-treated groups (Figure 3). However, administration of different doses of C. nitida significantly reduced renal ALT when compared with control group. In addition, the groups treated with 100 mg-400 mg significantly reduced renal AST and ALT activities when compared with furosemide-treated group (Figure 4).
Figure 3.
Effect of methanolic extract of Cola nitida on plasma AST (A) and ALT (B) in male Wistar rats. Data are expressed as mean ± S.E.M. n=6. Data were analyzed by one-way ANOVA followed by Bonferroni post hoc test. (*p<0.05 vs Control; #p<0.05 vs FRD).
Figure 4.
Effect of methanolic extract of Cola nitida on renal AST (A) and renal ALT (B) in male Wistar rats. Data are expressed as mean ± S.E.M. n=6. Data were analyzed by one-way ANOVA followed by Bonferroni post hoc test. (*p<0.05 vs Control; #p<0.05 vs FRD).
Effect of methanolic extract of Cola nitida on renal function test in male Wistar rats
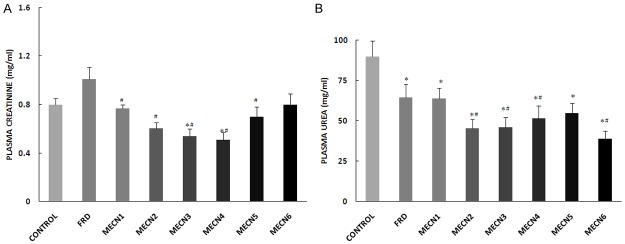
Elevated level of plasma creatinine and urea are potent biomarkers of renal dysfunction. Administration of different doses of C. nitida reduced the plasma levels of creatinine and urea when compared with control and furosemide-treated groups. The reduction was significant when compared with control- or furosemide-treated group except the 600 mg-treated group that showed no significant reduction in plasma urea level (Figure 5).
Figure 5.
Effect of methanolic extract of Cola nitida on plasma craetinine (A) and urea (B) in male Wistar rats. Data are expressed as mean ± S.E.M. n=6. Data were analyzed by one-way ANOVA followed by Bonferroni post hoc test. (*p<0.05 vs Control; #p<0.05 vs FRD).
Discussion
The current study investigated diuretic, natriuretic and kaliuretic activities of methanolic extract of C. nitida in male Wistar rats. Our results demonstrated that treatment with different doses of MECN significantly increased urine volume, urine electrolytes, which are associated with reduced plasma AST/ALT, creatinine, urea and renal AST/ALT activities. The treatment also altered body weight gain and water intake but not food intake compared with control group. In addition, there were no alterations in plasma electrolyte concentration.
C. nitida has been previously shown to lessen fatigue, prevent hunger pangs [5], increase mental activity, reduce the need for sleep and may even prevent metabolic disorder [16], and these effects have been attributed to caffeine-theobromine components of C. nitida [16,17]. However, there was paucity of information regarding the renal effects of MECN.
The present results that sub-chronic treatment with different doses of MECN significantly altered body weight gain when compared with control group was consistent with previous observation that aqueous and ethanolic extract of C. nitida causes a reduction in body weight gain in the treated group compared to control group [18,19]. The weight reduction has been attributed to the presence of caffeine as one of the active component of C. nitida [18]. Also, weight loss has been earlier reported in the habitual user of C. nitida [20,21]. Although C. nitida and its active constituent, caffeine have been reported as appetite-suppressant [21], but the present observation showed no significant alteration in food intake compared to control group. This implied that the inhibitory effect of MECN on food intake may be dose-dependent. In addition, our results showed a significant alteration in water intake, which is in line with earlier study [21].
The results that sub-chronic treatment with different doses of MECN significantly increased urine output except 100 mg/kg b.w was an indication that doses from 200 mg-600 mg/kg elicited diuretic activities, since diuresis is known as increased in urine production. Also, diuretic indices of different doses (Table 2) using the method of Lipschitz et al. [14] suggested that 100 mg-600 mg/kg of MECN in this present study elicited diuretic activities. In addition, high doses from 300 mg-600 mg/kg showed potent diuretic effect with diuretic indices >1.5 [14,15], whereas low doses 100/200 mg/kg- showed moderate diuretic effect with diuretic indices <1.5 [15]. This observation provided additional information to earlier studies that aqueous extract of C. nitida induces diuresis [19]. The spectrum of biological and pharmacological effect including diuresis of C. nitida has been attributed to its caffeine constituent. The doses of 300 mg-600 mg/kg produced more urine compared with furosemide-treated group. This implied that the high doses seemed to be more potent than the furosemide.
The observation that treatment with different doses except 100 mg/kg of MECN led to significant increase in urine electrolytes concentration; Na+, K+ and Cl- suggested that C. nitida dose-dependently elicited natriuretic and kaliuretic activities. This observation was similar to previous observation that aqueous extract of C. nitida induced a significant increase in urine concentrations of Na+, K+, Cl- in the experimental group compared with control group [19]. These effects could also be attributable to caffeine constituent of C. nitida [17]. Specifically 250 mg of caffeine has been reported to increase urine volume and sodium excretion [22], and theophylline another potent constituent of C. nitida [22] has been demonstrated to increase glomerular filtration rate (GFR) or renal blood flow [23]; this could reduce tubular reabsorption of water and sodium which may lead to diuresis and natriuresis [22]. However, the present doses of C. nitida did not alter plasma Na+, K+ and Cl- concentration when compared with control group except the group treated with 100 mg/kg b.w of C. nitida that significantly decreased plasma K+ concentration compared with control and perhaps due to inability or ineffectiveness of 100 mg/kg dose to maintain plasma-urine potassium balance. The impairment of potassium homeostasis attributable to significant loss of K+ through urine which subsequently led to significant decrease in circulating level of K+. Although the exact mechanism was not observed in the present study but our observation seems consistent with previous study that low dose of caffeine (main constituent of C. nitida) significantly reduced plasma K+ compared with control [24]. The present finding that some doses (200-600 mg/kg) of MECN did not alter the circulating levels of electrolytes were also in consonance with earlier study that treatment with ethanolic extract of C. nitida did not cause any significant change in plasma electrolytes [18]. It has been earlier documented that chronic consumption of caffeine containing beverages is an integral part of normal lifestyle and no electrolytes imbalance or dehydration has been reported [25]. In addition, 500 mg- and 600 mg-treated groups of C. nitida significantly increased plasma Cl- and K+ concentration compared with furosemide-treated group. This observation suggested that higher doses of methanolic extract of C. nitida like 500 mg/600 mg/kg- seemed to be associated with electrolyte imbalance. Therefore, the present results provided additional information that the maintenance of electrolyte balance during administration of C. nitida is dose-dependent.
Plasma/tissue AST or ALT are potent biomarkers of tissue toxicity ranging from hepatic, nephrotic, cardiac, cerebral to neuronal toxicities [26,27]. Our present results showed a significant reduction in plasma AST and ALT when compared with both the control and furosemide-treated groups, except the groups treated with 500 mg and 600 mg of C. nitida that did not alter the plasma AST compared to control and furosemide-treated groups. This suggested that treatment with current doses of C. nitida did not induce hepatotoxicity except the higher doses (500 mg/600 mg/kg). In addition, a significant reduction was observed in renal ALT when compared with control group, and likewise a reduction in renal AST when compared with furosemide-treated group except 500/600 mg/kg- dose that showed no alteration in renal AST when compared with control and furosemide-treated groups. This implied that treatment with low-moderate doses of MECN (100-400 mg/kg) did not induce nephrotoxicity. These findings were consistent with earlier report that treatment with doses lower or equal to 400mg over a short period of time could not induce toxicity [18].
Elevated levels of plasma creatinine and urea have been reported to indicate nephrotoxicity [28]. Our present findings showed a reduction in the circulating levels of creatinine and urea when compared with control and furosemide-treated groups. The reduction was significant when compared with control- or furosemide-treated group except the 600 mg- treated group that showed no significant reduction in plasma urea level. This finding suggested that treatment with MECN did not induce renal dysfunction except at higher dose like 600 mg/kg-. This observation was consistent and provided additional information to earlier studies that chronic consumption of caffeine or kolanut at higher doses may result in renal dysfunctions and development of nephritis [29,30]. Therefore, our present findings demonstrate that the renal effects of MECN are dose-dependent.
Conclusion
Our study suggests that methanolic extract of C. nitida elicits diuretic, natriuretic, kaliuretic activities without causing electrolyte impairment, hepatotoxicity and nephrotoxicity. These effects are dose-dependent.
Disclosure of conflict of interest
None.
References
- 1.Hatasaka H, Goldstain M. Kola nut as a symbol and its effect on the central nervous system. J Sep. 2001;257:2133–2136. [Google Scholar]
- 2.Ratsch C. The encyclopedia of psychoactive plants: ethnopharmacology and its applications. Rochester: Park Street Press; 1998. [Google Scholar]
- 3.Keay RWJ, Onochie CFA, Stanfield DP. “Nigerian trees”. Federal Department of Forest Research. 1964 [Google Scholar]
- 4.Purseglove JW. “Tropical crops: dicotyledons”. London: Longman Green & Co Ltd; 1977. [Google Scholar]
- 5.Esimone CO, Adikwu MU, Nworu CS, Okoye FBC, Odimegwu DC. Adaptogenic potentials of camellia sinesis leaves, garcinia kola and kola nitida seeds. Sci Res Essay. 2007;2:232–237. [Google Scholar]
- 6.Newall CA, Anderson LA, Philipson JD. Herbal medicine: a guide for health care professionals. Pharmarceutical Press; 1996. pp. 199–200. [Google Scholar]
- 7.Russel TA. The kola nut of West Africa. World Crop. 1995;7:221–225. [Google Scholar]
- 8.Javies G, editor. “The rise and fall of cocaine cola”. http://www.unz.org/Pub/Lew Rock well 2002: 00039.
- 9.Agunu A, Abdurahamn EM, Andrew GO, Muhammed Z. Diuretic activity of stem bark extracts of Steganotaenia araliaceae Hoechst [Apiaceae] . J Ethnopharmacol. 2005;96:471–5. doi: 10.1016/j.jep.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 10.Singh RG, Singh RP, Usha KP. Experimental evaluation of diuretic action of herbal drug (Tribulusterrestris Linn. ) on albino rats. J Res Edu Indian Med. 1991;3:19–21. [Google Scholar]
- 11.Alberto M. Should be the first choice in patients with essential hypertension? Am Nephrol. 2005;16:70–73. doi: 10.1681/asn.2004110964. [DOI] [PubMed] [Google Scholar]
- 12.Iqbal J, Javaid M. Diuretic resistance and its management. Br J Hos Med (Lond) 2014;75:C103–107. doi: 10.12968/hmed.2014.75.sup7.c103. [DOI] [PubMed] [Google Scholar]
- 13.Eshaghian S, Horwich T, Fonarow G. Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol. 2006;97:1759–64. doi: 10.1016/j.amjcard.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 14.Lipschitz WL, Hadidian Z, Kerpcsar A. Bioassay of diuretics. J Pharmacol Exp Ther. 1943;79:97–110. [Google Scholar]
- 15.Abdala S, Martin-Herrera D, Benjumea D, Perez-Paz P. Diuretic activity of Smilax canariensis, an endemic Canary Island species. J Ethnopharmacol. 2008;1:12–16. doi: 10.1016/j.jep.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 16.Olsen J. Public health epidemiology-what next? Scand J Public Health. 2005;33:81–83. doi: 10.1080/14034940510006067. [DOI] [PubMed] [Google Scholar]
- 17.Osim EE, Arthur Sk, Etta KM. Influence of Kola nuts (cola nitida alba) on in vivo secretion of gastric acid in cats. Int J Pharmacogn. 1991;29:215–220. [Google Scholar]
- 18.Salahdeen HM, Omoaghe AO, Isehunwa GO, Murtala BA, Alada AR. Gas chromatography mass spectrometry (GCMS) analysis of ethanolic extracts of kolanut (Cola nitida) (vent) and its toxicity in rats. Journal of Medicinal Plant Research. 2015;9:56–70. [Google Scholar]
- 19.Ashibuogwu MN, Adeosun OI, Akomolafe RO, Sanni DO, Olukiran OS. Diuretic activity and toxicity study of the aqueous extract of Cola nitida seed on markers of renal function and electrolytes in rats. Journal of Complementary and Integrative Med. 2015;13:79–89. doi: 10.1515/jcim-2015-0115. [DOI] [PubMed] [Google Scholar]
- 20.Jessen AB, Toubro S, Astrup A. Effect of chewing gum containing nicotine and caffeine on energy expenditure and substrate utilization in men. Am J Clin. 2003;77:1442–1447. doi: 10.1093/ajcn/77.6.1442. [DOI] [PubMed] [Google Scholar]
- 21.Jessen A, Buemann B, Toubro S, Skovgaard IM, Astrup A. The appetite-supperssant effect of nicotine is enhanced by caffeine. Diabetes Obes Metab. 2005;7:327–333. doi: 10.1111/j.1463-1326.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 22.Brater DC, Kaojarern S, Chennavasin P. Pharmacodynamics of the diuretic effects of aminophylline and acetazolamide alone and combined with furosemide in normal subjects. J Pharmacol Exp Ther. 1983;227:92–97. [PubMed] [Google Scholar]
- 23.Cheul DJ, Chan PN, Jun JS, Hyun CK, Hwa PI, Kwon SK, Woong KS. Changes of the blood chemistry components in serum of the rat after oral administration of caffeine. Korean J Vet Serv. 1997;20:297–306. [Google Scholar]
- 24.Tsimihodimos V, Kakaidi V, Elisaf M. Cola-induced hypokalaemia: pathophysiological mechanisms and clinical implications. Int J Clin Pract. 2009;63:900–902. doi: 10.1111/j.1742-1241.2009.02051.x. [DOI] [PubMed] [Google Scholar]
- 25.Neuhäuser Berthold, Beine S, Verwied SC, Lührmann PM. Coffee consumption and total body water homeostasis as measured by fluid balance and bioelectrical impedance analysis. Ann Nutr Metab. 1997;41:29–36. doi: 10.1159/000177975. [DOI] [PubMed] [Google Scholar]
- 26.Mitruka BM, Rawnsley H. Clinical, biochemical and haematological references values in normal experimental animals. Masson Publishing USA Inc. 1977:53–54. [Google Scholar]
- 27.Wang CS, Chang TT, Yao WJ, Wang ST, Chou P. Impact of increasing alanine aminotransferase levels within normal range on incident diabetes. J Formos Med Assoc. 2012;111:201–8. doi: 10.1016/j.jfma.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Abdel BMA. Some aspects of pharmacotoxicity of Goro (Cola nitida) in rats. Sudan University of Science and Technology (Thesis) 2013 [Google Scholar]
- 29.Ikegwuonu FI, Aire TA, Ogwuegbu SO. Effect of kola-nut extract administration on the liver, kidney, brain, testis and some serum constituents of the rats. J Appl Toxicol. 1981;1:292–294. doi: 10.1002/jat.2550010603. [DOI] [PubMed] [Google Scholar]
- 30.Jossa F, Krigh V, Farinaro E. Coffee and serum lipids: findings from the Olivetti heart study. Ann Epidemiol. 1993;3:250–5. doi: 10.1016/1047-2797(93)90027-2. [DOI] [PubMed] [Google Scholar]