ABSTRACT
Acrolein is a highly reactive electrophile causing toxic effects, such as DNA and protein adduction, oxidative stress, endoplasmic reticulum stress, immune dysfunction, and membrane damage. This Opinion/Hypothesis provides an overview of endogenous and exogenous acrolein sources, acrolein’s mode of action, and its metabolic fate. Recent reports underpin the finding that gut microbial glycerol metabolism leading to the formation of reuterin is an additional source of endogenous acrolein. Reuterin is an antimicrobial multicomponent system consisting of 3-hydroxypropionaldehyde, its dimer and hydrate, and also acrolein. The major conclusion is that gut microbes can metabolize glycerol to reuterin and that this transformation occurs in vivo. Given the known toxicity of acrolein, the observation that acrolein is formed in the gut necessitates further investigations on functional relevance for gut microbiota and the host.
KEYWORDS: endogenous acrolein, glycerol metabolism, gut microbiota, reuterin, toxicity
EXPOSURE, TOXICITY, AND FATE OF ACROLEIN
Human exposure to acrolein.
Acrolein, 2-propenal, is the simplest α,β-unsaturated aldehyde and a ubiquitous contaminant found in the environment and food. Exogenous sources of acrolein include tobacco smoke, exhaust gas emission, wood combustion, and deep-fat frying (1). As a result, acrolein can be detected in air, surface water, and various kinds of food. For example, 6.9 to 29.8 µg m−3 acrolein has been detected in the indoor air in restaurants in Germany (1). In addition, acrolein was recovered from food and beverages such as whisky (0.7 to 11.1 µg liter−1) and red wine (3,800 µg liter−1) (1). Levels reported for alcoholic beverages exceed minimal risk levels (4 µg kg−1 body weight day−1 for intermediate-duration oral exposure) (2) and the chronic oral dose (0.05 µg kg−1 body weight day−1) (2).
Endogenous formation of acrolein by chemical reactions and mammalian enzymatic activity has been investigated in depth (3). Myeloperoxidase, a heme enzyme excreted by human neutrophils, converts hydroxy-amino acids, e.g., threonine, to acrolein in the presence of H2O2 and a chlorine ion (Table 1). Acrolein can also be produced by copper-dependent amine oxidation of spermidine and spermine, followed by spontaneous retro-Michael-type cleavage (3). In addition, catabolism of oxazaphosphorine drugs, such as cyclophosphamide, produces acrolein (4). Moreover, Uchida et al. described lipid peroxidation as an important source of acrolein (5). While exposure to exogenous acrolein sources might be transient, acrolein can constantly be formed endogenously, raising the concern about its chronic toxicity.
TABLE 1 .
Overview of endogenous sources of acrolein
Mechanisms of acrolein toxicity.
Acrolein is a highly reactive electrophile that modifies cellular nucleophiles, giving rise to adverse responses involving multiple molecular mechanisms (Table 2). Acrolein can form cyclic DNA adducts by addition to the 1 and N2 positions of deoxyguanosine (6). Frequency and distribution of these acrolein-DNA adducts along the tumor suppressor gene p53 in human bronchial epithelial cells matched p53 mutations in cigarette smoking-related lung cancer (7). Binding of acrolein to amines of amino acid residues can lead to protein dysfunction. Uchida et al. found that lysine and histidine residues of low-density lipoprotein (LDL) can be modified by acrolein by covalent binding (5). Using Nα-acetyl-lysine and Nα-acetyl-histidine as model molecules, Nα-acetyl-Nε-(3-formyl-3,4-dihydropyridine)-lysine and Nα-acetyl-Nim-propanalhistidine were identified as the major adducts. These adducts may contribute to the dysfunction of the antiatherogenic apolipoprotein E (8). Reaction of acrolein with cysteine thiols of proteins leads to the formation of beta-propanal adducts through Michael addition, which can inactivate important enzymes. Besides having direct effects on biomolecular function from covalent modification of DNA or proteins, acrolein can induce indirect toxic effects by disrupting various signaling pathways. Acrolein induces apoptosis, endoplasmic reticulum stress, and oxidative stresses (for a review, see reference 9). Acrolein can decrease mitochondrial membrane potential and active apoptotic enzymes, such as caspase 9 and caspase 7 (9). Acrolein can trigger immune and inflammatory responses, such as the increased expression of nuclear factor kappa B (NF-κB), tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), or IL-8, contributing to endoplasmic reticulum stress (9). Moreover, acrolein decreased barrier function and increased permeability, potentially due to the downregulation of tight junction proteins ZO-1, occludin, and claudin-1 (10). Together, these mechanisms of acrolein toxicity contribute to the pathogenesis of various diseases and xenobiotic intoxication.
TABLE 2 .
Targets and modes of action of acrolein and their consequences
| Effects | Mode of action | Molecular event(s)a | Reference |
|---|---|---|---|
| Direct | |||
| DNA mutation | DNA adducts | Conjugation of DNA bases | 7 |
| Protein dysfunction | Amino acid adducts | Conjugation of amino acids bearing an amine/thiol group | 8 |
| Indirect | |||
| Apoptosis | Mitochondrial dysfunction | Mitochondrial membrane potential ↓, caspase 7/9↑, caspase 3↓ | 9 |
| Endoplasmic reticulum stress | Immune and inflammatory responses | NF-κB ↑, TNF-α ↑, IL-6 ↑, IL-8 ↑ | 9 |
| Intestinal barrier dysfunction | Downregulation of tight junction proteins | ZO-1 ↓, occludin ↓, claudin-1 ↓ | 10 |
Arrows represent upregulation (↑) and downregulation (↓).
Fate of acrolein.
The metabolism, distribution, and excretion of acrolein in vivo has been well characterized. In rats administered radiolabeled acrolein (2.5 mg kg−1 body weight) by oral gavage, urinary excretion, carbon dioxide expiration, and fecal excretion was 52 to 63%, 30 to 31%, and 13 to 15%, respectively. Residual radioactivity in tissue was minimal (<1.2%) (11). Similar excretion patterns were observed in rats dosed intravenously, with higher urinary excretion and CO2 expiration and lower fecal excretion (11). These observations suggest that acrolein was quickly taken up, metabolized, and excreted. The major metabolic pathway of acrolein is conjugation with glutathione (GSH), followed by enzymatic cleavage of γ-glutamic acid and glycine and acetylation of the resulting cysteine adduct, giving rise to S-(3-oxopropyl)-N-acetylcysteine (OMPA). Oxidation of OMPA produces carboxylethylmercapturic acid (CEMA). Minor products of direct acrolein metabolism include glyceraldehyde, oxalic acid, malonic acid, and 3-hydroxypropionic acid (3). Reduction of OMPA produces 3-hydroxylpropylmercapturic acid (3-HPMA), which is the major acrolein-derived metabolite in urine (3). 3-HPMA has been used as a biomarker of human exposure to tobacco smoking-derived acrolein (12). Intriguingly, urine samples of nonsmokers also contain 3-HPMA (12), suggesting that humans are constantly exposed to endogenous acrolein.
MICROBIAL GLYCEROL METABOLISM AND THE REUTERIN SYSTEM
Bacterial GDH reduces glycerol to 3-HPA, a key component of reuterin.
The human gut microbiota may be an as-yet-unrecognized source of endogenous acrolein. Using a combined analytic approach, we recently showed that acrolein is a product of bacterial glycerol metabolism (13). Bacterial vitamin B12-dependent glycerol/diol dehydratases (GDH) reduce glycerol to 3-hydroxypropionaldehyde (3-HPA) (13). A second substrate for these enzymes is 1,2-propanediol (1,2-PD), which is reduced to propanal, an intermediate of propionate formation (14). In aqueous solution, 3-HPA exists in an equilibrium with mainly its hydrate, 1,1,3-propanetriol, and its dimer, 2-(2-hydroxyethyl)-4-hydroxy-1,3-dioxane (13). This dynamic system (Fig. 1) has been called reuterin after Lactobacillus reuteri, the best-studied reuterin producer. 3-HPA also spontaneously dehydrates to acrolein, and at physiological conditions (pH 7, 37°C), acrolein is always present in solution, including in microbiological fermentation broth (13). These data suggest that acrolein should be considered an intrinsic component of the reuterin system (13).
FIG 1 .

Conversion of glycerol to 3-hydroxypropionaldehyde (3-HPA) by bacterial glycerol/diol dehydratases (GDH) and major components of the reuterin system.
Contribution of acrolein to antimicrobial activity and chemical reactions attributed to reuterin.
Reuterin exhibits inhibitory activity against a broad range of Gram-positive and Gram-negative bacteria, yeasts, molds, and protozoa (15). Conjugation of acrolein with GSH may cause depletion of thiol pools in cells, which is believed to contribute to the antimicrobial activity of reuterin (13). In addition to having an antimicrobial function, reuterin is implicated in the conjugation of food-derived carcinogenic heterocyclic amines (HCA), a process of potential relevance to the availability and carcinogenicity of HCA in the human gut (16–18). The HCA 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), an amino acid pyrolysis product formed when meat is cooked at high temperatures, is transformed to a glycerol conjugate metabolite, 7-hydroxy-5-methyl-3-phenyl-6,7,8,9-tetrahydropyrido[3′,2′:4,5]imidazo[1,2-α]pyrimidine-5-ium chloride (PhIP-M1) in the presence of L. reuteri or enterococci (16). Using the acrolein scavengers GSH and N-acetyl-l-cysteine (or PhIP as a reactive probe for acrolein), we showed that acrolein is the active compound of the reuterin system with regard to antimicrobial activity and PhIP transformation (13).
Human gut microbes produce reuterin.
Eleven percent of gut microbes are predicted to possess vitamin B12-dependent GDH and are therefore likely able to produce 3-HPA from glycerol (19). Reuterin-forming L. reuteri organisms have been isolated from human feces but occur in only low abundance in some humans (14). Screening fecal metagenomes of adult healthy humans, putative GDH-encoding genes were detected in members of the phyla Firmicutes (Eubacterium hallii, Blautia obeum, Ruminococcus gnavus, Flavonifractor plautii, Intestinimonas butyriciproducens, and Veillonella spp.) and Proteobacteria (Escherichia coli, Klebsiella spp., and Citrobacter spp.), indicating functional redundancy across phylogenetically different taxons (14). We used E. hallii as a gut-derived model organism to verify the predictions made by metagenome and genome analyses (the presence of a glycerol/diol dehydratase and vitamin B12 biosynthesis genes) in microbiological assays (14). These assays confirmed vitamin B12 synthesis and the formation of 3-HPA and propanal from glycerol and 1,2-PD, respectively, with propanal being further metabolized to propanol and propionate (14). In growing cultures of L. reuteri, a major proportion of 3-HPA is reduced to 1,3-propanediol (1,3-PD) by a NAD+-dependent oxidoreductase, allowing cofactor regeneration (20). E. hallii does not form 1,3-PD, which might lead to the accumulation of 3-HPA and acrolein and, ultimately, to the transformation of PhIP to PhIP-M1 (17). PhIP-M1 was also recovered from L. reuteri grown in the presence of glycerol despite a major proportion of 3-HPA being further metabolized to 1,3-PD, indicating that acrolein was released. Further gut microbes with predicted glycerol/diol dehydrates were shown to form 3-HPA from glycerol. Klebsiella and Citrobacter species produced 3-HPA during growth in the presence of glycerol in addition to 1,3-PD (21), and the ability of R. gnavus to form propionate from 1,2-propanediol (22) implies the presence of an active GDH and the potential of this species to metabolize glycerol to 3-HPA.
Bacterial formation of acrolein in the human intestine.
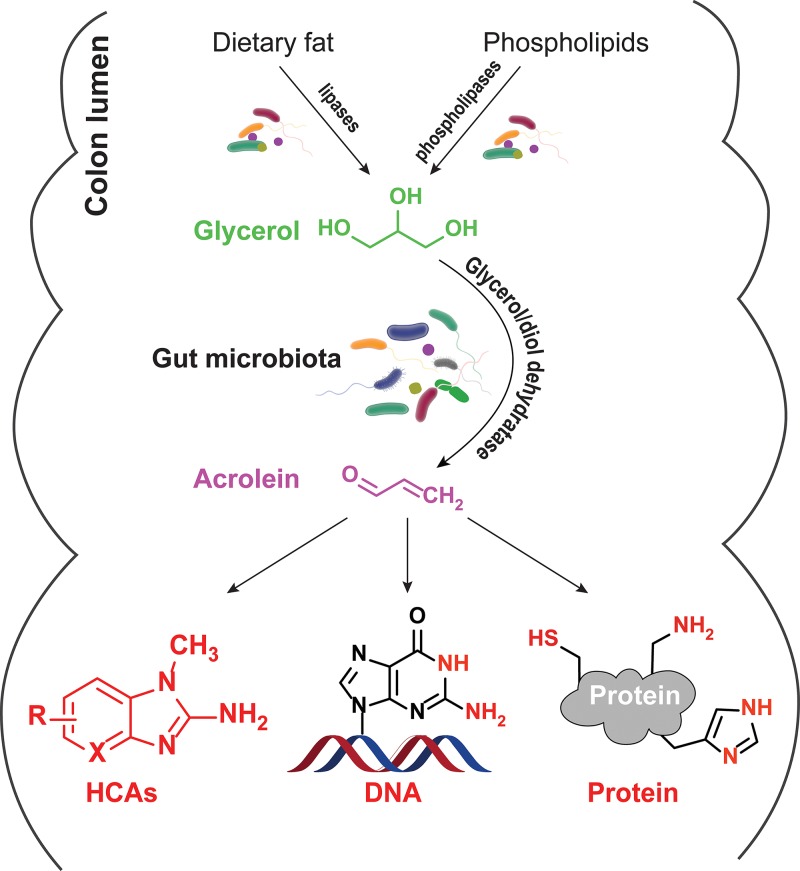
If there is any physiological relevance for the conversion of glycerol to acrolein in the human gut, it is essential that glycerol be present and that GDH be expressed by the human gut microbiota. Glycerol is a common additive in formulated foods, where it is used as a sweetener, humectant, and moisturizing or thickening agent, and glycerol can be liberated from tri-, di-, and mono-glycerides by digestive lipases in the small intestine (23). While glycerol is likely well absorbed in the small intestine, limited saturation of this process (24) leads to a portion of the chemical reaching the colon. Moreover, bacterial lipases of, for example, Prevotella intermedia (previously Bacteroides intermedius), Fusobacterium necrophorum, and Eubacterium combesii are active in the colon (Fig. 2) (23). Additionally, bacteria expressing phospholipases can hydrolyze phospholipids from the cell membrane to produce glycerol (Fig. 2). Thus, glycerol has been observed in human feces (25).
FIG 2 .
Overview of the endogenous precursors, formation, and potential targets of acrolein in the gut.
An anticipated fate of intestinal glycerol is its reduction to 3-HPA, based on the common presence of genes encoding GDH in metagenomes and its conversion to acrolein, supported by the observation of acrolein transformation products. The heterocyclic amines PhIP and MeIQx (2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline) are converted to PhIP-M1 and MelQx-M1 by complex colonic microbiota in the presence of glycerol (17, 18). Vanhaecke and coworkers reported that PhIP was transformed to PhIP-M1 by 18 fecal microbiota from individual donors, with PhIP transformation efficiencies ranging from 1.8% to 96% (16). Using an in vitro continuous fermentation model, PolyFermS, it could be shown that inactive microbiota can be made to significantly promote HCA transformation by addition of a reuterin-producing, GDH-bearing strain of E. hallii (17, 18). Finally, PhIP-M1 could be recovered from feces of consumers that obtained a single portion of cooked chicken meat containing PhIP (26). Variations in the occurrence and abundances of gut microbes with GDH among individuals and further metabolism of reuterin to 1,3-PD by some strains (20) might be a reason for interindividual variations in acrolein formation-respective HCA degradation proficiency and for susceptibility to the development of colorectal cancer.
CONCLUSIONS
The assertion that acrolein is produced from microbial glycerol metabolism in the human gut (Fig. 2) is supported by several strong points of evidence: (i) dietary HCAs are converted to acrolein conjugates by fecal and colon microbiota when glycerol is present (16, 17), (ii) a substantial proportion of 3-HPA is converted to acrolein under conditions prevailing in the human colon (13), and (iii) glycerol is present in the colon. Such production of acrolein in the human gut lumen may be regarded as a double-edged sword with regard to toxicological relevance. On one hand, acrolein conjugation of HCAs appears to be a detoxification process (18, 26), suggesting that microbially produced acrolein might attenuate carcinogenesis, but on the other hand, acrolein itself is toxic. This situation raises the question of whether chronic exposure to acrolein formed in the gut lumen by microbial metabolism has a net adverse influence on health or contributes in any beneficial manner. Moreover, since L. reuteri strains that are used as probiotics possess gdh and form reuterin and, potentially, acrolein, it may be prudent to reevaluate the safety of probiotic use of L. reuteri. Acrolein also is a broad-spectrum antimicrobial. However, to predict the antimicrobial impact of a highly reactive component, such as acrolein, may be very difficult in a complex ecosystem, such as the gut.
While further research is needed to define the physiological implications of acrolein for the gut microbiota and the host, gut microbial glycerol metabolism should be considered a relevant endogenous source of acrolein.
ACKNOWLEDGMENTS
This work was supported by ETH Zürich (grant ETH-41 16-1) and the Chinese Scholarship Council (grant 201406320209).
Footnotes
Citation Zhang J, Sturla S, Lacroix C, Schwab C. 2018. Gut microbial glycerol metabolism as an endogenous acrolein source. mBio 9:e01947-17. https://doi.org/10.1128/mBio.01947-17.
REFERENCES
- 1.International Agency for Research on Cancer 1995. Monograph of acrolein, p 335–372. International Agency for Research on Cancer, Lyon, France. [Google Scholar]
- 2.Agency for Toxic Substances and Disease Registry (ATSDR) 2007. Toxicological profile of acrolein, vol 16 Agency for Toxic Substances and Disease Registry, Atlanta, GA. [Google Scholar]
- 3.Stevens JF, Maier CS. 2008. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res 52:7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brock N, Stekar J, Pohl J, Niemeyer U, Scheffler G. 1979. Acrolein, the causative factor of urotoxic side-effects of cyclophosphamide, ifosfamide, trofosfamide and sufosfamide. Arzneimittelforschung 29:659–661. [PubMed] [Google Scholar]
- 5.Uchida K, Kanematsu M, Morimitsu Y, Osawa T, Noguchi N, Niki E. 1998. Acrolein is a product of lipid peroxidation reaction. Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J Biol Chem 273:16058–16066. doi: 10.1074/jbc.273.26.16058. [DOI] [PubMed] [Google Scholar]
- 6.Chung FL, Young R, Hecht SS. 1984. Formation of cyclic 1,N2-propanodeoxyguanosine adducts in DNA upon reaction with acrolein or crotonaldehyde. Cancer Res 44:990–995. [PubMed] [Google Scholar]
- 7.Feng Z, Hu W, Hu Y, Tang MS. 2006. Acrolein is a major cigarette-related lung cancer agent: preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc Natl Acad Sci U S A 103:15404–15409. doi: 10.1073/pnas.0607031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran TN, Kosaraju MG, Tamamizu-Kato S, Akintunde O, Zheng Y, Bielicki JK, Pinkerton K, Uchida K, Lee YY, Narayanaswami V. 2014. Acrolein modification impairs key functional features of rat apolipoprotein E: identification of modified sites by mass spectrometry. Biochemistry 53:361–375. doi: 10.1021/bi401404u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moghe A, Ghare S, Lamoreau B, Mohammad M, Barve S, McClain C, Joshi-Barve S. 2015. Molecular mechanisms of acrolein toxicity: relevance to human disease. Toxicol Sci 143:242–255. doi: 10.1093/toxsci/kfu233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen WY, Wang M, Zhang J, Barve SS, McClain CJ, Joshi-Barve S. 2017. Acrolein disrupts tight junction proteins and causes endoplasmic reticulum stress-mediated epithelial cell death leading to intestinal barrier dysfunction and permeability. Am J Pathol 187:2686–2697. doi: 10.1016/j.ajpath.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parent RA, Caravello HE, Sharp DE. 1996. Metabolism and distribution of [2,3-14C]acrolein in Sprague-Dawley rats. J Appl Toxicol 16:449–457. doi:. [DOI] [PubMed] [Google Scholar]
- 12.Carmella SG, Chen M, Zhang Y, Zhang S, Hatsukami DK, Hecht SS. 2007. Quantitation of acrolein-derived (3-hydroxypropyl)mercapturic acid in human urine by liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry: effects of cigarette smoking. Chem Res Toxicol 20:986–990. doi: 10.1021/tx700075y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engels C, Schwab C, Zhang J, Stevens MJ, Bieri C, Ebert MO, McNeill K, Sturla SJ, Lacroix C. 2016. Acrolein contributes strongly to antimicrobial and heterocyclic amine transformation activities of reuterin. Sci Rep 6:36246. doi: 10.1038/srep36246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engels C, Ruscheweyh HJ, Beerenwinkel N, Lacroix C, Schwab C. 2016. The common gut microbe Eubacterium hallii also contributes to intestinal propionate formation. Front Microbiol 7:713. doi: 10.3389/fmicb.2016.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vollenweider S, Lacroix C. 2004. 3-Hydroxypropionaldehyde: applications and perspectives of biotechnological production. Appl Microbiol Biotechnol 64:16–27. doi: 10.1007/s00253-003-1497-y. [DOI] [PubMed] [Google Scholar]
- 16.Vanhaecke L, Vercruysse F, Boon N, Verstraete W, Cleenwerck I, De Wachter M, De Vos P, van de Wiele T. 2008. Isolation and characterization of human intestinal bacteria capable of transforming the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Appl Environ Microbiol 74:1469–1477. doi: 10.1128/AEM.02064-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fekry MI, Engels C, Zhang J, Schwab C, Lacroix C, Sturla SJ, Chassard C. 2016. The strict anaerobic gut microbe Eubacterium hallii transforms the carcinogenic dietary heterocyclic amine 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). Environ Microbiol Rep 8:201–209. doi: 10.1111/1758-2229.12369. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Empl MT, Schwab C, Fekry MI, Engels C, Schneider M, Lacroix C, Steinberg P, Sturla SJ. 2017. Gut microbial transformation of the dietary imidazoquinoxaline mutagen MeIQx reduces its cytotoxic and mutagenic potency. Toxicol Sci 159:266–276. doi: 10.1093/toxsci/kfx132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Degnan PHH, Taga MEE, Goodman ALL. 2014. Vitamin B12 as a modulator of gut microbial ecology. Cell Metab 20:769–778. doi: 10.1016/j.cmet.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Ziney MG, Arneborg N, Uyttendaele M, Debevere J, Jakobsen M. 1998. Characterization of growth and metabolite production of Lactobacillus reuteri during glucose/glycerol cofermentation in batch and continuous cultures. Biotechnol Lett 20:913–916. doi: 10.1023/A:1005434316757. [DOI] [Google Scholar]
- 21.Vancauwenberge JE, Slininger PJ, Bothast RJ. 1990. Bacterial conversion of glycerol to β-hydroxypropionaldehyde. Appl Environ Microbiol 56:329–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crost EH, Tailford LE, Le Gall G, Fons M, Henrissat B, Juge N. 2013. Utilisation of mucin glycans by the human gut symbiont Ruminococcus gnavus is strain-dependent. PLoS One 8:e76341. doi: 10.1371/journal.pone.0076341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill MJ. 2004. Role of gut bacteria in human toxicology and pharmacology, p 131–142. Taylor & Francis, London, United Kingdom. [Google Scholar]
- 24.Yuasa H, Hamamoto K, Dogu SY, Marutani T, Nakajima A, Kato T, Hayashi Y, Inoue K, Watanabe J. 2003. Saturable absorption of glycerol in the rat intestine. Biol Pharm Bull 26:1633–1636. doi: 10.1248/bpb.26.1633. [DOI] [PubMed] [Google Scholar]
- 25.Weir TL, Manter DK, Sheflin AM, Barnett BA, Heuberger AL, Ryan EP. 2013. Stool microbiome and metabolome differences between colorectal cancer patients and healthy adults. PLoS One 8:e70803. doi: 10.1371/journal.pone.0070803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanhaecke L, Knize MG, Noppe H, De Brabander H, Verstraete W, Van de Wiele T. 2008. Intestinal bacteria metabolize the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine following consumption of a single cooked chicken meal in humans. Food Chem Toxicol 46:140–148. doi: 10.1016/j.fct.2007.07.008. [DOI] [PubMed] [Google Scholar]