Abstract
Objectives:
Linaclotide is a guanylate cyclase-C agonist approved in the United States, Canada, and Mexico at a once-daily 145-μg dose for the treatment of chronic idiopathic constipation (CIC); a once-daily 72-μg dose for CIC recently received FDA approval. The trial objective was to evaluate the efficacy and safety of a 72-μg linaclotide dose in CIC patients.
Methods:
This double-blind, placebo-controlled trial randomized patients with CIC (Rome III criteria) to once-daily linaclotide 72 μg or 145 μg, or placebo for 12 weeks. The primary endpoint, 12-week complete spontaneous bowel movement (CSBM) overall responder, required patients to have ≥3 CSBMs and an increase of ≥1 CSBM per week from baseline in the same week for ≥9 of 12 weeks of the treatment period. Secondary endpoints included 12-week change from baseline in bowel (SBM and CSBM frequency, stool consistency, straining) and abdominal (bloating, discomfort) symptoms, monthly CSBM responders, and 12-week CSBM responders among patients who averaged >1 SBM/week at baseline. Sustained response (12-week CSBM overall responders who met weekly criteria for 3 of the 4 final weeks (weeks 9–12) of treatment) was evaluated as an additional endpoint. Adverse events (AEs) were monitored.
Results:
The intent-to-treat population included 1,223 patients (mean age=46 years, female=77%, white=71%). The primary endpoint was met by 13.4% of linaclotide 72-μg patients vs. 4.7% of placebo patients (P<0.0001, odds ratio=3.0; statistically significant controlling for multiplicity). Sustained response was achieved by 12.4% of linaclotide 72-μg patients vs. 4.2% of placebo patients (nominal P<0.0001). Linaclotide 72-μg patients met 9-of-10 secondary endpoints vs. placebo (P<0.05; abdominal discomfort, P=0.1028). Patients treated with linaclotide 145 μg also improved CIC symptoms for the primary (12.4%) and sustained responder endpoint parameters (11.4%) and for all 10 of the secondary endpoint parameters including abdominal discomfort (P<0.05). Diarrhea, the most common AE, was mild in most instances and resulted in discontinuation of 0, 2.4%, and 3.2% of patients in the placebo, linaclotide 72-μg, and linaclotide 145-μg groups, respectively.
Conclusions:
Once-daily linaclotide 72 μg significantly improved CIC symptoms in both men and women with a low rate of discontinuation due to diarrhea over 12 weeks of treatment.
Introduction
Chronic idiopathic constipation (CIC) is a functional bowel disorder characterized by infrequent defecation, hard and difficult-to-pass stools, a sense of incomplete evacuation, and often, abdominal bloating and/or discomfort (1, 2, 3). Approximately 15% of the general population report experiencing symptoms consistent with CIC (4). The prevalence of CIC is higher in women than men and increases with age (5).
Linaclotide is a potent peptide agonist of the guanylate cyclase C (GC-C) receptor located on the luminal surface of the intestinal epithelium. Activation of GC-C increases cyclic guanosine monophosphate (cGMP) production, resulting in increases in lumenal chloride, bicarbonate, and fluid secretion (6, 7, 8). Linaclotide increases intestinal transit and reduces visceral hypersensitivity in animal models; in patients with irritable bowel syndrome with constipation (IBS-C), linaclotide improves abdominal pain and other abdominal symptoms, such as discomfort and bloating. These effects may be related to cGMP modulation of afferent nerve activity (9, 10, 11, 12, 13). Linaclotide is approved in the United States, Canada, and Mexico for the treatment of chronic idiopathic constipation (CIC) and IBS-C, and in the European Union (EU), Hong Kong, Japan, and Switzerland for the treatment of IBS-C (12, 13, 14).
A previous Phase 2b dose-range-finding study was conducted in 301 patients with CIC and evaluated once daily linaclotide doses of 72, 145, 290, and 580 μg. All four linaclotide doses were well tolerated and met the primary endpoint of change from baseline in SBM frequency over the 4-week treatment period (P<0.05 vs. placebo); in addition, an increasing treatment effect was observed with higher doses for key secondary endpoints (15). To ensure that an effective dose was identified for CIC patients, the 145-μg and 290-μg dose levels were evaluated in two large phase 3 trials (14). Both doses met the primary endpoint of a 12-week CSBM overall responder with little difference between the two doses in the secondary efficacy endpoints or in their adverse event profiles. Therefore, the lowest effective dose, 145 μg, was approved by the FDA for the treatment of CIC. A subsequent trial demonstrated that linaclotide (145 μg) was efficacious at treating men and women with CIC and prominent abdominal bloating (16). In order to ensure that patients with all levels of CIC severity (from mild to severe) have a dosing option with linaclotide and because patients differ in their response to linaclotide treatment, an additional dosing option of less than 145 μg is desirable. The 72-μg dose of linaclotide was selected as a potentially safe and effective option for CIC patients based on its demonstrated efficacy in the previous phase 2b study (15).
The primary objective of this trial was to investigate the efficacy and safety of linaclotide 72 μg administered once daily to patients with CIC.
Methods
Trial design
This 12-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group trial included patients at 105 clinical centers in the United States from October 2014 (first patient consented) through August 2015 (last patient completed). The trial was designed, conducted, and reported in compliance with the ethical principles that have their origins in the Declaration of Helsinki and the principles of Good Clinical Practice guidelines. Prior to their participation in the study, all patients reviewed and signed an informed consent form approved by an institutional review board. The trial is registered at ClinicalTrials.gov (registration number NCT02291679).
During an initial screening period of up to 21 days, patients provided blood and urine for routine testing and were instructed to discontinue any prohibited medications (eg, anti-cholinergics, opioids) for at least 14 days (24 h for non-steroidal anti-inflammatory drugs if taken for abdominal pain or discomfort, and for laxatives) before the start of baseline assessments. Eligible patients meeting the inclusion and exclusion criteria then entered the 14-day baseline period, during which they used an interactive voice response system (IVRS) to provide daily and weekly symptom assessments. Patients who continued to meet eligibility criteria were then randomized to linaclotide 72 μg, 145 μg, or placebo for 12 weeks.
In addition to a screening visit, study site visits occurred at the start of the baseline period (day −14) and throughout the treatment period (day 1 and at week 2, 4, 8, and 12 visits). All personnel involved in the design and implementation of the trial remained blinded until the database was locked and the results tables were generated.
Trial patients
Patients were men and women aged 18 years or older who met modified Rome III criteria for CIC (17). The criteria included <3 spontaneous bowel movements (SBMs: BMs occurring in the absence of laxative, suppository, or enema use during the preceding 24 h) per week and at least one of the following symptoms during ≥25% of BMs for at least 12 weeks (not necessarily consecutive) within the preceding 6 months: straining, lumpy or hard stools, and a sensation of incomplete evacuation. In addition, patients needed to report having an average of ≤6 SBMs and <3 complete SBMs (CSBMs: SBMs associated with a sensation of complete evacuation) per week during the 14-day baseline period. Patients also needed to demonstrate compliance with IVRS, requiring adequate completion of IVRS questions on ≥10 of 14 days of the baseline period. Patients were required to have met American Gastroenterological Association colonoscopy guideline requirements (18).
Patients were excluded if they reported loose or watery stools (Bristol Stool Form Scale (BSFS) (19) score of 6 or 7) in the absence of laxative, suppository, enema, or other prohibited medication use for >25% of BMs during the 12 weeks preceding the trial; mushy stool (BSFS score of 6) for>1 SBM, or a watery, liquid stool (BSFS score of 7) for any SBM during the baseline period; or met the Rome III criteria for irritable bowel syndrome (17). Other key exclusion criteria included structural GI abnormalities or conditions affecting GI motility; prior diagnosis of inflammatory bowel disease; a diagnosis or family history of a familial form of colorectal cancer; active peptic ulcer disease; a history of diverticulitis or any chronic condition that could be associated with abdominal pain or discomfort; a history of fecal impaction requiring hospitalization; or a history of cathartic colon, laxative or enema abuse, ischemic colitis, or pelvic floor dysfunction. Patients were excluded if they had: undergone bariatric surgery or any type of intestinal resection; abdominal or pelvic surgery 6 months prior to screening; appendectomy or cholecystectomy 60 days prior to screening; or other major surgery 30 days prior to screening.
Rescue medication (bisacodyl tablet or suppository) use was not allowed on the day before, day of, or day after randomization (protocol-defined bisacodyl rescue medication was otherwise allowed during the baseline and treatment periods, when ≥72 h had passed since the patient’s previous BM or when symptoms became intolerable). Patients who were on a stable dose of fiber, bulk laxatives, or stool softeners during the 30 days prior to screening were permitted to continue using these products during the trial.
Efficacy assessments and endpoints
Daily reports by patients via the IVRS included the number of BMs since the previous day’s call and whether rescue medication was used during that time. For each BM, patients then reported: whether the BM was associated with a sensation of complete emptying (yes/no); stool consistency (7-point BSFS; 1=“separate hard lumps like nuts [difficult to pass]” to 7=“watery, no solid pieces [entirely liquid]”); and severity of straining (5-point ordinal scale; 1=“not at all” to 5=“an extreme amount”). Daily patient reports also included rating of abdominal bloating, abdominal discomfort, and abdominal pain at its worst during the previous 24 h, each rated by the patient on an 11-point numerical rating scale (NRS; 0=“none”, 10=“very severe”).
Weekly IVRS assessments included constipation severity (5-point ordinal scale; 1=“none” to 5=“very severe”), adequate relief of constipation symptoms (yes/no), and degree of relief of constipation symptoms (7-point balanced scale; 1=“completely relieved” to 7=“as bad as I can imagine”).
Additional assessments were performed at specified patient visits to the study center. Treatment satisfaction was measured using a 5-point ordinal scale (1=“not at all satisfied”, 5=“very satisfied”) at weeks 2, 4, 8, and 12; and disease-specific quality of life was measured using the validated Patient Assessment of Constipation—Quality of Life (PAC-QOL) instrument (20).
Primary endpoint
The primary efficacy endpoint of the trial was the 12-week CSBM overall responder. A 12-week CSBM overall responder was a patient who was a CSBM weekly responder for ≥9 of the 12 weeks of the treatment period. A CSBM weekly responder for a treatment period week was a patient who had a CSBM weekly frequency rate that was ≥3 and increased by ≥1 from baseline, based on a minimum of four complete IVRS calls for that week. Bowel movements that occurred within 24 h following rescue medication use were not considered spontaneous and were therefore not counted for the efficacy endpoints.
Secondary and additional endpoints
Secondary endpoints were pre-specified, controlled for multiplicity, and compared the linaclotide 72-μg group with the placebo group. Secondary endpoints included 12-week average change from baseline in stool frequency (CSBM and SBM frequency rate), stool consistency, straining, abdominal bloating, and abdominal discomfort. Secondary responder endpoints included an analysis of the primary endpoint parameter (12-week CSBM overall responder) for the subgroup of patients who averaged >1 SBM per week during the baseline period, and monthly CSBM responders, in which responders were to meet weekly CSBM responder criteria for ≥3 of 4 weeks of the treatment month specified (month 1, month 2, or month 3).
Additional endpoints included: 12-week CSBM sustained responder (a sensitivity analysis for the primary endpoint), which required patients to be CSBM weekly responders for ≥9 of 12 weeks, including ≥3 of the last 4 weeks (Weeks 9–12) of the treatment period; 12-week degree of relief of constipation symptoms responder, which required patients to report their constipation symptoms as ‘somewhat’, ‘considerably’, or ‘completely’ relieved (i.e., ≤3) for all 12 weeks of the treatment period, or ‘considerably’ or ‘completely’ relieved (i.e., ≤2) for at least 6 of 12 weeks of the treatment period; 12-week change from baseline in constipation severity and in abdominal pain; and treatment satisfaction at week 12 and PAC-QOL (change from baseline to week 12).
Safety assessments
Treatment emergent adverse events were elicited/identified at each study visit using non-leading questions. Reports of diarrhea were all captured in a manner consistent with other spontaneous AEs, without consideration of other factors (i.e., stool consistency, perception of “bothersomeness”). The site investigator assessed all patient-reported adverse events (AEs) and serious AEs (SAEs) and determined their severity (mild, moderate, or severe) and relationship to study treatment (unrelated, unlikely, possible, probable, definite). Other safety evaluations included physical examinations, vital sign measurements, and standard clinical laboratory tests.
Statistical methods and data analysis
Eligible patients were stratified by baseline SBM frequency (i.e., those with >1 SBM/week and those with ≤1 SBM/week) and were randomly assigned to one of three double-blind treatments (72-μg or 145-μg linaclotide or matching placebo) in a 1:1:1 ratio using a block size of six through a central randomization. The treatment sequence was generated in SAS by a statistical programmer not assigned to this trial and provided securely to the IVRS vendor. Trial personnel used the IVRS to assign patients to treatment and inform study drug dispensing.
Corresponding with the trial’s primary objective, to determine the efficacy and safety of linaclotide 72 μg administered once daily to patients with CIC, the primary analysis compared the proportion of responders in the linaclotide 72-μg group with the proportion of responders in the placebo group by employing a 2-sided Cochran-Mantel-Haenszel (CMH) test stratified by baseline SBM frequency stratum (>1 per week vs. ≤1 per week) and geographical region.
For each of the change-from-baseline secondary efficacy parameters, the linaclotide 72-μg group was compared with the placebo group using an analysis of covariance (ANCOVA) model with fixed effect terms for treatment group, baseline SBM weekly frequency stratum, and geographical region and the corresponding baseline value as a covariate. The ANCOVA model included patients in all 3 treatment groups. In addition, comparison between the linaclotide 72-μg group and the placebo group on the primary efficacy parameter (12-week CSBM overall responder) within the baseline SBM frequency >1 per week stratum was conducted by employing a 2-sided CMH test stratified by geographical region alone. Lastly, for each of the monthly CSBM responder parameters, the proportion of responders in the linaclotide 72-μg group was compared with the proportion of responders in the placebo group using the same methods as the primary efficacy analysis.
The overall family-wise type I error rate for testing the primary and secondary efficacy endpoints was controlled at the 0.05 significance level using a five-step serial gate-keeping multiple comparisons procedure (MCP). The sample size of 400 patients per treatment arm provided 93% power to detect a difference between the placebo and linaclotide 72-μg groups for the primary efficacy endpoint. No direct comparisons between the linaclotide 72-μg and 145-μg dose groups were performed; however, comparisons between the linaclotide 145-μg and placebo groups were performed for all efficacy parameters, but were not controlled for multiplicity.
Patients were assumed not to have had BMs or to have taken rescue medications if the corresponding daily question was not answered. If a patient dropped out of the trial or otherwise did not report the efficacy data for a particular treatment-period week (patients were required to complete at least 4 IVRS calls during a treatment week), the patient was not considered a responder for that week. An observed-cases approach to missing data was applied to the change-from-baseline secondary endpoints, such that if a patient dropped out of the study or otherwise did not report data, the average of the non-missing data over the 12 weeks of the treatment period was the patient’s value. All P-values were based on two-sided tests.
Safety endpoints were summarized descriptively. Counts and percentages were utilized for treatment-emergent adverse events and potential clinically significant findings; n, mean, and standard deviation were computed for continuous safety measures.
All randomized patients who took at least 1 dose of study drug were included in safety analyses (safety population) and the intent-to-treat (ITT) population.
Results
Patient disposition, demographics, and baseline characteristics
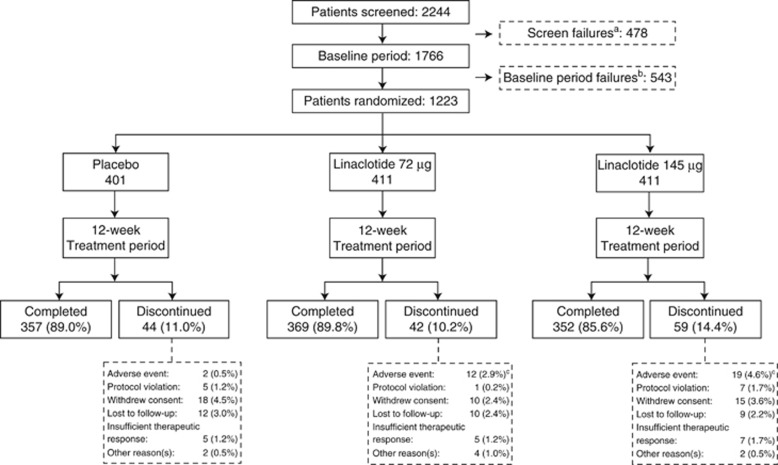
Of the 2,244 patients who provided informed consent, 1223 patients were randomized and received at least one dose of trial medication (Figure 1). Because all patients received the treatment to which they were randomized, the ITT and safety populations were identical. A total of 1,078 patients (88.1%) completed all 12 weeks of the treatment period. The treatment groups were generally balanced with respect to demographics, and baseline bowel and abdominal symptoms (Table 1). Mean compliance rate with study drug dosing (assessed by counting pills returned at study visits) up to study discontinuation/completion during the 12-week treatment period was >97%.
Figure 1.
Patient flow through the trial. (a) Patients who signed an informed consent but did not qualify for inclusion into the trial base on their screening visit evaluations. Patients who were re-screened and failed the second time during the screening period were only counted once. (b) Patients who signed an informed consent, entered the baseline period, but were not randomized into the trial. Patients who were re-screened and failed the second time during the baseline period were counted only in the pretreatment failure category. Patients who were re-screened and became randomized are not counted in either of the failure categories. (c) P=0.0123 for the linaclotide 72 μg group and P=0.0002 for the linaclotide 145 μg group vs. placebo (from pairwise comparisons with the placebo group using the Fisher’s exact test). The P-values for all other comparisons were >0.05.
Table 1. Summary of patient demographics and baseline characteristics (ITT population).
| Placebo (N=401) | Linaclotide 72 μg (N=411) | Linaclotide 145 μg (N=411) | |
|---|---|---|---|
| Demographic data | |||
| Age (years), mean (range) | 45.2 (14.7) | 45.8 (14.3) | 46.8 (14.0) |
| ≥65 years, n (%) | 39 (9.7) | 36 (8.8) | 43 (10.5) |
| Sex, n (%) | |||
| Female | 316 (78.8) | 312 (75.9) | 314 (76.4) |
| Male | 85 (21.2) | 99 (24.1) | 97 (23.6) |
| Race, n (%) | |||
| White | 276 (68.8) | 298 (72.5) | 294 (71.5) |
| Black | 102 (25.4) | 93 (22.6) | 94 (22.9) |
| Other | 23 (5.7) | 20 (4.9) | 23 (5.6) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 175 (43.6) | 178 (43.3) | 175 (42.6) |
| Not Hispanic or Latino | 226 (56.4) | 233 (56.7) | 236 (57.4) |
| BMI, mean (s.d.) | 29.3 (6.5) | 28.9 (6.0) | 29.3 (6.2) |
| SBM Stratum, n (%) | |||
| ≤1 SBM/week | 175 (43.6) | 167 (40.6) | 176 (42.8) |
| >1 SBM/week | 226 (56.4) | 244 (59.4) | 235 (57.2) |
| Baseline data, mean (s.d.) | |||
| CSBMs/week | 0.3 (0.5) | 0.2 (0.5) | 0.2 (0.4) |
| SBMs/week | 1.6 (1.2) | 1.7 (1.4) | 1.7 (1.4) |
| Stool consistencya | 2.0 (1.0) | 1.9 (0.9) | 2.0 (0.9) |
| Strainingb | 3.5 (0.9) | 3.6 (0.9) | 3.5 (0.8) |
| Constipation severityc | 3.7 (0.8) | 3.7 (0.8) | 3.6 (0.8) |
| Abdominal bloatingd | 5.3 (2.4) | 5.2 (2.6) | 5.3 (2.6) |
| Abdominal discomfortd | 4.8 (2.6) | 4.6 (2.7) | 4.7 (2.7) |
| Abdominal paind | 4.1 (2.8) | 4.1 (2.9) | 4.2 (2.9) |
| Stool softener/bulk laxative use, n (%)e | |||
| Bulk-forming laxatives | 1 (0.2) | 5 (1.2) | 3 (0.7) |
| Softeners, emollients | 2 (0.5) | 7 (1.7) | 5 (1.2) |
BMI, body mass index; CSBM, complete spontaneous bowel movement; SBM, spontaneous bowel movement.
Assessed using the BSFS: 1=separate hard lumps, like nuts (hard to pass); 2=sausage-shaped, but lumpy; 3=like a sausage but with cracks on its surface; 4=like a sausage or snake, smooth and soft; 5=soft blobs with clear cut edges (passed easily); 6=fluffy pieces with ragged edges, a mushy stool; 7=watery, no solid pieces (entirely liquid).
Assessed using a five-point ordinal scale: 1=not at all, 2=a little bit, 3=a moderate amount, 4=a great deal, 5=an extreme amount.
Assessed using a five-point ordinal scale: 1= none; 2=mild; 3=moderate; 4=severe; 5=very severe.
Assessed using a 11-point NRS: 0=none; 10=severe.
Use of fiber, bulk laxatives, or stool softeners was acceptable during the trial if the patient had been on a stable dose during the 30 days before the screening visit and planned to continue on a stable dose throughout the trial.
Efficacy results
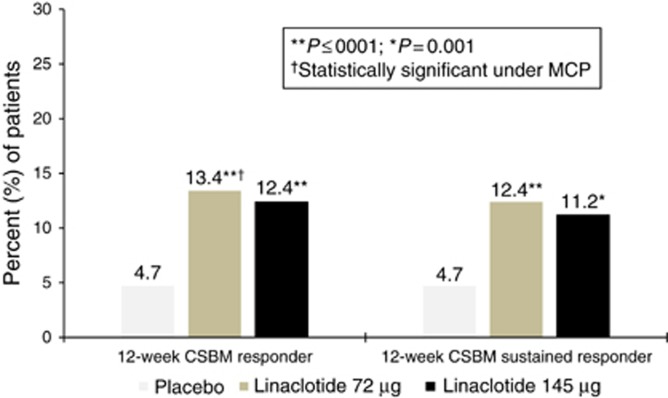
A total of 13.4% of patients receiving linaclotide 72 μg met the 12-week CSBM overall responder primary endpoint compared with 4.7% of patients receiving placebo (P<0.0001; odds ratio=3.0, 95% confidence interval=1.8, 5.2; Figure 2). These results were comparable to the approved linaclotide 145 μg dose group, in which 12.4% of patients met responder criteria (nominal P<0.0001; OR=2.8, 95% CI=1.6, 4.9). Nominal P-values are presented for the 145-μg dose, as these results were not controlled for multiplicity. Sustained response, defined as 12-week CSBM overall responder+weekly responder for ≥3 of the final 4 weeks of treatment, was significantly higher in the linaclotide 72-μg and 145-μg groups compared to placebo: 12.4, 11.2, and 4.7% of patients in the linaclotide 72, 145 μg, and placebo groups, respectively (Table 2).
Figure 2.
Percent of patients who were 12-week CSBM overall responders (primary endpoint) and sustained responders (additional endpoint). 12-week CSBM overall responder (primary endpoint): ≥3 CSBMs and an increase of ≥1 CSBM per week (weekly CSBM responder) for ≥9 of the 12 weeks of treatment period (P<0.0001 vs. placebo for both 72 μg and 145 μg doses, however only the 72 μg dose was evaluated under the multiple comparisons procedure (MCP)). Placebo, N=401; Linaclotide 72 μg, N=411; Linaclotide 145 μg, N=411. 12-week CSBM sustained responder (additional endpoint): 12-week CSBM overall responder who meets weekly CSBM responder criteria for ≥3 of the final 4 weeks of the treatment period (P=0.0001 and P=0.0010 vs. placebo for the 72 μg and 145 μg doses, respectively; this analysis was not controlled for multiplicity). Placebo, N=401; Linaclotide 72 μg, N=411; Linaclotide 145 μg, N=411. For both presented analyses, P-values were obtained from the CMH tests controlling for baseline SBM stratum and geographic region.
Table 2. Efficacy results during the 12-week treatment period (ITT population)Continued.
| Placebo (N=401) |
Linaclotide |
||||
| 72 μg (N=411) | P-value OR (95% CI) | 145 μg (N=411) | P-value OR (95% CI) | ||
| Primary endpoint a: 12-week CSBM overall responder | |||||
| b% 12-week CSBM overall responders: % of patients with ≥3 CSBMs/week and an increase of ≥1 CSBM/week (CSBM weekly responders) for ≥9 of 12 weeksc, d | 4.7 | 13.4 | <0.0001a 3.0 (1.8, 5.2) | 12.4 | <0.0001 2.8 (1.6, 4.9) |
| Secondary and additional endpoints | |||||
| CSBM | |||||
| Mean CSBMs/weekd | 1.0 | 1.8 | 2.0 | ||
| eChange from baseline in CSBMs/week, meand, f | 0.9 | 1.7 | <0.0001a | 1.9 | <0.0001 |
| e% CSBM weekly responders for ≥3 of 4 weeks in month 1 of treatmentc | 6.2 | 14.8 | <0.0001a 2.6 (1.6, 4.2) | 15.8 | <0.0001 2.8 (1.7, 4.6) |
| e% CSBM weekly responders for ≥3 of 4 weeks in month 2 of treatmentc | 9.5 | 18.7 | 0.0002a 2.2 (1.4, 3.3) | 19.0 | 0.0002 2.2 (1.5, 3.4) |
| e% CSBM weekly responders for ≥3 of 4 weeks in month 3 of treatmentc | 14.2 | 20.2 | 0.0342a 1.5 (1.0, 2.2) | 20.0 | 0.0385 1.5 (1.0, 2.2) |
| e% CSBM weekly responders for ≥9 of 12 weeks (>1 SBM/week at BL subpopulation)c, d | 7.1 | 17.2 | 0.0008a 2.7 (1.5, 4.9) | 17.4 | 0.0009 2.7 (1.5, 5.0) |
| Sustained responder | |||||
| % CSBM weekly responders for ≥9 of 12 weeks, including 3 of 4 final treatment weeksc, d | 4.7 | 12.4 | 0.0001 2.8 (1.6, 4.8) | 11.2 | 0.0010 2.5 (1.4, 4.4) |
| SBM | |||||
| Mean SBMs/weekd | 2.7 | 3.9 | 4.1 | ||
| eChange from baseline in SBMs/week, meand, f | 1.3 | 2.4 | <0.0001a | 2.6 | <0.0001 |
| Stool consistency (BSFS) | |||||
| Mean BSFS/weekd | 3.0 | 3.6 | 3.7 | ||
| eChange from baseline, meand, f | 1.1 | 1.7 | <0.0001a | 1.8 | <0.0001 |
| Straining | |||||
| Mean straining score (1 to 5-point ordinal scale)d | 2.7 | 2.5 | 2.3 | ||
| eChange from baseline, meand, f | −0.8 | −1.1 | <0.0001a | −1.2 | <0.0001 |
| Abdominal bloating | |||||
| Mean abdominal bloating score (11-point NRS)d | 4.2 | 3.8 | 3.7 | ||
| eChange from baseline, meand, f | −1.1 | −1.4 | 0.0063a | −1.5 | <0.0001 |
| Abdominal discomfort | |||||
| Mean abdominal discomfort score (11-point NRS)d | 3.6 | 3.3 | 3.3 | ||
| eChange from baseline, meand, f | −1.1 | −1.3 | 0.1028 | −1.4 | 0.0056 |
| Abdominal pain | |||||
| Mean abdominal pain score (11-point NRS)d | 3.2 | 2.9 | 2.9 | ||
| Change from baseline, meand, f | −1.0 | −1.2 | 0.0183 | −1.3 | 0.0029 |
| Constipation severity | |||||
| Mean constipation severity score (11-point NRS)d | 3.0 | 2.7 | 2.6 | ||
| Change from baseline, meand, f | −0.6 | −0.9 | <0.0001 | −1.0 | <0.0001 |
| Degree of relief of constipation | |||||
| % of patients at least ‘somewhat relieved’ for all 12 weeks or at least ‘considerably relieved’ for 6 of 12 weeks of treatmentc | 24.2 | 36.0 | 0.0004 | 36.0 | 0.0003 |
| Treatment satisfaction | |||||
| Mean treatment satisfaction at Week 12 (1 to 5-point ordinal scale)d | 2.9 | 3.4 | <0.0001 | 3.5 | <0.0001 |
| PAC-QOL—overall score | |||||
| Mean score at Week 12 (5-point scale)g | 1.4 | 1.2 | 1.1 | ||
| Change from baseline, meanf | −0.7 | −1.0 | <0.0001 | −1.0 | <0.0001 |
| Rescue medication use (bisacodyl) | |||||
| Mean percent of days during treatment | 8.7 | 9.5 | 9.4 | ||
| Change from baseline, meanf | −0.5 | <0.1 | 0.6752 | 0.3 | 0.5570 |
ANCOVA, analysis of covariance; BSFS, Bristol Stool Form Scale; CI, confidence interval; CSBM, complete SBM; ITT, intent to treat; MCP, multiple comparisons procedure; NRS, numerical rating scale; OR, odds ratio; PAC-QOL, Patient Assessment of Constipation—Quality of Life; SBM, spontaneous bowel movement.
Statistically significant under the MCP.
Primary endpoint: linaclotide 72 μg dose compared with placebo for 12-week CSBM overall responder evaluated under a multiple comparisons procedure (MCP); linaclotide 145 μg was evaluated for comparison purposes only and was not subjected to the MCP.
Odds ratios, 95% confidence intervals, and P values were based on a comparison of linaclotide vs. placebo using a Cochran-Mantel-Haenszel test controlling for SBM stratum (except for >1 SBM/week subpopulation analysis) and geographic region.
Means are over the 12-week treatment period.
Secondary endpoint: linaclotide 72 μg dose compared with placebo for the parameter specified, under the MCP; linaclotide 145 μg was evaluated for comparison purposes only and was not subjected to the MCP.
Changes from baseline are the least-squares means from an ANCOVA model; P values were based on a comparison of linaclotide vs. placebo using an ANCOVA model with treatment group, baseline SBM stratum, and geographic region as factors and corresponding baseline value as a covariate.
Composite of four 0- to 4-point subscales, using a last observation carried forward approach to missing data. Lower score indicates better outcome.
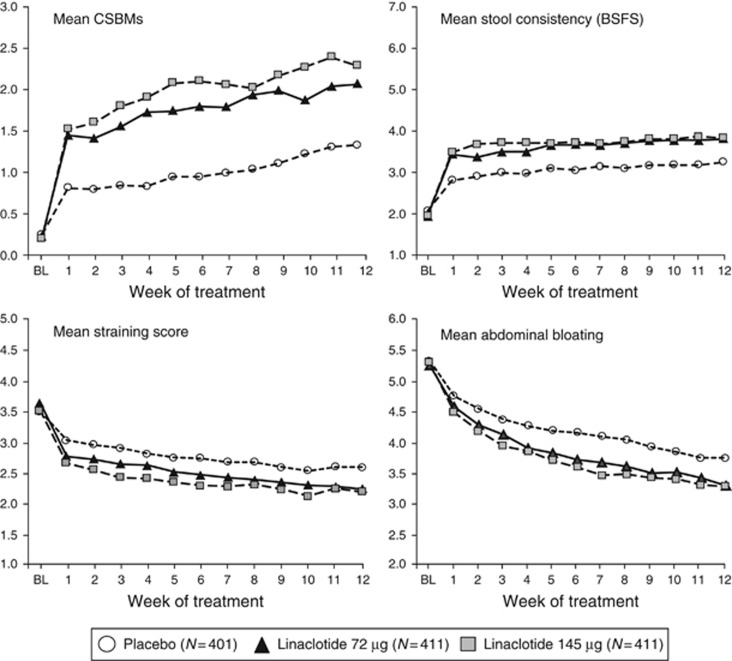
For five of the six secondary 12-week change-from-baseline endpoints, the linaclotide 72-μg group demonstrated statistically significant improvements compared with placebo (Table 2). The mean number of CSBMs and SBMs per week increased during treatment with linaclotide 72 μg by 1.7 and 2.4, respectively (both P<0.0001). Mean stool consistency score in the linaclotide 72-μg group was 3.6, an increase of 1.7 from baseline, compared to 3.0, an increase of 1.1 from baseline, for the placebo group (P<0.0001).The linaclotide 72-μg group also had 12-week-change-from-baseline improvements in abdominal symptoms (11-point NRS) relative to placebo, including improvement in abdominal bloating (−1.4 for 72 μg vs. −1.1 for placebo; P=0.0063) and abdominal pain (an additional study endpoint, −1.2 for 72 μg vs. −1.0 for placebo; nominal P=0.0183). For each of these parameters, improvement was seen during the first week of treatment and was sustained throughout the trial (Figure 3). Weekly change-from-baseline results over the 12 weeks of treatment for the linaclotide 145-μg group were similar to the 72-μg group, with modest dose ordering observed (Table 2, Figure 3).
Figure 3.
Weekly mean bowel and abdominal symptom scores over the 12-week Treatment Period. Mean CSBMs per week. For linaclotide 72 μg, P<0.001 for Weeks 1–9, P<0.01 for weeks 10–12; for linaclotide 145 μg, P<0.001 for all weeks. Mean stool consistency score by week based on the balanced, seven-point BSFS. For both linaclotide doses, P<0.001 for all weeks. Mean straining score by week based on a 5-point ordinal scale. For both linaclotide doses, P<0.001 for all weeks. Mean abdominal bloating score based on an 11-point NRS. For linaclotide 72 μg, P<0.05 for weeks 2, 4–9, 12; for linaclotide 145 μg, P<0.001 for weeks 3–9, P<0.05 for Weeks 1, 2 and 10–12. For the four presented analyses, P-values for linaclotide vs. placebo were obtained from an ANCOVA model with fixed effect terms for treatment group, baseline SBM stratum, and geographic region, and the baseline value as covariate.
For the remaining secondary endpoints, significantly higher percentages of linaclotide 72-μg patients met monthly CSBM responder criteria for months 1, 2, and 3 of the treatment period, respectively, compared with placebo patients (P<0.05). An analysis of the 12-week CSBM overall responder criteria in patients with less-severe CIC who averaged >1 SBM per week during the baseline period also showed significantly greater response for linaclotide 72-μg patients compared with placebo (P<0.0001), with responder rates that were higher than in the overall trial population (Table 2).
Among the additional endpoints, a higher percentage of patients in the linaclotide 72-μg group vs. the placebo group were relief of constipation symptoms responders. Linaclotide 72-μg patients reported improvements in constipation severity, abdominal pain, treatment satisfaction, and overall PAC-QOL scores compared with the placebo patients. For all efficacy parameters, results for the linaclotide 145-μg patients were similar to the linaclotide 72-μg patients, with the 145-μg group generally having slightly improved numerical values over the 72-μg group for the change-from-baseline parameters (Table 2). Across the treatment groups, mean rescue medication use was <10% of days (8.7–9.5%) during the treatment period.
Safety
During the 12-week treatment period, at least 1 treatment-emergent AE was reported by 107 patients (26.7%) in the placebo group, 143 patients (34.8%) in the linaclotide 72-μg group, and 145 patients (35.3%) in the linaclotide 145-μg group (72-μg odds ratio (OR)=1.47; confidence interval (CI) (1.09, 1.98) and 145-μg OR=1.50; CI (1.11, 2.02); Table 3. Most AEs were mild or moderate in severity; severe AEs were reported for 2.5%, 2.2%, and 3.4% of patients in the placebo, linaclotide 72-μg, and linaclotide 145-μg groups, respectively. Adverse events led to study discontinuation of 0.5% of placebo patients, 2.9% of linaclotide 72-μg patients, and 4.6% of linaclotide 145-μg patients.
Table 3. Adverse events and adverse events leading to discontinuation (safety population).
| Adverse event (preferred term)a | Placebo (N=401), n (%) | Linaclotide |
|
|---|---|---|---|
| 72 μg (N=411), n (%) | 145 μg (N=411), n (%) | ||
| Patients with at least 1 AE | 107 (26.7) | 143 (34.8) | 145 (35.3) |
| Diarrhea | 28 (7.0) | 79 (19.2) | 91 (22.1) |
| Abdominal distension | 2 (0.5) | 9 (2.2) | 5 (1.2) |
| Sinusitis | 1 (0.2) | 4 (1.0) | 8 (1.9) |
| Upper respiratory tract infection | 5 (1.2) | 6 (1.5) | 6 (1.5) |
| Flatulence | 5 (1.2) | 6 (1.5) | 3 (0.7) |
| Nasopharyngitis | 2 (0.5) | 2 (0.5) | 6 (1.5) |
| Patients with at least 1 AE leading to discontinuation | 2 (0.5) | 12 (2.9) | 19 (4.6) |
| Diarrhea | 0 | 10 (2.4) | 13 (3.2) |
AE, adverse event; n, number of patients with AEs.
Patients were counted only once within each preferred term.
Treatment-emergent adverse events (TEAEs) reported in ≥1% of patients in either linaclotide dose group and at an incidence greater than reported in placebo-treated patients during the treatment period.
The most common AE was diarrhea, which was reported by 28 (7.0%) placebo patients, 79 (19.2%) linaclotide 72-μg patients, and 91 (22.1%) linaclotide 145-μg patients (72-μg OR=3.17; CI (2.01, 5.00) and 145-μg OR=3.79; CI (2.42, 5.94)). Most diarrhea was reported to be mild or moderate in severity. Severe diarrhea was reported by 3 patients (0.7%) in the placebo group, 2 patients (0.5%) in the linaclotide 72-μg group, and 10 patients (2.4%) in the linaclotide 145-μg group. Of the patients who developed diarrhea, 10.7% of patients in the placebo group had onset within the first 7 days of treatment, compared with 54.4% in the linaclotide 72-μg group and 51.6% in the linaclotide 145-μg group; the median times to onset of the first episode of diarrhea were 33.5 days in the placebo group compared with 5.0 days and 7.0 days in the linaclotide 72-μg and 145-μg groups, respectively. No SAEs of diarrhea were reported during the trial. No clinically significant sequelae (e.g., orthostatic hypotension or dehydration) or occurrences of potentially clinically significant vital signs or laboratory values for sodium, potassium, blood urea nitrogen, or creatinine were reported in patients with diarrhea. Diarrhea was the most common AE leading to treatment discontinuation, occurring in no patients in the placebo group and 2.4% and 3.2% of patients in the 72-μg and 145-μg groups, respectively. Temporary dose interruptions due to diarrhea not leading to discontinuation were reported by 0.7%, 1.2%, and 1.5% of placebo, linaclotide 72-μg, and 145-μg patients, respectively.
Four serious adverse events (SAEs) were reported by 4 patients (1.0%) in the placebo group and included intraductal proliferative breast lesion, constipation, gastrointestinal hemorrhage, and asthma. Four SAEs were reported in three linaclotide 72-μg patients (0.7%): hypotension, azotemia with concurrent peptic ulcer, and colitis. Single SAEs were reported in two linaclotide 145-μg patients: colitis and inadequately controlled diabetes mellitus. One SAE, the colitis in the linaclotide 145 μg patient, was considered possibly related to study drug; none of the other SAEs were considered by the investigators to be treatment-related. There were no deaths reported in this trial.
There were no clinically significant differences between the linaclotide groups and the placebo group in the incidence of abnormal laboratory parameters or vital signs.
Discussion
In this trial of patients with chronic constipation, linaclotide 72 μg once daily achieved statistical significance (controlling for multiplicity) for the primary endpoint and 9 of 10 secondary endpoints when compared to placebo. The primary endpoint (12-week CSBM overall responder) required patients to have ≥3 CSBMs and an increase from baseline of ≥1 CSBM, within the same week, for ≥9 of 12 weeks. This is a particularly rigorous endpoint, when considering that the average baseline CSBM rate among patients in this trial was 0.2 per week and 76% reported 0 CSBMs. The linaclotide 72-μg dose also achieved statistical significance for the sustained responder endpoint, which required patients meeting the 12-week CSBM overall responder to also be weekly responders for ≥3 of the last 4 weeks of the treatment period. Monthly CSBM responders to 72-μg linaclotide increased numerically from month 1 to 3 and were significantly different from placebo for each month. Less symptomatic patients (i.e., those with >1 SBM/week during baseline) also showed a significant response to 72-μg linaclotide compared to placebo with a slightly higher response compared to the overall population. All secondary endpoints, with the exception of abdominal discomfort, showed statistically significant improvement with 72 μg of linaclotide compared to placebo.
Linaclotide 72 μg demonstrated efficacy that was similar to linaclotide 145 μg for the responder parameters and the 12-week change-from-baseline parameters. Nonetheless, for some of the change-from-baseline efficacy endpoints (e.g., CSBM frequency), there was a slightly greater effect with the 145-μg dose than with the 72-μg dose. This modest dose effect is consistent with dose-dependent increases in bowel frequency seen in the earlier phase 2b dose-ranging study of linaclotide (15).
Consistent with linaclotide’s pharmacology and established safety profile, diarrhea was the most common TEAE. Diarrhea infrequently led to discontinuation of treatment (2.4%, 3.2%, and 0 for linaclotide 72 μg, 145 μg, and placebo, respectively). Severe diarrhea was uncommon and was experienced by 0.5%, 2.4%, and 0.7% of linaclotide 72 μg, 145 μg, and placebo patients.
As in previous linaclotide studies, AEs were elicited from patients via non-leading questions and were all recorded and analyzed; “diarrhea” was not objectively defined as part of the AE reporting process. Thus, it is difficult to interpret whether diarrhea reported as an AE simply represented a significant change in stool consistency or frequency, as would be expected from a drug like linaclotide, or truly represented an adverse event. For patients with chronic constipation, looser stools may be welcome, and in linaclotide clinical trials are actually associated with higher treatment satisfaction (21). A better measure of the impact of diarrhea on patients than simple prevalence may be to look at dropout rates due to diarrhea, as this provides a measure of a patient’s willingness to continue therapy. In the present study, dropout rates due to diarrhea were low across all groups (<5%). Serious adverse events were infrequent and balanced across treatment groups (≤1% of patients in any group). In both trials, there were no clinically meaningful differences observed in clinical laboratory or vital signs parameters between the linaclotide and placebo groups. Therefore, when considering both treatment efficacy and tolerability in clinical practice, we hypothesize that patients with milder constipation or a greater responsiveness to GC-C agonism may obtain sufficient benefit with the 72-μg dose. Similarly, patients with more severe constipation and abdominal symptoms, or less sensitivity to GC-C agonism may benefit from the higher, 145-μg dose.
The results from this trial demonstrate that linaclotide at a daily dose of 72 μg for up to 12 weeks is safe and well tolerated, and may provide clinicians with an additional dosing option for treating the heterogeneous CIC patient population.
Study Highlights
Acknowledgments
We acknowledge the extensive contributions made by the late B. Joseph Lavins, MD. We are grateful for his tireless efforts in developing linaclotide and advancing guanylate cyclase research. He is dearly missed by his colleagues and friends.
Clinicaltrials.gov ID: NCT02291679.
Footnotes
Guarantor of the article: Michael L. Hall, MD.
Specific author contributions: A.J.L., B.E.L., P.S., and W.D.C. assisted in the interpretation of data, drafting, and critical revision of the manuscript for important intellectual content; CBK, MGC, MLH, SMF, and WB assisted in the interpretation of the data and critical revision of the manuscript for important intellectual content; CRO’D assisted in the interpretation of the data and drafting of the manuscript; DSR, KT, and RB provided statistical analysis and critical revision of the manuscript for important intellectual content; NDO coordinated acquisition of the data and trial supervision.
Financial support: This trial was funded by Forest Research Institute (an affiliate of Allergan, PLC) and Ironwood Pharmaceuticals, Inc.
Potential competing interests: Kenneth Tripp, David S. Reasner, Nicholas D. Omniewski, Christopher R. O’Dea, Mark G. Currie, and Michael L. Hall are employees of, and own stock/stock options in, Ironwood Pharmaceuticals. Carolyn B. Kurtz is a former employee of, and owns stock/stock option in, Ironwood Pharmaceuticals. Wieslaw Bochenek, Susan M. Fox, and Rick Blakesley are employees of, and own stock/stock options in, Allergan PLC. Philip Schoenfeld, Brian E. Lacy, William D. Chey, and Anthony J. Lembo are paid consultants to Ironwood Pharmaceuticals and Allergan.
References
- Johanson JF, Kralstein J. Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther 2007;25:599–608. [DOI] [PubMed] [Google Scholar]
- Koch A, Voderholzer WA, Klauser AG et al. Symptoms in chronic constipation. Dis Colon Rectum 1997;40:902–906. [DOI] [PubMed] [Google Scholar]
- Sandler RS, Drossman DA. Bowel habits in young adults not seeking health care. Dig Dis Sci 1987;32:841–845. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Liberman JN, Sandler RS et al. Epidemiology of constipation (EPOC) study in the United States: relation of clinical subtypes to sociodemographic features. Am J Gastroenterol 1999;94:3530–3540. [DOI] [PubMed] [Google Scholar]
- Lembo A, Camilleri M. Chronic constipation. N Engl J Med 2003;349:1360–1368. [DOI] [PubMed] [Google Scholar]
- Bryant AP, Busby RW, Bartolini WP et al. Linaclotide is a potent and selective guanylate cyclase C agonist that elicits pharmacological effects locally in the gastrointestinal tract. Life Sci 2010;86:760–765. [DOI] [PubMed] [Google Scholar]
- Busby RW, Bryant AP, Bartolini WP et al. Linaclotide, through activation of guanylate cyclase C, acts locally in the gastrointestinal tract to elicit enhanced intestinal secretion and transit. Eur J Pharmacol 2010;649:328–335. [DOI] [PubMed] [Google Scholar]
- Busby RW, Kessler MM, Bartolini WP et al. Pharmacological Properties, Metabolism and Disposition of Linaclotide, a Novel Therapeutic Peptide Approved for the Treatment of Irritable Bowel Syndrome with Constipation and Chronic Idiopathic Constipation. J Pharmacol Exp Ther 2013;344:196–206. [DOI] [PubMed] [Google Scholar]
- Castro J, Harrington AM, Hughes PA et al. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-c and extracellular cyclic GMP. Gastroenterology 2013;145:1334–1346.e1-11. [DOI] [PubMed] [Google Scholar]
- Eutamene H, Bradesi S, Larauche M et al. Guanylate cyclase C-mediated antinociceptive effects of linaclotide in rodent models of visceral pain. Neurogastroenterol Motil 2010;22:312–322. [DOI] [PubMed] [Google Scholar]
- Silos-Santiago I, Hannig G, Eutamene H et al. Gastrointestinal pain: Unraveling a novel endogenous pathway through uroguanylin/guanylate cyclase-C/cGMP activation. Pain 2013;154:1820–1830. [DOI] [PubMed] [Google Scholar]
- Chey WD, Lembo AJ, Lavins BJ et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol 2012;107:1702–1712. [DOI] [PubMed] [Google Scholar]
- Rao S, Lembo AJ, Shiff SJ et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol 2012;107:1714–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembo A, Schneier H, Lavins B. Results from two phase 3 clinical trials of Linaclotide in patients with chronic constipation (CC). N Engl J Med 2011;365:527–536. [DOI] [PubMed] [Google Scholar]
- Lembo AJ, Kurtz CB, MacDougall JE et al. Efficacy of linaclotide in patients with chronic constipation. Gastroenterol 2010;138:886–895. [DOI] [PubMed] [Google Scholar]
- Lacy BE, Schey R et al. Linaclotide in chronic idiopathic constipation patients with moderate to severe abdominal bloating: a randomized, controlled trial. PLoS ONE 2015;10:e0134349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drossman DA, Corazziari E, Delvaux N et al. Rome III: The functional gastrointestinal disorders 3 ed. Degnon Associates: McLean, VA. 2006. [Google Scholar]
- Winawer S, Feltcher R, Rex D. Colorectal cancer screening and surveillance: clinical guidelines and rationale—update based on new evidence. Gastroenterology 2003;124:5444–5460. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997;32:920–924. [DOI] [PubMed] [Google Scholar]
- Marquis P, De La Loge C, Dubois D et al. Development and validation of the Patient Assessment of Constipation Quality of Life questionnaire. Scand J Gastroenterol 2005;40:540–551. [DOI] [PubMed] [Google Scholar]
- Taylor DCA, Abel JL, Spiegel B et al. Impact of linaclotide and stool form on bowel movement satisfaction in patients with irritable bowel syndrome with constipation or chronic idiopathic constipation: results from the CONTOR study. Am J Gastroenterol 2016;111:S238. [Google Scholar]