Abstract
Whether photosynthetic pathway differences exist in the amplitude of nighttime variations in the carbon isotope composition of leaf dark-respired CO2 (δ13Cl) and respiratory apparent isotope fractionation relative to biomass (ΔR,biomass) in response to drought stress is unclear. These differences, if present, would be important for the partitioning of C3-C4 mixed ecosystem C fluxes. We measured δ13Cl, the δ13C of biomass and of potential respiratory substrates and leaf gas exchange in one C3 (Leymus chinensis) and two C4 (Chloris virgata and Hemarthria altissima) grasses during a manipulated drought period. For all studied grasses, δ13Cl decreased from 21:00 to 03:00 h. The magnitude of the nighttime shift in δ13Cl decreased with increasing drought stress. The δ13Cl values were correlated with the δ13C of respiratory substrates, whereas the magnitude of the nighttime shift in δ13Cl strongly depended on the daytime carbon assimilation rate and the range of nighttime variations in the respiratory substrate content. The ΔR,biomass in the C3 and C4 grasses varied in opposite directions with the intensification of the drought stress. The contribution of C4 plant-associated carbon flux is likely to be overestimated if carbon isotope signatures are used for the partitioning of ecosystem carbon exchange and the δ13C of biomass is used as a substitute for leaf dark-respired CO2. The detected drought sensitivities in δ13Cl and differences in respiratory apparent isotope fractionation between C3 and C4 grasses have marked implications for isotope partitioning studies at the ecosystem level.
Keywords: dark respiration, photosynthetic 13C discrimination, post-photosynthetic isotope fractionation, C3 species, C4 species, water stress
Introduction
Carbon isotope discrimination occurs during plant photosynthetic CO2 fixation, resulting in all higher plants being depleted in 13C in organic carbon relative to atmospheric CO2 (Farquhar and Sharkey, 1982). Because of the differences in anatomical structure and photosynthetic physiological processes, C4 photosynthesis discriminates less against 13CO2 than does C3 photosynthesis, which results in the 13C content in C4 plants (−6‰ to −19‰) being enriched compared to C3 species (−24‰ to −34‰) (Smith and Epstein, 1971; Farquhar et al., 1989). This large photosynthetic pathway difference in carbon isotope composition (δ13C) is useful for partitioning net ecosystem CO2 exchange into its components at both ecosystem and regional scales (Still et al., 2003; Zhang et al., 2006; Shimoda et al., 2009). In these applications, foliar δ13C is often used as a substitute for the δ13C of leaf dark-respired CO2. However, recent studies have shown that leaf dark-respired CO2 is often enriched in 13C compared to bulk biomass in both C3 and C4 plants (Bowling et al., 2008; Sun et al., 2010; Ghashghaie and Badeck, 2013). Because of high variability in the δ13C of leaf dark-respired CO2 (δ13Cl), the magnitude of 13C enrichment (respired CO2 vs. bulk biomass) varies substantially at the diel timescale and is highly sensitive to changes in environmental conditions (Ghashghaie et al., 2003; Sun et al., 2010; Werner and Gessler, 2011). This fact highlights the importance of understanding the mechanisms of short-term variations in δ13Cl and incorporating this phenomenon into ecosystem carbon exchange partitioning.
Large diel variations in δ13Cl (up to 14.8‰) have been observed in various plant functional types, including grasses, herbs and trees (Hymus et al., 2005; Prater et al., 2006; Werner et al., 2009). Several mechanisms have been proposed to explain short-term shifts in δ13Cl. First, daytime carbon assimilation is associated with a large variation in photosynthetic 13C discrimination, which may alter the carbon isotope composition of carbohydrates and subsequently influence δ13Cl (Ghashghaie et al., 2001; Sun et al., 2009). Second, shifts in the utilization of isotopically different respiratory substrates may alter the carbon isotope signature of leaf dark-respired CO2. For instance, respiratory substrate changes from 13C-enriched soluble carbohydrates to 13C-depleted lipids may cause 13C depletion in leaf dark-respired CO2 (Tcherkez et al., 2003). Third, the heterogeneous 13C distribution in hexose molecules (atoms 3 and 4 are more enriched in 13C than carbon atoms 1, 2, 5 and 6; Rossmann et al., 1991; Gleixner and Schmidt, 1997) and incomplete oxidation of glucose can cause up to a 4‰ shift in δ13Cl (Hobbie and Werner, 2004; Werner and Gessler, 2011). Glucose is converted to two molecules of pyruvate during glycolysis. Either the pyruvate can be completely oxidized to CO2 during the tricarboxylic acid (TCA) cycle or only two CO2 molecules are produced per glucose molecule and the molecules can be used for biosynthesis (e.g., acetyl-CoA). The CO2 produced in the latter case is enriched in δ13C (Rossmann et al., 1991; Gleixner and Schmidt, 1997). The magnitude of intra-molecular 13C differences in C3 species (on average, C-3 and C-4 are enriched in 13C by 6.2‰ compared to C-1, C-2, C-5 and C-6) is greater than that in C4 species (on average, C-3 and C-4 are enriched in 13C by 3.3‰ compared to C-1, C-2, C-5 and C-6), which may cause photosynthetic pathway differences in the magnitude of short-term variations in δ13Cl. Finally, changes in the carbon isotope signature in malate and the contribution of malate decarboxylation (release of 13C-enriched CO2) to total respiratory CO2 flux may also result in short-term variations in δ13Cl (Barbour et al., 2007a, 2011; Gessler et al., 2009). In a recent study, Lehmann et al. (2015) reported strong correlations between δ13Cl and the δ13C of malate during both daytime and nighttime in potato plants growing under various temperature and soil moisture conditions. The aforementioned mechanisms are responsible for not only short-term variations in δ13Cl but also changes in respiratory apparent isotope fractionation (Ghashghaie et al., 2001, 2003; Bowling et al., 2008).
The carbon isotope signature and the magnitude of the diel shift in δ13Cl (maximum δ13Cl value – minimum δ13Cl value) differ substantially among plant functional types and are sensitive to changes in environmental conditions, such as, drought (Duranceau et al., 1999; Ghashghaie et al., 2001; Priault et al., 2009; Sun et al., 2010). In a study of Phaseolus vulgaris, Duranceau et al. (1999) reported that progressive drought alters not only the δ13Cl value but also nighttime variations in δ13Cl. Compared to deep-rooted woody plants, δ13Cl in shallow-rooted grasses and herbs is more sensitive to seasonal variations in soil water availability in shallow soil layers (Sun et al., 2010). Plant functional type differences in the sensitivity of leaf gas exchange and plant growth to drought stress has been extensively reported, with deep-rooted trees and shrubs being less sensitive to drought than shallow-rooted herbs and grasses (Bucci et al., 2009; Comas et al., 2013). Recent studies have shown that the leaf net carbon assimilation rate in C4 grasses is equally or even more sensitive to drought stress compared to that in C3 grasses (Ripley et al., 2007, 2010). However, differences in drought sensitivity in the isotopic signatures of leaf dark-respired CO2 between C3 and C4 species remain largely unknown. This information is critical for the separation of C exchange in C3-C4 mixed ecosystems and assessing the drought sensitivity of component fluxes.
Using a pot experiment, we measured δ13Cl, the δ13C values of bulk leaf tissue and potential respiratory substrates (soluble sugars, starch and lipids), the pool size of labile C substrates and leaf gas exchange in one C3 (Leymus chinensis) and two C4 (Chloris virgata and Hemarthria altissima) grasses during a manipulated drought period. We focused on the impacts of drought on the trend and range of nighttime shifts in δ13Cl in these dominant species of the meadow steppe in Northeast China. The carbon isotope composition of leaf dark-respired CO2 and the magnitude of nighttime variation in δ13Cl are predicted to be sensitive to manipulated drought treatments in both C3 and C4 grasses. Compared to the C4 grasses, the studied C3 grass is likely to have greater nighttime variations in δ13Cl because the magnitude of the intra-molecular 13C differences in C3 species is greater than that in C4 species.
Materials and methods
Experimental design and treatments
The experiment was performed at the Grassland Ecological Research Station of Northeast Normal University, Jilin Province, China (44°40′-44°44′N, 123°44′-123°47′E). The research station has a semi-arid, continental climate with mean annual temperature ranged from 4.6 to 6.4°C (1950–2004). Mean annual precipitation ranged from 280 to 644 mm (1950–2014) with over 70% of the precipitation occurs from June to August. Potential evapotranspiration is approximately three times that of the annual precipitation. Vegetation is dominated by L. chinensis, a C3 perennial rhizomatous grass; Phragmites australis, C. virgata and H. altissima are also abundant. Soil is classified as a chernozem soil, with 2.0% soil organic carbon content and 0.15% soil total nitrogen content (Wang et al., 2015).
One C3 perennial grass (L. chinensis) and two C4 grasses (annual: C. virgata; perennial: H. altissima) that co-occur in the meadow steppe of the study area were selected as experimental plants. L. chinensis is a widespread dominant grass of arid and semi-arid steppe in northern China, eastern Mongolia and Transbaikalia, Russia (Wang and Ba, 2008) and has an ability to resist drought, cold and alkaline conditions (Shi and Wang, 2005). C. virgata is widely distributed on the Northeast China Plain and is ecologically and economically important because of its high protein content and seed production. In addition, it also grows rapidly and is highly tolerant of alkaline conditions (Yang et al., 2008; Lin et al., 2016). H. altissima is a perennial rhizomatous grass and is distributed in tropical, subtropical and temperate regions, especially in China and Southeast Asia. It has strong adaptability and stress resistance and can be used as a good soil and water conservation crop (Han et al., 2016).
On DOY 135 in 2013, seedlings of L. chinensis and H. altissima were transplanted to plastic pots (23.5 cm in diameter and 20 cm in height) filled with chernozem soil (8 kg soil pot−1). For C. virgata, plants were germinated from seeds and transplanted to plastic pots. All species were planted as monocultures (five individuals per pot). Before the initiation of the drought treatment, all the transplanted plants were manually watered (to field capacity) every 3 days. To ensure that plant growth was not limited by nutrient elements, each pot received 2 mg of nitrogen fertilizer in the form of NH4NO3 every week. All the pots were watered thoroughly on the date (DOY 165) prior to the initiation of the drought treatment. During the drought experiment period (DOY 166–172), we stopped watering the plants. Moreover, all the pots were placed under a plastic shed to exclude natural precipitation. Variations in the soil water content for the studied grasses are provided in the supplementary information (Figure S1). The measurements of leaf gas exchange and collection of both leaf dark-respired CO2 and fresh materials were conducted on DOY 166 (day 1 of the experiment), DOY 168 (day 3 of the experiment), DOY 170 (day 5 of the experiment) and DOY 172 (day 7 of the experiment). Before the initiation of the drought treatment, the studied grasses were in the stem elongation stage with average heights of 51.6, 62.9 and 42.4 cm for C. virgata, H. altissima and L. chinensis, respectively. The tiller densities of the studied grasses were 36 pot−1, 19 pot−1 and 17 pot−1 for C. virgata, H. altissima and L. chinensis, respectively. Each pot had total leaf areas of 1,045 cm2 pot−1, 618 cm2 pot−1 and 648 cm2 pot−1 for C. virgata, H. altissima and L. chinensis, respectively.
Leaf gas exchange measurements
Leaf gas exchange parameters (net CO2 assimilation rate, respiration rate, stomatal conductance, leaf-to-air vapor pressure deficit, leaf intercellular air space and ambient CO2 concentration) were measured every 3 h during a 24-h experimental cycle using an LI-6400 portable photosynthesis system (Li-Cor Biosciences, Lincoln, NE, USA). For each species, five pots were used for leaf gas exchange measurements. For each pot, two of the upper-most fully expanded leaves (the 2nd or 3rd leaf from the top) were measured for gas exchange parameters. The same leaves were marked and measured repeatedly throughout the experimental period. Before each measurement, the environmental conditions inside the leaf chamber (i.e., photosynthetically active radiation, air temperature, relative humidity and CO2 concentration) were set to match ambient conditions. The leaf respiration rate (R) was measured at 21:00, 00:00 and 03:00 h while the light intensity was set to zero.
Meteorological data and soil water content
Air temperature, photosynthetic photon flux density (PPFD), relative humidity and air saturation vapor pressure were obtained from an eddy tower approximately 2 km away from the experimental site on days 1, 3, 5 and 7 of the drought treatment. Volumetric soil water contents (SWC-V) were measured using an ECH2O soil moisture sensor (EC-5, Decagon Ltd., Pullman, WA, USA) and the data were collected with a ProCheck device (Decagon Ltd., Pullman, WA, USA).
Collection of leaf dark-respired CO2
Leaf dark-respired CO2 was collected using a 60-ml gas-tight syringe (Werner et al., 2007). Young and fully expanded leaves (comparable to those used for the leaf gas exchange measurements) were used for the collection of leaf dark-respired CO2. After the leaves (10–20 leaves) were placed inside the syringe, the syringe barrel was immediately flushed with CO2-free air five times by actuating the syringe plunger, and then, the leaf dark-respired CO2 was allowed to accumulate for 15 min in the syringe barrel. After the buildup of leaf-respired CO2, a 5-ml air sample containing leaf dark-respired CO2 was injected into a helium-flushed 12-ml vial (Presentation 1). Leaf dark-respired CO2 was collected during the nighttime period (21:00, 00:00 and 03:00 h) for each of the four sampling dates. For each sampling time, leaf dark-respired CO2 collection was repeated on five pots for each of the three studied grasses.
Extraction of lipids, soluble sugars and starch
For each sampling date, leaves comparable to those used for the leaf-respired CO2 sampling and gas exchange measurements were collected at 21:00, 00:00 and 03:00 h. The collected leaves were immediately flash frozen in liquid nitrogen to stop respiratory metabolic activities, temporarily stored in a deep freezer and then freeze-dried in a Labconco freeze drier (Labconco, Kansas City, MO, USA). The freeze-dried leaves were ground to a fine powder using a ball mill (MM 400 Retsch, Haan, Germany). Lipids, soluble sugars and starch were extracted using the protocols described by Wanek et al. (2001) and Göttlicher et al. (2006). In brief, the powdered leaf material (100 mg) was extracted with 1 ml of methanol/chloroform/water (MCW; 12:5:3, v/v/v) for 30 min at 70°C. After cooling, the samples were centrifuged at 10,000 g for 2 min. The supernatant (0.65 ml) was transferred to a new vial and phases were separated by adding 0.2 ml of chloroform and 0.7 ml of water. To determine the carbon isotope ratio of the lipids, 50 μl of the chloroform phase as pipetted into smooth tin capsules for liquids (4.75 × 11 mm, Santis Analytical AG, Teufen, Switzerland) and dried under a fume hood. Samples were then analyzed by an isotope ratio mass spectrometer (described below). Chlorophyll was also extracted with lipids, which may cause overestimation of the 13C content of lipids (Bidigare et al., 1991). The methanol/water phase (upper layer) was removed and processed for sugar isolation. Soluble sugars were isolated using an ion exchange column containing cation-exchange resin (Dowex 50 W × 8, Sigma Aldrich, St. Louis, MO, USA) and anion-exchange resin (Dowex 1 × 8, Sigma Aldrich, St. Louis, MO, USA). The columns were rinsed with deionized water, and effluent was dried in tin capsules for carbon isotope ratio measurement of soluble sugars. The pellet from the centrifugation was rinsed with deionized water, oven-dried after re-extraction with MCW three times, and then heated to 100°C to gelatinize the starch. After cooling to room temperature, a heat-stable α-amylase solution (A3306, Sigma-Aldrich, St. Louis, MO, USA) was added, and the samples were incubated at 85°C for 120 min. Thereafter, the samples were cooled to room temperature and centrifuged at 10,000 g for 3 min. The supernatant was transferred to pre-washed centrifugal ultrafiltration devices (Microcon YM-10, Millipore, Billerica, MA, USA) to remove the enzymes and other high molecular weight substances. The filtrates were then dried in a tin capsule to analyze the carbon isotope composition of the starch.
Isotope ratio mass analysis
All carbon isotope ratio analyses were performed using an isotope ratio mass spectrometer (Isoprime 100, Elementar, Langenselbold, Germany) coupled to an elemental analyzer (vario EL cube, Elementar, Langenselbold, Germany) for solid samples or a Trace Gas Pre-concentrator (Elementar, Langenselbold, Germany) for gaseous samples. The precision of repeated δ13C measurements on the working standards of solid and gaseous substrates was <0.1‰. The carbon isotope composition of the working standards was calibrated using reference materials from IAEA. The identical treatment principle was applied during the preparation of samples, checking standards and working standards. The natural abundance of 13C in the samples is reported relative to VPDB as follows:
| (1) |
δ13C of the leaf photosynthate pool
The δ13C of the cumulative photosynthate pool was estimated as follows:
| (2) |
where δ13Cpw is the assimilation-weighted, cumulative carbon isotopic value of the recently fixed photosynthates and Ai and δ13Cpi are the instantaneous net assimilation rate and the δ13C value of photosynthates at time i (06:00, 09:00, 12:00, 15:00 and 18:00 h), respectively. Carbon isotope composition of photosynthates (δ13Cp) was solved using the following equation:
| (3) |
where δ13Ca represents δ13C signature of atmospheric CO2 (assumed to be −8‰) and ΔP represents photosynthetic discrimination against 13C.
For C3 species, ΔP was estimated from the simplified linear model of Farquhar et al. (1982) as follows:
| (4) |
where a represents isotope fractionation occurring during diffusion through the air (4.4‰); b represents the net isotope fractionation associated with carboxylation reaction of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) (27‰); and Ci and Ca are the leaf intercellular air space and ambient CO2 concentrations, respectively. For the parameters in the Equation (4): a and b were obtained from literature (Farquhar et al., 1982) and Ci and Ca were measured using the LI-6400 portable photosynthesis system.
For the C4 species, Δp was estimated from a modified equation of Farquhar (1983) model of 13C discrimination during C4 plant photosynthesis as follows (Farquhar, 1983; Peisker and Henderson, 1992):
| (5) |
where a represents isotope fractionation associated with the diffusion of CO2 into the leaf (4.4‰); b4 represents net isotope fractionation associated with the dissolution of CO2 to (−7.9‰) and fixation by phosphoenolpyruvate carboxylase (PEPc, 2.2‰); 13C fractionation associated with the dissolution of CO2 to is temperature dependent; therefore, b4 was calculated for each time point using a temperature-correction equation provided by Mook et al. (1974); b3 represents isotope fractionation associated with Rubisco carboxylation (29‰); s represents isotope fractionation associated with the diffusion of CO2 out of the bundle sheath cells (1.8‰); φ represents the leakiness of the bundle sheath to CO2; and Ci and Ca represent the leaf intercellular air space and ambient CO2 concentrations, respectively. It has been reported that φ changes with plant water status (Buchmann et al., 1996; Williams et al., 2001; Tazoe et al., 2008; Sun et al., 2012); therefore, we assumed the leakiness on day 1 (optimum soil water condition) is 0.2 and on day 7 (severe drought stress) is 0.4 and generated a linear relationship between stomatal conductance and leakiness, which was used to estimate the leakiness on day 3 and 5. For the parameters in the Equation (5): a and b3 were obtained from literature (Farquhar, 1983), b4 and φ were calculated according to the environmental conditions and Ci and Ca were measured using the LI-6400 portable photosynthesis system.
Respiratory apparent 13C/12C fractionation
Respiratory apparent isotope fractionation relative to a particular substrate X (e.g., leaf bulk materials, starch, soluble sugars, lipids and cumulative photosynthate pool) was calculated as follows:
| (6) |
where δ13CX is the δ13C signature of substrate X and δ13Cl is δ13C of the leaf dark-respired CO2.
Measurements of the concentration of soluble sugars and transitory starch
Concentrations of soluble sugars and transitory starch were measured according to the microplate enzymatic method (Zhao et al., 2010). Briefly, soluble sugars were extracted by adding ethanol (EtOH) to powered plant tissue and heating in a water bath at 80°C. Each sample was extracted three times, and supernatants were purified by adding activated charcoal (Sigma C7606). After centrifugation, aliquots (20 μl) of soluble sugar extract were transferred to a 96-well microplate and placed in an oven to remove EtOH. Glucose, fructose and sucrose were assayed sequentially using the same sample. After the addition of 20 μl of DI water and a 100 μl mixture of the glucose assay reagent (Hexokinase, Sigma G3293), the microplates were incubated at 30°C for 15 min and measured for absorbance at 340 nm using a SpectraMax Plus Microplate Reader (Molecular Devices Corporation, Sunnyvale, CA, USA). The glucose concentration was calculated using a glucose standard curve. The fructose and sucrose concentrations were measured by changes in glucose concentration after adding 10 μl of phosphoglucose isomerase (Sigma P9544-0.25 EU) and 10 μl of invertase (Sigma I4504-83 EU) solution, respectively.
Starch was extracted by adding KOH to the sample residue remaining after the EtOH extraction of leaf soluble sugars and heating in a boiling water bath for 1 h. After the neutralization of KOH, Tris buffer and α-amylase solution (Sigma A3403) were added, and the test tube was incubated at 85°C for 30 min. To complete the starch hydrolysis, 1 ml of amyloglucosidase (Sigma A7095) was added, and the test tube was incubated at 55°C for 60 min and then placed in a boiling water bath for 4 min. Eventually, DI water was used to bring the test tube to a final volume of 6 ml, and the supernatant (centrifuged at 3,000 g for 10 min) was assayed for glucose as previously described. To account for water loss when glucose units are linked to form starch, the starch concentration in the sample was calculated according to the glucose concentration in the tissue and residue multiplied by a factor of 0.9.
Statistical analysis
One-way analysis of variance (ANOVA) was used to assess drought effects on diurnal average leaf gas exchange parameters, the carbon isotope composition of leaf dark-respired CO2 (δ13Cl), leaf soluble sugars (δ13Csoluble sugar), leaf starch (δ13Cstarch) and leaf lipids (δ13Clipid). Two-way ANOVA was used to assess nighttime variation and drought impacts on δ13Cl, δ13Csoluble sugar, δ13Cstarch and δ13Clipid, as well as on soluble sugars and starch concentrations. Linear regression analysis was used to assess correlations between δ13Cl and δ13Csoluble sugar, δ13Cstarch, δ13Clipid and δ13Cpw. Linear regression analysis was also conducted to evaluate dependence of the amplitude of nocturnal shifts in δ13Cl on the diurnal average net CO2 assimilation rate, nighttime average respiration rate, diurnal average stomatal conductance, diurnal average of the Ci/Ca ratio and diurnal average vapor pressure deficit, as well as on the magnitude of nighttime variations in leaf soluble sugars and starch concentrations. All analyses were carried out using SPSS software version 22 (SPSS Inc., Chicago, IL, USA). Average values are reported as arithmetic mean ± 1 SE.
Results
Leaf gas exchange
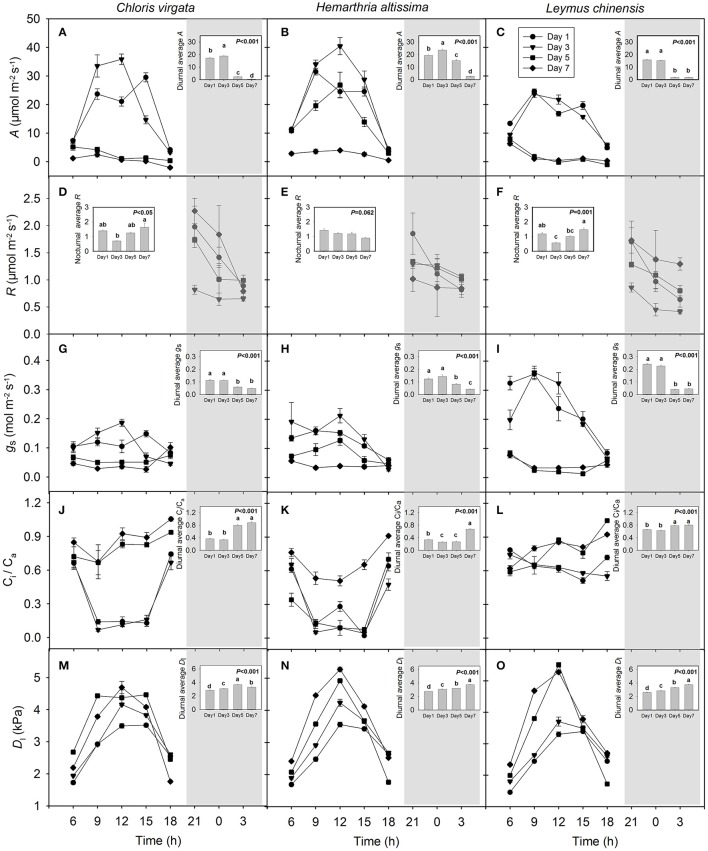
Large diurnal variations in the net carbon assimilation rate (A) were detected on days 1 and 3, but not on days 5 and 7, especially in the C4 annual grass C. virgata and the C3 perennial grass L. chinensis (Figures 1A–C). Diurnal average A decreased by 97.2%, 86.0% and 88.4% in C. virgata, H. altissima and L. chinensis, respectively, with the progress of drought stress and differed significantly among the measurement dates (Figures 1A–C). For each measurement date, the leaf nighttime respiration rate (R) decreased from 21:00 to 03:00 h (Figures 1D–F). For H. altissima, the nighttime average R decreased by 36.5% with the intensification of drought stress, whereas the nighttime average R in C. virgata and L. chinensis increased by 131.8% and 155.4%, respectively, from day 3 to 7. Strong diurnal variations in stomatal conductance (gs) were observed in L. chinensis on days 1 and 3, but not on days 5 and 7 (Figure 1I). The Ci/Ca ratio varied substantially on the diurnal timescale especially in C. virgata on days 1 and 3 and in H. altissima on days 1, 3 and 5 (Figures 1J–L). The diurnal average Ci/Ca ratio increased from 0.36 ± 0.02, 0.34 ± 0.02 and 0.65 ± 0.01 on day 1 to 0.88 ± 0.04, 0.67 ± 0.01 and 0.80 ± 0.01 on day 7 for C. virgata, H. altissima and L. chinensis, respectively (Figures 1J–L). The leaf-to-air vapor pressure deficit (Dl) varied substantially in all studied grasses, with maximum values detected between 09:00 and 12:00 h (Figures 1M–O). For all species, the diurnal average Dl (kPa) increased from 2.84 ± 0.03, 2.76 ± 0.01 and 2.61 ± 0.02 on day 1 to 3.30 ± 0.05, 3.76 ± 0.02 and 3.76 ± 0.01 on day 7 for C. virgata, H. altissima and L. chinensis, respectively (Figures 1M–O).
Figure 1.
Variations of (A–C) leaf net CO2 assimilation rate (A; μmol m−2 s−1), (D–F) respiration rate (R; μmol m−2 s−1), (G–I) stomatal conductance (gs; mol m−2 s−1), (J–L) Ci/Ca ratio (unitless) and (M–O) leaf-to-air vapor pressure deficit (Dl; kPa) on the day 1 (circle), day 3 (triangle), day 5 (square) and day 7 (diamond) of the drought treatment in Chloris virgata (annual C4), Hemarthria altissima (perennial C4) and Leymus chinensis (perennial C3). Diurnal average values are presented as an inset figure. Different lowercase letters in the inset figures indicate significant differences (P < 0.05) between the sampling dates (Tukey's test). Data are reported as the arithmetic mean ± 1 standard error (n = 5). The shaded area denotes the dark period.
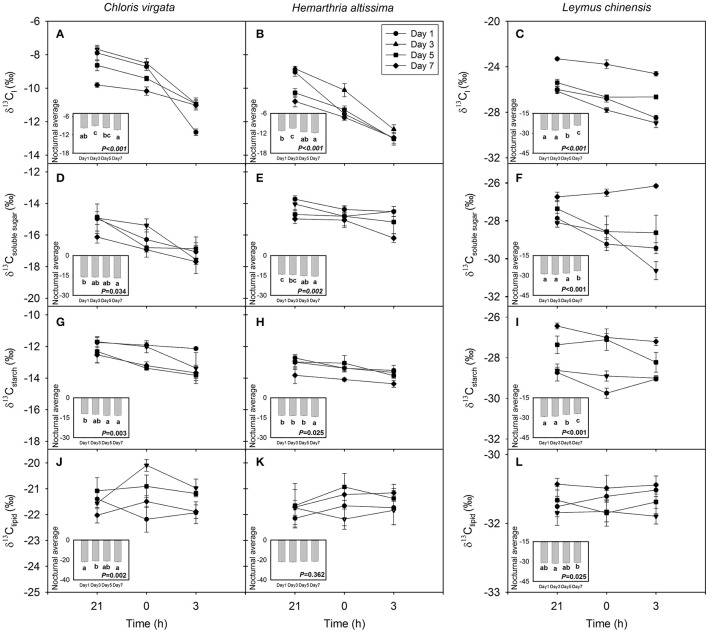
Variation in the δ13C of leaf dark-respired CO2
For all studied grasses, the 13C content in leaf dark-respired CO2 was depleted from 21:00 to 03:00 h; however, the magnitude of the nocturnal shift in the carbon isotope composition of leaf dark-respired CO2 (δ13Cl) decreased with the intensification of drought stress (Figures 2A–C). Nighttime average δ13Cl differed significantly among the sampling dates (Figures 2A–C; Table 1), with nighttime average δ13Cl values declining from −9.7 ± 0.2‰ and −11.2 ± 0.2‰ on day 1 to −10.3 ± 0.1‰ and −11.8 ± 0.1‰ on day 7 for C. virgata and H. altissima, respectively. For the C3 grass L. chinensis, nighttime average δ13Cl values increased from −27.1 ± 0.2‰ on day 1 to −23.9 ± 0.2‰ on day 7 (Figure 2C).
Figure 2.
Nighttime variations in the C isotope composition (‰) of (A–C) leaf dark-respired CO2 (δ13C l), (D–F) leaf soluble sugars (δ13Csoluble sugar), (G–I) leaf starch (δ13Cstarch) and (J–L) leaf lipids (δ13Clipid) on the day 1 (circle), day 3 (triangle), day 5 (square) and day 7 (diamond) of the drought treatment in Chloris virgata (annual C4), Hemarthria altissima (perennial C4) and Leymus chinensis (perennial C3). Diurnal average values are presented as an inset figure. Different lowercase letters in the inset figures indicate significant differences (P < 0.05) between the sampling dates (Tukey's test). Data are reported as the arithmetic mean ± 1 standard error (n = 5).
Table 1.
The df and P values from the two-way analysis of nighttime variation (N) and drought effects (D) in the C isotope composition of leaf-respired CO2 (δ13Cl), leaf soluble sugars (δ13Csoluble sugar), leaf starch (δ13Cstarch) and leaf lipids (δ13Clipid) in Chloris virgata (annual C4), Hemarthria altissima (perennial C4) and Leymus chinensis (perennial C3).
| Species | δ13Cl (‰) | δ13Csolublesugar (‰) | δ13Cstarch (‰) | δ13Clipid (‰) | |||||
|---|---|---|---|---|---|---|---|---|---|
| df | P | df | P | df | P | df | P | ||
| Chloris virgata | D | 3 | <0.001 | 3 | 0.158 | 3 | 0.001 | 3 | 0.002 |
| N | 2 | <0.001 | 2 | <0.001 | 2 | 0.032 | 2 | 0.33 | |
| D × N | 6 | <0.001 | 6 | 0.613 | 6 | 0.806 | 6 | 0.159 | |
| Hemarthria altissima | D | 3 | <0.001 | 3 | 0.003 | 3 | 0.005 | 3 | 0.391 |
| N | 2 | <0.001 | 2 | 0.037 | 2 | 0.012 | 2 | 0.66 | |
| D × N | 6 | 0.014 | 6 | 0.674 | 6 | 0.952 | 6 | 0.953 | |
| Leymus chinensis | D | 3 | <0.001 | 3 | <0.001 | 3 | <0.001 | 3 | 0.039 |
| N | 2 | <0.001 | 2 | 0.002 | 2 | 0.026 | 2 | 0.98 | |
| D × N | 6 | 0.008 | 6 | 0.023 | 6 | 0.144 | 6 | 0.817 | |
δ13C of potential respiratory substrates
The values of the carbon isotope composition of leaf soluble sugars (δ13Csoluble sugar) and starch (δ13Cstarch) decreased from 21:00 to 03:00 h except for δ13Csoluble sugar in L. chinensis on day 7 (Figures 2D–I). There were strong drought impacts on nighttime average δ13Csoluble sugar and nighttime average δ13Cstarch. Nighttime average δ13Csoluble sugar and nighttime average δ13Cstarch values decreased from −16.1 ± 0.2‰ and −11.9 ± 0.1‰ on day 1 to −16.9 ± 0.2‰ and −13.1 ± 0.3‰ on day 7 in C. virgata and from −14.1 ± 0.2‰ and −13.2 ± 0.2‰ on day 1 to −15.5 ± 0.2‰ and −14.1 ± 0.2‰ on day 7 in H. altissima. However, nighttime average δ13Csoluble sugar and nighttime average δ13Cstarch values increased from −28.8 ± 0.2‰ and −29.2 ± 0.1‰ on day 1 to −26.5 ± 0.1‰ and −26.9 ± 0.1‰ on day 7 in L. chinensis (Figures 2D–I; Table 1). No consistent nighttime variation patterns were observed in the δ13C of leaf lipids (δ13Clipid) (Figures 2J–L). Significant drought treatment impacts on the nighttime average δ13Clipid were detected in C. virgata and L. chinensis, but not in H. altissima (Table 1).
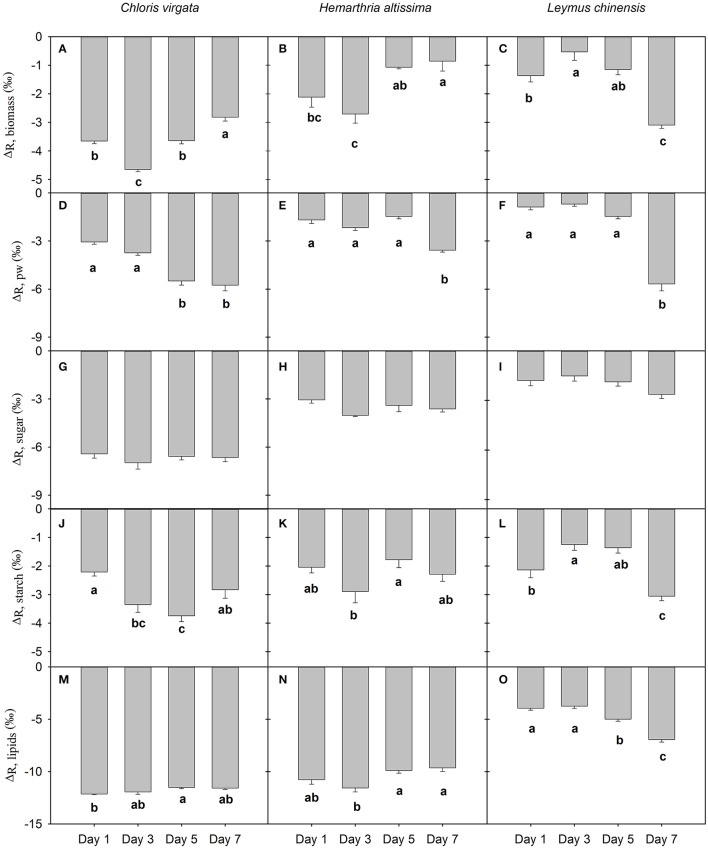
Respiratory apparent isotope fractionation
For the studied species, leaf dark-respired CO2 was enriched in 13C compared to biomass and other potential respiratory substrates (Figure 3). Respiratory apparent isotope fractionation relative to biomass (ΔR,biomass) differed significantly among the sampling dates for all studied species (Figures 3A–C). With the intensification of the water deficit, the magnitude of 13C enrichment in leaf dark-respired CO2 (relative to biomass) gradually decreased in the C4 grasses, whereas it increased in the C3 grass L. chinensis (Figure 3). Compared to the recently fixed photosynthates, leaf dark-respired CO2 was gradually enriched in 13C content in both C3 and C4 gasses as water deficit intensified (Figures 2D–F). Respiratory apparent isotope fractionation relative to photosynthates (ΔR,pw) changed from −3.1 ± 0.1‰, −1.7 ± 0.2‰ and −0.9 ± 0.2‰ on day 1 to −5.7 ± 0.4‰, −3.6 ± 0.1‰ and −5.7 ± 0.4‰ on day 7 for C. virgata, H. altissima and L. chinensis, respectively. There were no sampling date effects on respiratory apparent isotope fractionation relative to soluble sugars (ΔR,sugar); however, ΔR,sugar values differed significantly between the C3 and C4 grasses (Figures 3G–I). We detected significant differences in respiratory apparent isotope fractionation relative to starch (ΔR,starch) between the sampling dates; however, there were no apparent photosynthetic pathway differences (Figures 3J–L). For all studied species, respiratory apparent isotope fractionation relative to lipids (ΔR,lipid) differed significantly between the sampling dates. Moreover, the magnitude of 13C enrichment in leaf dark-respired CO2 (relative to lipids) was greater in the studied C4 grasses (average ΔR,lipid values of −11.8 ± 0.2‰ and −10.5 ± 0.5‰ for C. virgata and H. altissima, respectively) than in the C3 grass L. chinensis (an average ΔR,lipid value of −4.9 ± 0.6‰; Figures 3M–O).
Figure 3.
Respiratory apparent 13C/12C fractionation (‰) of leaf dark-respired CO2 relative to (A–C) biomass (ΔR, biomass), (D–F) recently fixed photosynthates (ΔR, pw), (G–I) soluble sugars (ΔR, sugar), (J–L) starch (ΔR, starch) and (M–O) lipids (ΔR, lipids) and on the days 1, 3, 5 and 7 of the drought treatment (D) in Chloris virgata (annual C4), Hemarthria altissima (perennial C4) and Leymus chinensis (perennial C3). The minus sign shows that the leaf dark-respired CO2 is 13C enriched compared to the potential respiratory substrates. Different lowercase letters indicate significant differences (P < 0.05) between the sampling dates (Tukey's test). Data are reported as the arithmetic mean ± 1 standard error (n = 5).
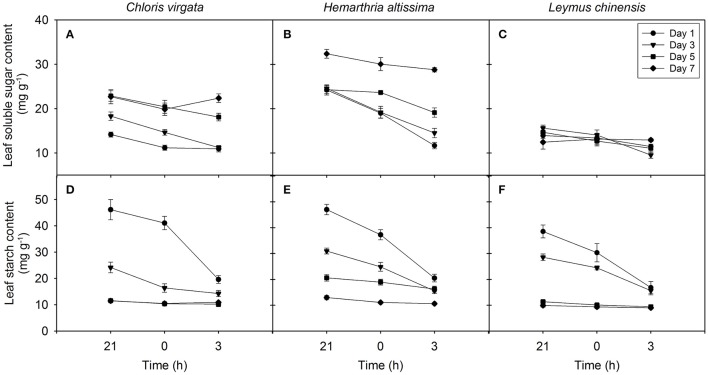
Changes in the concentrations of soluble sugars and transitory starch
Leaf soluble sugars showed a trend of decreasing from 21:00 to 03:00 h in all studied species; however, the range of nighttime variations in soluble sugars content (maximum value – minimum value) in the C4 grasses was greater than that in the C3 grass (Figures 4A–C). The impact of drought on the content of leaf soluble sugars was significant only in the C4 grasses (Table 2). For all studied species, the leaf starch content decreased from 21:00 to 0300 h, and the range of nighttime variations in starch content gradually diminished with the intensification of drought stress (Figures 4D–F). A significant drought effect on the leaf starch content was detected in all studied grasses (Table 2). Information on the content of the major sugars (glucose, fructose and sucrose) are provided in the supplementary section (Table S3).
Figure 4.
Nighttime variations in the content of leaf soluble sugars (A–C) and leaf starch (D–F) on the day 1 (circle), day 3 (triangle), day 5 (square), day 7 (diamond) of the drought treatment in Chloris virgata (annual C4), Hemarthria altissima (perennial C4), and Leymus chinensis (perennial C3). Data are reported as the arithmetic mean ± 1 standard error (n = 5).
Table 2.
The df and P values from the two-way analysis of nighttime variation (N) and drought effects (D) on leaf soluble sugars content (mg g−1) and leaf starch content (mg g−1) in Chloris virgata (annual C4), Hemarthria altissima (perennial C4) and Leymus chinensis (perennial C3).
| Species | Leaf soluble sugar (mg g−1) | Leaf starch (mg g−1) | |||
|---|---|---|---|---|---|
| df | P | df | P | ||
| Chloris virgata | D | 3 | <0.001 | 3 | <0.001 |
| N | 2 | <0.001 | 2 | <0.001 | |
| D × N | 6 | 0.032 | 6 | <0.001 | |
| Hemarthria altissima | D | 3 | <0.001 | 3 | <0.001 |
| N | 2 | <0.001 | 2 | <0.001 | |
| D × N | 6 | <0.001 | 6 | <0.001 | |
| Leymus chinensis | D | 3 | 0.985 | 3 | <0.001 |
| N | 2 | <0.001 | 2 | <0.001 | |
| D × N | 6 | 0.044 | 6 | <0.001 | |
Correlation between δ13Cl and the δ13C of respiratory substrates
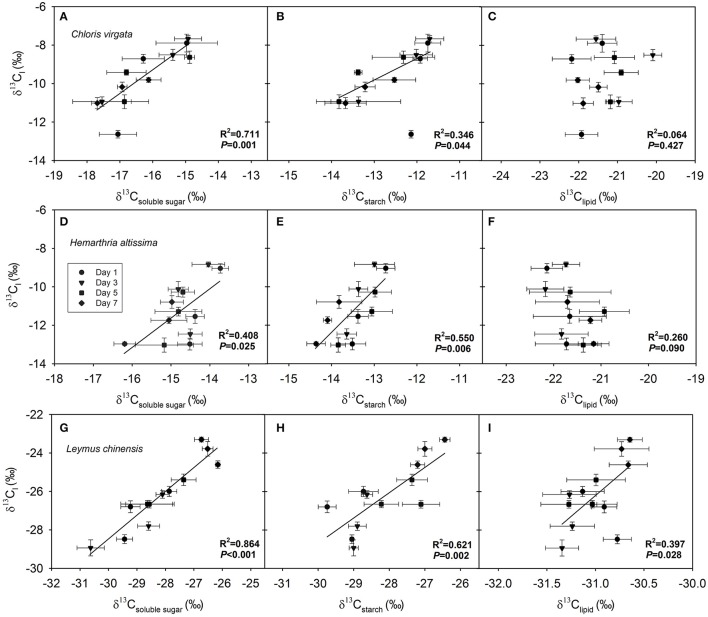
After pooling data across the four measurement dates, we detected a strong dependence of δ13Cl on δ13Csoluble sugar (Figures 5A,D,G) and δ13Cstarch (Figures 5B,E,H) in all studied grasses. However, a significant correlation between δ13Cl and δ13Clipid was detected only in the C3 species L. chinensis (Figure 5I), and not in the C4 species C. virgata and H. altissima (Figures 5C,F).
Figure 5.
Dependence of the C isotope composition of leaf dark-respired CO2 (δ13Cl) on (A,D,G) δ13C of leaf soluble sugars (δ13Csoluble sugar), (B,E,H) δ13C of leaf starch (δ13Cstarch) and (C,F,I) δ13C of leaf lipids (δ13Clipid) in Chloris virgata (annual C4), Hemarthria altissima (perennial C4) and Leymus chinensis (perennial C3). The P values and R2 of the linear relationship are provided.
Dependence of the magnitude of the nocturnal shift in δ13C on gas exchange parameters and variations in substrate availability
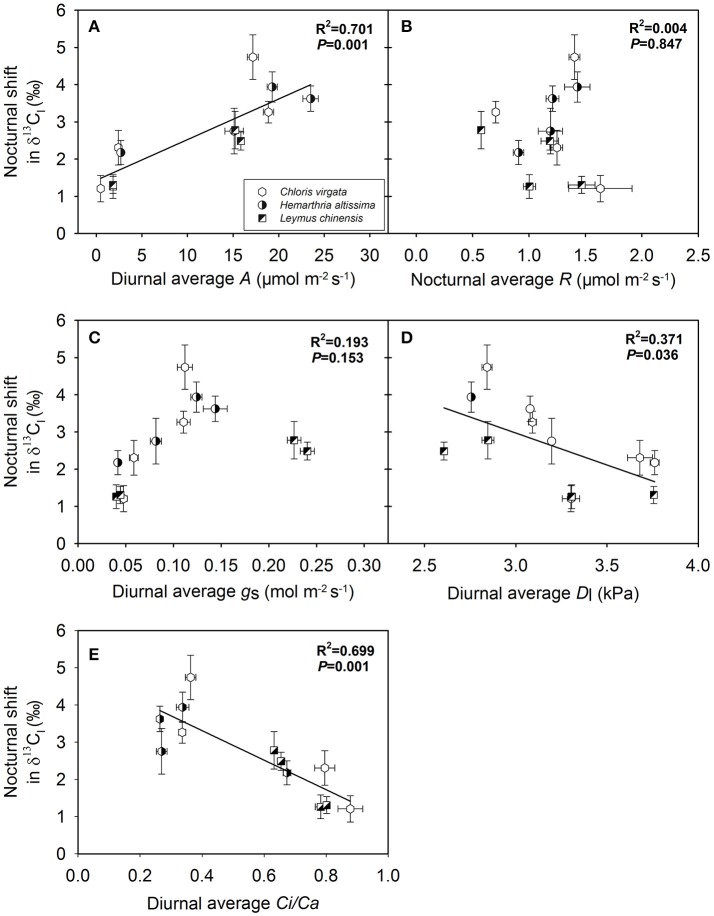
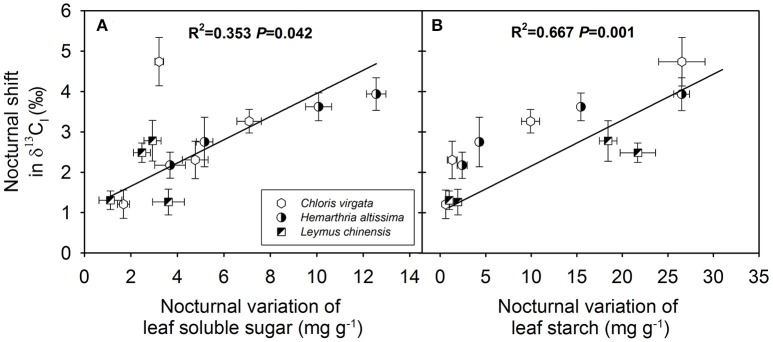
The magnitude of the nocturnal shift in δ13Cl (maximum δ13Cl value – minimum δ13Cl value) was positively correlated with the diurnal average A (Figure 6A) and negatively correlated with the diurnal average Dl (Figure 6D) and diurnal average Ci/Ca ratio (Figure 6E) across the studied C3 and C4 grasses. However, no significant correlations were observed between the magnitude of the nocturnal shift in δ13Cl and the nighttime average R (Figure 6B) or diurnal average gs (Figure 6C). We also detected positive correlations between the magnitude of the nocturnal shift in δ13Cl and the nighttime variations in the contents of leaf soluble sugars (Figure 7A) and leaf starch (Figure 7B).
Figure 6.
Dependence of the magnitude of the nocturnal shift in the C isotope composition of leaf dark-respired CO2 (δ13Cl) on (A) diurnal average leaf net CO2 assimilation rate (diurnal average A; μmol m−2 s−1), (B) nighttime average leaf respiration rate (nocturnal average R; μmol m−2 s−1), (C) diurnal average stomatal conductance (diurnal average gs; mol m−2 s−1), (D) diurnal average leaf-to-air vapor pressure deficit (diurnal average Dl; kPa) and (E) diurnal average of the Ci/Ca ratio (diurnal average of the Ci/Ca; unitless) in Chloris virgata (hexagon, annual C4), Hemarthria altissima (semi-filled circle, perennial C4), and Leymus chinensis (diagonal-filled square, perennial C3). The amplitude of nocturnal shift in δ13Cl was calculated as δ13Cl values at 21:00 h minus δ13Cl values at 03:00 h. The P values and R2 of the linear relationship are provided.
Figure 7.
Dependence of the magnitude of the nocturnal shift in the C isotope composition of leaf dark-respired CO2 (δ13Cl) on (A) nighttime variation in leaf soluble sugar (mg g−1) and (B) leaf starch (mg g−1) in Chloris virgata (hexagon), Hemarthria altissima (semi-filled circle) and Leymus chinensis (diagonal filled square). The P values and R2 of the linear relationship are provided.
Discussion
Nighttime variations in the δ13C of leaf dark-respired CO2
Large nighttime variations in the δ13C of leaf dark-respired CO2 (δ13Cl) were observed in both C3 (0.5–4.2‰ in L. chinensis) and C4 (0.5–5.9‰ in C. virgata and 1.3–4.9‰ in H. altissima) grasses, which may have resulted from changes in the carbon isotope signature of the primary respiratory substrates or shifts among substrates differing in 13C content (Ghashghaie et al., 2001; Sun et al., 2009). Daytime fluctuations in key environmental conditions (Brugnoli et al., 1988; Farquhar et al., 1989) and the rules of synthesis and remobilization of transitory starch (Zeeman et al., 2007) are likely to alter carbon isotope signature of nighttime respiratory substrates. The detected a strong dependence of δ13Cl on δ13Csoluble sugar (Figures 5A,D,G) highlights the importance of the controlling effects of changes in the carbon isotope signature of respiratory substrates on short-term variation in the δ13Cl.
Lipids are an important form of stored energy for plants to address stress conditions. In general, lipids are depleted in 13C more than the primary photosynthetic products (carbohydrates; Figure 2; Deniro and Epstein, 1977; Melzer and Schmidt, 1987). In a prolonged darkness study, δ13Cl in P. vulgaris decreased by 9‰ after 5 days of dark treatment (Tcherkez et al., 2003). This profound shift in δ13Cl was attributed primarily to the switching of respiratory substrates from soluble sugars to lipids. For the present study, we observed a strong correlation between δ13Cl and δ13Clipid in the C3 grass L. chinensis (Figure 5I), but not in the C4 grasses (Figures 5C,F). These observations suggest that the observed nocturnal depletion in 13C in leaf dark-respired CO2 may have also resulted from the shifting of the respiratory substrate toward 13C-depleted lipids with the progression of darkness (for the C3 grass) and potential differences in photosynthetic type in the use of lipids to address drought stress (Xu and Zhou, 2006). In future studies, the respiratory quotient should be measured to further confirm changes in the respiratory substrate (Tcherkez et al., 2003; Gessler et al., 2009).
Moreover, nocturnal shifts in δ13Cl may be caused by the heterogeneous carbon isotope distribution of hexose molecules (Rossmann et al., 1991; Gilbert et al., 2009, 2011) and C partitioning at key metabolic branch points (Hymus et al., 2005; Priault et al., 2009). This mechanism is discussed below.
13C enrichment in leaf dark-respired CO2
In the present study, we detected 13C enrichment in leaf dark-respired CO2 relative to bulk biomass or primary respiratory substrates in both C3 and C4 grasses (Figure 3), which is in agreement with the results of previous studies (Duranceau et al., 1999; Ghashghaie et al., 2001; Tcherkez et al., 2003; Huxman et al., 2005; Prater et al., 2006; Barbour et al., 2007b; Werner et al., 2007; Gessler et al., 2009; Priault et al., 2009; Cui et al., 2015). The apparent 13C/12C fractionation between respiratory substrates and CO2 is partly attributed to the heterogeneous 13C distribution within hexose molecules (Rossmann et al., 1991; Duranceau et al., 1999; Ghashghaie et al., 2001; Tcherkez et al., 2003) resulting from the isotope effects of aldolase involved in the formation of fructose-1,6-bisphosphate from triose phosphates (Gleixner and Schmidt, 1997; Schmidt, 2003). Theoretically, the heterogeneous 13C distribution and utilization of respiratory intermediates (oxidation vs. biosynthesis) could lead to 0–4.1‰ and 0–2.3‰ 13C enrichment in leaf dark-respired CO2 relative to primary respiratory substrates in C3 and C4 species, respectively (Ghashghaie et al., 2003; Hobbie and Werner, 2004). Contrary to our expectation, large respiratory apparent isotope fractionation compared to bulk biomass and potential respiratory substrates was detected in the C4 grasses, which cannot be explained by the non-statistical 13C distribution and variation in the utilization of respiratory intermediates. Other mechanisms are likely involved in the formation of leaf dark-respired CO2, such as malate decarboxylation, which will generate 13C-enriched CO2 (Lehmann et al., 2015). However, photosynthetic pathway differences in the contribution of malate decarboxylation to leaf dark-respired CO2 flux need to be further explored.
We also observed significant correlations of the magnitude of the nocturnal shift in δ13Cl with the daily average A (Figure 6A) and the magnitude of nighttime variations in primary substrates (Figure 7), which suggests that the observed nighttime variations in δ13Cl may have resulted from changes in substrate-availability-associated shifts in C partitioning and subsequent apparent isotope fractionation. However, the detected nighttime changes in δ13Cl are much greater than the maximum value that can be explained by the heterogeneous 13C distribution theory, especially in the studied C4 grasses. The results suggested other mechanisms, such as, changes in the carbon isotope signature of the primary respiratory substrates, may contribute to the nighttime variation in δ13Cl in the studied C4 grasses. Moreover, kinetic isotope effects of respiratory decarboxylating enzymes (pyruvate dehydrogenase, isocitrate dehydrogenase and 2-oxoglutarate dehydrogenase) may increase nocturnal variation in δ13Cl (Tcherkez and Farquhar, 2005; Werner et al., 2011). In a previous study, Werner (2010) reported that the combined isotope effects of respiratory decarboxylating enzymes, relative carbon flux changes through pyruvate dehydrogenase and the TCA cycle may theoretically induce more than a 9‰ shift in δ13Cl. The detected photosynthetic pathway differences in 13C-enrichment in leaf dark respired CO2 relative to biomass and respiratory substrates suggest cautions should be taken when δ13C of bulk biomass was used as a substitute of δ13Cl.
Drought-induced changes in the δ13C of leaf dark-respired CO2
For the studied C3 and C4 grasses, we detected significant drought impacts on the δ13C of leaf dark-respired CO2 and potential respiratory substrates (Table 1). However, the nighttime average δ13Cl on day 1 was not consistent with the variation pattern of the remaining measurement days in the two C4 grasses (Figure 2). These non-systematic drought effects may be attributed to the following: (1) The diurnal mean PPFD on day 1 was apparently lower than that on the remaining measurement days (Figure S1, Table S1), which may have strongly altered the photosynthetic discrimination of the studied C4 grasses and both the signature and magnitude of nighttime shifts in the δ13C of leaf dark-respired CO2 (Table S2). (2) On day 1, the volumetric soil water content for the C4 plant pots was >35% (Figure S1), which is beyond the upper limit of the optimum soil water content and may also have inhibited plant photosynthetic physiological processes. These effects can be seen from the gas exchange measurement (Figure 1).
The manipulated drought changed not only the 13C signature of leaf dark-respired CO2 but also the magnitude of nighttime variations in δ13Cl. The detected strong positive correlations between δ13Cl and respiratory substrates (Figure 5) suggests that short-term variation in δ13Cl is associated with photosynthetic discrimination and the 13C signature of the primary respiratory substrates. Drought-induced variations in photosynthetic discrimination and the carbon isotope composition of respiratory substrates have been extensively reported (Duranceau et al., 1999; Ghashghaie et al., 2001; Williams et al., 2001). A tight correlation between δ13Cl and the δ13C of the respiratory substrates throughout the drought period also indicated that carbohydrate pools in the studied species turned over quickly.
The magnitude of nighttime variations in δ13Cl decreased with the intensification of the drought treatment and was strongly dependent on the diurnal average net assimilation rate (Figure 6A), diurnal average leaf-to-air vapor pressure deficit (Figure 6D) and diurnal average of the Ci/Ca ratio (Figure 6E), as well as on nighttime variations in the contents of soluble sugars (Figure 7A) and starch (Figure 7B). The allocation (oxidation for energy production vs. biosynthesis of secondary compounds) of 13C-depleted respiratory intermediates (such as acetyl-CoA) depends on substrate availability. Changes in the availability and the magnitude of the nighttime shifts in primary respiratory substrates are the primary contributor to drought-induced variations in the range of the nighttime shift in δ13Cl.
For a coexisting C3 and C4 species ecosystem, photosynthetic pathways associated with contrasting carbon isotope signatures are useful for partitioning ecosystem carbon exchange (Lai et al., 2003; Still et al., 2003; Schnyder and Lattanzi, 2005; Shimoda et al., 2009). The foliar carbon isotope composition is often used as a substitute for δ13C in autotrophic respiration for the separation of C3 and C4 component fluxes (Lai et al., 2003; Still et al., 2003; Schnyder and Lattanzi, 2005; Shimoda et al., 2009). However, we detected that ΔR,biomass changed with plant water status and differed between the C3 and C4 plants (Table 2). More importantly, we observed that the magnitude of 13C enrichment in leaf dark-respired CO2 (relative to biomass) diminished in the C4 grasses, while it was enhanced in the C3 grass, with the intensification of the water stress (Table 2). These results suggest that the contribution of C4 plant-associated carbon flux is likely to be overestimated if the δ13C of biomass is used as a substitute for leaf dark-respired CO2. The studied C3 and C4 grasses demonstrated strong drought sensitivity in the carbon isotope signature and the magnitude of short term variations in leaf dark-respired CO2, which highlights the importance of incorporating these changes into the isotope partitioning studies.
Conclusions
For the studied C3 and C4 grasses, δ13Cl showed a decreasing trend from 21:00 to 03:00 h. The magnitude of the nighttime shift in δ13Cl decreased with increasing drought stress. The δ13Cl values were correlated with the δ13C of the respiratory substrates, which suggests that the drought treatment influences δ13Cl by affecting photosynthetic discrimination. The magnitude of the nighttime shift in δ13Cl was strongly dependent on the daytime carbon assimilation rate and the range of nighttime variations in substrate availability, which indicates that changes in respiratory substrate availability may alter the allocation (oxidation for energy production vs. biosynthesis of secondary compounds) of respiratory intermediates (such as acetyl-CoA) and subsequently affect δ13Cl. With the intensification of drought stress, leaf dark-respired CO2 in the C4 grasses was progressively depleted in 13C content, whereas leaf dark-respired CO2 in the C3 grass was enriched in 13C. Respiratory apparent isotope fractionation relative to biomass varied in opposite directions with the intensification of water stress between the C3 and C4 grasses. The contribution of C4 plant-associated carbon flux is likely to be overestimated if carbon isotope signatures are used for the partitioning of ecosystem carbon exchange and the δ13C of biomass is used as a substitute for leaf dark-respired CO2. The detected strong drought sensitivities in δ13Cl and differences in respiratory apparent isotope fractionation between the C3 and C4 grasses have marked implications for isotope partitioning studies at the ecosystem level.
Author contributions
WS, J-YM, YL and SZ conceived and designed the experiment, SZ, HC and YX conducted the experiment, WS and SZ analyzed data. WS and SZ wrote the manuscript. All authors helped drafting the manuscript and gave essential input to the work.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Chunge Liu helped with leaf dark-respired CO2 collection. This research was supported by the National Key Basic Research Program of China (2015CB150800), State Key Laboratory of Desert and Oasis Ecology, Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences (G2014-02-01), the National Natural Science Foundation of China (31270445, 31570470, 41671207), the National Key Technology Support Program (2013BAC09B03), the Fundamental Research Funds for the Central Universities (2412016KJ008), the Program of Introducing Talents of Discipline to Universities (B16011), Main Service Project of Characteristic Institute of Chinese Academy of Sciences (TSS-2015-014-FW-5-1), the 9th Thousand Talents Program of China (2013).
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2017.01996/full#supplementary-material
References
- Barbour M. M., Farquhar G. D., Hanson D. T., Bickford C. P., Powers H., McDowell N. G. (2007a). A new measurement technique reveals temporal variation in δ18O of leaf-respired CO2. Plant Cell Environ. 30, 456–468. 10.1111/j.1365-3040.2007.01633.x [DOI] [PubMed] [Google Scholar]
- Barbour M. M., Hunt J. E., Kodama N., Laubach J., McSeveny T. M., Rogers G. N. D., et al. (2011). Rapid changes in δ13C of ecosystem-respired CO2 after sunset are consistent with transient 13C enrichment of leaf respired CO2. New Phytol. 190, 990–1002. 10.1111/j.1469-8137.2010.03635.x [DOI] [PubMed] [Google Scholar]
- Barbour M. M., McDowell N. G., Tcherkez G., Bickford C. P., Hanson D. T. (2007b). A new measurement technique reveals rapid post-illumination changes in the carbon isotope composition of leaf-respired CO2. Plant Cell Environ. 30, 469–482. 10.1111/j.1365-3040.2007.01634.x [DOI] [PubMed] [Google Scholar]
- Bidigare R. R., Kennicutt M. C., II., Keeney-Kennicutt W. L. (1991). Isolation and purification of chlorophylls a and b for the determination of stable carbon and nitrogen isotope compositions. Anal. Chem. 63, 130–133. 10.1021/ac00002a008 [DOI] [Google Scholar]
- Bowling D. R., Pataki D. E., Randerson J. T. (2008). Carbon isotopes in terrestrial ecosystem pools and CO2 fluxes. New Phytol. 178, 24–40. 10.1111/j.1469-8137.2007.02342.x [DOI] [PubMed] [Google Scholar]
- Brugnoli E., Hubick K. T., Caemmerer S. V., Wong S. C., Farquhar G. D. (1988). Correlation between the carbon isotope discrimination in leaf starch and sugars of C3 plants and the ration of intercellualr and atmospheric partial pressures of carbon dioxide. Plant Physiol. 88, 1418–1424. 10.1104/pp.88.4.1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci S. J., Scholz F. G., Goldstein G., Meinzer F. C., Arce M. E. (2009). Soil water availability and rooting depth as determinants of hydraulic architecture of Patagonian woody species. Oecologia 160, 631–641. 10.1007/s00442-009-1331-z [DOI] [PubMed] [Google Scholar]
- Buchmann N., Brooks J. R., Rapp K. D., Ehleringer J. R. (1996). Carbon isotope composition of C4 grasses is influenced by light and water supply. Plant Cell Environ. 19, 392–402. 10.1111/j.1365-3040.1996.tb00331.x [DOI] [Google Scholar]
- Comas L. H., Becker S. R., Cruz V. M., Byrne P. F., Dierig D. A. (2013). Root traits contributing to plant productivity under drought. Front Plant Sci. 4:442. 10.3389/fpls.2013.00442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H. Y., Wang Y. B., Jiang Q., Chen S. P., Ma J. Y., Sun W. (2015). Carbon isotope composition of nighttime leaf-respired CO2 in the agricultural-pastoral zone of the Songnen Plain, Northeast China. PLoS ONE 10:e0137575. 10.1371/journal.pone.0137575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deniro M. J., Epstein S. (1977). Mechanism of carbon isotope fractionation associated with lipid-synthesis. Science 197, 261–263. 10.1126/science.327543 [DOI] [PubMed] [Google Scholar]
- Duranceau M., Ghashghaie J., Badeck F., Deléens E., Cornic G. (1999). δ13C of CO2 respired in the dark in relation to δ13C of leaf carbohydrates in Phaseolus vulgaris L. under progressive drought. Plant Cell Environ. 22, 515–523. 10.1046/j.1365-3040.1999.00420.x [DOI] [Google Scholar]
- Farquhar G. D. (1983). On the nature of carbon isotope discrimination in C4 species. Aust. J. Plant Physiol. 10, 205–226. 10.1071/PP9830205 [DOI] [Google Scholar]
- Farquhar G. D., Ehleringer J. R., Hubik K. T. (1989). Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Phys. 40, 503–537. 10.1146/annurev.pp.40.060189.002443 [DOI] [Google Scholar]
- Farquhar G. D., O'leary M. H., Berry J. A. (1982). On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust. J. Plant Physiol. 9, 121–137. 10.1071/PP9820121 [DOI] [Google Scholar]
- Farquhar G. D., Sharkey T. D. (1982). Stomatal conductance and photosynthesis. Annu. Rev. Plant Phys. 33, 317–345. 10.1146/annurev.pp.33.060182.001533 [DOI] [Google Scholar]
- Gessler A., Tcherkez G., Karyanto O., Keitel C., Ferrio J. P., Ghashghaie J., et al. (2009). On the metabolic origin of the carbon isotope composition of CO2 evolved from darkened light-acclimated leaves in Ricinus communis. New Phytol. 181, 374–386. 10.1111/j.1469-8137.2008.02672.x [DOI] [PubMed] [Google Scholar]
- Ghashghaie J., Badeck F. W. (2013). Opposite carbon isotope discrimination during dark respiration in leaves versus roots - a review. New Phytol. 201, 751–769. 10.1111/nph.12563 [DOI] [PubMed] [Google Scholar]
- Ghashghaie J., Badeck F. W., Lanigan G., Nogués S., Tcherkez G., Deléens E., et al. (2003). Carbon isotope fractionation during dark respiration and photorespiration in C3 plants. Phytochem. Rev. 2, 145–161. 10.1023/B:PHYT.0000004326.00711.ca [DOI] [Google Scholar]
- Ghashghaie J., Duranceau M., Badeck F. W. (2001). δ13C of CO2 respired in the dark in relation to δ13C of leaf metabolites: comparison between Nicotiana sylvestris and Helianthus annuus under drought. Plant Cell Environ. 24, 505–515. 10.1046/j.1365-3040.2001.00699.x [DOI] [Google Scholar]
- Gilbert A., Silvestre V., Robins R. J., Remaud G. (2009). Accurate quantitative isotopic 13C NMR spectroscopy for the determination of the intramolecular distribution of 13C in glucose at natural abundance. Anal. Chem. 81, 8978–8985. 10.1021/ac901441g [DOI] [PubMed] [Google Scholar]
- Gilbert A., Silvestre V., Robins R. J., Tcherkez G., Remaud G. (2011). A 13C NMR spectrometric method for the determination of intramolecular δ13C values in fructose from plant sucrose samples. New Phytol. 191, 579–588. 10.1111/j.1469-8137.2011.03690.x [DOI] [PubMed] [Google Scholar]
- Gleixner G., Schmidt H.-L. (1997). Carbon isotope effects on the fructose-1,6-bisphosphate aldolase reaction, origin for non-statistical 13C distributions in carbohydrates. J. Biol. Chem. 272, 5382–5387. 10.1074/jbc.272.9.5382 [DOI] [PubMed] [Google Scholar]
- Göttlicher S., Knohl A., Wanek W., Buchmann N., Richter A. (2006). Short-term changes in carbon isotope composition of soluble carbohydrates and starch: from canopy leaves to the root system. Rapid Commun. Mass Spectrom. 20, 653–660. 10.1002/rcm.2352 [DOI] [PubMed] [Google Scholar]
- Han W. J., Bai L. L., Li C. X., Cui Z., Yan J. W., Qin H. (2016). Effects of flooding on the photosynthetic response of Hemarthria altissima to drought. Acta Ecol. Sin. 36, 5712–5724. 10.5846/stxb201507181513 [DOI] [Google Scholar]
- Hobbie E. A., Werner R. A. (2004). Intramolecular, compound-specific, and bulk carbon isotope patterns in C3 and C4 plants: a review and synthesis. New Phytol. 161, 371–385. 10.1111/j.1469-8137.2004.00970.x [DOI] [PubMed] [Google Scholar]
- Huxman T. E., Wilcox B. P., Breshears D. D., Scott R. L., Snyder K. A., Small E. E., et al. (2005). Ecohydrological implications of woody plant encroachment. Ecology 86, 308–319. 10.1890/03-0583 [DOI] [Google Scholar]
- Hymus G. J., Maseyk K., Valentini R., Yakir D. (2005). Large daily variation in 13C -enrichment of leaf-respired CO2 in two Quercus forest canopies. New Phytol. 167, 377–384. 10.1111/j.1469-8137.2005.01475.x [DOI] [PubMed] [Google Scholar]
- Lai C. T., Schauer A. J., Owensby C., Ham J. M., Ehleringer J. R. (2003). Isotopic air sampling in a tallgrass prairie to partition net ecosystem CO2 exchange. J. Geophys. Res. 108:D18 10.1029/2002JD003369 [DOI] [Google Scholar]
- Lehmann M. M., Rinne K. T., Blessing C., Siegwolf R. T., Buchmann N., Werner R. A. (2015). Malate as a key carbon source of leaf dark-respired CO2 across different environmental conditions in potato plants. J. Exp. Bot. 66, 5769–5781. 10.1093/jxb/erv279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Shao S., Wang Y., Qi M., Lin L., Yan X. (2016). Germination responses of the halophyte Chloris virgata to temperature and reduced water potential caused by salinity, alkalinity and drought stress. Grass Forage Sci. 71, 507–514. 10.1111/gfs.12218 [DOI] [Google Scholar]
- Melzer E., Schmidt H.-L. (1987). Carbon isotope effects on the pyruvate dehydrogenase reaction and their importance for relative carbon-13 depletion in lipids. J. Biol. Chem. 262, 8159–8164. [PubMed] [Google Scholar]
- Mook W. G., Bommerso J. C., Staverma W. H. (1974). Carbon isotope fractionation between dissolved bicarbonate and gaseous carbon dioxide. Earth Plant Sci. Lett. 22, 169–176. 10.1016/0012-821X(74)90078-8 [DOI] [Google Scholar]
- Peisker M., Henderson S. A. (1992). Carbon: terrestrial C4 plants. Plant Cell Environ. 15, 987–1004. 10.1111/j.1365-3040.1992.tb01651.x [DOI] [Google Scholar]
- Prater J. L., Behzad M., Jeffery P. C. (2006). Diurnal variation of the δ13C R of pine needle respired CO2 evolved in darkness. Plant Cell Environ. 29, 202–211. 10.1111/j.1365-3040.2005.01413.x [DOI] [PubMed] [Google Scholar]
- Priault P., Wegener F., Werner C. (2009). Pronounced differences in diurnal variation of carbon isotope composition of leaf respired CO2 among functional groups. New Phytol. 181, 400–412. 10.1111/j.1469-8137.2008.02665.x [DOI] [PubMed] [Google Scholar]
- Ripley B. S., Frole K., Gilbert M. E. (2010). Differences in drought sensitivities and photosynthetic limitations between co-occurring C3 and C4 (NADP-ME) Panicoid grasses. Ann. Bot. Lond. 105, 493–503. 10.1093/aob/mcp307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley B. S., Gilbert M. E., Ibrahim D. G., Osborne C. P. (2007). Drought constraints on C4 photosynthesis: stomatal and metabolic limitations in C3 and C4 subspecies of Alloteropsis semialata. J. Exp. Bot. 58, 1351–1363. 10.1093/jxb/erl302 [DOI] [PubMed] [Google Scholar]
- Rossmann A., Butzenlechner M., Schmidt H.-L. (1991). Evidence for a nonstatistical carbon isotope distribution in natural glucose. Plant Physiol. 96, 609–614. 10.1104/pp.96.2.609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H.-L. (2003). Fundamentals and systematics of the non-statistical distributions of isotopes in natural compounds. Naturwissenschaften 90, 537–552. 10.1007/s00114-003-0485-5 [DOI] [PubMed] [Google Scholar]
- Schnyder H., Lattanzi F. A. (2005). Partitioning respiration of C3-C4 mixed communities using the natural abundance 13C approach - Testing assumptions on a controlled environment. Plant Biol. 7, 592–600. 10.1055/s-2005-872872 [DOI] [PubMed] [Google Scholar]
- Shi D., Wang D. (2005). Effects of various salt-alkaline mixed stresses on Aneurolepidium chinense (Trin.) Kitag. Plant Soil. 271, 15–26. 10.1007/s11104-004-1307-z [DOI] [Google Scholar]
- Shimoda S., Murayama S., Mo W., Oikawa T. (2009). Seasonal contribution of C3 and C4 species to ecosystem respiration and photosynthesis estimated from isotopic measurements of atmospheric CO2 at a grassland in Japan. Agric. Forest Meteorol. 149, 603–613. 10.1016/j.agrformet.2008.10.007 [DOI] [Google Scholar]
- Smith B. N., Epstein S. (1971). Two categories of 13C /12C ratios for higher plants. Plant Physiol. 47, 380–384. 10.1104/pp.47.3.380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Still C. J., Berry J. A., Ribas-Carbo M., Helliker B. R. (2003). The contribution of C3 and C4 plants to the carbon cycle of a tallgrass prairie: an isotopic approach. Oecologia 136, 347–359. 10.1007/s00442-003-1274-8 [DOI] [PubMed] [Google Scholar]
- Sun W., Resco V., Williams D. G. (2009). Diurnal and seasonal variation in the carbon isotope composition of leaf dark-respired CO2 in velvet mesquite (Prosopis velutina). Plant Cell Environ. 32, 1390–1400. 10.1111/j.1365-3040.2009.02006.x [DOI] [PubMed] [Google Scholar]
- Sun W., Resco V., Williams D. G. (2010). Nocturnal and seasonal patterns of carbon isotope composition of leaf dark-respired carbon dioxide differ among dominant plant species in a semiarid savanna. Oecologia 164, 297–310. 10.1007/s00442-010-1643-z [DOI] [PubMed] [Google Scholar]
- Sun W., Ubierna N., Ma J.-Y., Cousins A. B. (2012). The influence of light quality on C4 photosynthesis under steady-state conditions in Zea mays and Miscanthus × giganteus: changes in rates of photosynthesis but not the efficiency of the CO2 concentrating mechanism. Plant Cell Environ. 35, 982–993. 10.1111/j.1365-3040.2011.02466.x [DOI] [PubMed] [Google Scholar]
- Tazoe Y., Hanba Y. T., Furumoto T., Noguchi K., Terashima I. (2008). Relationships between quantum yield for CO2 assimilation, activity of key enzymes and CO2 leakiness in Amaranthus cruentus, a C4 dicot, grown in high or low light. Plant Cell Physiol. 49, 19–29. 10.1093/pcp/pcm160 [DOI] [PubMed] [Google Scholar]
- Tcherkez G., Farquhar G. D. (2005). Carbon isotope effect predictions for enzymes involved in the primary carbon metabolism of plant leaves. Funct. Plant Biol. 32, 277–291. 10.1071/FP04211 [DOI] [PubMed] [Google Scholar]
- Tcherkez G., Nogués S., Bleton J., Cornic G., Badeck F., Ghashghaie J. (2003). Metabolic origin of carbon isotope composition of leaf dark-respired CO2 in French bean. Plant Physiol. 131, 237–244. 10.1104/pp.013078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanek W., Heintel S., Richter A. (2001). Preparation of starch and other carbon fractions from higher plant leaves for stable carbon isotope analysis. Rapid Commun. Mass Spectrom. 15, 1136–1140. 10.1002/rcm.353 [DOI] [PubMed] [Google Scholar]
- Wang D., Ba L. (2008). Ecology of meadow steppe in northeast China. Rangeland J. 30, 247–254. 10.1071/RJ08005 [DOI] [Google Scholar]
- Wang Y., Jiang Q., Yang Z., Sun W., Wang D. (2015). Effects of water and nitrogen addition on ecosystem carbon exchange in a meadow steppe. PLoS ONE 10:e0127695. 10.1371/journal.pone.0127695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner C. (2010). Do isotopic respiratory signals trace changes in metabolic fluxes? New Phytol. 186, 569–571. 10.1111/j.1469-8137.2010.03248.x [DOI] [PubMed] [Google Scholar]
- Werner C., Gessler A. (2011). Diel variations in the carbon isotope composition of respired CO2 and associated carbon sources: a review of dynamics and mechanisms. Biogeosciences 8, 2437–2459. 10.5194/bg-8-2437-2011 [DOI] [Google Scholar]
- Werner C., Hasenbein N., Maia R., Beyschlag W., Maguas C. (2007). Evaluating high time-resolved changes in carbon isotope ratio of respired CO2 by a rapid in-tube incubation technique. Rapid Commun. Mass Spectrom. 21, 1353–1360. 10.1002/rcm.2970 [DOI] [PubMed] [Google Scholar]
- Werner C., Wegener F., Unger S., Nogués S., Priault P. (2009). Short-term dynamics of isotopic composition of leaf-respired CO2 upon darkening: measurements and implications. Rapid Commun. Mass Spectrom. 23, 2428–2438. 10.1002/rcm.4036 [DOI] [PubMed] [Google Scholar]
- Werner R. A., Buchmann N., Siegwolf R. T., Kornexl B. E., Gessler A. (2011). Metabolic fluxes, carbon isotope fractionation and respiration–lessons to be learned from plant biochemistry. New Phytol. 191, 10–15. 10.1111/j.1469-8137.2011.03741.x [DOI] [PubMed] [Google Scholar]
- Williams D. G., Gempko V., Fravolini A., Leavitt S. W., Wall G. W., Kimball B. A., et al. (2001). Carbon isotope discrimination by Sorghum bicolor under CO2 enrichment and drought. New Phytol. 150, 285–293. 10.1046/j.1469-8137.2001.00093.x [DOI] [Google Scholar]
- Xu Z. Z., Zhou G. S. (2006). Nitrogen Metabolism and photosynthesis in Leymus chinensis in response to long-term soil drought. J. Plant Growth Regul. 25, 252–266. 10.1007/s00344-006-0043-4 [DOI] [Google Scholar]
- Yang C., Jianaer A., Li C., Shi D., Wang D. (2008). Comparison of the effects of salt-stress and alkali-stress on photosynthesis and energy storage of an alkali-resistant halophyte Chloris virgata. Photosynthetica 46, 273–278. 10.1007/s11099-008-0047-3 [DOI] [Google Scholar]
- Zeeman S. C., Smith S. M., Smith A. M. (2007). The diurnal metabolism of leaf starch. Biochem J. 401, 13–28. 10.1042/BJ20061393 [DOI] [PubMed] [Google Scholar]
- Zhang J., Griffis T. J., Baker J. M. (2006). Using continuous stable isotope measurements to partition net ecosystem CO2 exchange. Plant Cell Environ. 29, 483–496. 10.1111/j.1365-3040.2005.01425.x [DOI] [PubMed] [Google Scholar]
- Zhao D., Mackown C. T., Starks P. J., Kindiger B. K. (2010). Rapid analysis of nonstructural carbohydrate components in grass forage using microplate enzymatic assays. Crop Sci. 50, 1537–1545. 10.2135/cropsci2009.09.0521 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.