Abstract
Purpose
The purpose of the study was to report the outcome of posterior approach white-line advancement surgery for severe involutional aponeurotic ptosis.
Patients and methods
This was a retrospective review of an interventional case series of all patients undergoing surgery for severe involutional aponeurotic ptosis during a 42-month period at a single center. The inclusion criteria were severe involutional ptosis (upper eyelid margin reflex distance (MRD) ≤1 mm) undergoing posterior approach surgery. There was minimum 3-month follow-up. The main outcome measures were type of ptosis (primary or recurrent), preoperative margin reflex distance, levator function and eyelid skin crease height, presence of visible iris sign (VIS), documented unusual intraoperative findings, postoperative complications, and follow-up time.
Results
Of the 836 procedures for ptosis, 122 procedures (76 patients) met the inclusion criteria for this study. Mean postoperative follow-up was 28 (median 18, range 12–98) weeks. Success rates were 80.3% (98/122) overall, 81.5% (66/81) in the non-VIS group, and 78% (32/41) in the VIS group. There was no significant difference between the two groups (P=0.411). Failures were due to undercorrection, with <2 mm MRD in 75% (18/24), overcorrection with >4.5 mm MRD in 16.7% (4/24), and inter-eyelid height asymmetry of >1 mm in 8.3% (2/122).
Conclusions
Outcomes of ptosis surgery for severe aponeurotic ptosis using a posterior approach white-line advancement are comparable to, and possibly better than, anterior approach in eyelids with VIS.
Introduction
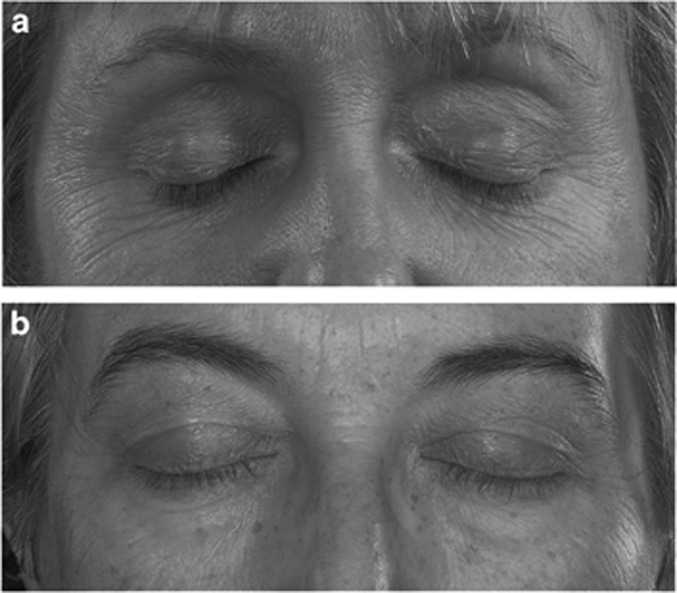
Severe involutional ptosis, including the visible iris sign (VIS, apparent visibility of the iris colour and/or part of the circumferential outline of the iris through a closed upper eyelid, with its overlying skin gently stretched),1 most likely reflects the severe end of the spectrum of clinicopathologic changes described for involutional ptosis1, 2, 3, 4, 5, 6 that include retraction of an attenuated, dehisced, or detached aponeurosis, retraction of orbital septum and preseptal orbicularis oculi muscle, stretching of the underlying Müller’s muscle, and age-related atrophy of soft tissue, all of which will result in increased translucency of the upper eyelid (Figures 1a and b).
Figure 1.
(a, b) Examples for visible iris sign (VIS)-positive and VIS-negative ptosis. (a) Visible iris sign left side: apparent visibility of the iris colour and/or part of the circumferential outline of the iris through a closed upper eyelid, with its overlying skin gently stretched both above and below. (b) Bilateral VIS-negative upper eyelid (Raman Malhotra, Corneoplastic Unit, Queen Victoria Hospital, NHS Foundation Trust).
Overall success rates for severe involutional ptosis using an anterior approach are ∼60–77% and lower where VIS exists (63.6% VIS vs 77.0% non-VIS) with failure due to undercorrection.1
Posterior approach ptosis surgery has traditionally been reserved for mild–moderate involutional blepharoptosis with good levator excursion with only one small series reporting outcomes in more severe ptosis.7 We present a retrospective review of an interventional case series, not reported in any previous study, of severe involutional ptosis and outcomes using a posterior approach white-line levator advancement technique.
Materials and methods
This was a retrospective review of an interventional case series of all patients undergoing surgery for severe involutional aponeurotic ptosis between July 2008 and December 2011 at the Queen Victoria Hospital, East Grinstead, UK.
Involutional aponeurotic ptosis had been diagnosed in patients with ptosis that was constant in all positions of gaze, good levator function, and a high or absent eyelid skin crease. In addition, in an attempt to match for severity, only cases with upper eyelid margin reflex distance ≤1 mm were included. That is to say, patients with mild–moderate involutional ptosis were excluded. Patients with congenital, myogenic, neurogenic, or traumatic aponeurotic ptosis, inadequate data, including postoperative follow-up data with photographs <3 months, and those who underwent anterior approach surgery were also excluded.
To define VIS positivity, the patient was instructed to gently close (but not squeeze) their eyes and two observers (usually the consultant, RM, and the fellow) were required to agree that there was apparent visibility of the iris colour and/or part of the circumferential outline of the iris through a closed upper eyelid, with its overlying skin gently stretched both above and below. In dark-skinned individuals or where the iris colour may be hard to define, the presence of the circumferential iris outline is determined by its contrast in relation to adjacent white sclera and VIS was considered positive if at least 2–3 clock hours of iris–sclera outline were discernible.
Data collected included type of ptosis (primary or recurrent), details of any previous ptosis surgery, preoperative margin reflex distance (MRD), levator function and eyelid skin crease height, documented unusual intraoperative findings, type of ptosis repair (anterior approach levator advancement or posterior approach levator advancement with or without Müller’s muscle resection), postoperative complications, and follow-up time.
Surgical success was defined as a postoperative MRD of ≥2 mm and ≤4.5 mm, inter-eyelid height asymmetry of ≤1 mm, and satisfactory eyelid contour. Institutional review board approval for this audit was obtained.
Surgical technique
All cases were performed under local anaesthesia with 1 ml subcutaneous infiltration,8 both along the eyelid skin crease and in the mid-pupil pretarsal region and 0.5 ml subconjunctival infiltration upon eyelid eversion using 0.5% bupivacaine with 1 : 200 000 adrenaline. Most patients also received a bolus injection of intravenous sedation immediately before local anaesthetic infiltration, but were alert for the remainder of the procedure.
The desired eyelid skin crease was marked and a 4-0 silk traction suture placed in the grey line of the upper eyelid that was then everted over a Desmarres retractor. Gentle diathermy was applied before a conjunctival incision made with a no. 15 Bard parker blade along but above the superior border of the tarsus. Müller’s muscle and conjunctiva was dissected off as a composite flap until the white line, which represents the posterior border of the levator aponeurosis, was identified. Dissection was then continued between the posterior surface of the levator aponeurosis and the conjunctiva to expose the post-aponeurotic fat pad9, 10 and the posterior surface of the levator palpebrae superioris (LPS) muscle. A double armed 5-0 vicryl suture was placed centrally through the most proximal white line at its junction with LPS, in a forehand manner and was then passed through the conjunctival surface of the tarsal plate, 1 mm below its superior border, and then through to the skin. The suture was captured through the skin in the region of the eyelid skin crease. The lid height and contour was assessed after tying this first suture in a bow and care was taken to ensure there was no slippage of the suture. If the eyelid position was deemed to be satisfactory, the suture was relaxed and a second suture was placed within 2–3 mm lateral to the first in the method described above. Both sutures were then tied. If the lid height was too low after the first suture, it was then relaxed and a second suture was passed higher through the white line and again through the tarsal plate and skin. If the upper eyelid contour appeared peaked after the first suture, then this was relaxed and a second suture was placed more central to the location of the peak. Using this method of altering the position of the second suture enabled minor adjustments to eyelid height and contour without the undue delay of removing the first suture in the majority of cases. In such situations, the first suture was gently tied so as to act as a ‘support’ rather than a ‘cardinal’ suture. Both Müller’s muscle and conjunctiva were left to heal spontaneously with no excision of these structures.
In cases with significant dermatochalasis, the procedure was combined with a blepharoplasty. This was carried out before eyelid eversion and white-line advancement. The technique involved either a skin only or skin-muscle blepharoplasty. The orbital septum and orbital fat were not violated and therefore the anterior surface of the aponeurosis was never exposed. The double armed 5-0 vicryl sutures came out within the wound but through the soft tissue overlying the anterior surface of the tarsal plate. By tying the sutures here they remained buried when skin closure was carried out, usually with continuous 6-0 vicryl rapide.
Patients were routinely reviewed at 1–2 weeks and 3 months postoperatively.
Statistical analysis
All analyses were performed using the SPSS Statistics 17.0 (Budapest, Hungary). Comparisons between preoperative and postoperative measurements from the VIS and non-VIS groups were statistically analysed using independent-samples t-test and Pearson’s χ2 test.
Results
Patients and demographics
A total of 514 patients undergoing 836 procedures for ptosis repair were identified, but 122 procedures (76 patients) were eligible for inclusion. Patients were further divided into two groups for comparison, those with VIS and those without VIS. In all, 41 procedures (31 patients) (40.8%, 31/76) had been identified as having VIS preoperatively.
A total of 76 patients (31 male, 45 female) underwent 122 ptosis procedures. Of these, 31 patients (10 male, 21 female) and 41 eyelids were identified as having VIS preoperatively. The majority of procedures were simultaneous bilateral ptosis repairs. The overall mean age at the time of surgery was 69.9±10.1 years, range 49–89 years.
Additional upper eyelid/brow procedures performed
Upper blepharoplasty was simultaneously performed in 70.7% of VIS (29/41) and 82.7% of non-VIS (67/81) cases.
Preoperative findings
Based on the t-test model there was neither statistically significant difference in the MRD levels in the two groups (VIS group mean MRD=0.74±0.42 mm compared with the non-VIS group mean MRD=0.55±0.53 mm, P=0.054) nor in preoperative levator function (VIS group mean levator function 13.65±1.92 mm compared with the non VIS group levator function 14.4±1.76 mm, P=0.052). There was statistically significant difference in eyelid skin crease height in the two groups, and eyelid skin crease was higher in the VIS group (mean: 10.85±1.67 mm) compared with the non-VIS group (mean: 9.58±1.77 mm) (P=0.023).
Outcomes
Mean postoperative follow-up was 28 (median 18, range 12–98) weeks.
There was no significant difference in postoperative MRD levels in the two groups (VIS group mean MRD=2.98±1.1 mm compared with the non-VIS group mean MRD=2.91±1.1 mm t-test P=0.74).
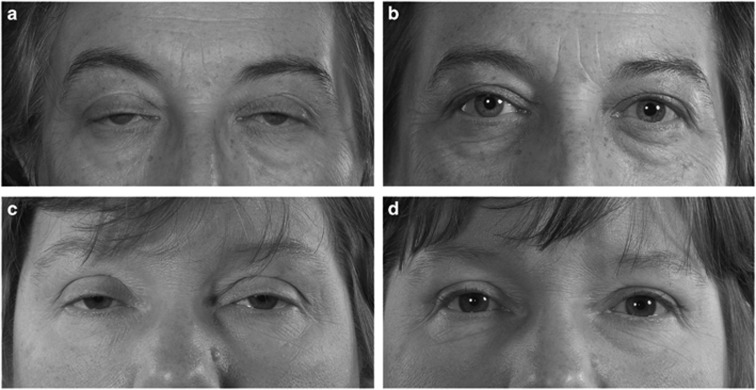
Success rates were 80.3% (98/122 procedures) overall, 81.5% (66/81) in the non-VIS group, and 78% (32/41) in the VIS group (Figure 2a–d). There was no significant difference between the two groups (χ2 test, P=0.411).
Figure 2.
(a–d) Pre- and postoperative pictures of VIS-negative and VIS-positive cases. (a) Bilateral VIS-negative severe aponeurotic ptosis preoperatively. (b) Following bilateral posterior approach white-line advancement (WLA) ptosis repair and upper blepharoplasty. (c) Bilateral VIS-positive severe aponeurotic ptosis preoperatively. (d) Following bilateral posterior approach white-line advancement (WLA) ptosis repair and upper blepharoplasty (Raman Malhotra, Corneoplastic Unit, Queen Victoria Hospital, NHS Foundation Trust).
Failures were due to undercorrection, with <2 mm MRD in 75% (18/24), overcorrection with >4.5 mm MRD in 16.7% (4/24), and inter-eyelid height asymmetry of >1 mm in 8.3% (2/122). In the VIS group failures were due to undercorrection with <2 mm MRD in 66.7% (6/9) and overcorrection with MRD >4.5 mm in 33.3% (3/9). In the non-VIS group failures were due to undercorrection with <2 mm MRD in 80% (12/15), overcorrection with MRD >4.5 mm in 6.7% (1/15), and inter-eyelid height asymmetry of >1 mm in 13.3% (2/15).
Postoperative complications
Amongst the ocular surface complications two patients developed corneal abrasions. One (non-VIS) developed a right-sided corneal abrasion at 2 weeks postoperatively that healed with the use of a bandage contact lens. In this case no exposed suture was visible. The other (VIS) developed a corneal abrasion due to a protruding posterior suture that resolved after suture lysis and removal of 1 suture. Ptosis correction remained successful with MRD 3 mm at 20 weeks, postoperatively.
Outcome of revisionary procedures
Of the 24 failures (15 non-VIS, 9 VIS) in 17 patients (10 non-VIS, 7 VIS), no revision procedures were performed in 9 patients (5 unilateral, 4 bilateral procedures): two were satisfied with their result, three because of medical reasons, and the decision for no revision was unclear in three patients. One patient was listed for lid lowering but, to date, has not attended for surgery.
Eight patients (5 unilateral, 3 bilateral) underwent revision surgery. Two required three revision procedures. One required two procedures. Four required one revision procedure (one bilateral). Anterior approach revision was performed in six patients (one bilateral) and selected in one case because of a prominent suture causing a corneal abrasion following the previous posterior approach. Of these, five required one procedure and one required two procedures.
Posterior revision was performed successfully in two patients (one bilateral). One patient underwent posterior levator recession because of overcorrection.
Discussion
Our study showed that the overall success rates for severe ptosis using a posterior approach for white-line advancement were comparable to those using an anterior approach.8 The two main reasons for this may be that, first, an attenuated and retracted levator aponeurosis deeper in the anterior orbit may be difficult to isolate during anterior approach surgery, potentially requiring unnecessary dissection and trauma. Isolating the posterior surface of the levator aponeurosis through a transconjunctival approach is relatively easy and avoids dissection of anterior eyelid structures. It simply requires the surgeon to dissect superiorly, along the conjunctiva–Müller’s flap and, occasionally, beyond Müller’s muscle to locate the healthier white line of the posterior surface of the levator aponeurosis. Second, anterior approach surgery for severe aponeurotic ptosis may be complicated by a thinned tarsus that is difficult to suture and prone to full-thickness suture passes. Posterior approach levator advancement therefore has a potential advantage where reattachment of the levator aponeurosis to the tarsus is carried out using full-thickness suture passes through the superior tarsus and onto the anterior surface, or skin, where they are tied. This eliminates any concern about unwanted full-thickness or inadequate partial-thickness tarsal bites during anterior approach ptosis repair. With the suture being passed full thickness through the upper tarsal plate 1 mm below the superior tarsal border, there lies a potential risk that an exposed suture may abrade the cornea. However, the free edge of the conjunctiva is re-draped and seems to remain over the superior edge of the tarsus. In addition, we have observed that once attached to the aponeurosis, the potentially exposed suture is rotated superiorly and the upper border of the tarsus rotates forward, reducing the potential to abrade the cornea. Only one previous small series7 reports outcomes of moderate-to-severe ptosis using a posterior approach technique. This also involved septal-sparing advancement of the aponeurosis in 27 predominantly Chinese patients. The authors sutured the aponeurosis to the anterior surface of the tarsus and avoided a full-thickness tarsal suture. Five of the six failures (all failures were undercorrected) had severe ptosis (MRD <1 mm). Some of these cases may have therefore been VIS positive and undercorrection would have been either because of early dehiscence of a partial-thickness tarsal suture (that would be avoided by a full-thickness tarsal suture) or inadequate posterior exposure and advancement of the aponeurosis and LPS.
We believe that more importantly, the effect of white-line advancement differs depending on whether the true posterior surface of the aponeurosis is advanced, in contrast to what we consider to be a ‘pseudo-white line’ that represents an attenuated aponeurosis continuing as the orbital septum. The levator aponeurosis has been shown to comprise two layers, of which the anterior layer is thick with less smooth muscle fibres, and reflects superiorly a few millimeters above the tarsus to become contiguous with the orbital septum. The posterior layer is thin with more smooth muscle fibres, and becomes confluent with the lower one third of the tarsal plate and subcutaneous tissue.11, 12
Occasionally, after placement of sutures in what appears to be a white line, there can be an undercorrection of ptosis. We have found that this usually arises from erroneous placement of sutures into the orbital septum (anterior layer of levator) that can occasionally appear as a white line. In such cases, the levator aponeurosis is often significantly thin and can be found by further dissection closer to the conjunctiva beyond a thin Müller’s muscle. As Müller’s muscle disappears, a thin white line can be identified. A useful anatomical landmark, lying on the surface of Müller’s muscle, is the post-aponeurotic fat pad.9, 10 Following this, further dissection above exposes a more healthy white sheet of the posterior surface of the levator aponeurosis and beyond that the posterior surface of LPS. Placement of sutures into this white sheet, that is to say the healthier posterior surface of the levator aponeurosis, will achieve the desired correction by a more effective advancement than simply plicating orbital septum to the tarsus.
When placing sutures into the white sheet of the healthier aponeurosis, care should be given to avoid incarcerating the orbital septum anteriorly. This would result in immediate undercorrection with limited levator excursion.
Anterior approach levator aponeurosis advancement is known for its potential to give rise to medial undercorrection in thinned eyelids.13, 14 In contrast, the posterior approach technique above consistently achieves a predictable normal-looking eyelid contour, similar to that reported with traditional posterior approach Müller’s resection surgery. We do not have exact figures for the number of cases in our series that required a second, more central suture because of contour peaking. Generally, the only time this would occur would be if the first suture is not placed centrally, in which case it is relaxed and not tied and a more central suture is then placed. The first suture can either be removed or left loosely tied. We consider this a learning-curve phenomenon in so far as once a surgeon encounters this, it is generally avoided in the future or quickly rectified with a central suture if seen to occur again.
Consistent with our previous findings,1 fewer adjunctive blepharoplasty procedures were performed in the VIS group, where dermatochalasis was not judged preoperatively to require treating. We believe this to be because of the predominance of upper sulcus hollowing in VIS ptosis, implying significant thinning and retraction as well as possibly greater secondary brow overcompensation. Following surgery, dermatochalasis in these patients can often appear unaddressed and consequently more noticeable.
We acknowledge the many limitations of this retrospective analysis of a prospective audit. Potential confounding factors include the performance of additional procedures at the time of ptosis repair that, although unlikely, could theoretically affect final eyelid height. That fewer patients in the VIS group were considered preoperatively to require a concomitant upper blepharoplasty reflects their greater upper sulcus hollow and the lack of apparent skin excess. Such patients are also considered to have a greater risk of corneal exposure-related symptoms. This was not apparent in our series as this would be a contraindication to ptosis surgery.
In conclusion, our results suggest that a posterior approach white-line levator advancement procedure is a suitable procedure for severe involutional ptosis.

Footnotes
The authors declare no conflict of interest.
References
- Malhotra R, Salam A, Then SY, Grieve AP. Visible iris sign as a predictor of problems during and following anterior approach ptosis surgery. Eye 2010; 25: 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherbarrow B, Blepharoptosis. In: Martin Dunitz. (ed). Oculoplastic Surgery. Taylor and Francis: London, UK, 2002, pp 21–43. [Google Scholar]
- Siddens DJ, Nesi FA. Acquired ptosis: classification and evaluation. In: Nesi F, Lisman R, Levine R (eds) Smith's Ophthalmic Plastic and Reconstructive Surgery. Mosby-Year Book Inc: St Louis, USA, 1998, pp 380–381. [Google Scholar]
- Martin PA, Rogers PA. Involutional ptosis: recognition and management. Aust N Z J Ophthalmol 1985; 13: 185–187. [DOI] [PubMed] [Google Scholar]
- Collin JR. Involutional ptosis. Aust N Z J Ophthalmol 1986; 14: 109–112. [DOI] [PubMed] [Google Scholar]
- Holmström H, Filip C. Aponeurotic repair of involutional blepharoptosis. Scand J Plast Reconstr Surg Hand Surg 2002; 36: 160–165. [DOI] [PubMed] [Google Scholar]
- Ng DS, Chan E, Ko ST. Minimal incision posterior approach levator plication for aponeurotic ptosis. Eye 2015; 29: 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Salam A, Malhotra R. Posterior approach white-line advancement ptosis repair: the evolving posterior approach to ptosis surgery. Br J Ophthalmol 2010; 94: 1513–1518. [DOI] [PubMed] [Google Scholar]
- Malhotra R, Mahadevan V, Leatherbarrow B, Barrett AW. The post-levator aponeurosis fat pad. Ophthal Plast Reconstr Surg 2015; 31: 313–317. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Nakano T, Ikeda H, Miyazaki H, Malhotra R, Kakizaki H. Post-levator aponeurosis fat pad. J Craniofac Surg 2016; 27: 2171–2172. [DOI] [PubMed] [Google Scholar]
- Fox SA. A modified Fasanella-Servat procedure for ptosis. Arch Ophthalmol 1975; 93: 639–640. [DOI] [PubMed] [Google Scholar]
- Kakizaki H, Zako M, Nakano T, Asamoto K, Miyaishi O, Iwaki M. The levator aponeurosis consists of two layers that include smooth muscle. Ophthal Plast Reconstr Surg 2005; 21: 379–382. [PubMed] [Google Scholar]
- Kakizaki H, Malhotra R, Selva D. Upper eyelid anatomy: an update. Ann Plast Surg 2009; 63: 336–343. [DOI] [PubMed] [Google Scholar]
- Ichinose A, Tahara S. Transconjunctival levator aponeurotic repair without resection of Müller's muscle. Aesthetic Plast Surg 2007; 31: 279–284. [DOI] [PubMed] [Google Scholar]