Abstract
Purpose
To investigate the microstructural differences of the inner retina in the peripapillary and macular areas in children with or without retinopathy of prematurity (ROP).
Methods
This prospective cohort study included school-age children with a history of ROP and age-matched healthy, full-term children. The macular ganglion cell complex (mGCC), peripapillary retinal nerve fiber layer (RNFL), refractive status, and ocular biometry were measured. The metrics of the mGCC and associated anatomical changes were the primary outcomes. Mann–Whitney U tests and chi-squared tests were used to compare variables between the two groups.
Results
A total of 41 eyes from 21 preterm children with ROP and 34 eyes from 17 full-term children were enrolled. ROP eyes had significantly thicker mGCC (P<0.001) with uneven distribution compared with full-term eyes. The RNFLs of ROP eyes were thicker in the temporal quadrants but thinner in the nasal quadrants (P=0.01 and.04, respectively). In addition, the ROP eyes had shallower anterior chamber depths (ACDs), thicker lenses, and higher degrees of refractive errors (all P<0.05) but similar axial lengths (ALs) (P=0.58) compared with full-term eyes.
Conclusions
The mGCC was thicker in children with ROP, and their inner retinal structures had a different distribution pattern than those in full-term children. The myopia of children with ROP was associated with the abnormal development of the anterior segment rather than long ALs. These alterations in inner retinal anatomy and optic components emphasize the importance of careful examinations to monitor the development of glaucoma or visual decline in children with ROP.
Introduction
Children with a history of retinopathy of prematurity (ROP) represent a unique population because they might have different ocular sequelae during development, even if the disease has regressed. The eyes of preterm children have more highly curved corneas, shallower anterior chambers, thicker lenses, and shorter axial lengths (ALs) than those of full-term children,1, 2 and these differences become more significant as the severity of ROP increases.3 Because these anatomical alterations can change refractive status, ROP has long been associated with the development of myopia, astigmatism, and anisometropia.4, 5 Although the overall prevalence of myopia in eyes with ROP remains stable across age,6 the proportion of high myopia, particularly myopia with diopters ≥8, continued to increase in the Early Treatment of ROP population up to 3 years of age.4
Preterm children with or without a history of ROP have a thinner retinal nerve fiber layer (RNFL) than full-term children.7, 8 Glaucoma is a progressive optic neuropathy characterized by RNFL thinning. Whether reduced RNFL thickness in preterm children is associated with glaucoma remains unknown; however, glaucoma was recognized as an important cause of visual decline in children with severe ROP after surgery.9 In contrast, myopia itself, especially high myopia, is strongly associated with glaucoma.10, 11 After adjusting for known glaucoma risk factors and intraocular pressure, the Blue Mountains Eye Study10 concluded that myopic individuals had a two- to threefold increased risk of glaucoma compared with nonmyopic individuals. The mechanical stretching of the ocular structure alone or insufficient ocular perfusion in people with myopia can lead to RNFL damage.11
Therefore, the interactions among ROP, myopia, and glaucoma remain complex and unclear. An analysis of the ganglion cell layer (GCL) might be a powerful predictor to diagnose and monitor glaucoma.12 The advancement of spectral-domain optical coherence tomography (SD-OCT) improved the ability to present an accurate segmentation in the retina and observe subtle changes.13 Different SD-OCT machines measure GCL using different parameters including the macular ganglion cell complex (mGCC) and the GCL-inner plexiform layer (IPL). Nevertheless, the results of GCL-IPL were inconsistent in different studies,13, 14 and a lack of mGCC measurements exist for children with a history of ROP in the literature.
Hence, the present study performed detailed measurements of the optic nerve head (ONH) and inner retinal structures (including the peripapillary RNFL and mGCC) in a large sample of school-age children with or without a history of ROP. The results might clarify the microstructural differences in ROP eyes and allow us to evaluate the likelihood of developing glaucoma. We also measured the refraction and biometry of related optical components to obtain a better understanding of the relationship between refractive errors and ocular structural changes.
Methods
Patients and grouping
This prospective, comparative study was conducted at Chang Gung Memorial Hospital, a referral medical center in Taiwan between October 2012 and September 2015. This study recruited two cohorts of children: The first cohort included children with a history of treatment-requiring ROP who received regular follow-up assessments at our hospital; the second cohort consisted of age-matched healthy controls who were recruited at our clinics. The control children were born at full term (gestational age [GA]≥37 weeks; birth weight [BW]≥2500 g), and they did not present with any ocular disease except refractive errors.
All patients had a normal-appearing posterior pole on ophthalmoscopy. ROP grade was categorized by the maximal severity in the acute stage.15 The indications for diode laser photocoagulation depended on the development of threshold disease defined by the previous CRYO-ROP study.16 The indications for intravitreal injection of bevacizumab (IVB; Avastin; Genentech Inc., San Francisco, California, USA) were patients with pre-threshold ROP, and the injected volume was 0.625 mg (0.025 ml) as described in our previous study.17 Both eyes of each child were examined and included in the study analysis.
Patients were excluded if they had stage 4 or 5 ROP and had undergone vitrectomy or scleral buckling. Patients with residual retinal sequelae of ROP, including retinal detachment or macular dragging or fold, were also excluded.
All procedures conformed to the tenets of the Declaration of Helsinki. The Institutional Review Board of Chang Gung Memorial Hospital approved the study protocol. Written informed consent was obtained from the parents of each child.
Ophthalmic examination
All study participants underwent thorough ophthalmic examinations, including slit lamp biomicroscopy, indirect ophthalmoscopy, and best-corrected visual acuity. Visual acuity was assessed via Landolt-C optotypes and converted into logarithms of minimum angle of resolution (LogMAR) units prior to data analysis. Biometry, including anterior chamber depth (ACD), lens thickness (LT), vitreous depth, and AL were measured using A/B mode ultrasound (Aviso, Quantel Medical, Cournon d'Auvergne Cedex, France). Any abnormality in pupil, iris, or lens clarity was documented.
Peripapillary RNFL, mGCC and ONH measurements via SD-OCT
An experienced technician blind to the patient’s group performed the SD-OCT scans (RTVue-100; software version 4.0; Optovue, Inc., Fremont, California, USA). The RTVue OCT uses a near-infrared light-source centered at 840 nm, with a 50-nm bandwidth. The system has an axial resolution of 5 μm and can perform 26 000A-scans per second. To measure the ONH, RNFL, and mGCC, the standard glaucoma module was used. The peripapillary RNFL thickness was measured at a diameter of 3.45 mm around the center of the optic disc. The mGCC consists of three layers: the RNFL, the GCL, and the IPL.18 The mGCC scan covered a 7 × 7 mm2 area in the macula and was centered 1 mm temporal to the fovea to improve coverage of the temporal macula. Four types of data (average, superior, inferior thicknesses, and superior-inferior difference) were used to analyze the mGCC. The mGCC global loss volume (GLV) and focal loss volume (FLV) metrics are designed to detect patterns of loss. The GLV detects diffuse loss, and the FLV detects localized loss over the mGCC map. The image quality was examined carefully following each acquisition. Poor-quality scans, defined as images with (1) a signal strength index (SSI) of <45; (2) RNFL or mGCC segmentation errors; or (3) a local weak signal caused by medial opacities, were excluded.
Statistics
Statistical analyses were performed using SAS software version 9.2 (SAS Inc., Cary, North Carolina, USA). The categorical variables are presented as frequencies and percentages, and the continuous variables are presented as means±standard deviations. Mann–Whitney U tests and chi-squared tests were used to compare variables between the ROP group and the control group, where appropriate. Spearman’s rank correlation coefficient was used to assess the relationship between two variables. A P value of <0.05 was considered to indicate statistical significance.
Results
Baseline characteristics
A total of 41 eyes of 21 preterm children and 34 control eyes of 17 full-term children were included in this study. The mean GA of children in the ROP group was 26.2±2.1 weeks (range, 23–36 weeks) and 39.0±1.7 weeks (range, 37–41 weeks) in the control group (P<0.001). The mean BW of the children in the ROP group was 919.4±260.8 g (range, 552–1530 g) and 3195.9±384.3 g (range, 2500–3878 g) in the control group (P<0.001). The mean age (years) at examination was 9.6±2.5 in the ROP group and 8.4±1.7 in control group (P=0.14). In the ROP group, all of the eyes (100%) were zone 2 ROP. Six eyes (14.6%) were stage 2 ROP plus disease, and 35 eyes (85.4%) were stage 3 ROP plus disease. A total of 37 (90.2%) eyes were treated with diode laser photocoagulation, and 4 eyes (9.8%) were treated with diode laser photocoagulation and IVB
All of the study participants (100%) had normal pupil shape and responded to light. No posterior synechiae or iris abnormality was noted. The lenses of all eyes were clear.
Refraction and biometry of optical components
The refractive status and optical component measurement means in the ROP and control groups are presented in Table 1. The children with a history of ROP showed poorer best-corrected visual acuity (P<0.001). The mean spherical powers of the ROP and control groups were −4.6±5.3 and −0.4±2.4 diopters (P=0.01), respectively. The mean cylindrical powers of the ROP and control groups were −2.6±1.4 and −1.3±0.8 diopters (P<0.001), respectively. A significantly greater spherical equivalent (refractive errors) was observed among the children with a history of ROP (P<0.001).
Table 1. Refractive Errors, Optical Components, and Macular Ganglion Cell Complex Parameters of Preterm Children with ROP and Age-matched Full-term Children.
| Variable | ROP group | Control group | P-valuea |
|---|---|---|---|
| Spherical power (D) | −4.6±5.3 | −0.4±2.4 | 0.01 |
| Cylindrical power (D) | −2.6±1.4 | −1.3±0.8 | <0.001 |
| Spherical equivalent (D) | −5.9±5.6 | −1.4±2.2 | <0.001 |
| Keratometry | |||
| K1 horizontal | 43.4±1.3 | 43.0±1.1 | 0.30 |
| K2 vertical | 45.5±1.6 | 44.6±1.1 | 0.04 |
| K1-K2 average | 44.5±1.3 | 43.8±1.0 | 0.12 |
| Uncorrected VA (logMAR) | 1.0±0.8 | 0.5±0.4 | 0.01 |
| Corrected VA (logMAR) | 0.1±0.1 | 0±0 | <0.001 |
| Axial length (mm) | 23.8±1.6 | 23.6±0.9 | 0.58 |
| Anterior chamber depth (mm) | 3.3±0.4 | 3.5±0.3 | 0.01 |
| Lens thickness (mm) | 4.0±0.2 | 3.5±0.2 | <0.001 |
| Vitreous depth (mm) | 16.7±1.6 | 16.4±1.0 | 0.71 |
| Intraocular pressure (mm Hg) | 13.8±2.2 | 13.8±2.7 | 0.97 |
| CCT (μm) | 553.1±38.6 | 542.8±32.9 | 0.29 |
| Average mGCC (μm) | 101.0±9.9 | 95.0±8.2 | <0.001 |
| Superior mGCC (μm) | 100.9±10.2 | 94.6±8.4 | <0.001 |
| Inferior mGCC (μm) | 101.0±10.0 | 95.4±8.3 | 0.01 |
| mGCC superior-inferior difference (μm) | −0.1±4.5 | −0.8±3.4 | 0.20 |
| FLV (%) | 1.4±2.0 | 0.6±0.8 | 0.05 |
| GLV (%) | 4.7±5.9 | 7.4±6.1 | 0.02 |
Abbreviations: CCT, central corneal thickness; D, diopters; FLV, focal loss volume; GLV, global loss volume; K1, Keratometry 1; K2, Keratometry 2; logMAR, logarithm of the minimum angle of resolution; mGCC, macular ganglion cell complex; VA, visual acuity.
Mann–Whitney U test.
Despite the similarity in average AL between the ROP group and the age-matched healthy controls (P=0.58), the children with a history of ROP had significantly shallower ACDs and thicker LTs (P=0.01 and<0.001, respectively). No difference was found in the vitreous depth between the two groups (P=0.71). Neither baseline intraocular pressure nor central corneal thickness differed between the two groups (P=0.97 and. 29, respectively).
Distribution of peripapillary RNFL thickness
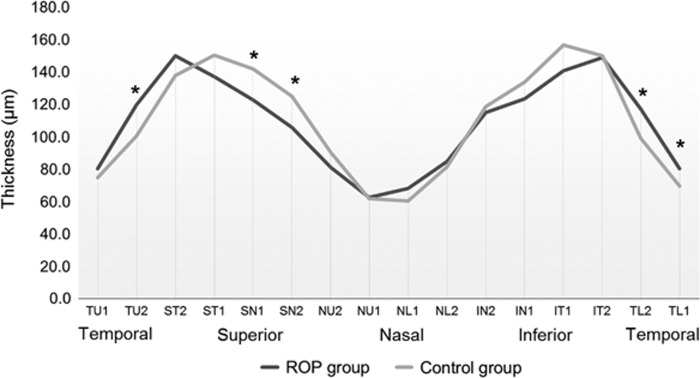
Figure 1 depicts the mean distribution of the global spatial profile of peripapillary RNFL thickness in the ROP and control groups. The peaks near the superior and inferior sectors showed a temporal deviation in the ROP group, which means that the superotemporal and inferotemporal sectors were thicker, and the superonasal and inferonasal sectors were thinner. The average RNFL thickness of children with ROP did not significantly differ from that of full-term children (108.4±16.0 μm and 109.5±12.1 μm, respectively, P=0.91).
Figure 1.
Peripapillary average RNFL thickness profiles (TSNIT graphs) of all study participants. The asterisks denote the sectors with significantly different RNFL thicknesses. Overall, the ROP group showed thicker RNFLs in the temporal quadrant and thinner RNFLs in the nasal quadrant (from left to right: P=0.01, 0.04, 0.01, 0.02, and 0.04, respectively). TU, temporal upper; ST, superior temporal; SN, superior nasal; NU, nasal upper; NL, nasal lower; IN, inferior nasal; IT, inferior temporal; TL, temporal lower.
mGCC metrics
The mGCC results are summarized in Table 1. The average, superior, and inferior mGCC thicknesses were all thicker in children with a history of ROP than healthy children (P<0.001,<0.001 and 0.01, respectively), but the mGCC difference between the superior and inferior areas was similar across these two groups (P=0.20). The eyes of children with a history of ROP had a lower percentage of global loss (mGCC-GLV) but a higher percentage of focal difference (mGCC-FLV) than those in healthy full-term children (P=0.02 and 0.05, respectively).
Correlation between refraction and biometry measurements and RNFL and mGCC measurements
The spherical powers, cylindrical powers, and spherical equivalent in all patients were positively correlated with RNFL thickness (Spearman’s correlation=0.32, 0.32, and 0.38, respectively; P=0.01, 0.01, and 0.001, respectively). The AL in all patients was negatively correlated with RNFL thickness (Spearman’s correlation=−0.35; P=0.01). The ACD in all patients was not correlated with RNFL thickness (Spearman’s correlation=0.24; P=0.09). In contrast, there was no significant correlation between mGCC thickness and spherical powers, cylindrical powers, spherical equivalent, AL, or ACD among all patients (Spearman’s correlation=0.09, 0.16, 0.04, −0.22, and −0.08, respectively; P=0.44, 0.17, 0.71, 0.10, and 0.58, respectively). The interaction between group status and refraction/biometry of optic components was insignificant, and subgroup analysis for either the ROP group or control group revealed similar correlations between refraction/biometry of optic components and RNFL/mGCC measurements (data not shown).
ONH metrics
Several ONH parameters were analyzed, including disc area, cup-to-disc ratio, rim or cup area, and rim or cup volume. None of these values significantly differed between the ROP and control groups (all P>0.05).
Discussion
Premature birth might disrupt normal ocular growth in different ways in both the posterior and anterior segment of the eye. Our results showed that preterm children with a history of ROP have poorer best-corrected visual acuity, shallower ACD, greater LT, changes in peripapillary RNFL distribution, and greater mGCC thickness with increased mGCC-FLV compared with full-term children. In contrast with the previous literature concerning the characteristics of RNFL or the microstructures of the inner retina in preterm children,8, 13 our study simultaneously combined detailed measurements of the ocular biometry of optical components, ONH, and retinal structures. We found that although the pattern of temporal deviation of RNFL peaks in children with a history of ROP was similar to the temporal shift of the RNFL peaks in patients with axial myopia, the mechanism was different. The greater inner retinal thickness with uneven distribution also suggests immature macula development in children with a history of ROP, highlighting the importance of vision and glaucoma surveillance during growth.
Myopia following preterm birth has many unique characteristics compared with myopia following full-term birth. School-age myopia is usually associated with long ALs. In contrast, myopia in preterm patients is usually associated with normal or short ALs.2, 19 Evidence also exists of arrested development in the anterior segment among premature patients, including microcornea,20 steep corneal curvature,2 shallow ACD,2, 19 and thickened lens.2, 19 These findings indicate that the development of myopia in premature patients is primarily influenced by the arrested development of the ocular anterior segment. The abnormal development and crowdedness of anterior segment structures results in a refractive myopia in preterm children, and not the axial myopia observed in healthy full-term children.
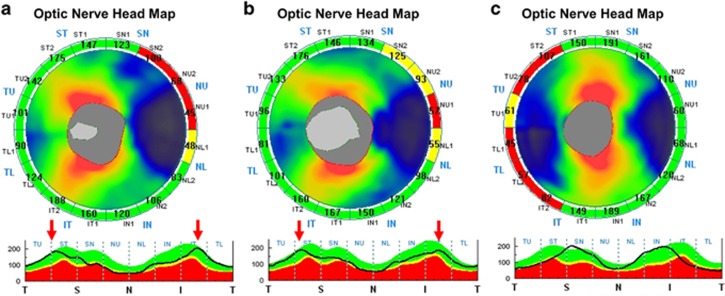
Myopic eyes with long ALs usually have thinner global RNFLs and show temporal shifting of the superotemporal and inferotemporal RNFL bundles.21 According to Wakitani et al,22 one possible reason for this difference is the increase in the diameter of the OCT scan circle projected onto the retina with increasing AL. Another explanation might be that a stretched, thinner peripapillary RNFL is present in longer eyes.23 Our study demonstrated that ROP eyes have significantly thinner RNFLs in the superonasal quadrants and thicker RNFLs in the temporal quadrants compared with control eyes (Figure 1). These results were consistent with previous reports.8, 13 Because the temporal quadrant is composed of papillomacular bundle axons, Pueyo et al13 hypothesized that a thicker temporal RNFL in children with a history of ROP indicates ganglion cell reorganization to preserve macular function. In addition, the optic disc parameters including disc area, cup-to-disc ratio, and rim area did not differ between preterm children with a history of ROP and healthy full-term children, indicating the similarity of the ganglion cell axon orientation in the peripapillary area. Therefore, the thicker temporal RNFL in preterm children should not be regarded as the temporal shifting of peak RNFL thickness associated with axial myopia. Figure 2 presents the representative peripapillary RNFL thickness profiles (TSNIT graphs) from an ROP child with myopia, an ROP child without myopia, and a full-term control child in our study.
Figure 2.
Representative peripapillary RNFL thickness profiles (TSNIT graphs) from (a) a preterm ROP child with myopia, (b) a preterm ROP child without myopia, and (c) a full-term control child. (a) This 11-year-old girl was born at 25 weeks GA and 716 g BW. Her refractive error was −4.0/−1.0 × 178, and her AL was 23.89 mm. (b) This 9-year-old girl was born at 27 weeks GA and 1070 g BW. Her refractive error was 0/−0.25 × 165, and her AL was 23.57 mm. (c) This 12-year-old girl was born at 40 weeks GA and 3100 g BW. Her refractive error was −0.25/−0.5 × 150, and her AL was 23.92 mm. The thickened RNFL over the temporal sector is typically observed in preterm children (arrows). Nevertheless, the thickening was not explained by temporal shifting of the peak RNFL thickness associated with axial myopia, as in full-term children.
Regardless of myopia, preterm children also have a thinner peripapillary RNFL than their full-term counterparts,7, 8, 24, 25 and the RNFL thickness in preterm children was correlated with the stage of ROP.24 The disruption of the autoregulation of ocular blood flow and the rise in carbon dioxide tension in preterm infants might interfere with the normal maturation of the optic disc.26, 27 Therefore, recent studies have suggested that preterm birth is associated with subclinical optic nerve hypoplasia.8, 24 By using handheld SD OCT, many studies have described the development of the optic nerve head and peripapillary area in preterm and full-term infants.25, 28, 29 Tong et al found that preterm infants had larger vertical cup diameters and vertical cup-to-disc ratios than term infants at 37–42 weeks postmenstrual age.28 In addition, Patel et al studied a group of 352 full-term children aged between 1 day and 13 years.29 They found the mean disc and cup diameters increased by 30% and 40%, respectively, between birth and 13 years of age. The peripapillary temporal RNFL demonstrated an initial decrease of nearly 35% between birth and 18 months of age, followed by a slow increase up to 12 years of age. These studies suggested preterm birth negatively affects optic nerve or RNFL development at a very early age rather than a later development change.25 Finally, the majority of prior studies7 and the current study noted that children with a history of ROP had received laser treatment. Laser photocoagulation is well documented to affect the axons of ganglion cells and induce the progressive thinning of the nerve fiber layer over the long term.30
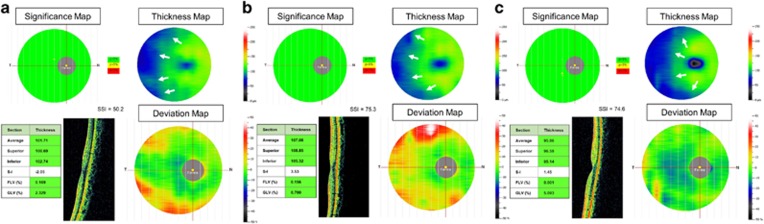
Human foveal development is a complex process that includes the centrifugal displacement of the inner retinal cells and the centripetal displacement of photoreceptors.31 In contrast with the adult fovea, the following signs of immaturity are observed after premature birth: shallow foveal pit, the persistence of inner retinal layers, and a thin photoreceptor layer.32 Dubis et al found that the persistence of inner retinal layers was seen in 93% premature born neonates and subclinical cystoid macular oedema was noted in 54% premature born neonates, and these abnormal changes were found in patients independent of ROP stage.33 Vinekar et al also reported cystoid macular oedema in 23 of 79 eyes (29.1%) with stage 2 ROP despite clinically normal foveae and found no cystoid macular oedema in patients with stage 0 or 1.34 Some reasons have been proposed to explain the abnormal macular changes:33, 34 (1) a response to higher concentrations of vascular endothelial growth factor, (2) mechanical traction exerted on the macula, or (3) concurrent systemic diseases, such as hypoxia or sepsis. The development of the macula begins during gestational weeks 24 through 26 and is not completed until ~4 years of age.19 Nevertheless, our study enrolled a group of 10-year-old (on average) preterm children and found that their mGCCs, a surrogate parameter for the inner retinal layer, remained thicker than those of age-matched full-term children (P=0.0019). Figure 3 shows representative mGCC maps of our ROP and healthy full-term samples. The foveal depression was shallower in children with a history of ROP. In the control population, a trend was observed such that the mGCC of the nasal side was thicker than that of the temporal side; furthermore, the mGCC became gradually thinner towards the periphery.35 In contrast, this trend was not present in children with a history of ROP. Two explanations are proposed to explain our findings. First, despite the foveal development that is reported to continue after premature birth,32 prematurity disrupts the peripheral migration and maturation of inner retinal cells. Alternatively, laser treatment that seeks to destroy avascular retina might contribute to these changes (as reported by our previous study19) given that thicker perifovea and parafovea were found in treated children with a history of ROP than in children whose ROP spontaneously regressed. The normative RTVue database consists of 861 healthy eyes of participants of various ethnicities, with ages ranging between 19 and 82 years.36 Thus, interpretations of the significance map in young children should be made with caution. Available studies concerning the inner retinal structure changes in preterm children with previous ROP are summarized in Table 2.
Figure 3.
Representative mGCC maps and macular cross-section scanning from the same patients shown in Figure 2. (a) A preterm ROP child. The average mGCC thickness was 101 μm. The mGCC-FLV was 0.169%. The SSI was 50.2. (b) A preterm ROP child. The average mGCC thickness was 107 μm. The mGCC-FLV was 0.156%. The SSI was 75.3. (c) A full-term control child. The average mGCC thickness was 95 μm. The mGCC-FLV was 0.001%. The SSI was 74.6. The foveal depression was shallower in children with a history of ROP. The mGCC showed more thickening on the thickness map and more uneven distribution on the distribution map in children with a history of ROP. In the normal population, a trend shows that the mGCC on the nasal side was thicker than that on the temporal side, and the mGCC grew gradually thinner towards the periphery.35 By contrast, this transition was less obvious in children with a history of ROP (white arrows).
Table 2. Literature Review of Inner Retinal Measurement via SD-OCT in Preterm Children with ROP.
| Country | Age at Assessment (yrs) | No. of Eyes | GA | BW | ROP Stage (0/1/2/3) | Treatment Modality | SE of Refractive Error (D) | Normal Control | Inner Retinal Layer Measurement | |
|---|---|---|---|---|---|---|---|---|---|---|
| Yanni et al14 | USA | 10.1±3.0 | 24a | 25.6±2.0 | 739±184 | 4/5/5/10 | Laserb | −0.6±4.7 | Yes | Thicker GCL-IPL in prematurity |
| Pueyo et al13 | Spain | 8.6±2.7 | 10 | 27.8±1.2 | 1,070±284 | N/A | Laser | −1.6±5.3 | Yes | Thinner GCL-IPL in prematurity |
| Current study, 2017 | Taiwan | 9.6±2.5 | 41 | 26.2±2.1 | 919±260 | 0/0/6/35 | Laser (±IVB) | −5.9±5.6 | Yes | Thicker mGCC in prematurity |
Abbreviations: D, diopters; GCL-IPL, ganglion cell layer and inner plexiform layer; IVB, intravitreal injection of Bevacizumab; mGCC, macular ganglion cell complex; ROP, retinopathy of prematurity; SD-OCT, spectral domain optical coherence tomography; SE, spherical equivalent; yrs, years.
Including no ROP (n=4) and regressed ROP (n=20).
Only 15 eyes received laser treatment.
Another notable finding in our study was that the mGCC-FLV was significantly increased in children with a history of ROP compared with full-term children (Figure 3). Increased mGCC-FLV is a powerful predictor of conversion from glaucoma suspect or preperimetric glaucoma to perimetric glaucoma.12 Nevertheless, this estimation is usually made under conditions of reduced overall mGCC thickness due to ganglion cell axon injury in patients with glaucoma. Our study showed that eyes with a history of ROP had a significantly thicker mGCCs and less general loss of mGCC than healthy eyes. The increased mGCC-FLV in ROP eyes might represent a heterogeneous distribution and a disrupted maturation process of the inner retina and not a true finding of future glaucomatous progression. Long-term surveillance of glaucoma development is needed to evaluate the predictive value of this variable.
The strength of the current study is that we measured the biometry of optical components, optic disc morphology, and mGCC/RNFL in children with ROP and age-matched full-term controls, thereby providing strong evidence to clarify the relationships among inner retinal structural alterations, refractive errors, and ocular structural changes. Our study also has limitation. First, patients with different stages of ROP and different treatment modalities might have different microstructural changes in the retina, and the participants in the present study were mostly zone 2, stage 3 patients (85.4%) who underwent laser photocoagulation (90.2%). Therefore, subgroup analyses of different stages of children with ROP or different treatment effects (laser vs IVB) were not possible. Previous studies reported that IVB was associated with less myopia than either laser photocoagulation or combined laser and bevacizumab treatment for type 1 ROP,37, 38 and treatment-requiring ROP eyes were susceptible to more severe myopia with age compared with eyes without ROP or those with spontaneously regressed ROP.39 The intraretinal layer thicknesses in high myopic eyes varied significantly from emmetropic eyes.40 In addition, laser treatment might alter the thickness of the inner layer of the retina,41 and such changes should be considered in ROP patients after laser photocoagulation treatment. Finally, we lacked medical records from the neonatal period of our ROP cohort because the study was conducted at a referred tertiary centre. Hypoxia and systemic inflammation might influence the activation of microglial cells and neuronal apoptosis,13, 42 thereby inducing damage to the ganglion cells and changes to the inner retinal layers. These neonatal conditions might lead to bias in our study.
In conclusion, school-aged children with a history of ROP showed a crowded anterior segment of the eyes and different patterns of RNFL and inner retinal structures compared with children born at term. These changes in ocular growth and microstructures in the retina might influence visual development and the occurrence of glaucoma in the future. Children with a history of ROP should receive careful examinations and regular long-term follow-up assessments, even after the disease has regressed.
Acknowledgments
Chang Gung Memorial Hospital Research Grants CMRPG3F0191–3 and CMRPG3E0521–2 and the National Science Council Research Grant (MOST 104-2314-B-182A-100-MY2) supported this study. These funding organizations played no role in the design or conduct of this research.
Footnotes
The authors declare no conflict of interest.
References
- Cook A, White S, Batterbury M, Clark D. Ocular growth and refractive error development in premature infants without retinopathy of prematurity. Invest Ophthalmol Vis Sci 2003; 44(3): 953–960. [DOI] [PubMed] [Google Scholar]
- Chen TC, Tsai TH, Shih YF, Yeh PT, Yang CH, Hu FC et al. Long-term evaluation of refractive status and optical components in eyes of children born prematurely. Invest Ophthalmol Vis Sci 2010; 51(12): 6140–6148. [DOI] [PubMed] [Google Scholar]
- Cook A, White S, Batterbury M, Clark D. Ocular growth and refractive error development in premature infants with or without retinopathy of prematurity. Invest Ophthamol Vis Sci 2008; 49(12): 5199–5207. [DOI] [PubMed] [Google Scholar]
- Quinn GE, Dobson V, Davitt BV, Hardy RJ, Tung B, Pedroza C et al. Progression of myopia and high myopia in the early treatment for retinopathy of prematurity study–Findings to 3 years of age. Ophthalmology 2008; 115(6): 1058–1064. [DOI] [PubMed] [Google Scholar]
- Laws D, Shaw DE, Robinson J, Jones HS, Ng YK, Fielder AR. Retinopathy of prematurity – a prospective-study – review at 6 months. Eye 1992; 6: 477–483. [DOI] [PubMed] [Google Scholar]
- Quinn GE, Dobson V, Kivlin J, Kaufman LM, Repka MX, Reynolds JD et al. Prevalence of myopia between 3 months and 5 1/2 years in preterm infants with and without retinopathy of prematurity. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology 1998; 105(7): 1292–1300. [DOI] [PubMed] [Google Scholar]
- Akerblom H, Holmstrom G, Eriksson U, Larsson E. Retinal nerve fibre layer thickness in school-aged prematurely-born children compared to children born at term. Br J Ophthalmol 2012; 96(7): 956–960. [DOI] [PubMed] [Google Scholar]
- Wang J, Spencer R, Leffler JN, Birch EE. Characteristics of peripapillary retinal nerve fiber layer in preterm children. Am J Ophthalmol 2012; 153(5): 850–855, e851. [DOI] [PubMed] [Google Scholar]
- Hartnett ME, Gilbert MM, Hirose T, Richardson TM, Katsumi O. Glaucoma as a cause of poor vision in severe retinopathy of prematurity. Graef Arch Clin Exp 1993; 231(8): 433–438. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Hourihan F, Sandbach J, Wang JJ. The relationship between glaucoma and myopia – the blue mountains eye study. Ophthalmology 1999; 106(10): 2010–2015. [DOI] [PubMed] [Google Scholar]
- Hsu CH, Chen RI, Lin SC. Myopia and glaucoma: sorting out the difference. Curr Opin Ophthalmol 2015; 26(2): 90–95. [DOI] [PubMed] [Google Scholar]
- Zhang X, Loewen N, Tan O, Greenfield DS, Schuman JS, Varma R et al. Predicting development of glaucomatous visual field conversion using baseline fourier-domain optical coherence tomography. Am J Ophthalmol 2016; 163: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueyo V, Gonzalez I, Altemir I, Perez T, Gomez G, Prieto E et al. Microstructural changes in the retina related to prematurity. Am J Ophthalmol 2015; 159(4): 797–802. [DOI] [PubMed] [Google Scholar]
- Yanni SE, Wang J, Chan M, Carroll J, Farsiu S, Leffler JN et al. Foveal avascular zone and foveal pit formation after preterm birth. Br J Ophthalmol 2012; 96(7): 961–966. [DOI] [PubMed] [Google Scholar]
- International Committee for the Classification of Retinopathy of P. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol 2005; 123(7): 991–999. [DOI] [PubMed] [Google Scholar]
- Elsas FJ, Collins M, Jones J, Kimble JA, Kline L, Witherspoon D et al. Multicenter trial of cryotherapy for retinopathy of prematurity – ophthalmological outcomes at 10 years. Arch Ophthalmol 2001; 119(8): 1110–1118. [DOI] [PubMed] [Google Scholar]
- Wu WC, Kuo HK, Yeh PT, Yang CM, Lai CC, Chen SN. An updated study of the use of bevacizumab in the treatment of patients with prethreshold retinopathy of prematurity in Taiwan. Am J Ophthalmol 2013; 155(1): 150–158. [DOI] [PubMed] [Google Scholar]
- Tan O, Chopra V, Lu AT, Schuman JS, Ishikawa H, Wollstein G et al. Detection of macular ganglion cell loss in glaucoma by Fourier-domain optical coherence tomography. Ophthalmology 2009; 116(12): 2305–2314, e2301-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WC, Lin RI, Shih CP, Wang NK, Chen YP, Chao AN et al. Visual acuity, optical components, and macular abnormalities in patients with a history of retinopathy of prematurity. Ophthalmology 2012; 119(9): 1907–1916. [DOI] [PubMed] [Google Scholar]
- Kelly SP, Fielder AR. Microcornea associated with retinopathy of prematurity. Br J Ophthalmol 1987; 71(3): 201–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Lee EJ, Kim TW. Peripapillary retinal nerve fibre layer thickness profile in subjects with myopia measured using the Stratus optical coherence tomography. Br J Ophthalmol 2010; 94(1): 115–120. [DOI] [PubMed] [Google Scholar]
- Wakitani Y, Sasoh M, Sugimoto M, Ito Y, Ido M, Uji Y. Macular thickness measurements in healthy subjects with different axial lengths using optical coherence tomography. Retina J Ret Vit Dis 2003; 23(2): 177–182. [DOI] [PubMed] [Google Scholar]
- Bendschneider D, Tornow RP, Horn FK, Laemmer R, Roessler CW, Juenemann AG et al. Retinal nerve fiber layer thickness in normals measured by spectral domain OCT. J Glaucoma 2010; 19(7): 475–482. [DOI] [PubMed] [Google Scholar]
- Park KA, Oh SY. Retinal nerve fiber layer thickness in prematurity is correlated with stage of retinopathy of prematurity. Eye 2015; 29(12): 1594–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman AL, Sevilla MB, Mangalesh S, Gustafson KE, Edwards L, Cotten CM et al. Thinner retinal nerve fiber layer in very preterm versus term infants and relationship to brain anatomy and neurodevelopment. Am J Ophthalmol 2015; 160(6): 1296–1308 e1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan DW. Failure of autoregulation and intraventricular haemorrhage in preterm infants. Lancet 1980; 1(8174): 896–898. [DOI] [PubMed] [Google Scholar]
- Holmes JM, Zhang SC, Leske DA, Lanier WL. Carbon dioxide-induced retinopathy in the neonatal rat. Curr Eye Res 1998; 17(6): 608–616. [PubMed] [Google Scholar]
- Tong AY, El-Dairi M, Maldonado RS, Rothman AL, Yuan EL, Stinnett SS et al. Evaluation of optic nerve development in preterm and term infants using handheld spectral-domain optical coherence tomography. Ophthalmology 2014; 121(9): 1818–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Purohit R, Lee H, Sheth V, Maconachie G, Papageorgiou E et al. Optic nerve head development in healthy infants and children using handheld spectral-domain optical coherence tomography. Ophthalmology 2016; 123(10): 2147–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muqit MMK, Wakely L, Stanga PE, Henson DB, Ghanchi FD. Effects of conventional argon panretinal laser photocoagulation on retinal nerve fibre layer and driving visual fields in diabetic retinopathy. Eye 2010; 24(7): 1136–1142. [DOI] [PubMed] [Google Scholar]
- Hendrickson AE, Yuodelis C. The morphological development of the human fovea. Ophthalmology 1984; 91(6): 603–612. [DOI] [PubMed] [Google Scholar]
- Maldonado RS, O'Connell RV, Sarin N, Freedman SF, Wallace DK, Cotten CM et al. Dynamics of human foveal development after premature birth. Ophthalmology 2011; 118(12): 2315–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubis AM, Subramaniam CD, Godara P, Carroll J, Costakos DM. Subclinical macular findings in infants screened for retinopathy of prematurity with spectral-domain optical coherence tomography. Ophthalmology 2013; 120(8): 1665–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinekar A, Avadhani K, Sivakumar M, Mahendradas P, Kurian M. Braganza S et al. Understanding clinically undetected macular changes in early retinopathy of prematurity on spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci 2011; 52(8): 5183–5188. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Akagi T, Hangai M, Kimura Y, Suda K, Hasegawa T et al. Effect of axial length on macular ganglion cell complex thickness and on early glaucoma diagnosis by spectral-domain optical coherence tomography. J Glaucoma 2016; 25(5): e481–e490. [DOI] [PubMed] [Google Scholar]
- Garas A, Vargha P, Hollo G. Diagnostic accuracy of nerve fibre layer, macular thickness and optic disc measurements made with the RTVue-100 optical coherence tomograph to detect glaucoma. Eye 2011; 25(1): 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang CK, Hubbard GB, Hutchinson AK, Lambert SR. Outcomes after intravitreal bevacizumab versus laser photocoagulation for retinopathy of prematurity: a 5-year retrospective analysis. Ophthalmology 2015; 122(5): 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JM, Shin DH, Kim SJ, Ham DI, Kang SW, Chang YS et al. Outcomes after laser versus combined laser and bevacizumab treatment for type 1 retinopathy of prematurity in zone I. Retina 2017; 37(1): 88–96. [DOI] [PubMed] [Google Scholar]
- Kuo HK, Sun IT, Chung MY, Chen YH. Refractive error in patients with retinopathy of prematurity after laser photocoagulation or bevacizumab monotherapy. Ophthalmologica 2015; 234(4): 211–217. [DOI] [PubMed] [Google Scholar]
- Liu X, Shen M, Yuan Y, Huang S, Zhu D, Ma Q et al. Macular thickness profiles of intraretinal layers in myopia evaluated by ultrahigh-resolution optical coherence tomography. Am J Ophthalmol 2015; 160(1): 53–61 e52. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kang TS, Kwak BS, Jo YJ, Kim JY. Long-term effect of panretinal photocoagulation on spectral domain optical coherence tomography measurements in diabetic retinopathy. Curr Eye Res 2017; 1–5. [DOI] [PubMed]
- Tremblay S, Miloudi K, Chaychi S, Favret S, Binet F, Polosa A et al. Systemic inflammation perturbs developmental retinal angiogenesis and neuroretinal function. Invest Ophthalmol Vis Sci 2013; 54(13): 8125–8139. [DOI] [PubMed] [Google Scholar]