Abstract
Prior research has shown that infants learn statistical regularities in action sequences better than they learn non-action event sequences. This is consistent with current theories claiming that the same mechanism guides action observation and action execution. The current eye-tracking study tested the prediction, based on these theories, that infants’ ability to learn statistical regularities in action sequences is modulated by their own motor abilities. Eight- to eleven-month-old infants observed an action sequence containing two deterministic action pairs (i.e., action A always followed by action B) embedded within an otherwise random sequence. One pair was performed with a whole-hand grasp. The second pair was performed with a pincer grasp, a fine motor skill that emerges around 9 months of age. Infants were then categorized into groups according to which grasp was dominant in their motor repertoire. Predictive looks to correct upcoming actions during the deterministic pairs were analyzed to measure whether infants learned and anticipated the sequence regularities. Findings indicate that infants learned the statistical regularities: across motor groups, they made more correct than incorrect predictive fixations to upcoming actions. Overall, learning was not significantly modulated by their dominant grasping abilities. However, infants with a dominant pincer grasp showed an earlier increase in correct predictions for the pincer grasp pair and not the whole-hand grasp. Likewise, infants with a dominant whole-hand grasp showed an early increase in correct predictions for the pair performed with a whole-hand grasp, and not the pincer grasp. Together, these findings suggest that infants’ ability to learn action sequences is facilitated when the observed action matches their own action repertoire. However, findings cannot be explained entirely by motor accounts, as infants also learned the actions less congruent with their own abilities. Findings are discussed in terms of the interplay between the motor system and additional non-motor resources during the acquisition of new motor skills in infancy.
Keywords: statistical learning, motor development, eye-tracking, infant cognition, action prediction
Introduction
Within the first months of life, infants begin to demonstrate remarkable abilities to form expectations about the actions they observe others perform. Infants readily anticipate the outcomes of observed actions and the trajectories of an actor’s movements as they unfold (Falck-Ytter et al., 2006; Ambrosini et al., 2013). For instance, they can predict that an adult will bring a cup to her mouth upon grasping it, long before they themselves can grasp mugs and drink from them (Hunnius and Bekkering, 2010). The mechanisms that support this ability have recently been a focus of intense study (for a review, see Hunnius and Bekkering, 2014). This body of work has centered around understanding how infants learn to anticipate observed actions based on observational experiences and their own developing action experiences.
Observational experiences create opportunities for infants to learn the statistical regularities in their environment. A recent surge of empirical work has provided convincing evidence that infants can detect multiple types of statistical regularities in different sensory domains from early in life (Aslin et al., 1998; Fiser and Aslin, 2001; Kirkham et al., 2002; Teinonen et al., 2009; Slone and Johnson, 2015). For instance, at 8 months of age, infants can segment novel auditory sequences into word-like units based on the transitional probabilities between syllables (Saffran et al., 1996). They can also form visual associations between objects and spatial locations based on their recurring co-occurrence (Kirkham et al., 2002) and can anticipate where an object will appear next based on those learned associations (Marcus et al., 1999).
Recent evidence has shown that statistical learning abilities extend to the action domain as well. Human action contains inherent sequential structure within a seemingly complex stream of motion (Baldwin and Baird, 2001; Zacks, 2004). From early in life, infants are sensitive to regularities in the actions they observe. For instance, 8-month-old infants segment observed action streams into separate units similarly to how they segment auditory sequences into words (Stahl et al., 2014). Within the first year of life, infants can learn to associate actions and the effects they produce, both for actions that they observe (Paulus et al., 2012) and those that they perform themselves (Verschoor et al., 2013). These findings add to a growing body of work which demonstrates that both observational and motor experiences contribute to infants’ emerging skills for processing and performing goal-directed actions.
Recently, researchers have begun to investigate whether, beyond segmentation, infants can also predict upcoming actions based on statistical learning. In a recent experiment, 18-month-old toddlers observed continuous action sequences with either deterministic or random transitional probabilities between actions. In a control condition, another group of toddlers observed the same sequence that featured self-propelled events rather than a human actor (Monroy et al., 2017b). Proportions of correct predictive looks preceding the deterministic actions increased over trials for the toddlers who observed the human actor, but not for those who observed the non-action visual events. These findings provided evidence that observing actions benefits action prediction above and beyond observing non-action perceptual sequences. One possible explanation is that prior motor experiences with the observed actions contributed to the enhanced learning, as these actions were all within the motor capabilities of toddlers.
Support for this hypothesis comes from a growing body of evidence illustrating that action prediction is tightly coupled to infants’ motor proficiency (Gredebäck and Kochukhova, 2010; Kanakogi and Itakura, 2011; Cannon et al., 2012; Gerson et al., 2015). For instance, in one recent experiment, infant and adult participants watched videos of other infants either crawling or walking across a room while their eye movements were recorded. Infants for whom crawling was their dominant form of locomotion predicted crawling more accurately than walking. In contrast, infants for whom walking was the dominant form of locomotion (and adults) were equally accurate at predicting both actions (Stapel et al., 2016). Training studies—in which infants are given novel experience with actions they have never yet performed—immediately alter how infants subsequently perceive those actions (Sommerville et al., 2005; Gerson and Woodward, 2014). Together, these findings suggest that infants process actions more efficiently once the actions are more strongly established in their own motor repertoire.
Current theories propose that activation of the motor system during action observation is the most likely mechanism underlying efficient action prediction (Flanagan and Johansson, 2003; Falck-Ytter et al., 2006). In line with this claim, findings from neuroimaging studies reveal that motor regions in the brain are activated when infants observe others’ actions. Activity in these regions is greater in response to actions with which infants have more motoric experience, and are therefore more dominant in their motor repertoire, relative to actions with which they have less or no motoric experience (Southgate et al., 2010; Gerson et al., 2015). In adults, motor activation is causally linked to predictive eye movements: introducing a competing motor task inhibits the ability to anticipate observed actions. Likewise, disrupting activity in the motor cortex via transcranial magnetic stimulation (TMS) impairs predictive eye movements, further suggesting that the motor system may even be necessary to successfully predict ongoing actions (Elsner et al., 2013).
Together, the current research shows that both observational statistical learning and action experience are central to infants’ action understanding (Hunnius and Bekkering, 2014). To date, a few studies have attempted to compare the relative contributions of self-produced and observed actions on infants’ action understanding (Gerson and Woodward, 2014) but none have examined how these two forms of experience might interact. We aimed to address this gap by asking whether newly acquired motor experience with grasping actions modulated infants’ abilities to learn statistical regularities between those same actions when they were viewed in continuous sequences. In other words, if infants recruit motor representations when they observe actions they can perform, does this help them to more easily detect the sequential regularities between those actions during observation?
To tackle this question, we exploited infants’ natural acquisition of a pincer grasp, a fine motor skill that emerges in the second half of the first year of life. Results from a prior study in our own lab indicated that the pincer grasp emerges between 8 and 11 months of age (Meyer et al., 2016). We thus expected 8- to 11-month-old infants to vary in the degree to which a whole hand (i.e., palmar) grasp and a pincer grasp were more dominant in their motor repertoire. In an eye-tracking experiment, infants were shown a video of an action sequence comprised of six possible object-directed actions. Within this sequence, there were two deterministic pairs in which one action always followed a second action with 100% probability and was followed by an effect. All other actions occurred in a random order. If infants learned the statistical structure of the action pairs, they should, in principle, make visual anticipations to the locations of the second action upon observing the first action (cf. Monroy et al., 2017b). Both actions of one pair were performed with a pincer grasp, whereas both actions of the second pair were performed with a whole-hand grasp. Following the video, a grasp test was conducted to assess each infant’s grasping proficiency.
We expected that, if infants learned the action pairs, they would make more visual anticipations toward the second action and/or its effect than to any other object during the first action of an action pair (i.e., a predictive time window). We also expected they would demonstrate an increase in correct visual anticipations to the second actions as the sequence progressed (Shafto et al., 2012; Monroy et al., 2017b). Finally, we hypothesized that infants would be better at learning the statistical regularities for the action pairs more dominant in their current motor repertoire. For example, we expected that those infants performing more whole-hand grasps, but not yet performing pincer grasp actions, would anticipate the second action of the whole hand pair more reliably than the pincer grasp pair. Infants for whom both actions are equally dominant in their motor repertoire should not show preferential learning for one pair over the other.
Materials and Methods
Participants
Forty-eight infants from 8 to 11 months of age were included in the final sample (Table 1). Infants were recruited from a database of interested families from the surrounding region who volunteered to participate. Seven additional infants were tested but excluded from the final sample due to calibration errors (n = 1) or failure to complete the observation phase due to excessive fussiness (n = 5). One infant made zero fixations on any of the trials of interest (i.e., the action pairs) and was also excluded from analyses. The study was approved by the ethical committee of behavioral science at the Faculty of Social Sciences in Nijmegen (Ethische Commissie Gedragswetenschappelijk Onderzoek; ECG2012-1301-006). All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Table 1.
Characteristics of the final sample.
| Sample characteristics |
Grasp test measures |
||||||
|---|---|---|---|---|---|---|---|
| Motor group | n | Mean age in months (SD) | Gender (f:m) | Mean prop. pincer grasps (SD) | Mean prop. transitional grasps (SD) | Mean prop. hand grasps (SD) | Mean span in months∗ (SD) |
| Pincer-dominant | 11 | 10.86 (0.63) | 6:5 | 0.65 (0.17) | 0.17 (0.11) | 0.18 (0.13) | 2.65 (1.58) |
| Transitional | 22 | 10.07 (0.94) | 6:16 | 0.21 (0.16) | 0.45 (0.30) | 0.35 (0.25) | 1.93 (1.10) |
| Hand-dominant | 15 | 9.94 (0.97) | 7:8 | 0.06 (0.08) | 0.13 (0.11) | 0.80 (0.10) | 1.65 (0.90) |
∗Mean span in months = number of months since infant first used a pincer grasp, per parent report (for descriptive purposes only; not used in analyses).
Stimuli
Video stimuli were created featuring a toy with multiple objects that could be manipulated in distinct ways (Figure 1A). An adult actor performed a continuous action sequence with the various objects on the toy. For each action, the actor’s hand entered the screen nearest to the object upon which she would act, performed one action with that object (3 s), and then left the screen. This was followed by a brief pause (1 s) before the next action began. Only the actor’s hand was visible during each action.
FIGURE 1.

(A) Example frame from the video stimuli, in which an adult performed a continuous sequence of actions with the six possible objects on the toy. (B) Following observation, infants’ grasping abilities were assessed using the pictured apparatus, which required them to extract the bead from the wooden board. (C) Schematic illustrating the statistical structure of the action sequence containing two deterministic pairs that caused a light effect: one pair was performed with a pincer grasp and the second with a whole-hand grasp. Numbers represent transitional probabilities between paired and random actions. Dotted lines underneath the first action of a pair depict the 4 second period preceding the target actions in which predictive gaze fixations were analyzed.
Videos were divided into four blocks, with the viewing angle of the toy stimulus alternating between blocks to ensure that spatial location did not become a predictable cue. Attention-getter clips (4 s) were played between blocks followed by a still frame (1 s) of the toy (with no hand visible) to help the infant reorient to the new perspective. The entire sequence lasted approximately 7 minutes. Engaging background music accompanied the video stimuli and was unrelated to the stimulus presentation.
Action Sequence
The action sequence was structured as follows: two deterministic pairs were embedded within an otherwise pseudorandomized order of six object-directed actions. One pair was performed with a pincer grasp (Pincer pair) and consisted of the actions ‘slide’ followed by ‘open’; the second pair was performed with a whole-hand grasp (Hand pair) and consisted of ‘bend’ followed by ‘push’ (Figure 1C). The second actions of the pairs were labeled the target actions, as these were the actions that infants could learn to predict as they observed the unfolding sequence. Both pairs caused an action-effect, which was a green or a pink colored light in the center of the toy that turned on at the midpoint of the target actions. The light’s two colors (green and pink) always corresponded to the same pairs within one sequence. This matching was randomly counterbalanced across infants: one group always saw the Pincer pair activate a green light and the Hand pair activate a pink light, and the second group saw the reverse.
No action or pair occurred more than three times consecutively, and all elements (pairs and random actions) occurred with equal frequency. Target actions also occurred elsewhere in the sequence as random actions, to ensure that the effect only occurred after the two-step action pair and could not be independently associated with the target actions. Action sequences consisted of 96 total actions with 12 trials of each pair (Pincer and Hand).
Grasp Test Device
Infants’ grasping proficiency was assessed using a wooden apparatus (Figure 1B). A small and a large bead (3 and 5 mm diameter) were attached to strings that were threaded through removable wooden panels which fit into the apparatus frame.
Procedure
The testing procedure consisted of an action observation phase followed by the grasp test. Infants were seated on a caretaker’s lap throughout both phases. During the action observation phase, eye movements were recorded continuously with a Tobii T120 eye-tracker (Tobii Technologies, Inc.) at 60 Hz. Gaze was calibrated using a standard nine-point calibration procedure until at least eight points were acquired or a maximum of three attempts. Immediately following calibration, infants were shown the video stimuli. Caretakers were requested to avert their gaze during calibration and to refrain from influencing their child during the observation phase.
After the sequence was completed (or until infants became too fussy to continue the observation task), caretakers and infants moved to a nearby table for the grasp test (adapted from the procedure of Meyer et al., 2016). The experimenter placed the test apparatus in front of the infant and performed a single demonstration of how to grasp the bead and pull it out. After returning the bead to its original position (Figure 1B), infants were given 1 minute to pull out each bead. Each time they removed the bead, the experimenter replaced it and the infant could try again. This phase was videotaped from a camera placed with full view of the infant for offline behavioral analysis. In addition, a parental questionnaire was administered prior to the testing session with questions about infants’ grasping history1.
Data Analysis
Eye-Tracking Data
Raw eye-tracking data was separated into discrete fixations using a custom software program (GSA; Philip van den Broek, Donders Institute) with a spatial filter of 30 pixels and a temporal filter of 100 ms. Regions of interest (ROIs) of equal size were defined around each object (i.e., action location) and around the action-effect (250 and 130 square pixels, respectively). Predictive time windows were defined as the 4 s from the first frame in which the hand appeared to perform the first action of a pair until the final frame just before the hand reappeared to perform the target (second) action (dashed bars; Figure 1C).
Calculation of Proportions of Predictive Fixations
Fixations to the target object and to the action-effect locations during predictive time windows were considered correct, whereas fixations to any other object were considered incorrect. Fixations to the location of ongoing actions were always excluded. We first calculated the proportions of correct (Eq. 1) and incorrect predictive fixations (Eq. 2) across all trials for each pair, divided the sum by the total fixations made to all ROIs. Total incorrect fixations were divided by four to yield the average number of fixations to an incorrect region; this measure has also been described elsewhere as an estimate of chance (Tummeltshammer and Kirkham, 2013). Proportions of correct and incorrect fixations were compared for each action pair, representing infants’ preference for anticipating a correct upcoming action and/or its effect relative to the other object locations. Proportions of correct fixations (Eq. 1) were also calculated per trial and analyzed using Generalized Estimating Equations (GEEs) to examine the emergence of predictive gaze over the course of the experiment. GEE analyses do not apply list-wise exclusion of cases and are thus advantageous for analyzing data with repeated measures that contain missing points, such as trials in which no anticipatory fixations occur (Zeger et al., 1988).
| (1) |
| (2) |
Equations 1 and 2: Calculations of the proportion measures. ‘All ROIs’ refers to the six objects and the action-effect.
Grasp Test
Infants’ ability to pull the beads out of the panels served as our measure of grasp proficiency (Figure 2). Video recordings of the grasp test phase were coded offline by a coder who was blind to the aims of the study. Each attempt to extract the bead from the device was coded as hand grasp, a transitional (i.e., inferior pincer) grasp, or a pincer grasp. Next, we calculated the proportion of times infants used each grasp type out of the total number of times he or she successfully extracted the beads, collapsed across small and large beads. Unsuccessful attempts were not coded.
FIGURE 2.
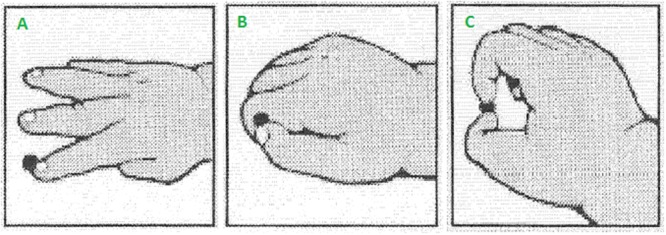
Illustrations depicting classification of grasping actions into whole hand (A), transitional (B), or pincer (C) grasps during the grasp test. Only (C) was considered a true pincer grasp as this action requires opposition of the thumb and forefinger. Image modified with permission from Erhardt (1994).
Almost all infants could extract the bead from the device, while demonstrating different levels of grasping competence to do so. We thus classified infants according to the type of grasp they used most frequently to extract the bead, reasoning that this would reflect the motor ability most dominant in their current repertoire. Rather than acquiring new motor skills in sudden steps, infants’ motor skills emerge in a gradual, graded way (see Ambrosini et al., 2013 for another non-binary scoring method). Each infant was classified as Pincer-dominant, Hand-dominant, or Transitional depending on which grasp they used most (Table 1). For instance, if the relative proportions of an infant’s grasping actions were 0.58, 0.25, and 0.17 (pincer, transitional, and hand, respectively) this infant would be classified as a Pincer-dominant infant. Infants whose relative proportions were evenly distributed across grasp types—such as 0.33, 0.33, and 0.34—were also classified as Transitional infants. To avoid confusion, infant groups (Pincer-dominant, Hand-dominant, and Transitional) are presented in italics and action pairs (Pincer and Hand) in non-italics.
Results
Age Effects
A one-way ANOVA with age as dependent variable and Motor Group as a factor indicated that mean age differed significantly among the three motor groups, F(2,47) = 3.89, p = 0.03 (see Table 1). Pairwise comparisons revealed that the Pincer-dominant infants were significantly older than the Hand-dominant groups (mean difference [MD] = 27.96 days, p = 0.01) and the Transitional group (MD = 24.00 days; p = 0.02). There were no differences between the Hand-dominant and the Transitional infants (MD = 3.96, p = 0.66).
Visual Attention
There were no differences between infant groups in overall looking time to all ROI throughout the entire video, or in the total number of fixations during predictive time windows (ps > 0.25). Thus, infants with different levels of motor experiences did not demonstrate different visual attention to the action sequence. Across groups, infants made anticipatory fixations to the target actions on 28.3% of the experimental trials for which gaze data was obtained across both pairs. This rate of anticipatory looks is typical for infants in this age range (Tummeltshammer and Kirkham, 2013).
To assess rates of anticipations over the course of the experiment, we conducted a linear, model-based General Estimating Equations (GEE) with an unstructured working correlation matrix. Each of the 12 trials from each pair (Pincer and Hand) was assigned a 1 if it included anticipation and a 0 if not. Trials were then collapsed into four time bins with three trials in each bin. Pair (Pincer and Hand) and Time Bin (T1, T2, T3, and T4) were entered as within-subjects repeated measures and Motor Group (Pincer-dominant, Transitional, and Hand-dominant) was entered as a between-subjects factor. Across all infants, rates of anticipation decreased significantly over the course of the experiment, χ2(3) = 51.14, p < 0.001. Pairwise comparisons revealed a consistent statistically significant decrease in the proportion of trials containing predictive fixations from each Time Bin to the next (e.g., from T1 to T2, from T2 to T3, and from T3 to T4). There were no other main effects or interactions (ps > 0.17), indicating that rates of visual attention did not differ across infants based on age or motor abilities.
Correct vs. Incorrect
A one-sample Kolmogorov–Smirnov test revealed that proportion scores did not differ from a normal distribution for all dependent variables (ps > 0.05). A Levene’s test confirmed that the variances between the motor groups did not significantly differ from one another, p > 0.05.
To assess whether infants anticipated the next events in the sequence, we first compared proportions of correct fixations (Eq. 1) relative to incorrect fixations (Eq. 2) across the duration of the experiment. If infants learned the action pairs, proportions of fixations to correct locations should be higher than proportions to incorrect locations. In this analysis, the first trial was always excluded from calculations, as infants should not be able to correctly predict on the very first observation of each pair.
We first compared correct and incorrect fixations across all infants to assess whether learning occurred at all. An ANOVA with Prediction (Correct vs. Incorrect) and Pair (Pincer and Hand) as within-subject factors and age as a covariate revealed a marginally significant main effect of Prediction, F(1,43) = 3.04, = 0.07, p = 0.09 and a significant effect of age, p = 0.04. Without age as a covariate, the main effect of Prediction was significant, F(1,46) = 22.08, = 0.32, p < 0.001 (Figure 3). Across pairs, correct proportions were higher than incorrect proportions (MD = 0.14, SEM = 0.03, p < 0.001)2. There was no main effect of pair (p = 0.64) nor was there an interaction between Pair and Prediction (p = 0.64).
FIGURE 3.

The mean proportions of correct and incorrect gaze fixations collapsed across motor groups. Bars represent standard errors of the mean.
We next added Motor Group (Pincer-dominant, Transitional, and Hand-dominant) as a between-subjects factor to assess whether correct and incorrect fixations varied among motor groups with age as a covariate. This yielded no main effects or interactions with Motor Group (ps > 0.60). Thus, as a group, infants selectively anticipated the correct action and its effects more frequently than they incorrectly anticipated other objects on the screen across all trials, and this did not significantly differ between pairs or motor groups.
Learning Over Time
To further probe infants’ predictive gaze behaviors, we examined correct gaze proportions over time. We expected proportions of correct fixations (Eq.1) to increase over trials, as infants learned the sequence regularities. Correct fixations were entered into a linear, model-based GEE with an unstructured Working Correlation Matrix. Pair (Pincer and Hand) and Time Bin (T1, T2, T3, and T4) were entered as within-subjects repeated measures and Motor Group (Pincer-dominant, Transitional, and Hand-dominant) was entered as a between-subjects factor. Age was included as a covariate. The GEE revealed a significant main effect of Time Bin, χ2(3) = 31.00, p < 0.001, a significant interaction between Pair and Time Bin, χ2(3) = 15.047, p = 0.002, a significant interaction between Motor Group and Time Bin, χ2(6) = 23.33, p = 0.001, and a significant three-way interaction between Pair, Motor Group, and Time Bin, χ2(6) = 22.98, p = 0.001. There was no main effect of age, p = 0.16.
To assess whether the time-course of learning differed among motor groups, pairwise comparisons were conducted to follow up on the significant three-way interaction between Pair, Motor Group, and Time Bin. This interaction effect is illustrated in Figure 4. Based on our a priori hypotheses, we expected the largest differences in predictive gaze between Pincer-dominant and Hand-dominant infants. Therefore, we first focus on the results from follow-up comparisons between these two groups, before turning to the results from the Transitional group.
FIGURE 4.

The mean proportion of correct gaze fixations over the four time bins of the experiment plotted separately for the Pincer pair (above) and the Hand pair (below). Lines represent the three infant motor groups. Bars represent standard errors.
For the Pincer-dominant motor group, correct predictions to the Pincer pair sharply increased from T1 to T2 (MD = 0.34, SEM = 0.12, p = 0.003) and then subsequently decreased from T2 to T4 (MD = 0.32, SEM = 0.10, p = 0.002). In contrast, the Hand-dominant group demonstrated no such increase in correct proportions from T1 to T2 (MD = 0.12, SEM = 0.10, p = 0.23). However, there were no differences between Pincer- and Hand-dominant groups for the Pincer pair at either T1 or T2 (ps > 0.14). It was not the case that the Hand-dominant infants showed no evidence for learning of the Pincer pair, as they did improve from T1 to T4 (MD = 0.33, SEM = 0.10, p = 0.001), but this increase was slower than that of the Pincer-dominant infants and did not emerge until the final quarter of the trials.
For the Hand pair, the pattern was reversed: the Pincer-dominant infants showed no difference in correct predictions from T1 to T2 (MD = 0.08, SEM = 0.12, p = 0.53) whereas correct predictions increased marginally for the Hand-dominant infants (MD = 0.19, SEM = 0.10, p = 0.07). There was no difference between Pincer-dominant and Hand-dominant groups for the Hand pair at T1 (MD = 0.03, SEM = 0.10, p = 0.78); however, they did differ significantly at T2 (MD = 0.29, SEM = 0.12, p = 0.017). The Hand-dominant infants subsequently showed a decrease in correct predictions from T2 to T3 (MD = 0.24, SEM = 0.10, p = 0.016) and no significant gain across the experiment (T1 to T4; MD = 0.02, SEM = 0.09, p = 0.80). The Pincer-dominant infants also showed no significant gain across the experiment for the Hand pair (MD = 0.02, SEM = 0.12, p = 0.85).
The Transitional group demonstrated a pattern in between that of the Pincer-dominant and the Hand-dominant group. For the Pincer pair, the transitional group also increased their correct fixations from T1 to T2 like the Pincer-dominant group (MD = 0.27, SEM = 0.08, p = 0.001), followed by a decrease from T2 to T3 (MD = -0.41, SEM = 0.08, p < 0.001). However, they again showed a second rise in correct predictions from T3 to T4 (MD = 0.51, SEM = 0.06, p < 0.001), like the Hand-dominant group, and overall their correct predictions increased from T1 to T4 (MD = 0.37, SEM = 0.08, p < 0.001). For the Hand pair, the transitional group closely followed the pattern of the Hand-dominant infants, with an initial gain in correct anticipations from T1 to T2 (MD = 0.24, SEM = 0.08, p = 0.003) followed by a decrease from T2 to T3 (MD = -0.22, SEM = 0.07, p = 0.002), and no significant change across the entire experiment (T1 to T4; MD = 0.03, SEM = 0.08, p = 0.70).
To sum up, the infants with more dominant pincer grasp abilities quickly detected the pair structure for actions performed with a pincer grasp and correctly anticipated the upcoming action or its effect within the first few observations. This was then followed by a decrease in correct predictions in later trials. Similarly, the infants with a dominant hand grasp showed a faster improvement in predictions for the actions performed with a hand grasp, followed by a decline in performance. The Transitional infants, whose motor experience fell between Hand-dominant and Pincer-dominant infants, showed fixation patterns which shared characteristics with both groups.
Discussion
Observational statistical learning and motor experiences are both key pathways to infants’ developing action understanding and social-cognitive abilities (Hunnius and Bekkering, 2014). In the current eye-tracking experiment, we aimed to shed light on how these processes interact with one another during action observation. Infants observed an action sequence containing two deterministic pairs, one performed with a pincer grasp and the other with a whole-hand grasp. Predictive gaze to the second actions of each pair and their associated effects were measured as an indicator of statistical learning. Following observation, infants’ motor performance on a grasp test was used to determine their dominant grasp type. Our central hypothesis was that learning would be modulated by infants’ level of motor proficiency with the observed grasp type.
Findings revealed that infants, as a group, learned the transitional probabilities within the observed action sequences. Across pairs and motor groups, infants made more fixations to the correct upcoming actions and/or their effects than to other action locations on the screen. Consistent with prior findings with older infants (Monroy et al., 2017b), these results show that 8- to 11-month-olds can predict upcoming actions and their effects by learning transitional probabilities within an action sequence. Further, the general ability to predict upcoming actions was not driven by the specific motor action observed or by infants’ levels of motor proficiency.
A direct link between infants’ action perception and action production has previously been demonstrated for simple, isolated actions such as reaching and grasping (e.g., Gredebäck and Kochukhova, 2010; Kanakogi and Itakura, 2011). In the current experiment, we examined whether this link extends to situations in which infants need to use their statistical learning skills to predict upcoming sequential actions. This would be consistent with motor-based accounts of action understanding, which hypothesize that the motor system guides the generation of action predictions (Kilner et al., 2007).
Our data provide partial support for the notion that recent motor experiences influence infants’ statistical learning for action sequences. However, they do not provide conclusive support for the hypothesis that motor-based learning is essential for action predictions. Results showed that infants with a dominant pincer-hand grasp demonstrated an early increase in correct anticipations for the pincer grasp but not the hand grasp, indicating faster learning for the action dominant in their current motor repertoire. Likewise, infants with a dominant whole-hand grasp similarly demonstrated an early increase in correct anticipations for the hand grasp, but not the pincer grasp. The transitional infants—who likely had similar levels of proficiency with both actions—shared similar patterns with both groups. In sum, infants’ ability to learn action pairs based on statistical regularities was faster for the actions that are dominant in their current motor repertoire.
Faster learning for the action pair matching infants’ own motor abilities may reflect the influence of motor experiences on the ability to predict upcoming sequential actions, as we had hypothesized. According to motor-based accounts, the motor system combines prior knowledge with incoming sensory input to generate a prediction (Kilner et al., 2007). Motor experiences with the observed act are one important source of prior knowledge and allow the motor system to generate more precise predictions (Stapel et al., 2016). According to these views, the current data show that infants more readily predicted the actions for which they could recruit an established motor representation.
Unexpectedly, infants with a dominant whole-hand grasp, who had little experience performing a pincer grasp, still demonstrated learning for the pincer grasp pair. These findings suggest that the motor system was not the critical driving factor in infants’ action processing. However, there were three principal differences between the current experiment and the previous evidence for a closer link between motor skills and action prediction skills (e.g., Gredebäck and Kochukhova, 2010; Kanakogi and Itakura, 2011; Ambrosini et al., 2013). First, our paradigm featured sequential actions that differed in both statistical likelihood and type of grasp, whereas previous studies featured isolated reaching actions that differed only in the observed grasp. A recent study with adults revealed that, when action sequences contain varying degrees of predictability between actions, non-motor neural networks are activated that are traditionally involved in processing uncertainty within probabilistic perceptual input (Ahlheim et al., 2014). In a similar vein, perceptually difficult conditions engage additional brain regions beyond those typically activated during action observation (Lingnau and Petris, 2013). Thus, under uncertainty, domain-general regions outside the action-observation network become involved. One possibility is that additional non-motor processes became involved when infants’ own motor system does not have enough knowledge or sensory information to generate a precise prediction.
Secondly, prior research investigating anticipatory gaze and motor abilities have measured anticipations to the target, or end-point, of the actor’s reach-to-grasp actions. For instance, in Ambrosini et al. (2013), fixations during an actor’s reaching movement were recorded and anticipations were defined as any fixation to the object before the hand made contact. In this study, infants with faster anticipations to the object were considered more predictive than infants who anticipated later (see also Falck-Ytter et al., 2006; Gredebäck and Kochukhova, 2010; Kanakogi and Itakura, 2011; Rosander and von Hofsten, 2011). Some have interpreted such findings as evidence for the influence of an internal motor program, indicated by faster visual anticipations during the movement trajectory of observed action (i.e., a ‘gaze advantage,’ Rosander and von Hofsten, 2011). In the current experiment, we assessed anticipations to a future action step, rather than speed of gaze latencies during a reaching phase in which multiple motor cues are immediately available—such as movement velocity, hand shape and trajectory. Possibly, the difference between our findings and those of the aforementioned studies could indicate that motor experiences have a lesser impact on predicting the identity of an upcoming action step that cannot be predicted solely based on incoming motor information.
A third important difference between the current study and most prior research is our method of classifying motor ability. Here, we classified infants based on the relative dominance of each grasp type, rather than a binary classification of whether infants could in general perform the action or not (e.g., van Elk et al., 2008). This method more closely mirrors how infants’ motor development naturally unfolds (Ambrosini et al., 2013). Infants accumulate both visual and motor experiences with the fine-grained kinematics of an action—such as a certain muscle movement—before piecing together the entire action skill (Senna et al., 2016). Thus, although the whole-hand infants do not yet readily or voluntarily perform a pincer grasp, they may be able to take advantage of finer-grained motor cues for movements that they can do, such as the actor’s hand shape (Ambrosini et al., 2013). Indeed, some motor-based accounts claim that the motor system can predict even those actions well outside our own physical abilities—such as a bird’s flight—by approximating the link between the observed act and the motor system’s internal model (Schubotz, 2007).
Recent studies have further probed the influence of developing motor abilities on infants’ visual attention to actions and the objects and effects related to them. Importantly, shifts in visual attention may relate to the nature of what is attended to, rather than simply the overall amount. Though we observed no differences in global attention to objects between motor groups, there may have been differences in the microstructure of infants’ gaze shifts which could have led them to receive altered visual inputs according to the congruency between the observed action pair and their own motor expertise. For instance, Smith and Yu (2008) and Yu and Smith (2011) used microanalytic techniques to show that infants’ learning outcomes related to fine-grained patterns of gaze shifts between objects, and could be modeled by a simple associative learning model. Although in the current study we restricted our analyses to only predictive gaze, further analysis of the relations between fine-grained measures of visual attention and motor abilities would be an interesting avenue for future research and may shed additional light on the observed patterns.
Along these same lines, in the current study we considered predictions to upcoming actions and their effects as correct. We did not investigate whether infants only predicted the effect instead of the next upcoming action. Prior research has shown that infants of a similar age range rely on cues from action-effects to learn about sequential outcomes (Verschoor et al., 2010; Monroy et al., 2017a). It has also been suggested that the motor system predicts the effects of our own actions and those that we observe (Elsner et al., 2002). Thus, though it was not the focus of the current study, it would be interesting to further investigate whether the presence of action-effects might be an important aspect of the relation between motor experiences and statistical learning.
Surprisingly, following the initial rise in correct predictions for actions matching their own motor abilities, correct predictions subsequently declined. This decline was consistent across all infants and across action pairs, and showed that predictions did not follow a stable pattern over time. One simple explanation is a loss of attention to the stimuli. The proportions of trials containing predictive gaze fixations steadily decreased over the course of the experiment, indicating that infants made fewer predictions during later trials. Infants may have simply stopped anticipating after successfully making a few correct predictions, given that no new information was offered by subsequent repetitions of the action pairs. They may instead have begun to engage in other visual behaviors such as tracking the actor’s hands or exploring the visual scene in search of novel information. Some paradigms use so-called ‘occluders’ to encourage participants to make visual anticipations (e.g., Hunt and Aslin, 2001; Johnson et al., 2003; Falck-Ytter et al., 2006; Paulus et al., 2011). In contrast, in our study, all objects were freely visible throughout the entire demonstration which, though more ecologically valid, may also have ‘discouraged’ anticipatory gaze. The conditions under which infants reliably and consistently anticipate actions, particularly in naturalistic, live contexts, are an important avenue for future research which we are currently pursuing.
Conclusion
Given the accumulating evidence for the role of the motor system in facilitating action processing, can motor accounts explain the current findings? We propose that infants were engaging their motor systems as they processed the action sequence: when the observed action pairs were congruent with the grasping action most dominant in their motor repertoire, infants demonstrated a rapid increase in correct predictions. However, differences between motor groups were subtle. Learning was not tightly constrained by infants’ level of motor expertise, suggesting that additional cognitive processes come into play when infants need to use their statistical learning skills to generate action predictions. These findings further demonstrate that infants’ action prediction abilities cannot solely be explained by motor accounts, but likely reflect the recruitment of both motor and non-motor strategies when prediction requires learning statistical regularities in action sequences.
Author Contributions
CM led the writing of this manuscript. CM, SG, and SH designed the experimental paradigm. CM collected the data and conducted the analyses. SG and SH both provided supervision and discussion regarding the analyses. SG and SH reviewed and contributed edits to this manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors sincerely thank our research assistants for their hard work with coding, Angela Khadar for recruiting infant participants, Janne Rinke, Anneloes Hulsman, and Lisanne Schröer for testing participants and coding, and the Baby BRAIN lab in Nijmegen for constructive feedback and support.
Funding. This work was supported by an Initial Training Network (ITN) of the People Marie Curie Actions—Seventh Research Program (FP7) of the European Union (grant number FP7ITN2011-289404).
This was collected as a secondary measure to support the data from the grasp test and was not used in the current analyses.
Due to the uneven and relatively small sizes of our participant groups and relatively small samples, we repeated this analysis using a non-parametric Wilcoxon Signed Ranks test to compare Correct and Incorrect fixations across pairs and motor groups. This analysis confirmed that the mean proportion of Correct fixations was significantly greater than the proportion of Incorrect fixations, z = -3.91, p < 0.001, r = -0.56.
References
- Ahlheim C., Stadler W., Schubotz R. (2014). Dissociating dynamic probability and predictability in observed actions-an fMRI study. Front. Hum. Neurosci. 8:273. 10.3389/fnhum.2014.00273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosini E., Reddy V., de Looper A., Costantini M., de Looper A., Sinigaglia C. (2013). Looking ahead: anticipatory gaze and motor ability in infancy. PLOS ONE 8:e67916. 10.1371/journal.pone.0067916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslin R., Saffran J., Newport E. (1998). Computation of conditional probability statistics by 8-month-old infants. Psychol. Sci. 9 321–324. 10.1111/1467-9280.00063 10193055 [DOI] [Google Scholar]
- Baldwin D., Baird J. (2001). Discerning intentions in dynamic human action. Trends Cogn. Sci. 5 171–178. 10.1016/S1364-6613(00)01615-6 [DOI] [PubMed] [Google Scholar]
- Cannon E. N., Woodward A. L., Gredeback G., von Hofsten C., Turek C. (2012). Action production influences 12-month-old infants’ attention to others’ actions. Dev. Sci. 15 35–42. 10.1111/j.1467-7687.2011.01095.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsner B., Hommel B., Mentschel C., Drzezga A., Prinz W., Conrad B., et al. (2002). Linking actions and their perceivable consequences in the human brain. Neuroimage 17 364–372. 10.1006/nimg.2002.1162 [DOI] [PubMed] [Google Scholar]
- Elsner C., D’Ausilio A., Gredebäck G., Falck-Ytter T., Fadiga L. (2013). The motor cortex is causally related to predictive eye movements during action observation. Neuropsychologia 51 488–492. 10.1016/j.neuropsychologia.2012.12.007 [DOI] [PubMed] [Google Scholar]
- Erhardt R. (1994). “The erhardt developmental prehension assessment,” in Developmental Hand Dysfunction: Theory, Assessment, and Treatment 2nd Edn ed. Snowshoe Ct. E. (Maplewood, MN: Erhardt Developmental Products; ) [Google Scholar]
- Falck-Ytter T., Gredebäck G., von Hofsten C. (2006). Infants predict other people’s action goals. Nat. Neurosci. 9 878–879. 10.1038/nn1729 [DOI] [PubMed] [Google Scholar]
- Fiser J., Aslin R. (2001). Unsupervised statistical learning of higher-order spatial structures from visual scenes. Psychol. Sci. 12 499–504. [DOI] [PubMed] [Google Scholar]
- Flanagan J. R., Johansson R. S. (2003). Action plans used in action observation. Nature 424 769–771. 10.1038/nature01861 [DOI] [PubMed] [Google Scholar]
- Gerson S. A., Bekkering H., Hunnius S. (2015). Short-term motor training, but not observational training, alters neurocognitive mechanisms of action processing in infancy. J. Cogn. Neurosci. 27 1207–1214. 10.1162/jocn [DOI] [PubMed] [Google Scholar]
- Gerson S. A., Woodward A. L. (2014). The joint role of trained, untrained, and observed actions at the origins of goal recognition. Infant Behav. Dev. 37 94–104. 10.1016/j.infbeh.2013.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gredebäck G., Kochukhova O. (2010). Goal anticipation during action observation is influenced by synonymous action capabilities, a puzzling developmental study. Exp. Brain Res. 202 493–497. 10.1007/s00221-009-2138-1 [DOI] [PubMed] [Google Scholar]
- Hunnius S., Bekkering H. (2010). The early development of object knowledge: a study of infants’ visual anticipations during action observation. Dev. Psychol. 46 446–454. 10.1037/a0016543 [DOI] [PubMed] [Google Scholar]
- Hunnius S., Bekkering H. (2014). What are you doing? How active and observational experience shape infants’ action understanding. Philos. Trans. R. Soc. B Biol. Sci. 369:20130490. 10.1098/rstb.2013.0490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt R. H., Aslin R. (2001). Statistical learning in a serial reaction time task: access to seperable statistical cues by individual learners. J. Exp. Psychol. Gen. 130 658–680. 10.1037/0096-3445.130.4.658 [DOI] [PubMed] [Google Scholar]
- Johnson S. P., Amso D., Slemmer J. A. (2003). Development of object concepts in infancy: evidence for early learning in an eye-tracking paradigm. Proc. Natl. Acad. Sci. U.S.A. 100 10568–10573. 10.1073/pnas.1630655100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanakogi Y., Itakura S. (2011). Developmental correspondence between action prediction and motor ability in early infancy. Nat. Commun. 2:341. 10.1038/ncomms1342 [DOI] [PubMed] [Google Scholar]
- Kilner J. M., Friston K., Frith C. (2007). Predictive coding: an account of the mirror neuron system. Cogn. Process. 8 159–166. 10.1007/s10339-007-0170-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham N. Z., Slemmer J., Johnson S. P. (2002). Visual statistical learning in infancy: evidence for a domain general learning mechanism. Cognition 83 B35–B42. 10.1016/S0010-0277(02)00004-5 [DOI] [PubMed] [Google Scholar]
- Lingnau A., Petris S. (2013). Action understanding within and outside the motor system: the role of task difficulty. Cereb. Cortex 23 1342–1350. 10.1093/cercor/bhs112 [DOI] [PubMed] [Google Scholar]
- Marcus G. F., Vijayan S., Rao S. B., Vishton P. M. (1999). Rule learning by seven-month-old infants. Science 283 77–80. 10.1126/science.283.5398.77 [DOI] [PubMed] [Google Scholar]
- Meyer M., Braukmann R., Stapel J. C., Bekkering H., Hunnius S. (2016). Monitoring others’ errors: the role of the motor system in early childhood and adulthood. Br. J. Dev. Psychol. 34 66–85. 10.1111/bjdp.12101 [DOI] [PubMed] [Google Scholar]
- Monroy C., Gerson S. A., Domínguez-Martínez E., Kaduk K., Hunnius S., Reid V. (2017a). Sensitivity to structure in action sequences: an infant event-related potential study. Neuropsychologia. 10.1016/j.neuropsychologia.2017.05.007 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Monroy C., Gerson S., Hunnius S. (2017b). Toddlers’ action prediction: statistical learning of continuous action sequences. J. Exp. Child Psychol. 157 14–28. 10.1016/j.jecp.2016.12.004 [DOI] [PubMed] [Google Scholar]
- Paulus M., Hunnius S., Elk M. Van, Van Elk M., Bekkering H. (2012). How learning to shake a rattle affects 8-month-old infants’ perception of the rattle’s sound: electrophysiological evidence for action-effect binding in infancy. Dev. Cogn. Neurosci. 2 90–96. 10.1016/j.dcn.2011.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus M., Hunnius S., van Wijngaarden C., Vrins S., van Rooij I., Bekkering H. (2011). The role of frequency information and teleological reasoning in infants’ and adults’ action prediction. Dev. Psychol. 47 976–983. 10.1037/a0023785 [DOI] [PubMed] [Google Scholar]
- Rosander K., von Hofsten C. (2011). Predictive gaze shifts elicited during observed and performed actions in 10-month-old infants and adults. Neuropsychologia 49 2911–2917. 10.1016/j.neuropsychologia.2011.06.018 [DOI] [PubMed] [Google Scholar]
- Saffran J., Aslin R., Newport E. (1996). Statistical learning by 8-month-old infants. Science 274:1926 10.1126/science.274.5294.1926 [DOI] [PubMed] [Google Scholar]
- Schubotz R. I. (2007). Prediction of external events with our motor system: towards a new framework. Trends Cogn. Sci. 11 211–218. 10.1016/j.tics.2007.02.006 [DOI] [PubMed] [Google Scholar]
- Senna I., Addabbo M., Bolognini N., Longhi E., Macchi Cassia V., Turati C. (2016). Infants’ visual recognition of pincer grip emerges between 9 and 12 months of age. Infancy 22 389–402. 10.1111/infa.12163 [DOI] [PubMed] [Google Scholar]
- Shafto C. L., Conway C. M., Field S. L., Houston D. M. (2012). Visual sequence learning in infancy: domain-general and domain-specific associations with language. Infancy 17 247–271. 10.1111/j.1532-7078.2011.00085.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slone L. K., Johnson S. P. (2015). Infants’ statistical learning: 2- and 5-month-olds’ segmentation of continuous visual sequences. J. Exp. Child Psychol. 133 47–56. 10.1016/j.jecp.2015.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L., Yu C. (2008). Infants rapidly learn word-referent mappings via cross-situational statistics. Cognition 106 1558–1568. 10.1016/j.cognition.2007.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerville J. A., Woodward A. L., Needham A. (2005). Action experience alters 3-month-old infants’ perception of others’ actions. Cognition 96 B1–B11. 10.1016/j.cognition.2004.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate V., Johnson M. H., Karoui I. E., Csibra G. (2010). Motor system activation reveals infants’ on-line prediction of others’ goals. Psychol. Sci. 21 355–359. 10.1177/0956797610362058 [DOI] [PubMed] [Google Scholar]
- Stahl A. E., Romberg A. R., Roseberry S., Golinkoff R. M., Hirsh-Pasek K. (2014). Infants segment continuous events using transitional probabilities. Child Dev. 85 1821–1826. 10.1111/cdev.12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapel J. C., Hunnius S., Meyer M., Bekkering H. (2016). Motor system contribution to action prediction: temporal accuracy depends on motor experience. Cognition 148 71–78. 10.1016/j.cognition.2015.12.007 [DOI] [PubMed] [Google Scholar]
- Teinonen T., Fellman V., Näätänen R., Alku P. (2009). Statistical language learning in neonates revealed by event-related brain potentials. BMC Neurosci. 10:21. 10.1186/1471-2202-10-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummeltshammer K. S., Kirkham N. Z. (2013). Learning to look: probabilistic variation and noise guide infants’ eye movements. Dev. Sci. 16 760–771. 10.1111/desc.12064 [DOI] [PubMed] [Google Scholar]
- van Elk M., van Schie H. T., Hunnius S., Vesper C., Bekkering H. (2008). You’ll never crawl alone: neurophysiological evidence for experience-dependent motor resonance in infancy. Neuroimage 43 808–814. 10.1016/j.neuroimage.2008.07.057 [DOI] [PubMed] [Google Scholar]
- Verschoor S. A., Spapé M., Biro S., Hommel B. (2013). From outcome prediction to action selection: developmental change in the role of action–effect bindings. Dev. Sci. 16 801–814. 10.1111/desc.12085 [DOI] [PubMed] [Google Scholar]
- Verschoor S., Weidema M., Biro S., Hommel B. (2010). Where do action goals come from? Evidence for spontaneous action-effect binding in infants. Front. Psychol. 1:201. 10.3389/fpsyg.2010.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Smith L. B. (2011). What you learn is what you see: using eye movements to study infant cross situational word learning. Dev. Sci. 14 165–180. 10.1111/j.1467-7687.2010.00958.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks J. (2004). Using movement and intentions to understand simple events. Cogn. Sci. 28 979–1008. 10.1016/j.cogsci.2004.06.003 [DOI] [Google Scholar]
- Zeger S. L., Liang K. Y., Albert P. S. (1988). Models for longitudinal data: a generalized estimating equation approach. Biometrics 44 1049–1060. 10.2307/2531734 [DOI] [PubMed] [Google Scholar]


