Abstract
In fragile X syndrome (FXS), sensory hypersensitivity and impaired habituation is thought to result in attention overload and various behavioral abnormalities in reaction to the excessive and remanent salience of environment features that would normally be ignored. This phenomenon, termed sensory defensiveness, has been proposed as the potential cause of hyperactivity, hyperarousal, and negative reactions to changes in routine that are often deleterious for FXS patients. However, the lack of tools for manipulating sensory hypersensitivity has not allowed the experimental testing required to evaluate the relevance of this hypothesis. Recent work has shown that BMS-204352, a BKCa channel agonist, was efficient to reverse cortical hyperexcitability and related sensory hypersensitivity in the Fmr1-KO mouse model of FXS. In the present study, we report that exposing Fmr1-KO mice to novel or unfamiliar environments resulted in multiple behavioral perturbations, such as hyperactivity, impaired nest building and excessive grooming of the back. Reversing sensory hypersensitivity with the BKCa channel agonist BMS-204352 prevented these behavioral abnormalities in Fmr1-KO mice. These results are in support of the sensory defensiveness hypothesis, and confirm BKCa as a potentially relevant molecular target for the development of drug medication against FXS/ASD.
Introduction
Fragile X syndrome (FXS) is the most common inherited form of mental retardation and a leading known cause of autism spectrum disorder (ASD). A CGG trinucleotide repeat expansion in the promoter region of the FMR1 gene is responsible for its transcriptional silencing (Verkerk et al, 1991). The resulting absence or reduced expression of the Fragile X Mental Retardation Protein (FMRP) induces a complex phenotype that often includes intellectual disability, social and communication impairment, stereotypic behavior, attention deficits, hyperactivity, hyperarousal, and sensory abnormalities (Hagerman, 2006; Merenstein et al, 1996).
Several lines of evidence suggest that altered sensory processing may participate in the generation of major behavioral problems in FXS. In normal individuals, sensory habituation is a progressively reduced neuronal response to repeated exposure to the same sensory stimulation, so that irrelevant stimuli can be progressively ignored and attention focused on the most salient and relevant aspects of the environment. In contrast, sensory hypersensitivity and deficit in habituation are prominent features of FXS, causing enhanced and persistent responses to stimuli of various sensory modalities (Andrea et al, 2013; Castrén et al, 2003; Ethridge et al, 2016; Miller et al, 1999; Schneider et al, 2013), and adverse behavioral responses to otherwise neutral sensory stimuli. This phenomenon, termed sensory defensiveness, correlates with the expression of repetitive motor patterns and behavioral rigidity in autistic children (Baranek et al, 1997; Miller et al, 1999), and has been proposed as the primary cause of hyperactivity, hyperarousal, and various deleterious behaviors such as stereotypies and even self-injury in FXS patients when confronted to change in their habits (Baranek et al, 1997; Contractor et al, 2015; Hagerman, 2006; Merenstein et al, 1996; Miller et al, 1999; Symons et al, 2003). The hypothesis that altered neuronal sensory processing may participate in the generation of deleterious behavior is attractive and may offer physiological targets for therapeutic intervention in FXS. However, this is yet mostly based on correlative clinical observations and it remains to be demonstrated that restoring normal sensory sensitivity can also rescue behavioral response to novelty or to changes in habits. Recent advances in preclinical research using the Fmr1-knockout (KO) mouse model of FXS offer tools to test this hypothesis.
The best-characterized rodent model of FXS is the Fmr1-KO mouse that lacks FMRP because of a disruption in the Fmr1 gene (The Dutch-Belgian Fragile X Consortium, 1994; Mientjes et al, 2006). Fmr1-KO mice reproduce many phenotypes of FXS patients, including impaired social interactions, repetitive behavior, hyperactivity, and cognitive deficits (Hébert et al, 2014; Kazdoba et al, 2014; Michalon et al, 2012; Oddi et al, 2015; Peier et al, 2000; Pietropaolo et al, 2011). Cognitive rigidity and reduced flexibility in paradigms that involve task reversal have been reported in Fmr1-KO mice (Kazdoba et al, 2014; Kramvis et al, 2013), but behavioral aversion to novelty has not been much studied in this model. Interestingly, sensory hypersensitivity and impaired habituation have been shown at both behavioral and neuronal levels in Fmr1-KO mice (Arnett et al, 2014; Lovelace et al, 2016; Moon et al, 2006; Restivo et al, 2005; Zhang et al, 2014). There is clear evidence that the neocortex of Fmr1-KO mice is hyperexcitable (Gibson et al, 2008; Goncalves et al, 2013; Rotschafer and Razak, 2013; Zhang et al, 2014), pointing to a causative role for neocortical circuit defects in sensory hypersensitivity in FXS. In fact, previous work has shown that independently of its function as a translation regulator, FMRP also interacts with the β4 regulatory subunit of big conductance voltage and Ca2+-activated K+ channels (BKCa) in hippocampal and cortical excitatory neurons that influence action potential duration and neurotransmitter release (Deng and Klyachko, 2016; Deng et al, 2013; Myrick et al, 2015). Accordingly, recent work has shown that specific alterations in potassium channels are responsible for increased cortical excitability and sensory hypersensitivity in Fmr1-KO mice (Zhang et al, 2014). In this study, a dysfunction of dendritic HCN1-containing channels (responsible for Ih) as well as of dendritic and somatic BKCa in pyramidal neurons of the primary somatosensory cortex was found to result in increased intrinsic excitability by increasing input resistance, augmenting the spatial and temporal summation of excitatory synaptic potentials and by promoting the efficacy by which action potentials backpropagate into the dendrites and trigger dendritic spikes. Moreover, BKCa channel agonists such as BMS-191011 or BMS-204352 were efficient in reversing cortical hyperexcitability in vitro and increased acoustic startle in behaving Fmr1-KO mice, a widely recognized behavioral readout of sensory sensitivity previously used to assess sensory hypersensitivity in Fmr1-KO mice (Michalon et al, 2012; Nielsen et al, 2002).
In the present study, we have investigated the behavioral responses of Fmr1-KO mice exposed to novel and familiar environments differing from their home cage, and found that BMS-204352 was effective in preventing the major behavioral disturbances expressed by Fmr1-KO mice when removed from their usual environment. These results provide support for the sensory defensiveness hypothesis, and confirm BKCa as a pertinent molecular target for the development of drug medication against FXS/ASD.
Materials and methods
Animals
Naive 12–14-week-old second-generation Fmr1-KO mice (n=37) and their wild-type (WT, n=44) littermates were used in our study, bred and housed as in prior study from our laboratory (Zhang et al, 2014). Compared with the original Fmr1−/y mouse line (The Dutch-Belgian Fragile X Consortium et al, 1994), second-generation Fmr1 knockout (Fmr1−/− Fmr1−/y) mice (Mientjes et al, 2006) are deficient for both FMR1 RNA and FMR protein. WT and KO male mice, socially housed in standard cages enriched with Nestlets, were individually isolated in similar standard cages 1 week before the starting point of the experimental protocol. Animals were maintained on a 12 h:12 h light/dark schedule and provided with ad libitum access to food and water. Pharmacological testing included intraperitoneal injection of either vehicle (type-1, vehicle for BMS-204352: 0.9% NaCl, 1.25% DMSO, 1.25% Tween-80, 10 ml per kg of body weight; type-2, vehicle for DZ: 0.9% NaCl, 0.2% alcohol, 10 ml per kg of body weight), the BKCa agonist BMS-204352 (Tocris, 2 mg per kg of body weight, dissolved in type-1 vehicle solution), as in prior studies using BMS-204352 (Hébert et al, 2014; Zhang et al, 2014), or the anxiolytic diazepam (DZ, Sigma-Aldrich, 1.5 mg/kg, dissolved in type-2 vehicle solution). All experiments were performed during the light period under constant mild luminosity (60–70 Lux). All experimental procedures were performed in accordance with the EU directives regarding the protection of animals used for experimental and scientific purposes, 86/609/EEC and 2010/63/EU. All experiments were performed in accordance with the French law and approved by the Ethical committee CEEA50 (saisine 5012024-A).
Locomotor Activity
At 30 min after injection of either vehicle or BMS-204352, the animal was individually introduced into an empty open-field chamber (45 × 33 cm arena, surrounded by 50 cm high walls and wiped clean with 70% ethanol before introduction of each animal) for behavioral monitoring. Continuous video recording was performed at a rate of 25 frames/s with a video camera (Logitech HD Webcam C270) placed 1 m above the platform, and processed offline with Ethovision XT software (Noldus Technology, Wageningen, The Netherlands) for animal tracking (X–Y coordinates of the body center). Parameters analyzed were: total distance moved, total number of rotations (ie, turn angle >180° within 1 cm during locomotion periods at speed >2 cm/s), total time resting (speed <1 cm/s for at least 2 s) and ratios of total time spent in center (distance from the walls >10 cm) vs total time in the session, and time resting in center vs total time resting in the session. Self-grooming (back, belly, nose, and ears) was identified offline and tagged manually by a trained experimenter blinded to the genotype, so that we could quantify the durations of individual episodes of each type of grooming. In a series of additional control experiments, video monitoring was performed of animals placed in a new cage with nest building material and pretreated (15 min before recording) with either vehicle or the anxiolytic DZ. Locomotion was then quantified as the total distance run during the initial 1 h recording period.
Nest Building
Nest building behavior was assessed in home cage (dimensions 20 × 10 × 15 cm) 1 week after individual housing, in a new environment (new and wider cage, dimensions 30 × 15 × 20 cm, fresh litter, located in a different room) 4 days after testing in the home cage, and in the open field chamber after familiarization (1 h per day during 10–11 days). Nest building scoring was performed by a trained experimenter blinded to the genotype at different delays (1 to 7 h) after placing the animal in the presence of nesting material (Nestlet, 2.7 g, 2.5 × 2.5 cm and 5 mm thick compressed cotton), using the following standardized scoring scale (Deacon, 2006): 1: Nestlet not noticeably shredded; 2: Nestlet 10 to 50% shredded, not used as a nest; 3: Nestlet shredded 50 to 90%, but the shredded material remains scattered in the cage and is not used as a nest; 4: Nestlet shredded >90%, and shredded material used as a flat nest; and 5: Nestlet shredded >90% and used as a rounded nest with sides covering the mouse. In a series of control experiments performed to validate this scaling method with an objective measure, the nest building score was additionally quantified as the normalized weight of the remaining unshredded nesting material using the following standardized scoring scale: 1: above 85% of Nestlet unshredded; 1.5: between 65 and 85% of Nestlet unshredded; 2: between 47.5 and 65% of Nestlet unshredded; 2.5: between 30 and 47.5% of Nestlet unshredded; 3: between 22.5 and 30% of Nestlet unshredded; 3.5: between 15 and 22.5% of Nestlet unshredded; 4: between 7.5 and 15% of Nestlet unshredded; and 5: <7.5% of Nestlet unshredded. As illustrated in Supplementary Figure S1, both methods for evaluation of nest building provided very similar results (correlation coefficient=0.91, p<0.001, n=66 nests from 8 WT and 14 Fmr1-KO mice).
Statistics
Data processing was performed with homemade scripts and functions from the Matlab statistics toolbox (Mathworks). Descriptive statistics for all experiments are presented in Supplementary Tables S1–S3. Data were systematically tested for normal distribution with the Lilliefors test, a modification of the Kolmogorov–Smirnov test recommended for small sample sizes (Razali and Bee Wah, 2011). Data following a normal distribution were analyzed using parametric tests: ANOVA with genotype and drug condition as factors (and post hoc Turkey’s test) for independent data sets, and Student’s t-test for paired data. Data following nonnormal distributions were analyzed using nonparametric tests: Kruskal–Wallis (and post hoc Mann–Whitney U-test) for independent data sets and Wilcoxon signed-ranks test for paired data. Differences were considered statistically significant for values of p<0.05. Data are presented as mean±SEM.
Results
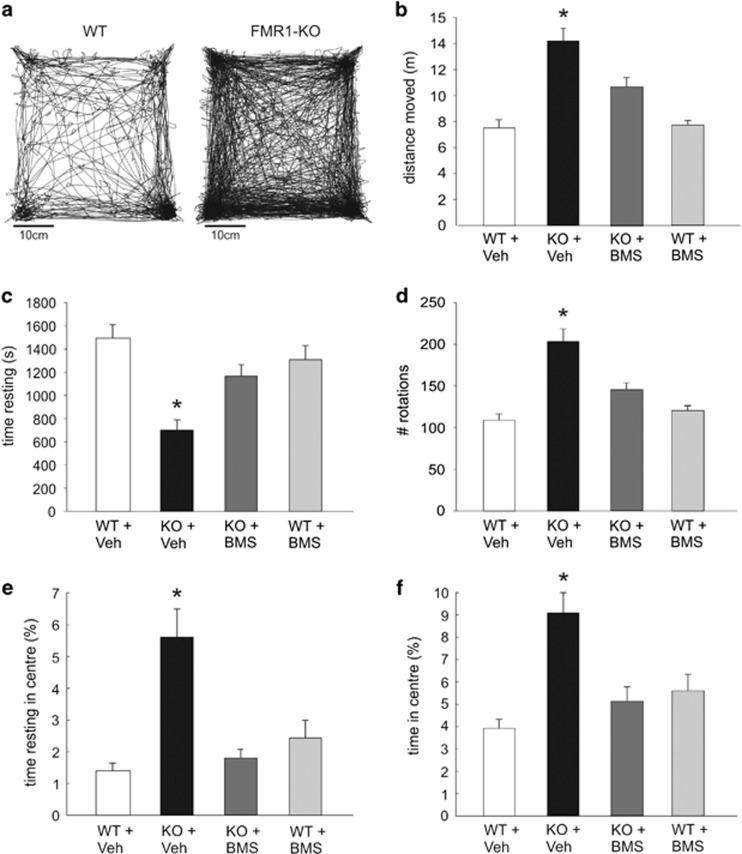
In order to test the hypothesis that reversing sensory hypersensitivity may help prevent various deleterious behaviors such as stereotypies or self-injury expressed by FXS individuals when confronted to changes in their habits, we have evaluated the potential behavioral improvement of reversing sensory hypersensitivity in Fmr1-KO mice exposed to a novel environment. Control (WT) and Fmr1-KO mice pretreated with either vehicle or BMS-204352 (2 mg/kg), a drug recently shown to restore normal cortical excitability and sensory sensitivity in Fmr1-KO mice, were exposed for 1 h to an open-field arena that they had never visited before (novel environment). Compared with WT, Fmr1-KO mice placed in the novel open field showed increased total distance moved (ANOVA, Fgenotype(1, 53)=49.64, p<0.001),decreased total resting time (Kruskal–Wallis χ2(3, 53)=22.17, p<0.001), and increased number of rotations (Fgenotype(1, 53)=38.86, p<0.001), indicative of hyperactivity. Fmr1-KO mice also spent more time in the center of the arena (total time: Fgenotype(1, 53)=11.93, p<0.001; resting time: χ2(3, 53)=14.63, p=0.001), possibly indicating reduced anxiety. As illustrated in Figure 1a and b, all these parameters were significantly rescued by BMS-204352 (distance moved, Fdrug(1, 53)=5.72, p=0.006; total time resting, χ2(3, 53)=22.17, p=0.001; number of rotations, Fdrug(2, 53)=5.89, p<0.001; total time in center, Fdrug(2, 41)=11.4, p=0.013; time resting in center, χ2(3, 53)=14.63, p=0.045), being fully restored to WT values for time resting in center χ2(3, 53)=14.63, p=0.75) and time in center (Fgenotype × drug(1, 53)=17.17, p=0.59). BMS-204352 had no significant effect on WT mice.
Figure 1.
BMS-204352 rescue of the hyperactivity phenotype of Fmr1-KO mice in a novel environment. (a) Single animal (left, WT; right, Fmr1-KO) total trajectory during a 1 h exposure to an open field never visited before. (b–f) Summary plots of locomotor activity during the initial 1 h spent in a novel open field for WT and Fmr1-KO mice pretreated with either vehicle or BMS-204352 (IP injection 30 min before introduction into the open field). WT+Veh, n=16, KO+Veh, n=13, KO+BMS-204352, n=13, WT+BMS-204352, n=15. (b) Total distance moved; (c) total time resting; (d) number of rotations (angle >180°); (e) total time resting in center; and (f) total time in center. *Statistically significant difference compared with all other groups (p<0.05). Note the general hyperactivity of KO mice, rescued by BMS-204352 treatment.
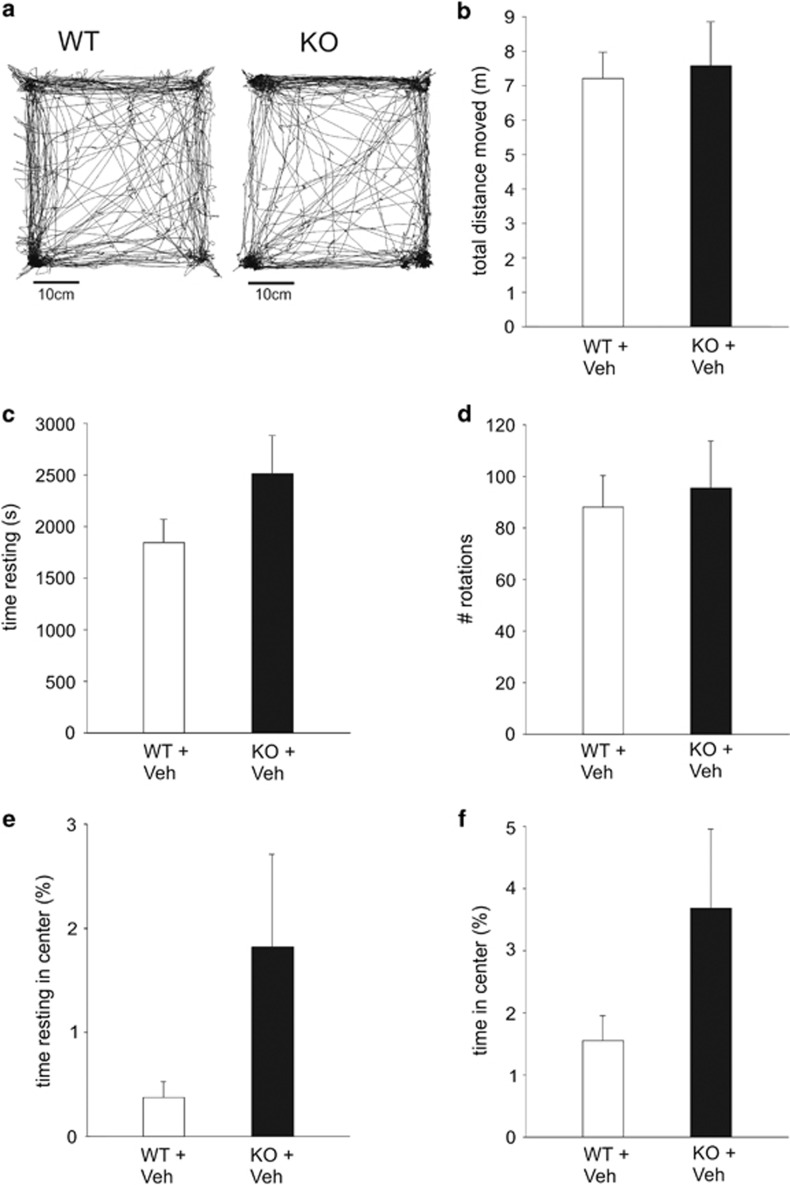
From the literature it is not clear whether hyperactivity, one of the most consistently reported features in FXS studies (The Dutch-Belgian Fragile X Consortium, 1994; Dansie et al, 2013; de Diego-Otero et al, 2008; Kramvis et al, 2013; Michalon et al, 2012; Mineur et al, 2002; Oddi et al, 2015; Olmos-Serrano et al, 2011; Peier et al, 2000; Pietropaolo et al, 2011; Restivo et al, 2005; Spencer et al, 2011; The Dutch-Belgian Fragile X Consortium et al, 1994) is a permanent behavioral phenotype of Fmr1-KO mice or an adverse reaction to novelty or change in habits (due for instance to being taken away from the home cage for testing). As illustrated in Figure 2, when Fmr1-KO mice were exposed to the open-field chamber after a familiarization of 5 days, 1 h/day, they did not show any increase in total distance moved (F(2, 21)=1.89), total time resting (F(2, 21)=3.84), number of rotations (F(2, 21)=2.88), total time in center (F(2, 19)=1.43), or total time resting in center (F(2, 19)=1.45). This suggests that hyperactivity is not a permanent phenotypic character of Fmr1-KO mice but rather a reaction to change. Altogether, these data suggest that restoring normal sensory sensitivity with the BKCa agonist BMS-204352 provides a rescue from the hyperactivity observed in Fmr1-KO mice in reaction to their exposure to a novel environment.
Figure 2.
Fmr1-KO mice are not hyperactive in a familiar environment. (a) Single animal (left, WT; right, Fmr1-KO) total trajectory during a 1 h exploration session of an open field after familiarization (1 h/day during 5 days). (b–f) Summary plots of locomotor activity during the 1 h recording period in the familiar open field for WT and Fmr1-KO mice. WT, n=8, KO, n=8. (b) Total distance moved; (c) total time resting; (d) number of rotations (angle >180°); (e) total time resting in center; and (f) total time in center. No statistically significant effect of genotype.
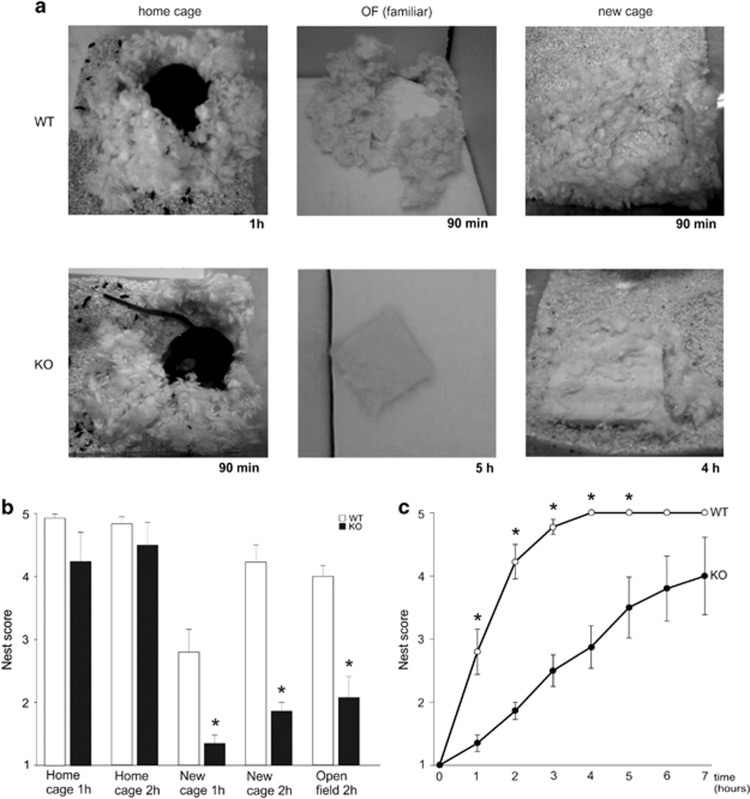
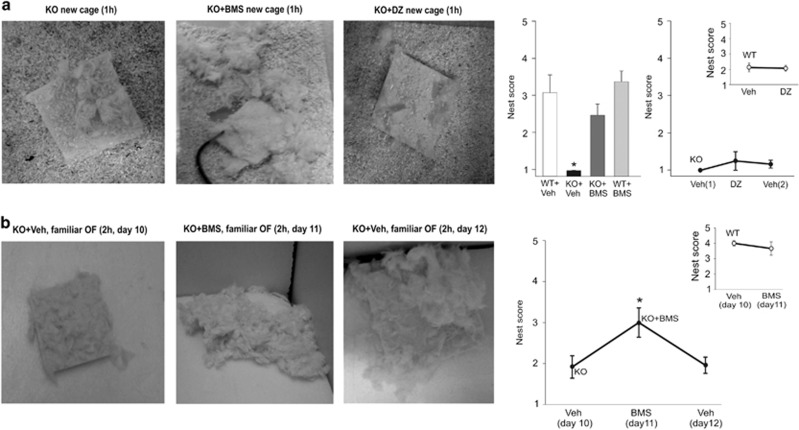
Beside general locomotor activity, nest building has been proposed as a highly sensitive and well-characterized assay for well-being and ability to perform activities of daily living in mice (Deacon, 2012; Jirkof, 2014). We have therefore used this test to evaluate the global behavioral perturbation induced by confronting Fmr1-KO mice to a change in routine (ie, either transferring them to a new cage or to a familiar environment but distinct from their home cage), and the potential benefice of restored sensory sensitivity for restoring normal behavior and well-being. As illustrated in Figure 3, WT and Fmr1-KO mice provided with nest building material in their home cage performed elaborated nests within a couple of hours (no difference between WT and Fmr1-KO in nest building score after 2 h, Mann–Whitney, U=80.0, p=0.66). On the other hand, whereas WT mice were minimally perturbed in nest building when tested in a new cage or in the familiar open-field chamber (being exposed 1 h/day for 10 days before testing), Fmr1-KO mice appeared strongly affected by changing environment as they performed poorly in nest building in both the new cage (after 1 h, W=145, p=0.002; after 2 h, W=183.5, p<0.001) and familiar open field (cf. Figure 3b). As illustrated in Figure 4a, nest building performance in a new cage was significantly rescued in Fmr1-KO mice after pretreatment with BMS-204352, injected 15 min before testing (after 1 h, χ2(3, 26)=24.09, p=0.008, after 2 h, χ2(3, 26)=21.50, p=0.002), so that Fmr1-KO mice then performed as well as WT (after 1 h χ2(3, 26)=24.09, p=0.84, after 2 h, χ2(3, 26)=21.5, p=0.76). On the other hand, no improvement in nest building was observed in Fmr1-KO mice injected with DZ (after 1 h, W=1, p=1; after 2 h, W=10, p=0.13) at a dose and timing promoting anxiolytic rather than sedative effects (Dailly et al, 2002), as verified by the absence of statistically significant difference in locomotion between pretreatment with Vehicle or DZ (total distance run, WT+Veh vs WT+DZ, t(6)=0.80, p=0.45; KO+Veh vs KO+DZ, t(7)=−1.51, p=0.17), suggesting that their impaired performance was not due to increased anxiety. In order to evaluate more precisely the potential of BMS-204352 for preventing the behavioral perturbations induced by a change in routine, Fmr1-KO mice were tested for nest building on 3 consecutive days in the familiar open field. As illustrated in Figure 4b, Fmr1-KO mice pretreated with vehicle performed poorly compared with WT for nest building in the open field after 10 days of familiarization (1 h/day) (WT+Veh vs Fmr1-KO+Veh on day 10: after 2 h, U=130.0, p<0.001). Their performance increased significantly when tested again the next day, after pretreatment with BMS-204352 (Fmr1-KO+Veh day 10 vs Fmr1-KO+BMS day 11: after 2 h, paired Wilcoxon signed-ranks test, W=0, p=0.007) that, on the other hand, had no significant effect on WT mice (WT+Veh day 10 vs WT+BMS day 11: after 2 h, Wilcoxon signed-ranks, W=9, p=0.25). The beneficial effect of BMS-204352 on Fmr1-KO mice was reversible as this improvement was no longer observed upon further testing for a third day, after pretreatment of the same animals with vehicle (Fmr1-KO+Veh day 10 vs Fmr1-KO+Veh day 12, after 2 h, W=19.5, p=0.75).
Figure 3.
Impaired nest building for Fmr1-KO mice outside home cage. (a) Illustration of nest building performance for individual WT and Fmr1-KO mice in their home cage (left), familiar open field (OF, familiarization 1 h/day during 9 days), and new cage (right). The pictures were taken at the time indicated (between 1 h and 5 h after introduction of the nest building material). Note that the WT mouse had terminated nest building after 90 min in all tested conditions, whereas the KO mouse performed well in its home cage but not in the familiar open field or in a new cage. (b) Summary plots of nest building scores in home cage, new cage, and familiar open field for WT and Fmr1-KO mice. (c) Summary plot of nest building over time for WT and Fmr1-KO mice in a new cage. Note that WT mice complete nest building within 2 to 3 h in any tested condition, whereas KO mice have delayed nest building. (b, c) *Statistically significant effect of genotype (p<0.05).
Figure 4.
BMS-204352 but not diazepam rescue of nest building in familiar open field and new cage. (a) Illustration of nest building performance of Fmr1-KO mice after 1 h in a new cage after vehicle (left), BMS-204352 (middle), or diazepam (DZ, right) injection. Summary plots of nest building performance after 1 h, following treatment with either vehicle or BMS-204352 (left, 5 WT+Veh, 5 WT+BMS, 12 Fmr1-KO+Veh, and 9 Fmr1-KO+BMS). The effect on nest building of the anxiolytic DZ, tested against that of vehicle (Veh) on the same animals but on consecutive days (8 WT and 6 Fmr1-KO mice tested consecutively with vehicle, DZ, and for Fmr1-KO mice vehicle again), is illustrated on the right plots. *P<0.05 compared with all other groups. (b) Illustration of nest building performance of Fmr1-KO mice treated either with vehicle (days 10 and 12) or BMS-204352 (day 11) after 2 h in a familiar (1 h/day during 9 days) open field. Summary plot of nest building performance on days 10 (treatment=vehicle), 11 (BMS-204352), and 12 (vehicle) for Fmr1-KO (n=15) mice. Inset, nest building performance on days 10 (treatment=vehicle) and 11 (BMS-204352) for WT mice (n=12). *P<0.05 compared with Veh (day 10) and Veh (day 12). Note the reversible rescue of nest building performance in Fmr1-KO mice with the BMS-204352 treatment.
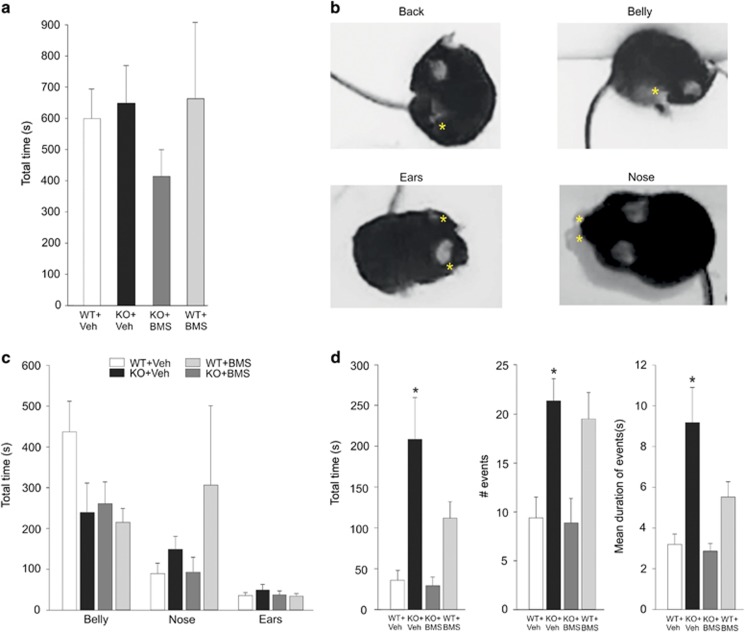
Therefore, Fmr1-KO mice are deeply perturbed by changes in environment or in daily routine, as FXS patients in whom such situations promote repetitive behavior and even often self-injury. Because repetitive behavior in mice can be expressed as excessive self grooming, we compared the amount of time that WT and Fmr1-KO mice exposed to a novel environment dedicated to grooming activity. As illustrated in Figure 5, we found no effect of genotype (WT vs Fmr1-KO) or drug (Veh vs BMS) on total grooming time (χ2(3, 28)=3.04). On the other hand, when we discriminated between distinct types of self-grooming (ie, back, belly, nose, and ears), we did observe that Fmr1-KO mice had excessive grooming of the back, both in terms of number and duration of grooming episodes (total time Fgenotype(1, 28)=2.5, p=0.001; number of events χ2(3, 28)=12.92, p=0.047; mean duration of events Fgenotype(1, 28)=2.79, p=0.001). As illustrated in Figure 5 and Supplementary Figure S2, this increase was specific to grooming the back, because there was no difference in the number or duration of belly-, nose-, or ears-grooming episodes (belly: total time, χ2(3, 28)=6.16; number of events, Fgenotype(1, 28)=0.91, mean duration of events, Fgenotype(1, 28)=0.69; nose: total time, χ2(2, 18)=3.66, number of events, Fgenotype(1, 28)=0.25, mean duration of events, χ2(2, 18)=5.41; ears: total time, Fgenotype(1, 28)=0.7, number of events, Fgenotype(1, 28)=0.47, mean duration of events, χ2(2, 18)=6.04). Moreover, increased grooming of the back was rescued to normal levels by pretreatment with BMS-204352 (Fmr1-KO vs Fmr1-KO+BMS: total time, Fdrug(1, 28)=3.28, p=0.001; number of events, χ2(3, 28)=12.93, p=0.037; mean duration of events Fdrug(1, 28)=4.03, p<0.001; WT vs Fmr1-KO+BMS: total time, Fgenotype × drug(1, 28)=20.18, p=0.998; number of events, χ2(3, 28)=12.93, p=0.999; mean duration of events, Fgenotype × drug(1, 28)=18.9, p=0.995). Even though the effect did not reach statistical significance, there was a tendency for increased grooming of the back following pretreatment with BMS-204352 in WT mice (WT+Veh vs WT+BMS: total time, Fdrug(1, 28)=3.28, p=0.25; number of events, χ2(3, 28)=12.93, p=0.08; mean duration of events, Fdrug(1, 28)=4.03, p=0.37). This side effect does not account for the recovery exerted by BMS-204352 pretreatment in Fmr1-KO mice, with which grooming of the back was reduced to WT level.
Figure 5.
BMS-204352 prevents excessive self-grooming of the back in Fmr1-KO mice exposed to a novel environment. Wild-type (WT) and Fmr1-KO mice (KO) treated either with vehicle (Veh) or BMS-204352 (BMS) were exposed for 1 h to a novel open field. Total grooming time (a) or specific grooming of the back, belly, nose, or ears (b–d) were quantified (number of animals: 8 WT, 8 Fmr1-KO). (a) Total self-grooming time. (b) Pictures showing the four different types of self-grooming activities quantified in (c, d) (*position of paws). (c) Total time spent in grooming the belly, nose, or ears. (d) Total time (left plot), number (middle), and mean duration (right) of events for grooming the back. *P<0.05 compared with WT+Veh and KO+BMS. Note the excessive grooming of the back in Fmr1-KO mice, an effect fully rescued by BMS-204352.
Discussion
The main objective of this study was to experimentally address the hypothesis, derived from clinical observations, that specific physiological deficits in cortical circuits, responsible for sensory hypersensitivity and lack of habituation, are also involved in the sensory defensiveness and major behavioral disturbances expressed by FXS patients exposed to unusual environmental situations or changes in their routine. Even though mouse studies do not always allow to predict the outcome of human studies, we have used Fmr1-KO mice as a model of FXS, and the BKCa agonist BMS-204352 as a tool to restore normal cortical excitability and sensory sensitivity in Fmr1-KO mice. Treated and untreated animals were exposed to changes in their daily routine by being transferred to a new environment or removed from their home cage for 1 to 2 h. We found that in such cases, Fmr1-KO mice expressed a range of behavioral perturbations. Normalizing neuronal excitability with the BKCa channel agonist BMS-204352, at a dose previously shown to rescue behaviorally measured sensory hyperresponsiveness (Zhang et al, 2014), prevented these behavioral disorders in Fmr1-KO mice. These results lend support to the sensory defensiveness hypothesis, by considering a relationship between altered sensory sensitivity and alterations in behaviors only indirectly related to sensory processing. Nevertheless, the case of a patient carrying a point mutation in FMR1 (R138Q), which was shown in FMR1-KO mice to result in impaired FMRP-mediated modulation of BKCa channels (Myrick et al, 2015), emphasizes the complexity of FXS. This patient presented a history of intellectual disability and intractable epilepsy, but not the other maladaptive behaviors commonly associated with FXS or autism, such as stereotypic behaviors, hyperactivity, impulsivity, physical aggressiveness, difficulty with changes or transitions, or problems with sleeping or eating. Although this observation suggests a direct link between BKCa impairment, severe neurodevelopmental deficits and dysregulation in circuit excitability, it also points at the involvement of other factors, potentially interacting with BKCa impairment, in the variety of phenotypic traits associated with FXS. Moreover, it is important to note that BKCa channels are expressed ubiquitously. Consequently, the beneficial effects of BMS-204352 may also be mediated by a variety of physiological targets independent of cortical excitability and sensory sensitivity. Further studies will be necessary to evaluate in which respect other deficits reversed in Fmr1-KO mice by the restoration of BKCa function, such as altered hippocampal physiology, impaired social interactions, and spatial memory (Deng and Klyachko, 2016; Hébert et al, 2014), involve or not the reversal of cortical hyperexcitability and sensory hypersensitivity. Altogether, our results provide further evidence for BKCa as a potentially important molecular target for the development of drug medication against FXS/ASD. The BKCa channel agonist BMS-204352 has been approved for human use, having no toxicity or adverse effects (Jensen, 2002). Provided that our observations prove relevant to humans, it is an interesting candidate for clinical trials involving FXS patients, as a complement or alternative to approaches targeting mGluR1/5 that proved effective in mice but not yet in patients (Krueger and Bear, 2011; Michalon et al, 2012).
Some concerns have been expressed in the literature regarding dissimilarities between sensory processing alterations in FXS patients and Fmr1-KO mice. Acoustic startle is often considered a behavioral readout of sensory sensitivity and prepulse inhibition (PPI) of acoustic startle to reflect sensory gating. Increased startle and decreased PPI are consistently observed in FXS patients (Frankland et al, 2004; Kazdoba et al, 2014; Perry et al, 2007; Yuhas et al, 2011), reflecting increased sensory sensitivity and decreased gating. On the other hand, both increased and decreased startle responses have been reported in Fmr1-KO mice, and PPI has often but not always been reported to be increased in Fmr1-KO mice, as discussed in a recent review (Kazdoba et al, 2014). A potential cause of discrepancy is that startle and PPI in mice seem to depend on stimulus intensity, with increased startle and decreased PPI at low intensities but the opposite with loud auditory stimuli (Nielsen et al, 2002). Decreased PPI in Fmr1-KO mice has thus been proposed to result from increased perception of the weak prestimulus (Chen and Toth, 2001). Moreover, all studies directly measuring spontaneous cortical activity or the neuronal response to sensory stimulations in Fmr1-KO mice reported increased neuronal excitability (Goncalves et al, 2013; Zhang et al, 2014) and sensory responses (Arnett et al, 2014; Rotschafer and Razak, 2013; Zhang et al, 2014) that can even lead to audiogenic seizures (Chen and Toth, 2001; Dansie et al, 2013; Musumeci et al, 2000). In addition, studies in which habituation has been tested directly, either from behavior (Moon et al, 2006; Restivo et al, 2005) or neuronal (Lovelace et al, 2016) readout, have reported impaired habituation in Fmr1-KO mice. Therefore, sensory hypersensitivity and reduced habituation seem clearly established in both Fmr1-KO mice and clinical FXS. Furthermore, pharmacological interventions using the Fmr1-KO mouse have demonstrated predictive validity for this model (Kazdoba et al, 2014), as the results from several drug studies in Fmr1-KO mice have paralleled findings from human FXS treatment trials (eg, minocycline (Paribello et al, 2010) and lithium (Berry-Kravis et al, 2008)). We therefore believe that our results may have translational value for defining pharmacological intervention to treat FXS patients.
The behavioral readouts of our study include locomotor activity, nest building, and self-grooming. The preclinical literature contains some uncertainty regarding whether hyperactivity is a reaction to novelty (Kramvis et al, 2013) or a permanent phenotype (de Diego-Otero et al, 2008) of FXS. Our results suggest that this is a reaction to novelty, because we did not observe an hyperactive phenotype after familiarization. It is interesting to note that Fmr1-KO mice were previously found to be still hyperactive after 24 h in the testing environment (de Diego-Otero et al, 2008), suggesting that habituation is less stressful for Fmr1-KO mice when performed progressively over several days (1 h/day for 5 to 10 days in our study).
Expression of increased grooming has been reported in Fmr1-KO mice as an emotional response to cognitive or social challenge (McNaughton et al, 2008; Moon et al, 2008). In keeping with a previous study on Fmr1-KO mice exposed to a novel open field (Mineur et al, 2002), our results do not show any difference in total self-grooming time. However, analysis of distinct grooming types revealed specific excessive grooming of the back in Fmr1-KO mice exposed to the novel open field, a phenotype reversed by pretreatment with BMS-204352. Previous work has shown that the behavioral microstructure of self-grooming in rodents may serve as a sensitive marker of stress levels, even without significant change in overall grooming time (Song et al, 2016). Increased caudal self-grooming has been reported as a response to relatively moderate aversive conditions such as bright light and novelty exposure (Meshalkina and Kalueff, 2016; van Erp et al, 1994). We therefore suggest that in our mouse line, grooming of the back may be a relevant index of FXS-related repetitive behavior, and that this sign of discomfort can be alleviated by BMS-204352, a treatment rescuing cortical hyperexcitability, sensory hypersensitivity, and behavioral hyperarousal.
Nest building has been proposed as an index of well-being and the ability to perform activities of daily living (Jirkof, 2014) that is finally one of the most important objectives of clinical intervention. The fact that Fmr1-KO mice showed a clear impairment in nest building suggests that they are highly perturbed when exposed to novelty or even to situations out of their daily routine, as also are FXS or ASD patients. The behavioral rescue provided by BMS-204352 is compatible with the clinical hypothesis that restoring normal sensory sensitivity may indeed be a decisive way of restoring well-being in FXS or ASD, and points to BKCa as a potentially relevant physiological target for therapeutic drug development. Our protocol of alternation of vehicle and BMS-204352 treatment on 3 consecutive days nevertheless shows that the effects of BMS-204352 on nest building performance are transitory. Further studies are awaited to evaluate the potential beneficial effects of chronic BMS-204352 delivery, as well as the development of more stable molecules allowing long-term corrective action on BKCa channels.
Funding and disclosure
The authors declare no conflict of interest.
Acknowledgments
We thank Nora Abrous and Guillaume Lucas for feedback on the manuscript. This work was supported by funding from INSERM, CNRS, and Agence Nationale pour la Recherche (ANR). We thank the Animal Housing and Genotyping facilities, supported by funding from INSERM and LabEX BRAIN (ANR-10-LABX-43). MICM was supported by an international PhD fellowship (ANR-10-IDEX-03-02). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
XL conceived the project. MICM, MG, AF, and XL planned the research. MICM, FM, MCM, EA, CD, ES, GB, MG, and XL performed data acquisition. MICM, FM, EA, CD, SP, GB, AM, and XL analyzed the data. MICM and XL wrote the paper and all other authors provided feedback.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Andrea S, Jacena LM, Patrick A, Rawi N, Tasleem C, John O et al (2013). Electrocortical changes associated with minocycline treatment in fragile X syndrome. J Psychopharmacol (Oxford, England) 27: 956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett MT, Herman DH, McGee AW (2014). Deficits in tactile learning in a mouse model of fragile X syndrome. PLoS ONE 9: e109116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranek GT, Foster LG, Berkson G (1997). Tactile defensiveness and stereotyped behaviors. Am J Occup Ther 51: 91–95. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Sumis A, Hervey C, Nelson M, Porges SW, Weng N et al (2008). Open-label treatment trial of lithium to target the underlying defect in fragile X syndrome. J Dev Behav Pediatr 29: 293–302. [DOI] [PubMed] [Google Scholar]
- Castrén M, Pääkkönen A, Tarkka IM, Ryynänen M, Partanen J (2003). Augmentation of auditory N1 in children with fragile X syndrome. Brain Topogr 15: 165–171. [DOI] [PubMed] [Google Scholar]
- Chen L, Toth M (2001). Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience 103: 1043–1050. [DOI] [PubMed] [Google Scholar]
- Contractor A, Klyachko VA, Portera-Cailliau C (2015). Altered neuronal and circuit excitability in fragile X syndrome. Neuron 87: 699–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailly E, Hascoët M, Colombel MC, Jolliet P, Bourin M (2002). Relationship between cerebral pharmacokinetics and anxiolytic activity of diazepam and its active metabolites after a single intra-peritoneal administration of diazepam in mice. Hum Psychopharmacol 17: 239–245. [DOI] [PubMed] [Google Scholar]
- Dansie LE, Phommahaxay K, Okusanya AG, Uwadia J, Huang M, Rotschafer SE et al (2013). Long-lasting effects of minocycline on behavior in young but not adult Fragile X mice. Neuroscience 246: 186–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Diego-Otero Y, Romero-Zerbo Y, Bekay RE, Decara J, Sanchez L, Fonseca FR-D et al (2008). [alpha]-Tocopherol protects against oxidative stress in the fragile X knockout mouse: an experimental therapeutic approach for the Fmr1 deficiency. Neuropsychopharmacology 34: 1011–1026. [DOI] [PubMed] [Google Scholar]
- Deacon R (2012). Assessing burrowing, nest construction, and hoarding in mice. J Vis Exp: e2607. [DOI] [PMC free article] [PubMed]
- Deacon RMJ (2006). Assessing nest building in mice. Nat Protoc 1: 1117–1119. [DOI] [PubMed] [Google Scholar]
- Deng PY, Klyachko VA (2016). Genetic upregulation of BK channel activity normalizes multiple synaptic and circuit defects in a mouse model of fragile X syndrome. J Physiol 594: 83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Rotman Z, Blundon JA, Cho Y, Cui J, Cavalli V et al (2013). FMRP regulates neurotransmitter release and synaptic information transmission by modulating action potential duration via BK channels. Neuron 77: 696–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Dutch-Belgian Fragile X Consortium, Bakker CE, Verheij C, Willemsen R, van der Helm R, Oerlemans F et al (1994). Fmr1 knockout mice: a model to study fragile X mental retardation. Cell 78: 23–33. [PubMed] [Google Scholar]
- Ethridge LE, White SP, Mosconi MW, Wang J, Byerly MJ, Sweeney JA (2016). Reduced habituation of auditory evoked potentials indicate cortical hyper-excitability in fragile X syndrome. Transl Psychiatry 6: e787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Wang Y, Rosner B, Shimizu T, Balleine BW, Dykens EM et al (2004). Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Mol Psychiatry 9: 417–425. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Bartley AF, Hays SA, Huber KM (2008). Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J Neurophysiol 100: 2615–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves JT, Anstey JE, Golshani P, Portera-Cailliau C (2013). Circuit level defects in the developing neocortex of Fragile X mice. Nat Neurosci 16: 903–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ (2006). Lessons from fragile X regarding neurobiology, autism, and neurodegeneration. J Dev Behav Pediatr 27: 63–74. [DOI] [PubMed] [Google Scholar]
- Hébert B, Pietropaolo S, Même S, Laudier B, Laugeray A, Doisne N et al (2014). Rescue of fragile X syndrome phenotypes in Fmr1 KO mice by a BKCa channel opener molecule. Orphanet J Rare Dis 9: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen BS (2002). BMS-204352: a potassium channel opener developed for the treatment of stroke. CNS Drug Rev 8: 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirkof P (2014). Burrowing and nest building behavior as indicators of well-being in mice. J Neurosci Methods 234: 139–146. [DOI] [PubMed] [Google Scholar]
- Kazdoba TM, Leach PT, Silverman JL, Crawley JN (2014). Modeling fragile X syndrome in the Fmr1 knockout mouse. Intractable Rare Dis Res 3: 118–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramvis I, Mansvelder H, Loos M, Meredith R (2013). Hyperactivity, perseveration and increased responding during attentional rule acquisition in the fragile X mouse model. Front Behav Neurosci 7: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger DD, Bear MF (2011). Toward fulfilling the promise of molecular medicine in fragile X syndrome. Annu Rev Med 62: 411–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovelace JW, Wen TH, Reinhard S, Hsu MS, Sidhu H, Ethell IM et al (2016). Matrix metalloproteinase-9 deletion rescues auditory evoked potential habituation deficit in a mouse model of Fragile X Syndrome. Neurobiol Dis 89: 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton CH, Moon J, Strawderman MS, Maclean KN, Evans J, Strupp BJ (2008). Evidence for social anxiety and impaired social cognition in a mouse model of fragile X syndrome. Behav Neurosci 122: 293–300. [DOI] [PubMed] [Google Scholar]
- Merenstein SA, Sobesky WE, Taylor AK, Riddle JE, Tran HX, Hagerman RJ (1996). Molecular-clinical correlations in males with an expanded FMR1 mutation. Am J Med Genet 64: 388–394. [DOI] [PubMed] [Google Scholar]
- Meshalkina DA, Kalueff AV (2016). Commentary: Ethological evaluation of the effects of social defeat stress in mice: beyond the social interaction ratio. Front Behav Neurosci 10: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalon A, Sidorov M, Ballard Theresa M, Ozmen L, Spooren W, Wettstein Joseph G et al (2012). Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron 74: 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mientjes EJ, Nieuwenhuizen I, Kirkpatrick L, Zu T, Hoogeveen-Westerveld M, Severijnen L et al (2006). The generation of a conditional Fmr1 knock out mouse model to study Fmrp function in vivo. Neurobiol Dis 21: 549–555. [DOI] [PubMed] [Google Scholar]
- Miller LJ, McIntosh DN, McGrath J, Shyu V, Lampe M, Taylor AK et al (1999). Electrodermal responses to sensory stimuli in individuals with fragile X syndrome: a preliminary report. Am J Med Genet 83: 268–279. [PubMed] [Google Scholar]
- Mineur YS, Sluyter F, Wit S, Oostra BA, Crusio WE (2002). Behavioral and neuroanatomical characterization of the Fmr1 knockout mouse. Hippocampus 12: 39–46. [DOI] [PubMed] [Google Scholar]
- Moon J, Beaudin AE, Verosky S, Driscoll LL, Weiskopf M, Levitsky DA et al (2006). Attentional dysfunction, impulsivity, and resistance to change in a mouse model of fragile X syndrome. Behav Neurosci 120: 1367–1379. [DOI] [PubMed] [Google Scholar]
- Moon J, Ota KT, Driscoll LL, Levitsky DA, Strupp BJ (2008). A mouse model of fragile X syndrome exhibits heightened arousal and/or emotion following errors or reversal of contingencies. Dev Psychobiol 50: 473–485. [DOI] [PubMed] [Google Scholar]
- Musumeci SA, Bosco P, Calabrese G, Bakker C, De Sarro GB, Elia M et al (2000). Audiogenic seizures susceptibility in transgenic mice with fragile X syndrome. Epilepsia 41: 19–23. [DOI] [PubMed] [Google Scholar]
- Myrick LK, Deng P-Y, Hashimoto H, Oh YM, Cho Y, Poidevin MJ et al (2015). Independent role for presynaptic FMRP revealed by an FMR1 missense mutation associated with intellectual disability and seizures. Proc Natl Acad Sci USA 112: 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen DM, Derber WJ, McClellan DA, Crnic LS (2002). Alterations in the auditory startle response in Fmr1 targeted mutant mouse models of fragile X syndrome. Brain Res 927: 8–17. [DOI] [PubMed] [Google Scholar]
- Oddi D, Subashi E, Middei S, Bellocchio L, Lemaire-Mayo V, Guzman M et al (2015). Early social enrichment rescues adult behavioral and brain abnormalities in a mouse model of fragile X syndrome. Neuropsychopharmacology 40: 1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos-Serrano JL, Corbin JG, Burns MP (2011). The GABA-A receptor agonist THIP ameliorates specific behavioral deficits in the mouse model of fragile X syndrome. Dev Neurosci 33: 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paribello C, Tao L, Folino A, Berry-Kravis E, Tranfaglia M, Ethell IM et al (2010). Open-label add-on treatment trial of minocycline in fragile X syndrome. BMC Neurol 10: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peier AM, McIlwain KL, Kenneson A, Warren ST, Paylor R, Nelson DL (2000). Over)correction of FMR1 deficiency with YAC transgenics: behavioral and physical features. Hum Mol Genet 9: 1145–1159. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Lopez B, Maron L, Lincoln A (2007). Sensorimotor gating deficits in adults with autism. Biol Psychiatry 61: 482–486. [DOI] [PubMed] [Google Scholar]
- Pietropaolo S, Guilleminot A, Martin B, D’Amato FR, Crusio WE (2011). Genetic-background modulation of core and variable autistic-like symptoms in Fmr1 knock-out mice. PLoS ONE 6: e17073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razali NM, Bee Wah Y (2011). Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson-Darlingtests. J Stat Model Analyt 2: 21–33. [Google Scholar]
- Restivo L, Ferrari F, Passino E, Sgobio C, Bock J, Oostra BA et al (2005). Enriched environment promotes behavioral and morphological recovery in a mouse model for the fragile X syndrome. Proc Natl Acad Sci USA 102: 11557–11562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotschafer S, Razak K (2013). Altered auditory processing in a mouse model of fragile X syndrome. Brain Res 1506: 12–24. [DOI] [PubMed] [Google Scholar]
- Schneider A, Leigh MJ, Adams P, Nanakul R, Chechi T, Olichney J et al (2013). Electrocortical changes associated with minocycline treatment in fragile X syndrome. J Psychopharmacol 27: 956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Berridge KC, Kalueff AV (2016). 'Stressing' rodent self-grooming for neuroscience research. Nat Rev Neurosci 17: 591–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer CM, Alekseyenko O, Hamilton SM, Thomas AM, Serysheva E, Yuva-Paylor LA et al (2011). Modifying behavioral phenotypes in Fmr1KO mice: genetic background differences reveal autistic-like responses. Autism Res 4: 40–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons FJ, Clark RD, Hatton DD, Skinner M, Bailey DB (2003). Self-injurious behavior in young boys with fragile X syndrome. Am J Med Genet A 118A: 115–121. [DOI] [PubMed] [Google Scholar]
- van Erp AMM, Kruk MR, Meelis W, Willekens-Bramer DC (1994). Effect of environmental stressors on time course, variability and form of self-grooming in the rat: handling, social contact, defeat, novelty, restraint and fur moistening. Behav Brain Res 65: 47–55. [DOI] [PubMed] [Google Scholar]
- Verkerk AJMH, Pieretti M, Sutcliffe JS, Fu Y-H, Kuhl DPA, Pizzuti A et al (1991). Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65: 905–914. [DOI] [PubMed] [Google Scholar]
- Yuhas J, Cordeiro L, Tassone F, Ballinger E, Schneider A, Long JM et al (2011). Brief report: sensorimotor gating in idiopathic autism and autism associated with fragile X syndrome. J Autism Dev Disord 41: 248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bonnan A, Bony G, Ferezou I, Pietropaolo S, Ginger M et al (2014). Dendritic channelopathies contribute to neocortical and sensory hyperexcitability in Fmr1(−/y) mice. Nat Neurosci 17: 1701–1709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.