Abstract
Stem cells are critical for normal tissue homeostasis and injury‐induced regeneration, but how wounding affects tissue regeneration at the cellular and molecular levels is not yet fully understood. A new study by Yui et al (2018) demonstrates that the extracellular matrix (ECM) remodeling modulates intestinal stem cells in tissue repair and regeneration via activation of the Hippo pathway effectors YAP/TAZ.
Subject Categories: Development & Differentiation, Signal Transduction, Stem Cells
The epithelium of the mammalian intestine has a short half‐life and undergoes constant regeneration under physiological conditions. The intestinal stem cells (ISCs) residing at the crypt base are critical for normal intestine homeostasis. Upon tissue damage, ISCs rapidly expand and differentiate into mature intestinal epithelial cells to restore tissue architecture (Baker, 2014). Previous work has shown that induced pluripotent stem cells (iPSCs) can develop into fetal‐like intestinal stem cells that can be propagated in vitro when Wnt signaling is inhibited (Fordham et al, 2013). These fetal‐like stem cells can contribute to wound repair, replenishment of lost stem cells, and restoration of tissue architecture (Blanpain & Fuchs, 2014). A new study by Yui et al (2018) shows that intestinal tissue damage can also induce fetal‐like stem cells through ECM remodeling, increased FAK/Src signaling, and YAP/TAZ activation, thereby contributing to intestinal epithelial tissue repair (Fig 1).
Figure 1. YAP/TAZ mediate extracellular signals to intestinal stem cell activation and tissue repair.
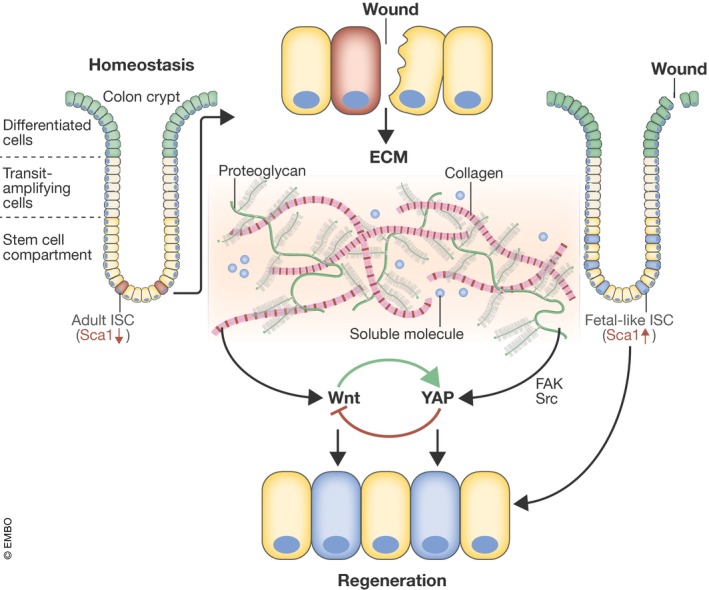
Upon tissue damage, remodeling of extracellular matrix such as collagen/Wnt could activate FAK/Src and YAP/TAZ, leading to stem cell activation and tissue regeneration.
Yui et al (2018) found that the mouse repairing epithelium (RE) exhibits high expression of the fetal‐like ISC marker SCA1, compared to homeostatic tissue. The SCA1high cells also show low expression of some markers of adult ISCs. Notably, Sca1 is transcriptionally upregulated in fetal but not adult epithelium organoids. The authors also examined clinical samples and observed that fetal progenitor cell markers, such as ANXA1 and TROP2, were highly expressed in inflammatory regions of the diseased intestine, where the epithelium was in a state of regeneration, compared to non‐inflamed regions. Thus, the RE expressed primitive fetal‐associated markers and displayed characteristics of fetal ISCs (Fig 1).
The authors then explored the molecular mechanism underlying the ISC rejuvenation of the repairing epithelium, comparing the expression profiles of homeostatic and repairing tissues. Some ECM components, such as collagen, were highly elevated in the RE. Moreover, the regenerating tissue showed increased levels of collagen receptor β1 integrin subunit and FAK at the basolateral cell surface, as well as enhanced levels of phosphorylated SRC (pSRC) and loss of polarized F‐actin filaments. These results suggest that RE reprogramming might be due to exposure to the altered environmental stimuli and mechanical properties at the wound bed. To further clarify whether the altered ECM environment of RE affects tissue repair, the authors treated the tissue with FAK and Src inhibitors during the transition from ulceration to the reparation phase. They found that inhibiting either FAK or SRC delayed repair following injury. Consistent with these observations, another recent study using genetic deletion of Csk, a negative regulator of SRC, also supported positive roles for SRC and FAK in intestinal epithelial homeostasis (Imada et al, 2016). Thus, the ECM‐mediated FAK pathway signaling is important for colonic repair.
To identify environmental cues that regulate the RE, Yui et al (2018) used a 3D culture system. The authors found that combined stimulation of the canonical Wnt pathway and collagen type I reproduced a RE‐like phenotype in vitro. Importantly, inhibiting FAK and SRC reduced organoid growth in collagen type I. In addition, inhibitors affecting signaling downstream of integrins via Rho kinase (i.e., C3 toxin mevastatin) and actin polymerization (cytochalasin D) also significantly reduced growth in collagen type I. Thus, ECM‐mediated integrin signaling is important for organoid growth.
Finally, the authors asked whether YAP/TAZ are involved in RE remodeling. To address this question, they compared YAP subcellular localization and target gene expression between homeostatic and repairing tissues. They found that the numbers of cells with nuclear YAP were highly elevated during the repairing phase and the well‐characterized YAP/TAZ target gene signature was also significantly increased in the RE. Blockade of FAK and SRC signaling or Wnt signaling reduced the number of cells with nuclear YAP during tissue regeneration. These results indicate that YAP/TAZ act downstream of ECM and Wnt signaling in repairing epithelial cells. Further evidence from studies with YAP/TAZ gain‐ or loss‐of‐function experiments showed that YAP/TAZ were necessary and sufficient to promote the RE‐like state in vitro and tissue regeneration in vivo. Yui et al (2018) also demonstrated that regeneration was a reversible process between a homeostatic and repair‐like state with the collagen type I culture system.
Taken together, the study by Yui et al (2018) sheds new light on the mechanisms of stem cell rejuvenation and tissue regeneration in response to injury. Remodeling of the ECM activates the focal adhesion signaling pathway and cytoskeletal re‐arrangement, therefore leading to YAP/TAZ activation that contributes to transition of the fetal‐like ISCs during tissue repair. Mechanistically, YAP/TAZ integrate hormonal signals, such as Wnt, and mechanical signals, such as the stiffness and composition of ECM, during intestinal epithelial repair to promote fetal‐like stem cells and tissue regeneration (Fig 1).
The study by Yui et al (2018) reveals a critical role of YAP/TAZ in cellular reprogramming, particularly in fetal‐like ISCs during wound healing. This knowledge may aid future therapy, particularly for patients with inflammatory diseases such as ulcerative colitis and Crohn's disease, where the intestines undergo constant tissue repair. However, some key questions still remain: For instance, the exact role of YAP/TAZ in ISCs is not yet fully resolved. It has been reported that YAP restricts the expansion and regeneration of ISCs by inhibiting Wnt signaling, which has a prominent positive role in ISC maintenance and tissue homeostasis (Barry et al, 2013). Moreover, previous studies have shown that YAP could reprogram the Lgr5(+) intestinal stem cells by inhibiting the Wnt homeostatic program and thus promote the renewal of intestinal epithelium through the regulation of stem/progenitor cell proliferation and differentiation (Gregorieff et al, 2015; Imajo et al, 2015). Collectively, these reports all support a critical role of YAP/TAZ in intestinal stem cell function and tissue regeneration, but more studies are needed to clarify the exact role of YAP/TAZ in ISCs. Notably, Wnt activates YAP/TAZ, whereas YAP/TAZ exert a negative feedback to inhibit Wnt signaling (Azzolin et al, 2012; Barry et al, 2013; Gregorieff et al, 2015; Park et al, 2015). One possible model is that a balanced activity of both Wnt and YAP/TAZ controls ISCs and tissue regeneration (Fig 1). Too much YAP/TAZ activity (e.g., overexpression of active YAP/TAZ or knockout of upstream inhibitors of Hippo components) may cause a significant inhibition of Wnt and thus interfere with the function of Wnt in stem cells. On the other hand, too little YAP/TAZ activity (e.g., genetic deletion of YAP/TAZ) may also impair intestinal homeostasis and regeneration, since YAP/TAZ are required for stem cell maintenance. Other open questions to consider: what controls the remodeling of the extracellular matrix? How do YAP/TAZ regulate the fate between homeostasis (adult ISCs) and repair tissue (fetal‐like ISCs)? As YAP/TAZ are downstream effectors of the Hippo pathway, it remains to be addressed whether upstream Hippo pathway kinases, such as LATS1/2, are involved in the rejuvenation and expansion of fetal‐like stem cells in the regenerating intestine. In addition, more specific and potent YAP/TAZ inhibitors would be valuable to aid functional study of these transcription co‐activators in order to circumvent the effects of feedback regulation because genetic manipulation is long term and can be complicated by physiological feedback regulation.
Conflict of interest
K.L.G. is a co‐founder and has an equity interest in Vivace Therapeutics, Inc. The terms of this arrangement have been reviewed and approved by the University of California, San Diego, in accordance with its conflict of interest policies.
See also: S Yui et al (January 2018)
References
- Azzolin L, Zanconato F, Bresolin S, Forcato M, Basso G, Bicciato S, Cordenonsi M, Piccolo S (2012) Role of TAZ as mediator of Wnt signaling. Cell 151: 1443–1456 [DOI] [PubMed] [Google Scholar]
- Baker N (2014) Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol 15: 19–33 [DOI] [PubMed] [Google Scholar]
- Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, Ogino S, Kuo CJ, Camargo FD (2013) Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature 493: 106–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E (2014) Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science 344: 1242281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordham RP, Yui S, Hannan NR, Soendergaard C, Madgwick A, Schweiger PJ, Nielsen OH, Vallier L, Pedersen RA, Nakamura T, Watanabe M, Jensen KB (2013) Transplantation of expanded fetal intestinal progenitors contributes to colon regeneration after injury. Cell Stem Cell 13: 734–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL (2015) Yap‐dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature 526: 715–718 [DOI] [PubMed] [Google Scholar]
- Imada S, Murata Y, Kotani T, Hatano M, Sun C, Konno T, Park JH, Kitamura Y, Saito Y, Ohdan H, Matozaki T (2016) Role of Src family kinases in regulation of intestinal epithelial homeostasis. Mol Cell Biol 36: 2811–2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imajo M, Ebisuya M, Nishida E (2015) Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nat Cell Biol 17: 7–19 [DOI] [PubMed] [Google Scholar]
- Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW, Meng Z, Lin KC, Yu FX, Alexander CM, Wang CY, Guan KL (2015) Alternative Wnt signaling activates YAP/TAZ. Cell 162: 780–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yui S, Azzolin L, Maimets M, Terndrup Pedersen M, Fordham RP, Hansen SL, Larsen HL, Guiu J, Alves MRP, Rundsten CF, Johansen JV, Li Y, Madsen CD, Nakamura T, Watanabe M, Nielsen OH, Schweiger PJ, Piccolo S, Jensen KB (2018) YAP/TAZ‐dependent reprogramming of colonic epithelium links ECM remodeling to tissue regeneration. Cell Stem Cell 22: 35–49.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]


