Abstract
The extended synaptotagmins (E‐Syts) are endoplasmic reticulum (ER) proteins that bind the plasma membrane (PM) via C2 domains and transport lipids between them via SMP domains. E‐Syt1 tethers and transports lipids in a Ca2+‐dependent manner, but the role of Ca2+ in this regulation is unclear. Of the five C2 domains of E‐Syt1, only C2A and C2C contain Ca2+‐binding sites. Using liposome‐based assays, we show that Ca2+ binding to C2C promotes E‐Syt1‐mediated membrane tethering by releasing an inhibition that prevents C2E from interacting with PI(4,5)P2‐rich membranes, as previously suggested by studies in semi‐permeabilized cells. Importantly, Ca2+ binding to C2A enables lipid transport by releasing a charge‐based autoinhibitory interaction between this domain and the SMP domain. Supporting these results, E‐Syt1 constructs defective in Ca2+ binding in either C2A or C2C failed to rescue two defects in PM lipid homeostasis observed in E‐Syts KO cells, delayed diacylglycerol clearance from the PM and impaired Ca2+‐triggered phosphatidylserine scrambling. Thus, a main effect of Ca2+ on E‐Syt1 is to reverse an autoinhibited state and to couple membrane tethering with lipid transport.
Keywords: C2 domain, extended synaptotagmin, lipid transfer, phosphatidylserine scrambling, SMP domain
Subject Categories: Membrane & Intracellular Transport
Introduction
Endoplasmic reticulum (ER)‐plasma membrane (PM) contact sites represent a general feature of all eukaryotic cells (Friedman & Voeltz, 2011; Gallo et al, 2016; Saheki & De Camilli, 2017a). Their occurrence reflects the presence of proteins that tether the two membranes and mediate cross talk between them. One such class of tethers is the extended synaptotagmins (E‐Syts), resident proteins of the ER membrane that are evolutionarily conserved from unicellular organisms to all metazoans (Craxton, 2001, 2007; Manford et al, 2012; Toulmay & Prinz, 2012; Giordano et al, 2013; Levy et al, 2015; Perez‐Sancho et al, 2015). In mammals, they are encoded by three different genes, E‐Syt1, E‐Syt2, and E‐Syt3 (Min et al, 2007), which form homo‐ and heterodimers. All three E‐Syts comprise an N‐terminal hydrophobic hairpin through which they are anchored to the ER (Giordano et al, 2013; Saheki et al, 2016). This region is followed by a synaptotagmin‐like mitochondrial lipid‐binding protein (SMP) domain (Lee & Hong, 2006; Kopec et al, 2010; Toulmay & Prinz, 2012; Schauder et al, 2014) and multiple C2 domains, five in E‐Syt1 and three in E‐Syt2 and E‐Syt3 (see Fig 1A; Min et al, 2007; Saheki & De Camilli, 2017b). As shown by the crystal structure, the first two C2 domains of E‐Syt2 (C2AB) are arranged in tandem (Schauder et al, 2014; Xu et al, 2014) and a similar arrangement is predicted for the C2AB domains of E‐Syt1 and E‐Syt3. The last C2 domain (C2E for E‐Syt1 and C2C for E‐Syt2 and E‐Syt3) is characterized by a positively charged surface (Idevall‐Hagren et al, 2015), and the additional C2 domains unique to E‐Syt1 (C2CD) are similar to C2AB and most likely represent a duplication of this pair (Min et al, 2007). The recruitment of E‐Syts to ER‐PM contact sites requires PI(4,5)P2 in the PM and occurs constitutively in the case of E‐Syt2 and E‐Syt3, while it requires elevation of cytosolic Ca2+ in the case of E‐Syt1 (Chang et al, 2013; Giordano et al, 2013; Fernandez‐Busnadiego et al, 2015; Idevall‐Hagren et al, 2015).
Figure 1. E‐Syt1cyto specifically binds to PI(4,5)P2‐containing membranes in a C2E‐dependent way in the absence of Ca2+ and C2C, but not C2A, stimulates this binding in the presence of Ca2+ .

-
ADomain structures of E‐Syts (left) and E‐Syt1 constructs (right) used for the liposome tethering assays shown in the figure. Slanted white lines indicate the mutations in the Ca2+ binding sites in C2A and C2C domains.
-
B–DLiposome aggregation, due to tethering of anchoring and target liposomes in the presence of E‐Syt1 constructs (protein:lipid ratio 1:1,000) at RT, as assessed by increase in turbidity (OD 405 nm). Time‐courses are at left, and bar graphs showing quantification of OD405 increases at the end of the incubation (arrows in the left panel) are at right. (B) Effect of the lipid composition and C2E on liposome tethering by E‐Syt1cyto in the absence of Ca2+. A cocktail of EGTA, imidazole, and proteinase K (Cocktail) was added after 10 min. (C) Effect of the absence or presence of Ca2+ on liposome tethering by E‐Syt1cyto with or without mutations in the Ca2+‐binding sites in C2A and C2C. (D) Effect of the absence or presence of Ca2+ and of PI(4,5)P2 in the target liposomes on liposome tethering by E‐Syt1cyto lacking the C2E domain. Mean and SD of three independent experiments. P‐values from t‐test with Bonferroni corrections are quoted on the graphs. ****P < 0.0001; n.s., not significant.
The defining feature of the E‐Syts among proteins with multiple C2 domains is the presence of the SMP domain (Lee & Hong, 2006), a member of the TULIP domain superfamily (Kopec et al, 2010). A shared characteristic of TULIP domains, which are present both in extracellular and intracellular proteins, is the property to harbor lipids within a hydrophobic cavity and, at least in many cases, to transport them (Oram et al, 2003; Qiu et al, 2007; Kopec et al, 2011; Schauder et al, 2014; AhYoung et al, 2015; Alva & Lupas, 2016; Jeong et al, 2016; Saheki et al, 2016; Yu et al, 2016; Lees et al, 2017; Liu et al, 2017). SMP domains are typically found in intracellular proteins that act at membrane contact sites (Kornmann et al, 2009; Toulmay & Prinz, 2012; Reinisch & De Camilli, 2016; Lees et al, 2017; Liu et al, 2017). Structural and biochemical studies of the SMP domain of E‐Syt2 revealed that it dimerizes to form a 90‐Å‐long cylinder with a deep hydrophobic groove that runs along its main axis and that contains glycerophospholipids (Schauder et al, 2014). Consistent with these properties, purified recombinant E‐Syt1 transfers glycerolipids between two populations of liposomes that mimic the ER and the PM, respectively (Saheki et al, 2016; Yu et al, 2016), without selectivity for a specific headgroup (Schauder et al, 2014; Hoglinger et al, 2017).
Genome‐edited cells lacking all the three E‐Syts showed no major differences in the steady‐state glycerolipids compositions of the PM. However, delayed clearance of the transient accumulation of diacylglycerol (DAG) at the PM produced by phospholipase C (PLC)‐dependent PI(4,5)P2 hydrolysis was observed (Saheki et al, 2016), suggesting a role of E‐Syts in the homeostatic response that follows a stimulus. This phenotype was rescued by expression of E‐Syt1, but not by E‐Syt1 lacking the SMP domain. Additionally, rescue required an elevation in cytosolic Ca2+ (Saheki et al, 2016). Whether other changes at the PM occur in response to stimuli in the E‐Syts KO cells remain unclear.
Of the five C2 domains in E‐Syt1, two (C2A and C2C) bind Ca2+ (Min et al, 2007). Ca2+ binding to the C2C domain is responsible for ER tethering to the PM, as mutations that impair its Ca2+ binding were sufficient to abolish Ca2+‐dependent recruitment of E‐Syt1 to the PM (Chang et al, 2013; Giordano et al, 2013). The mechanism involved in this effect is not fully understood yet. It may be mediated by (i) a direct interaction of the C2C domain with the PI(4,5)P2‐rich PM (Giordano et al, 2013), (ii) the release by Ca2+ of an inhibitory action of the C2C domain on the binding of C2E to the PI(4,5)P2‐rich PM (Idevall‐Hagren et al, 2015), or (iii) both mechanisms. In addition, mutations in the Ca2+‐binding sites of either the C2A or the C2C domain reduced the lipid transfer activity of E‐Syt1 in vitro (Yu et al, 2016). However, the function of C2A in SMP domain‐dependent lipid transfer remains elusive. Interestingly, the C2A domain of E‐Syt2 and E‐Syt3, two proteins whose binding to the PM is not regulated by Ca2+, also contains Ca2+‐binding sites. A plausible scenario is that the C2A domain of the E‐Syts may play a regulatory role in lipid transfer independent of tethering. Goal of this study was to test this hypothesis.
Here, we find that the Ca2+‐dependent properties of the C2A and C2C domains of E‐Syt1 have different functions at membrane contact sites. Using a liposome‐based assay, we confirm that Ca2+ binding to the C2C domain acts primarily by enabling the binding of the C2E domain to PI(4,5)P2‐rich membranes. Importantly, we show that Ca2+ binding to the C2A domain enables lipid transfer by E‐Syt1 via the release of an autoinhibitory intramolecular interaction of this domain with the SMP domain. We also show that Ca2+ binding to the C2A and C2C domains plays important roles in the E‐Syt1‐dependent regulation of lipid homeostasis at the PM in living cells.
Results
C2C and C2E domains of E‐Syt1 cooperate in membrane tethering
The contributions of individual C2 domains in E‐Syt1‐mediated membrane tethering were assessed using an in vitro liposome turbidity assay (Saheki et al, 2016). To this aim, purified cytosolic fragment of human E‐Syt1, in which the N‐terminal region (including the hydrophobic hairpin) was replaced by a His‐tag (E‐Syt1cyto, Fig 1A), was added to the mixture of two populations of liposomes. One liposome population (anchoring liposomes, ER‐like in composition) comprised phosphatidylcholine (PC), a nickel‐conjugated lipid [DGS‐NTA(Ni)] that functions as a binding site for the His‐tagged proteins, and NBD‐phosphatidylethanolamine (PE). The other liposome population (target liposomes, PM‐like in composition) comprised PC and two acidic phospholipids, phosphatidylserine (PS) and PI(4,5)P2. The increase in turbidity, which reflects clustering of liposomes into larger particles, was measured as optical density at 405 nm.
Addition to the liposome mixture of E‐Syt1cyto in a buffer devoid of Ca2+ resulted in an increase in optical density (turbidity; Fig 1B). Upon addition of a “cocktail” of EGTA, imidazole (to disrupt the nickel His‐tag interaction) and proteinase K at the end of incubation, the increase in optical density was reversed, ruling out the fusion of liposomes as the cause of the increase in optical density (Fig 1B). No change in optical density was observed in the absence of E‐Syt1cyto, of DGS‐NTA(Ni) in the anchoring liposomes, or of the target liposomes (Fig 1B). We conclude that although E‐Syt1 is primarily diffuse throughout the ER when expressed alone, under in vitro conditions, it can tether ER‐like to PM‐like liposomes even in the absence of Ca2+. Actually, low level of E‐Syt1‐dependent ER‐PM contact sites can be observed at resting Ca2+ concentration in the cells (Giordano et al, 2013; Fernandez‐Busnadiego et al, 2015).
It was shown that the C2E domain of E‐Syt1 shares the properties of the C2C domains of E‐Syt2 and E‐Syt3, which in these two proteins mediate constitutive PI(4,5)P2‐dependent PM binding (Giordano et al, 2013; Fernandez‐Busnadiego et al, 2015; Idevall‐Hagren et al, 2015). All these three C‐terminal C2 domains lack a Ca2+‐binding site and have a highly basic surface in common (Idevall‐Hagren et al, 2015). Accordingly, and consistently with studies in cells (Fernandez‐Busnadiego et al, 2015; Idevall‐Hagren et al, 2015), a mutant E‐Syt1cyto that lacks the C2E domain (SMP‐C2ABCD, Fig 1A) failed to tether target liposomes in the absence of Ca2+ (Fig 1B). Lack of PI(4,5)P2 in the target liposomes, or replacement of the 5% PI(4,5)P2 with 20% PS in these liposomes, also dramatically reduced E‐Syt1cyto‐mediated Ca2+‐independent membrane tethering (Fig 1B). These results indicate that in the liposome‐based system, the cytosolic domain of E‐Syt1 mediates tethering of the two classes of liposomes in a C2E‐dependent way in the absence of Ca2+.
We next investigated the role of Ca2+ in liposome tethering using the same liposome‐based assay. Addition of Ca2+ (100 μM) resulted in a strong increase in the basal tethering by E‐Syt1cyto observed in the Ca2+‐free buffer (Fig 1C). E‐Syt1 contains putative Ca2+‐binding sites in its C2A and C2C domains (Min et al, 2007; Giordano et al, 2013; Xu et al, 2014). Mutations of key residues that mediate Ca2+ binding in the C2C domain of the construct (E‐Syt1cyto C2Cx, Fig 1A) only showed a slightly increased Ca2+‐dependent membrane tethering (Fig 1C). In contrast, mutations in the Ca2+‐binding sites in the C2A domain (E‐Syt1cyto C2Ax, Fig 1A) still produced a significant increase in Ca2+‐dependent liposome tethering (Fig 1C). Both of these mutants had a similar level of Ca2+‐independent basal liposome tethering (Fig 1C). These findings were consistent with the previous reports that the C2C domain is required for Ca2+‐dependent ER‐PM tethering of E‐Syt1 in intact cells (Chang et al, 2013; Giordano et al, 2013).
A lower degree of Ca2+‐dependent tethering occurred also with an E‐Syt1cyto fragment lacking the C2E domain (SMP‐C2ABCD, Fig 1A and D). This tethering did not require PI(4,5)P2 in target liposomes (Fig 1D) and occurred at a similar level for both constructs harboring Ca2+‐binding mutations in either the C2A domain (SMP‐C2AxBCD) or the C2C domain (SMP‐C2ABCxD; Fig EV1A), although binding of SMP‐C2ABCxD occurred with slower kinetics (Fig EV1B). In addition, a similar dependence on Ca2+ concentration [half maximal effective concentration (EC50)] was observed for the binding of SMP‐C2AxBCD and SMP‐C2ABCxD to membranes, as revealed by the liposome tethering assay (Fig EV1D). Given that E‐Syt1cyto C2Ax showed a much stronger Ca2+‐dependent stimulation of tethering than E‐Syt1cyto C2Cx (Fig 1C), and C2E does not bind Ca2+, we conclude that the increased E‐Syt1cyto‐mediated liposome tethering in the presence of Ca2+ mainly relies on a synergistic effect between Ca2+‐bound C2C and C2E. This most likely occurs via the release of an inhibitory action of C2C affecting C2E binding to the PI(4,5)P2‐rich membranes, since an E‐Syt1 construct lacking the C2ABCD region (SMP‐C2E, Fig EV1A) tethered the liposomes at a higher level than E‐Syt1cyto in the absence of Ca2+ (Fig EV1C). This inhibition did not completely abolish the membrane binding of C2E in the absence of Ca2+, as C2E‐dependent and Ca2+‐independent liposome tethering by E‐Syt1cyto could be observed (Fig 1B).
Figure EV1. Ca2+ binding to C2C promotes E‐Syt1cyto‐mediated liposome tethering by releasing an inhibitory action of C2C on the membrane binding of C2E.

-
ADomain structures of E‐Syt1 constructs used for the liposomes tethering assays shown in (B and C). Asterisks indicate the mutations in the membrane binding surface of C2A domain.
-
B, CTethering of anchoring and target liposomes in the presence of E‐Syt1 constructs at RT as assessed by increase in turbidity (OD 405 nm). In each of the panels, time‐courses are at left and bar graphs showing quantification of OD405 increases at the end of the incubation (arrows in the left panels) are at right. (B) Effect of the absence or presence of Ca2+ on liposome tethering by SMP‐C2AxBCD, SMP‐C2ABCxD, and SMP‐C2AmBCxD. Mean and SD of three independent experiments. P‐values from t‐test with Bonferroni corrections are quoted on the graphs. ****P < 0.0001; n.s. not significant. (C) Liposome tethering by E‐Syt1cyto with or without C2ABCD domains in the absence of Ca2+. Mean and SD of three independent experiments. P‐values from two‐tailed Student's t‐test are quoted on the graphs. ***P < 0.001.
-
DSMP‐C2ABCxD and SMP‐C2AxBCD bind to liposomes in a Ca2+‐dependent manner, as revealed by liposome tethering. OD405 readings were normalized to the maximum value. Mean and SD of three independent experiments.
-
ELeft: Purified C2CD and C2E were incubated in the presence of the cross‐linker BS3 and in the absence or presence of Ca2+ and liposomes. The left lane shows molecular weight markers, with sizes indicated in kilodaltons. The cross‐linked heterodimer, as confrmed by mass spectrometry, is indicated by the red arrowhead. Right: Normalized intensity of cross‐linked heterodimer was plotted. Mean and SD of three independent experiments. P‐values from t‐test with Bonferroni corrections are quoted on the graphs. **P < 0.01; n.s., not significant.
-
FSequence of C2CD and C2E used for cross‐linking assay. The peptides identified by mass spectrometry are highlighted in red.
To confirm that the inhibitory action of C2C on C2E is through a direct interaction, we purified C2CD and C2E, respectively, and performed cross‐linking experiments. After incubation with the cross‐linker BS3 [bis(sulfosuccinimidyl) suberate], a band with MW corresponding to that expected for a C2CD‐C2E heterodimer appeared (Fig EV1E, red arrowhead, lanes 6, 7, and 8). The heterodimer of C2CD‐C2E was confirmed by mass spectrometry analysis of the band, which reveled peptides of both C2CD and C2E, covering nearly their entire sequences (Fig EV1F). Importantly, formation of this band in response to the cross‐linker was strongly reduced when the two protein fragments were incubated with both Ca2+ and liposomes, that is, when C2CD can bind to membranes (lane 9, see right panel for quantification). The band was only weak, but this was not unexpected as in the intact protein the C2CD and the C2E domain are part of the same polypeptide and thus are in close proximity (which will increase the efficiency of heterodimer formation), while in our case, they are dispersed in solution. This result strongly supports the hypothesis that C2CD inhibits the binding of C2E to a PI(4,5)P2‐containing membrane through a direct interaction, which will be released by Ca2+ binding to C2C to redirect it to the membrane.
Altogether, these results demonstrate that both PI(4,5)P2 binding by C2E and Ca2+ regulation of C2C play the dominant role in the Ca2+ stimulation of E‐Syt1‐dependent liposome tethering.
C2A domain of E‐Syt1 participates in the Ca2+ regulation of lipid transfer in vitro
In view of the lack of a relevant impact of the Ca2+‐binding properties of C2A on Ca2+‐dependent liposome tethering by E‐Syt1 (Fig 1C), we explored whether these properties play a role in lipid transfer using a fluorescence resonance energy transfer (FRET)‐based assay (Saheki et al, 2016; Yu et al, 2016). The assay involves the same liposomes described above (with the addition of NBD‐PE to the anchoring liposomes, henceforth defined as donor liposomes). The fluorescence of NBD, which is partially self‐quenched in these liposomes, increases as a result of dequenching since NBD‐PE is transferred to the target liposomes (henceforth defined as acceptor liposomes). Consistent with previous reports (Saheki et al, 2016; Yu et al, 2016), Ca2+ strongly stimulated lipid transfer by E‐Syt1cyto, as revealed by NBD‐PE dequenching (Fig 2B). However, this stimulation was completely abolished by the Ca2+‐binding mutations in C2A (E‐Syt1cyto C2Ax), in spite of very little impact of these mutations on liposome tethering (Fig 2B).
Figure 2. Ca2+ binding to C2A is required for lipid transfer by the SMP domain when flanked by the C2AB domain.

-
ADomain structures of constructs used for the lipid transfer assays shown in the figure. PH = PH domain of rat PLCδ. Slanted white lines indicate the mutations in the Ca2+ binding sites in C2A and C2C domains. Asterisks indicate the mutations in the membrane binding surface of C2A domain.
-
BLeft: Time‐course of normalized fluorescence signals of liposomes mixtures containing 1.5% NBD‐PE in the donor liposomes in the absence or presence of Ca2+ at RT. E‐Syt1 constructs were added at time 0 (protein:lipid ratio 1:1,000). Right: Quantification of the increase in NBD fluorescence at the end of the incubation (arrow in left).
-
C–ELipid transfer between donor and acceptor liposomes in the presence of E‐Syt1 constructs (protein:lipid ratio 1:400) at 37°C as assessed by dequenching of NBD‐PE fluorescence. In each of the panels, time‐courses are at the top and bargraphs showing quantification of NBD fluorescence at the end of the incubation (arrows in the upper panels) are at the bottom. (C) Effect of C2ABCDE‐dependent liposome tethering on lipid transfer mediated by the SMP domain. (D) Effect of the Ca2+‐binding property of C2A on the lipid transfer activity of the SMP domain between C2ABCDE‐tethered liposomes. (E) Effect of Ca2+ on the lipid transfer activity of the SMP domain alone or SMP‐C2AB on liposomes tethered by a PH domain.
SMP domain of E‐Syt1 alone can transfer lipids in a Ca2+‐independent manner between tethered membranes
To investigate the interplay of the SMP domain and C2 domains in SMP domain‐dependent lipid transfer, His‐tagged SMP domain of human E‐Syt1 (SMP, Fig 2A) was generated and tested for its activity in the in vitro lipid transfer assay. No increase in turbidity (consistent with lack of tethering; Fig EV2A) or in lipid transfer was observed upon incubation of the SMP domain alone with donor and acceptor liposomes, even in the presence of Ca2+ (Fig 2C). While in principle even the SMP domain alone could mediate some lipid transfer during random encounters between liposomes, the rate of such transfer may be too low to be detected during the assay period (30 min) in the absence of tethering. However, when both SMP and a His‐tagged C2ABCDE fragment of E‐Syt1 (C2ABCDE, Fig 2A) were added together to the mixtures of donor and acceptor liposomes, SMP domain‐dependent lipid transfer was observed in the presence of Ca2+ (Fig 2C), that is, conditions under which the C2ABDCE can mediate tethering (Fig EV2A). Ca2+ appeared to be needed only to facilitate tethering, as a similar degree of lipid transfer by SMP domain was observed irrespective of the presence of Ca2+, when the two sets of liposomes were connected by another tether, His‐tagged PHPLCδ, which binds to PI(4,5)P2 (Garcia et al, 1995; Hammond & Balla, 2015) in the acceptor liposomes in a Ca2+‐independent way (Figs 2E and EV2B). These experiments further demonstrate that a key role of Ca2+ in E‐Syt1‐dependent lipid transfer is to mediate tethering. Additionally, these results also reveal that the additional importance of Ca2+ binding to the C2A domain in enabling lipid transfer is only manifested when the SMP and C2A are part of the same polypeptide.
Figure EV2. Both C2ABCDE and PHPLC δ can tether liposomes, although C2ABCDE, but not PHPLC δ requires Ca2+ .

-
A, BTethering of anchoring and target liposomes in the presence of the constructs indicated (see Fig 4A, protein:lipid ratio 1:400) at 37°C as assessed by increase in turbidity (OD 405 nm). In each of the panels, time‐courses are at left and bar graphs showing quantification of OD405 increases at the end of the incubation (arrows in the left panels) are at right. (A) Effect of the absence or presence of Ca2+ on liposome tethering by SMP or C2ABCDE. Mean and SD of three independent experiments. P‐values from t‐test with Bonferroni corrections are quoted on the graphs. ****P < 0.0001; n.s. not significant. (B) Effect of the absence or presence of Ca2+ on liposome tethering by PHPLCδ. Mean and SD of three independent experiments. P‐values from two‐tailed student's t‐test are quoted on the graphs. n.s. not significant.
C2A domain of E‐Syt1 inhibits the activity of SMP domain in the absence of Ca2+ via an intramolecular interaction
To further analyze the property of the C2A domain of E‐Syt1 in lipid transfer, His‐tagged SMP‐C2AB and His‐tagged SMP domain were tested in parallel. When added to donor and acceptor liposomes, no transfer was observed with the SMP domain alone (see above, Figs 2C and EV3A), while the SMP‐C2AB construct induced some liposome tethering and lipid transfer, but only in the presence of Ca2+ (Fig EV3). The lipid transfer properties of the SMP‐C2AB fragment were much more pronounced, and only in the presence of Ca2+, when liposomes were additionally tethered by the His‐tagged C2ABCDE fragment (Fig 2D). However, mutations in C2A domain that make SMP‐C2AB incapable of binding Ca2+ (SMP‐C2AxB, Fig 2A) abolished its lipid transfer activity even in the presence of Ca2+ and of C2ABCDE (Fig 2D). Under these conditions (presence of C2ABCDE and Ca2+), a robust lipid transfer was achieved by SMP alone (see above, Fig 2C and D), although it was lower than SMP‐C2AB (Fig 2D), which is possibly due to a higher basal lipid transfer by SMP‐C2AB compared to SMP alone in the presence of Ca2+ (Fig EV3A).
Figure EV3. The partial membrane tethering activity of SMP‐C2AB relative to E‐Syt1cyto correlates with a lower Ca2+‐dependent lipid transfer activity.

- Lipid transfer between donor and acceptor liposomes in the presence of E‐Syt1 constructs (see Fig 4A, protein:lipid ratio 1:1,000) at RT as assessed by dequenching of NBD‐PE fluorescence. Time‐courses are at left, and bar graphs showing quantification of NBD fluorescence at the end of the incubation (arrow in the left panel) are at right.
- E‐Syt1cyto and SMP‐C2AB were added to the mixture of anchoring and target liposomes (protein:lipid ratio 1:1,000) in the absence or presence of Ca2+. Time‐courses are at left, and bar graphs showing quantification of OD405 increases at the end of the incubation (arrow in the left panel) are at right.
These findings suggested that (i) the C2A domain may block lipid transfer activity of the SMP domain via an intra‐ or intermolecular autoinhibitory interaction, (ii) the surface of C2A involved in this autoinhibitory interaction is not the one harboring the Ca2+‐binding sites, and (iii) this inhibition is released upon binding of Ca2+ to the C2A domain. To test this hypothesis, we assessed lipid transfer using His‐tagged PHPLCδ to tether donor and acceptor liposomes. In the presence of PHPLCδ, SMP‐C2AB transferred lipids in the presence of Ca2+, while its lipid transfer activity was barely detectable in the absence of Ca2+ (Fig 2E). Given that the SMP domain alone can transport lipids in Ca2+‐independent manner when liposomes are tethered by the PHPLCδ (see above, Fig 2E), these results support the hypothesis that Ca2+ binding to the C2A domain enhances SMP domain‐dependent lipid transfer by releasing an autoinhibition.
We next tested the importance of membrane binding for the property of Ca2+ to release the inhibitory effect of C2A on lipid transfer. Based on a.a. sequence alignment, C2A is predicted to have the same hydrophobic loop that in the C2A and C2B domain of Syt1, which participates in membrane binding (Martens et al, 2007). Introduction of mutations into bulky hydrophobic residues (V348A/L351A) of this predicted C2A loop in the SMP‐C2ABCxD construct (SMP‐C2AmBCxD), that is, a construct whose binding to membranes in response to Ca2+ is entirely dependent upon the C2A domain, abolished its Ca2+‐dependent binding to the liposomes (Fig EV1A and B). When introduced into full‐length E‐Syt1cyto (E‐Syt1cyto C2Am, Fig 2A), the same mutations only partially impaired Ca2+‐dependent lipid transfer activity, in contrast to the complete block of activity produced by the Ca2+‐binding mutant (E‐Syt1cyto C2Ax; Fig 2B). These results show that Ca2+ binding to C2A can release its inhibitory action on the SMP domain irrespective of membrane binding, but also demonstrate that Ca2+‐dependent membrane binding enhances this effect.
To gain insight into the mechanisms underlying autoinhibition, we inspected the reported crystal structure of the SMP‐C2AB domains of E‐Syt2 (Schauder et al, 2014), which is predicted to be very similar to the corresponding region of E‐Syt1. In the crystal structure, the SMP domain forms a dimer flanked by two interfaces with the C2A domains of the monomers: an intramolecular interface and an intermolecular interface (Fig 3A). Both interfaces involve charged residues, which are conserved in E‐Syt1 (Figs 3B and EV4A). The importance of these two interfaces for the lipid transport function of E‐Syt1 was assessed by mutating positively charged residues to negatively charged residues in the SMP domain. The R227E mutation in the SMP domain at the intermolecular interface (Fig 3B right) had no impact on lipid transfer ability of SMP‐C2AB (Fig 3C). In contrast, the R266E/R267E double mutation in the SMP at the intramolecular interface (Fig 3B left) abolished the inhibitory effect of the C2A domain on the lipid transfer activity in the absence of Ca2+ when using PHPLCδ‐tethered liposomes (Fig 3C). Furthermore, when the in vitro lipid transfer assay was performed in the presence of high salt (500 mM NaCl), that is, conditions expected to disrupt the salt bridge between the SMP and C2A, SMP‐C2AB had a higher lipid transfer activity than in more physiological salt conditions (100 mM NaCl). Conversely, lipid transfer by the SMP domain alone (without the flanking C2AB domain) was the same in both conditions (Fig 3D). These results suggest that an intramolecular salt bridge drives an autoinhibitory interaction of the C2A domain with the SMP domain.
Figure 3. C2A domain inhibits the lipid transfer activity of the SMP domain in the absence of Ca2+ via an intramolecular interaction.

-
ARibbon representation of the crystal structure of the SMP‐C2AB of human E‐Syt2 dimer in different orientations (PDB code 2DMG). One monomer is shown in regular color and the other in pale colors. The SMP domain is in yellow and the C2AB domain pairs in red. Lipid molecules are represented as stick in dark orange.
-
BLeft: Intramolecular interface between the SMP domain and the C2A domain. Right: Intermolecular interface between SMP domain and C2A domain.
-
C, DLipid transfer between donor and acceptor liposomes in the presence of E‐Syt1 constructs (protein:lipid ratio 1:400) at 37°C as assessed by dequenching of NBD‐PE fluorescence. In each of the panels, time‐courses are at left and bar graphs showing quantification of NBD fluorescence at the end of the incubation (arrows in the left panels) are at right. (C) Effect of mutations in the SMP domain at either its intramolecular or intermolecular interface. (D) Effect of the salt concentration on the lipid transfer activity of SMP or SMP‐C2AB on liposomes tethered by a PH domain. Mean and SD of three independent experiments. P‐values from t‐test with Bonferroni corrections are quoted on the graphs. *P < 0.05; **P < 0.01; n.s., not significant.
Figure EV4. The C2CD domains pair has no direct impact on the lipid transfer activity of the SMP domain.
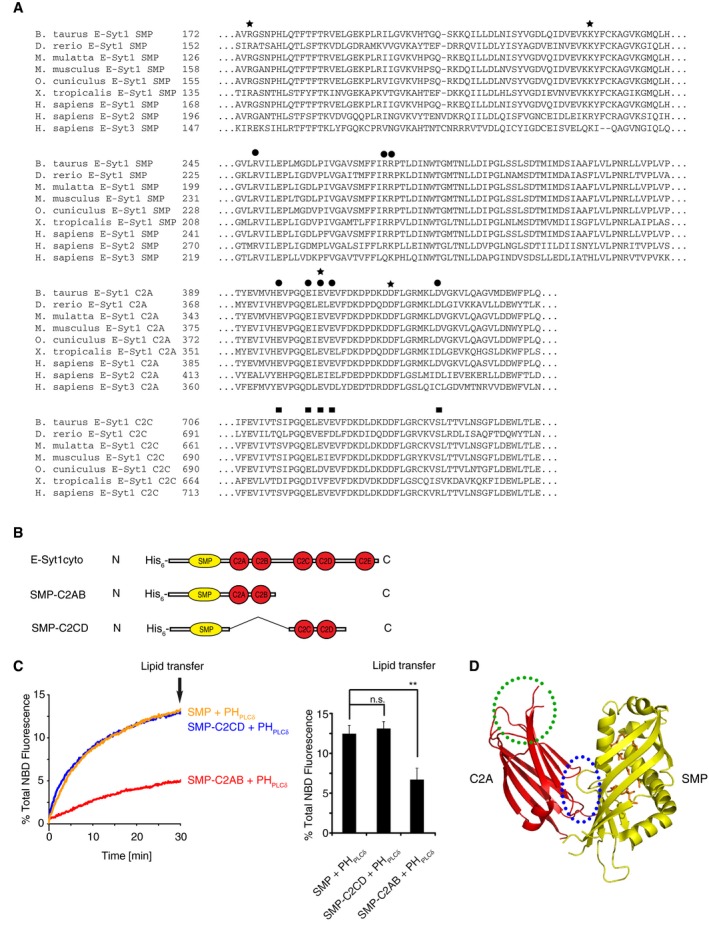
- Sequence alignment of the three E‐Syts from various species showing conserved amino acid residues implicated in intramolecular (black circles) and intermolecular (black stars) interfaces between the SMP domain and C2A domains in the crystal structure of E‐Syt2. The residues of C2C corresponding to the residues of C2A involved in the SMP‐C2A interaction are indicated by black squares. Note that these residues are not highly conserved.
- Domain structures of E‐Syt1 used for the lipid transfer assays shown in (C).
- Impact of the C2AB domain pair, but not of the C2CD domain pair, on lipid transfer mediated by the indicated proteins between PHPLCδ ‐tethered donor and acceptor liposomes as determined by dequenching of NBD‐PE in donor liposomes at 37°C. Time‐courses are shown at left, and bar graphs showing quantification of % total NBD fluorescence at the end of the incubation (arrow in the left panel) are shown at right. Mean and SD of three independent experiments. P‐values from t‐test with Bonferroni corrections are quoted on the graphs. **P < 0.01; n.s., not significant.
- C2A binds Ca2+ and the SMP domain via different surfaces. The intramolecular interface between the SMP domain (yellow) and C2A domain (red) of E‐Syt2 is indicated by a blue dashed circle. The Ca2+‐binding surface of C2A is indicated by a green dashed circle. Lipid molecules are shown in orange.
Finally, replacing the C2AB domain pair in the SMP‐C2AB construct with the C2CD domain pair of E‐Syt1(SMP‐C2CD, Fig EV4B) did not inhibit SMP domain‐mediated lipid transfer (Fig EV4C). This was consistent with the lack of conservation between C2A and C2C in the surface of C2A that mediates the intramolecular interaction with the SMP domain (Fig EV4A). Such surface is opposite to the Ca2+‐binding sites, which is also the bilayer‐binding surface of the C2A domain (Xu et al, 2014; Fig EV4D). Based on crystallographic studies of the C2A domain of E‐Syt2, Ca2+ binding to C2A results in a decrease in the negative potential of the surface of C2A that interacts with the SMP domain, most likely decreasing the strength of the interaction (Xu et al, 2014). We hypothesize that Ca2+ functions as a switch that shift the C2A domain from an SMP‐binding state to a bilayer‐binding state, thus releasing the inhibition of SMP‐mediated lipid transfer. As C2A binding to a bilayer does not require presence of acidic phospholipids (Min et al, 2007), bilayer binding of C2A domain may occur either “in cis” or “in trans”.
Collectively, these results point to occurrence of an autoinhibitory intramolecular interaction between the SMP domain and the C2A domain of E‐Syt1. This autoinhibition impairs lipid transfer activity and is released by Ca2+.
Role of Ca2+ binding to C2A and C2C in the property of E‐Syt1 to clear DAG from the PM
Having validated the importance of Ca2+ binding to the C2A domain of E‐Syt1 in lipid transfer in vitro, we next tested the role of this Ca2+ binding on the action of E‐Syt1 in living cells. A previous study (Saheki et al, 2016) showed that cells lacking the two major E‐Syts (E‐Syt1 and E‐Syt2; double KO, DKO) or all three E‐Syts (triple KO, TKO) have a partial defect in the clearance of PLC‐dependent DAG accumulation from the plasma membrane, as monitored by the fluorescence of the DAG probe C1PKC‐mCherry (Codazzi et al, 2001). In WT cells overexpressing the muscarinic M1 receptor (M1R), plasma membrane DAG rapidly increases in response to the M1R agonist Oxo‐M [which stimulates PLC (Willars et al, 1998; Suh et al, 2004; Horowitz et al, 2005)], and such increase is persistent in the presence of a DAG kinase inhibitor (DGKi) even after reversal of M1R activation with atropine (Fig 4A; Saheki et al, 2016). However, excess DAG was rapidly cleared in WT cells upon the addition of ionomycin (Fig 4A), a drug that increases cytosolic Ca2+ (Morgan & Jacob, 1994) and thus results in the acute recruitment of E‐Syt1 to the plasma membrane (Fig EV5B and C; Saheki et al, 2016). Such clearance was not observed in E‐Syts TKO cells (Fig 4A and B), and this phenotype was rescued by re‐expression of EGFP‐E‐Syt1 (Figs 4A and B, and EV5A), but not of EGFP‐E‐Syt1 with mutations in the Ca2+‐binding sites of its C2C domain (EGFP‐E‐Syt1 C2Cx; Fig 4A and B), that is, a mutant construct that is not recruited to the PM in response to Ca2+ elevation (Fig EV5B and C; Giordano et al, 2013).
Figure 4. E‐Syts‐dependent DAG extraction from the PM in intact cells requires Ca2+ binding to C2 domains.

- Time‐course of normalized mCherry signal at the PM of WT and E‐Syts TKO cells expressing the DAG reporter C1PKC‐mCherry, as assessed by TIRF microscopy, in response to the indicated compounds.
- Quantification of F/F0 at the end of the experiment (arrow in A).
Figure EV5. EGFP‐E‐Syt1 C2Ax is recruited to the PM but does not rescue the defect in DAG extraction from the PM in E‐Syts TKO cells.

- TIRF microscopy images of E‐Syts TKO cells expressing WT or C2Ax mutant EGFP‐E‐Syt1, together with the DAG probe C1PKC‐mCherry, and exposed to the indicated compounds at the indicated time. Note loss of mCherry signal in response to ionomycin in cells expressing WT E‐Syt1. Scale bars, 10 μm.
- Upper: Time‐course of normalized EGFP signal at the PM of E‐Syts TKO cells expressing the WT or EGFP‐E‐Syt1 C2Ax, as assessed by TIRF microscopy, in response to the indicated compounds. Bottom: Quantification of F/F0 at 9.5 min (arrow in the upper panel). Mean and SEM, n = 22 cells (EGFP‐E‐Syt1), n = 11 cells (EGFP‐E‐Syt1 C2Ax), n = 10 cells (EGFP‐E‐Syt1 C2Cx); P‐values from t‐test with Bonferroni corrections are quoted on the graphs. n.s., not significant; ****P < 0.0001.
- Confocal images of E‐Syts TKO cells expressing the indicated constructs before and during stimulation with 2 μM ionomycin as indicated. Scale bars, 10 μm.
- Left: Time‐course of normalized mCherry signal at the PM of E‐Syts TKO cells expressing the DAG reporter C1PKC‐mCherry, as assessed by TIRF microscopy, in response to the indicated compounds. Right: Quantification of F/F0 at the end of the experiment (arrow in the left panel). Mean and SEM, n = 31 cells (TKO + EGFP‐E‐Syt1), n = 35 cells (TKO + EGFP‐E‐Syt1 C2Ax), n = 18 cells (TKO + EGFP‐E‐Syt1 C2Am); P‐values from t‐test with Bonferroni corrections are quoted on the graphs. n.s., not significant; ****P < 0.0001.
Importantly, in agreement with our in vitro lipid transfer results, a construct harboring mutations in the Ca2+‐binding sites of the C2A domain of EGFP‐E‐Syt1 (EGFP‐E‐Syt1 C2Ax) also failed to rescue the DAG clearance defect in E‐Syts TKO cells (Figs 4A and B, and EV5A), although loss of Ca2+ binding by the C2A domain was shown not to have an obvious impact on PM recruitment upon elevation of cytosolic Ca2+ (Fig EV5B and C; Chang et al, 2013). Expression of EGFP‐E‐Syt1 C2Am also could not rescue the DAG clearance defect in E‐Syts KO cells (Fig EV5D), even if E‐Syt1cyto C2Am had partial lipid transfer activity in vitro (Fig 2B), demonstrating a more severe effect of this mutation in the context of a living cell.
Ca2+ binding to both C2A and C2C is required for a role of E‐Syt in enabling PS scrambling
During a systematic investigation of PM properties that may be affected in cells lacking E‐Syts, we found that such cells have a defect in the externalization of PS in response to ionomycin (Figs 5A and B, EV6A and B). The increase in the surface exposure of PS, which is normally concentrated in the inner leaflet of the PM, can be measured by monitoring the recruitment to the cell surface of fluorescent annexin V [a PS‐binding protein (Heemskerk et al, 1997; Lhermusier et al, 2011)], which is added in the extracellular medium (Williamson et al, 1995; Zwaal et al, 2005). Conversely, loss of PS from the inner PM leaflet can be monitored lactadherin C2 domain fused with mCherry, a PS‐binding probe [Lact‐C2‐mCherry (Yeung et al, 2008)].
Figure 5. E‐Syts KO cells are defective in Ca2+‐dependent PS scrambling.

-
A, BConfocal images of WT (A) and E‐Syt1/2 double KO (DKO) cells (B) before and 15 min after stimulation with 2 μM ionomycin. PS scrambling was detected with confocal microscopy by the binding to the cell surface of Cy3‐labeled annexin V (annexin V‐Cy3) present in the medium. Scale bars, 10 μm.
-
CDomain structures of E‐Syt1 constructs used for the rescue experiments.
-
D–FNormalized cell surface‐associated annexin V‐Cy3 fluorescence in response to 2 μM ionomycin, as assessed by confocal microscopy, for WT and E‐Syt1/2/3 triple KO (TKO) cells with and without rescue with transfected E‐Syt1 constructs. Time‐courses are at left, and bar graphs showing quantification of F/F0 at the time point (arrow in the left panels) are at right. (D) PS scrambling within the time frame indicated is abolished in E‐Syts TKO cells, and rescued by expression of EGFP‐E‐Syt1 alone or EGFP‐E‐Syt1 together with Myc‐E‐Syt2, but not by expression of EGFP‐E‐Syt1 ∆SMP [mean and SEM, n = 20 cells (WT), n = 20 cells (TKO), n = 16 cells (TKO + EGFP‐E‐Syt1 + Myc‐E‐Syt2), n = 24 cells (TKO + EGFP‐E‐Syt1), n = 17 cells (TKO + EGFP‐E‐Syt1 ∆SMP); P‐values from t‐test with Bonferroni corrections are quoted on the graphs. n.s., not significant; ****P < 0.0001]. (E) Defective rescue of PS scrambling in E‐Syts TKO cells transfected with EGFP‐E‐Syt1 carrying SMP domain mutations as indicated [mean and SEM, n = 20 cells (TKO), n = 24 cells (TKO + EGFP‐E‐Syt1), n = 20 cells (TKO + EGFP‐E‐Syt1 V169W/L308W); P‐values from t‐test with Bonferroni corrections are quoted on the graphs. n.s., not significant; ****P < 0.0001]. (F) Defective rescue of PS scrambling in E‐Syts TKO cells transfected with E‐Syt1 harboring Ca2+‐binding mutations in C2A or C2C [mean and SEM, n = 10 cells (TKO), n = 18 cells (TKO + EGFP‐E‐Syt1), n = 15 cells (TKO + EGFP‐E‐Syt1 C2Ax), n = 18 cells (TKO + EGFP‐E‐Syt1 C2Cx); P‐values from t‐test with Bonferroni corrections are quoted on the graphs. n.s., not significant; ****P < 0.0001].
Figure EV6. E‐Syt1/2 DKO cells are defective in Ca2+‐dependent PS exposure.

-
A–DConfocal images of WT (A and C) and E‐Syt1/2 DKO (B and D) cells incubated with FITC‐labeled annexin V (annexin V‐FITC, A and B) and expressing Lact‐C2‐mCherry (C and D) before and during stimulation with 2 μM ionomycin as indicated. Note the progressive accumulation of annexin V‐FITC signals (A) and decrease in Lact‐C2‐mCherry signals (C, insets) at the PM of WT cells, but not of DKO cells (B and D). Scale bars, 10 μm.
-
ELeft: Time‐course of normalized Lact‐C2‐mCherry fluorescence, as assessed by confocal microscopy, in response to 2 μm ionomycin, from WT and E‐Syt1/2 DKO cells. For overexpression and rescue experiments, WT and E‐Syts DKO cells were transfected with EGFP‐E‐Syt1 together with Myc‐E‐Syt2 as indicated. Right: Quantification of F/F0 at the end of the experiment (arrow in the left panel). Mean and SEM, n = 13 cells (WT), n = 13 cells (WT + EGFP‐E‐Syt1&Myc‐E‐Syt2), n = 16 cells (DKO), n = 10 cells (DKO + EGFP‐E‐Syt1&Myc‐E‐Syt2); P‐values from t‐test with Bonferroni corrections are quoted on the graphs. n.s., not significant; ****P < 0.0001.
-
FConfocal images of E‐Syt1/2 double KO (DKO) cells expressing the indicated constructs before and during stimulation with 2 μM ionomycin at the indicated time. PS scrambling was detected with confocal microscopy by the binding to the cell surface of annexin V‐Cy3 present in the medium. Note that the two EGFP‐E‐Syt1 constructs are recruited to the PM in response to Ca2+. Scale bars, 10 μm.
-
GTime‐course of normalized annexin V‐Cy3 fluorescence, as assessed by confocal microscopy, in response to 2 μm ionomycin, from WT and E‐Syt1/2 DKO cells. For overexpression and rescue experiments, WT and E‐Syts DKO cells were transfected with either EGFP‐E‐Syt1 together with Myc‐E‐Syt2, or EGFP‐E‐Syt1 alone, or EGFP‐E‐Syt1 ΔSMP as indicated. Mean and SEM (quantification in H).
-
HQuantification of F/F0 at the end of the experiment (arrow in G). Mean and SEM, n = 8 cells (WT), n = 12 cells (WT + EGFP‐E‐Syt1&Myc‐E‐Syt2), n = 14 cells (WT + EGFP‐E‐Syt1), n = 11 cells (DKO), n = 13 cells (DKO + EGFP‐E‐Syt1&Myc‐E‐Syt2), n = 15 cells (DKO + EGFP‐E‐Syt1), n = 13 cells (DKO + EGFP‐E‐Syt1 ΔSMP); P‐values from t‐test with Bonferroni corrections are quoted on the graphs. n.s., not significant; **P < 0.01, ****P < 0.0001.
-
IRescue with C2 domain Ca2+‐binding‐deficient mutants of E‐Syt1. Overexpression and rescue with C2C domain mutant and C2A domain mutant are shown. Quantification of annexin V‐Cy3 F/F0 at the end of the experiment similar to (G). Mean and SEM, n = 13 cells (WT), n = 11 cells (WT + EGFP‐E‐Syt1 C2Ax), n = 15 cells (WT + EGFP‐E‐Syt1 C2Cx), n = 12 cells (DKO), n = 10 cells (DKO + EGFP‐E‐Syt1), n = 15 cells (DKO + EGFP‐E‐Syt1_C2Ax), n = 12 cells (DKO + EGFP‐E‐Syt1 C2Cx); P‐values from t‐test with Bonferroni corrections are quoted on the graphs. n.s., not significant; ***P < 0.001.
-
JDetection of PM lipid scrambling with FM1‐43. Time‐courses of normalized FM1‐43 fluorescence, as assessed by confocal microscopy, from WT, E‐Syt DKO, and E‐Syt TKO cells, are on the left. Bar graphs showing quantification of F/F0 at the end of the experiment (arrow in left panel) are on the right. Mean and SEM, n = 34 cells (WT), n = 28 cells (TKO), n = 24 cells (DKO); P‐values from t‐test with Bonferroni corrections are quoted on the graphs. ****P < 0.0001.
The ionomycin (Ca2+)‐dependent PS externalization process itself remains poorly understood. There is evidence that such scrambling can be affected by the lipid composition of the bilayer (Contreras et al, 2010; Brown & Conboy, 2013). We exploited this phenotype to further validate the physiological importance of E‐Syts and of the Ca2+‐binding sites of the C2A and C2C domains of E‐Syt1.
Incubation of WT cells with ionomycin induced a rapid increase in fluorescently tagged annexin V associated with the cell surface, as detected by confocal microscopy (Figs 5A and EV6A). This increase was paralleled by a decrease in the Lact‐C2‐mCherry signal associated with the PM, which is consistent with the scrambling of PS from the inner to the outer leaflet of the PM (Fig EV6A, C and E). In E‐Syts KO cells, binding of annexin V to the cell surface was dramatically impaired (Figs 5B and D, and EV6B and G–I), and loss of Lact‐C2‐mCherry signal from the PM did not occur (Fig EV6B and D). Addition to cells of FM1‐43, another probe that detects externalized PS and other acidic phospholipids (Zweifach, 2000), confirmed the robust defect in lipid scrambling in E‐Syts KO cells (Fig EV6J). As shown by the annexin V‐Cy3 fluorescence assays, the lipid scrambling defect could be rescued by re‐expression of E‐Syt1 together with E‐Syt2 (Figs 5C and D, and EV6F–H), and to a lower extent by re‐expression of E‐Syt1 alone (Figs 5D–F and EV6G–I), but not by an E‐Syt1 construct lacking the SMP domain (EGFP‐E‐Syt1 ΔSMP; Figs 5C and D, and EV6F–H). Additionally, an E‐Syt1 construct harboring bulky hydrophobic residues in the hydrophobic cavity of the SMP domain (V169W/L308W), and therefore defective in lipid transport (Saheki et al, 2016), had much reduced rescue activity (Fig 5E). These results confirmed that the phenotype observed was dependent upon the loss of the lipid transport activity of the E‐Syts.
Importantly, E‐Syt1 constructs harboring mutations that impair Ca2+ binding to either the C2C or to C2A domains failed to rescue PS scrambling (Figs 5C and F, and EV6I), indicating that both Ca2+‐dependent properties of E‐Syt1, recruitment to the PM and stimulation of lipid transport, are required for the rescue.
Discussion
Our results provide new insight into the molecular mechanisms underlying Ca2+‐dependent regulation of the lipid transport and membrane tethering properties of E‐Syt1. We propose that these two properties are regulated by its C2A and C2C domain, that is, its two Ca2+‐binding domains, respectively. C2A binds the SMP domain via a charge‐based intramolecular interaction that inhibits its lipid transport function, and Ca2+ binding releases this inhibition. C2C mediates Ca2+‐regulated tethering of the ER to the PM via an action that is heavily dependent on the property of C2E to bind the PM in a PI(4,5)P2‐dependent way. Importantly, our results also show that these functions are physiologically relevant, as E‐Syt1 constructs with mutations in the Ca2+‐binding sites of either C2 domains fail to rescue defects in lipid dynamics at the PM in E‐Syts KO cells.
Using a liposome‐based turbidity assays, we demonstrate that although E‐Syt1cyto‐dependent tethering is strongly stimulated by Ca2+, tethering occurs also in the absence of Ca2+, and that this tethering requires its C2E domain. This result is in agreement with the previous findings that low level of E‐Syt1‐dependent ER‐PM contacts occurs in living cells in the absence of Ca2+ elevation (Giordano et al, 2013; Fernandez‐Busnadiego et al, 2015) and that the C2E domain, when expressed alone, binds the PM at resting Ca2+ concentration (Idevall‐Hagren et al, 2015). In fact, analysis of the structural homology between C2E domain of E‐Syt1 and other classical C2 domains of synaptotagmins (Fukuda et al, 1995; Chapman et al, 1998; Fernandez‐Chacon et al, 2001) predicts a polybasic patch at the surface of the C2E domain [i.e., a patch expected to bind the PI(4,5)P2‐rich PM] but no Ca2+‐binding sites (Idevall‐Hagren et al, 2015).
Our demonstration that stimulation by Ca2+ of E‐Syt1cyto‐mediated liposome tethering is dependent on Ca2+ binding to its C2C domain is consistent with studies of overexpressed full‐length E‐Syt1 in living cells (Chang et al, 2013; Giordano et al, 2013). Unlike our previous conclusion that Ca2+‐bound C2C domain itself was responsible for binding to the PI(4,5)P2‐rich PM (Giordano et al, 2013), our current findings strongly suggest that, in addition to this function, a main action of Ca2+ on the C2C domain is to release its inhibitory action on the binding of C2E to membranes, corroborating a previous suggestion from studies of semi‐permeabilized cells (Idevall‐Hagren et al, 2015). We note that E‐Syt2 and E‐Syt3, which lack a central C2CD domain pair (Min et al, 2007; see Fig 1A), but have a C‐terminal C2 domain similar to the C‐terminal C2 domain of E‐Syt1 (C2E; Idevall‐Hagren et al, 2015), are constitutively localized at the PM even at basal Ca2+ concentration, further supporting an inhibitory role of C2C in C2E PM binding. Thus, we propose that the recruitment of E‐Syt1 to the PM is spatially and temporally controlled by the cooperation of the C2C and C2E domains.
Primary amino acid sequence similarity suggests that the C2CD domain pair may represent a duplication of the C2AB domain pair (Min et al, 2007). However, we found that mutations in the Ca2+‐binding sites of C2A have very little effect on E‐Syt1‐mediated liposome tethering, although a low level of Ca2+‐dependent increase in liposomes tethering was observed when incubating liposomes with SMP‐C2ABCxD or SMP‐C2AB (see Figs EV1B and EV3B). This low level increase in liposome tethering is consistent with the property of the very similar C2AB domains of E‐Syt2 to bind liposomes in a Ca2+‐dependent way (Min et al, 2007). Most likely, in the context of full‐length E‐Syt1, this contribution to tethering is negligible. These point mutations, however, completely abolished the lipid transfer activity of E‐Syt1, which was confirmed by (i) reduced dequenching of NBD‐PE (i.e., lipid transport between the two liposomes) in the liposome‐based lipid transfer assay, and (ii) loss of rescue of the defects in lipid dynamics at the PM observed in E‐Syts KO living cells.
An open question prior to this study was how Ca2+ binding to C2A contributes to the lipid transfer activity of the SMP domain. We showed here that a construct comprising the SMP domain and the flanking C2AB domain pair had negligible lipid transport activity when liposomes were tethered in the absence of Ca2+. However, the bilayer‐anchored SMP domain alone, that is, without flanking C2 domains, can directly transfer lipids between liposomes, although tethering of liposomes was required to make lipid transfer efficient enough to be detected. The tether did not need to be part of the SMP domain‐containing construct. Additionally, the level of lipid transfer mediated by the SMP domain alone was similar in the absence or presence of Ca2+ when the liposomes were tethered by a Ca2+‐independent module (e.g., PHPLCδ).
We propose that Ca2+ binding to C2A enhances lipid transfer activity by releasing an intramolecular interaction with the SMP domain that keeps the SMP domain in an autoinhibited state. Inspection of the crystal structure of the Ca2+‐free cytosolic fragment of human E‐Syt2 comprising the SMP domain and C2A and C2B (Schauder et al, 2014) supports this possibility. In the structure, which based on primary sequence similarity is expected to be very similar for the corresponding fragment of E‐Syt1, a negatively charged surface of C2A, which is adjacent to its Ca2+‐binding site, interacts with positively charged residues of the SMP domain located next to the entry of the hydrophobic groove. Presence of the C2AB next to the groove may prevent the access of the SMP domain to membranes by steric hindrance (Fig 3A and B). Upon Ca2+ binding, the electrostatic potential of C2A becomes neutral or positive (Xu et al, 2014), thus most likely weakening the interaction with the SMP domain and re‐orienting the C2A domain for binding to the membrane (either the ER or the PM). This change, in turn, would allow the SMP domain to efficiently extract and transfer lipids (Fig 6). The importance of Ca2+ binding to C2A for lipid transport implies that even if E‐Syt2 is localized constitutively at ER‐PM contacts when not heterodimerized to E‐Syt1 (Giordano et al, 2013), its lipid transport activity may be Ca2+ dependent. Thus, E‐Syts may mediate lipid transport at ER‐PM contacts only in response to Ca2+ elevation, irrespective of their localization at rest.
Figure 6. Model for Ca2+‐mediated releasing of an autoinhibitory conformation of E‐Syt1 to couple ER‐PM tethering with lipid transport.

Ca2+ binding to C2A and C2C promotes their interaction with membranes: in trans or possibly in cis in the case of C2A, and in trans in the case of C2C. The main effects of these Ca2+ bindings are to release the interaction of C2A with the SMP domain that impairs lipid transport and to enhance the binding of C2E to PI(4,5)P2 in the PM that is partially inhibited by C2C in the absence of Ca2+. The Ca2+ ions are shown as small blue circles. Double‐headed red arrows indicate lipid transfer mediated by the SMP domain. Lipid transport is likely to be mediated by the shuttling of the SMP domain between the two membranes (Reinisch & De Camilli, 2016).
An unexpected finding reported in this study is that absence of the E‐Syts impairs Ca2+‐induced PS scrambling. The mechanisms underlying this change, which may be explained by abnormalities of the PM bilayer (Contreras et al, 2010; Brown & Conboy, 2013) as a result of the absence of the E‐Syts‐mediated lipid transfer between ER and PM, remain to be further explored. We believe that the E‐Syts are not directly involved in PS scrambling, but that they produce this effect indirectly, for example, by an effect on some properties of the PM bilayer resulting from their lipid transport properties. One reason to support this idea is that upon ionomycin addition the externalization of PS continues to increase at 15 min (Fig 5D) when EGFP‐S‐Syt1 presence at the PM has already returned to background levels [the signal of at the PM returns to the background level within 3 min after adding ionomycin (Fig EV5B)]. We exploited this defect of E‐Syts TKO cells, as well as the previously described delay in the clearance from the PM of acutely produced DAG (Saheki et al, 2016), to assess the property of Ca2+ binding to the C2A and C2C domain of E‐Syt1 with rescue experiments. Both phenotypes could be rescued by re‐expressing E‐Syt1, but not E‐Syt1 with mutations in the SMP domain, C2A or C2C. Thus, both the property of E‐Syt1 to transfer lipids in response to Ca2+ and the property to be recruited to the PM in response to Ca2+ are required for the rescue. Collectively, these findings further demonstrate the importance of both Ca2+‐bound C2 domains for the function of the E‐Syts. They also support the hypothesis that the lipid transport functions of the E‐Syts are involved, most likely indirectly, in enabling the PM to support PS scrambling in response to ionomycin, at least in our in vitro conditions. Thus, they provide new evidence for a role of the E‐Syts in the control of lipid homeostasis at the PM. The role(s) of the E‐Syts in the control of PM lipid homeostasis may be partially redundant with those of other lipid transfer proteins, given the lack of major defects in development, viability, or fertility in E‐Syts KO mice (Sclip et al, 2016; Tremblay & Moss, 2016). As the E‐Syts are highly conserved in evolution from unicellular organisms to mammals, their actions may become critical only under specific functional states that need to be further investigated.
In summary, we report here that elevations of cytosolic Ca2+ not only control the localization of E‐Syt1, but also enable its lipid transfer activity and that at least some of these actions are mediated by the release of autoinhibitory mechanisms. Autoinhibition by a C2 domain was reported for other proteins, for example, Munc13 (Michelassi et al, 2017), E3 ubiquitin protein ligase SMURF2 (Wiesner et al, 2007), and protein kinase C βII (Antal et al, 2015). Additionally, release of a C2 domain‐dependent inhibitory action mediates some of the effects of Ca2+ on synaptotagmin 1 in neurotransmitter release (Zhou et al, 2017). Thus, Ca2+‐dependent release of inhibitory interactions may be a common feature of a variety of C2 domain‐containing proteins.
Materials and Methods
Chemicals
Chemicals were from the following sources: Isopropyl‐β‐D‐thiogalactoside (IPTG; AmericanBio), Oxo‐M and atropine (Sigma‐Aldrich), ionomycin (Sigma‐Aldrich), annexin V‐FITC (BD and BioVision), annexin V‐Cy3 (BioVision), DGK inhibitor (R 59‐022, Tocris Bioscience), proteinase K (Sigma‐Aldrich), FM1‐43 (Molecular Probes/Life technologies), BS3 (Thermo Fisher Scientific). The following concentrations of chemicals are used in all of the experiments unless noted otherwise: Oxo‐M, 10 μM; atropine, 50 μM; ionomycin, 2 μM (except in Fig EV5A and D at 6 μM); DGK inhibitor, 50 μM; BS3, 500 μM. All lipids were obtained from Avanti Polar Lipids: 1,2‐dioleoyl‐sn‐glycero‐3‐phosphocholine (DOPC), 850375; 1‐palmitoyl‐2‐oleoyl‐sn‐glycero‐3‐phospho‐L‐serine (POPS), 840034; L‐α‐phosphatidylinositol‐4,5‐bisphosphate [PI(4,5)P2], 840046; 7‐nitrobenzoxadiazole (NBD)‐1,2‐dipalmitoyl‐sn‐glycero‐3‐phosphoethanolamine (DPPE), 810144; 1,2‐dioleoyl‐sn‐glycero‐3‐[(N‐(5‐amino‐1‐carboxypentyl) iminodiacetic acid) succinyl] [DGS‐NTA(Ni)], 790404.
Plasmids
A plasmid encoding the M1 muscarinic acetylcholine receptor (M1R) was a kind gift from Bertil Hille (University of Washington; Suh et al, 2004). Lact‐C2‐mCherry is from Sergio Grinstein (University of Toronto; Yeung et al, 2008). C1PKC‐mCherry, untagged E‐Syt1, Myc‐E‐Syt2 [Myc‐E‐Syt2S (Giordano et al, 2013) was used for this study], EGFP‐E‐Syt1, EGFP‐E‐Syt1 C2Cx [called mCherry‐E‐Syt1‐mut in (Giordano et al, 2013)], EGFP‐E‐Syt1 ΔSMP, and EGFP‐E‐Syt1 V169W/L308W (EGFP‐E‐Syt1 SMPmut) were previously described (Giordano et al, 2013; Saheki et al, 2016).
The regions coding for residues 93–1,104 (E‐Syt1cyto), 93–941 (SMP‐C2ABCD), 93–634 (SMP‐C2AB), 315–1,104 (C2ABCDE), 624–973 (C2CD), and 936–1,104 (C2E) of human E‐Syt1 were amplified by PCR and cloned using AscI and NotI sites into the pCMV6‐An‐His vector (OriGene) for Expi293 cell expression. SMP domain (93–327) of human E‐Syt1 was cloned using NdeI and SalI sites, and PH domain (11–140) of rat PLCδ was cloned using NheI and XhoI sites into pET‐28a vector (Novagen). SMP‐C2E (93–327 and 898–1,104) and SMP‐C2CD (93–327 and 634–973) were generated by overlap PCR and cloned into the pET‐28a vector.
EGFP‐E‐Syt1 C2Ax (Ca2+‐binding‐deficient C2A) was generated as follows: the two aspartic acid residues at a.a. position 406 and 410, which are predicted to confer Ca2+‐binding properties to the C2A domain of E‐Syt1 (Xu et al, 2014), were mutated to alanine using site‐directed mutagenesis. The following primers were used: E1_C2A_Mut_F, GATTGAAGTGGAGGTGTTCGACAAGGcTCCAGATAAAGcTGACTTTCTGGGCAGAATGAAGCTGG; E1_C2A_Mut_R, CCAGCTTCATTCTGCCCAGAAAGTCAgCTTTATCTGGAgCCTTGTCGAACACCTCCACTTCAATC.
EGFP‐E‐Syt1 C2Am (membrane‐binding‐deficient C2A) was generated as follows: the valine at a.a. position 348 and leucine at a.a. position 351 were mutated to alanine using site‐directed mutagenesis. The following primers were used: E1_C2A_Mut2_F, GTTCCAAGGACAAATATGctAAGGGCgcaATTGAGGGCAAGTCAGAC; E1_C2A_Mut2_R, GTCTGACTTGCCCTCAATtgcGCCCTTagCATATTTGTCCTTGGAAC
Protein expression and purification
Expression in eukaryotic cells
Fragments of human E‐Syt1 were expressed in Expi293 cells with an N‐terminal His6‐tag, as described previously (Saheki et al, 2016). Cells were harvested and lysed in buffer A [25 mM Tris–HCl, pH 8.0, 300 mM NaCl, 10 mM imidazole, 1× complete EDTA‐free protease inhibitor cocktail (Roche), 0.5 mM TCEP] by three freeze–thawing cycles using liquid nitrogen. The lysates were clarified by centrifugation at 17,000 × g for 30 min, and the protein was purified by a Ni‐NTA column (Clontech) and was further purified by gel filtration (Superdex 200, GE Healthcare) in buffer B (25 mM Tris–HCl, pH 8.0, 100 mM NaCl, 0.5 mM TCEP). Fractions containing E‐Syt1 were pooled and concentrated to ~1 mg/ml.
Expression in bacteria
Constructs encoding E‐Syt1 fragments were transformed into BL21 (DE3) RIL Codon Plus (Agilent) Escherichia coli cells. Cells were grown in Super Broth medium at 37°C to an OD600 of 0.6, and the expression was induced by addition of 0.5 mM IPTG for 20 h at 18°C. The cells were harvested and lysed by sonication in buffer A. The lysates were clarified by centrifugation at 30,000 × g for 1 h, and the protein was isolated by a Ni‐NTA column, and further purified by gel filtration in buffer B. Fractions containing E‐Syt1 were pooled and concentrated to ~1 mg/ml.
Liposome preparation
Anchoring/donor liposomes: 78.5:1.5:20 mole percent DOPC:NBD‐DPPE:DGS‐NTA(Ni) or 98.5:1.5 mole percent DOPC:NBD‐DPPE.
Target/acceptor liposomes: 85:10:5 mole percent DOPC:POPS: PI(4,5)P2 or 90:10 mole percent DOPC:POPS or 70:30 mole percent DOPC:POPS.
Lipid mixtures were dissolved in chloroform in glass tubes and then dried under a stream of N2 gas followed by further drying in vacuum for 2 h. The lipid films were then hydrated with buffer B. Liposomes were formed by 10 freeze–thaw cycles in liquid N2 and 37°C water and extrusion through polycarbonate filters with a pore size of 50 nm (Avanti Polar Lipids).
Lipid transfer assays
All in vitro lipid transfer assays were performed as previously described with slight changes (Saheki et al, 2016). In brief, the reactions were performed in 50 μl volumes. The final lipid concentration in the reaction was 0.5 mM, with donor and acceptor liposomes added at a 1:1 ratio. Reactions were initiated by the addition of protein (protein:lipid ratio 1:1,000 or 1:400) in a 96‐well plate (Corning). The fluorescence intensity of NBD was monitored with an excitation of 460 nm and emission of 538 nm every 10 s over 30 min at 37°C or room temperature (RT, see figure legends) by using SpectraMax M5 Microplate Reader (Molecular Devices). All data were corrected by setting the data point at 0 min to zero, and subtracting the baseline values obtained in the absence of protein. The data were expressed as a percentage of the maximum fluorescence, determined after adding 10 μl of 2.5% n‐dodecyl‐β‐D‐maltopyranoside (DDM, Avanti Polar Lipids) to the reactions after 30 min. All experiments were repeated three times, and a representative trace is shown. Bars represent average fluorescence values from the three‐pooled readings.
Liposome tethering assays
Liposome tethering assays were performed as for the lipid transfer assays, except that absorbance at 405 nm was measured to assess turbidity. Reactions were initiated by the addition of protein in a 96‐well plate (Corning) using SpectraMax M5 Microplate Reader (Molecular Devices). The data were expressed as absolute absorbance values subtracted by the absorbance prior to protein addition. All experiments were repeated three times, and a representative trace is shown. Bars represent average 405 nm absorbance values from the three‐pooled readings.
Cross‐linking assay
For cross‐linking with BS3 (Thermo Fisher Scientific), 15 μM indicated proteins were incubated with or without Ca2+ or liposomes with 500 μM BS3 at RT for 0.5 h in 25 mM HEPES, pH 7.4, 150 mM NaCl, 0.5 mM TCEP. The reactions were quenched with 50 mM Tris–HCl, pH 8.0. All samples were analyzed by SDS–PAGE, and Coomassie blue‐stained gels were analyzed using ImageJ (NIH). The putative heterodimer band was dissected and analyzed by Mass Spectrometry by the Yale Keck Biotechnology Resource Laboratory.
Cell culture and transfection
HeLa cells were cultured in Dulbecco's modified essential Eagle medium (DMEM; Life Technologies) supplemented with 20% (v/v) fetal bovine serum (FBS, Life Technologies) at 37°C and 5% CO2. Transfection of plasmids was carried out with Lipofectamine 2000 (Life Technologies), according to the manufacturer's instructions. Wild‐type (WT) as well as genome‐edited HeLa cell lines were verified as free of mycoplasma contamination by a PCR‐based method. Genome‐edited cells were clonally isolated, and mutations or indels were identified by sequencing. E‐Syt1/2 double KO cells (DKO) were clones 6–8, and E‐Syt1/2/3 triple KO cells (TKO) were clone 5 described in Saheki et al (2016). No cell lines used in this study were found in the database of commonly misidentified cell lines that is maintained by ICLAC and NCBI Biosample. The same clones, either the DKO: 6–8 cell lines for DKO cells or the TKO: 5 cell lines for TKO cells, were used for all experiments throughout this study. All cell‐based experiments were repeated at least two times.
Fluorescence microscopy
For imaging experiments, cells were plated on 35‐mm glass‐bottomed dishes at low density (MatTek Corp, Ashland, MA, USA). Live cell imaging was carried out 1 day after transfection. Spinning disk confocal (SDC) microscopy was performed using the Improvision UltraVIEW VoX system (Perkin‐Elmer) built around a Nikon Ti‐E inverted microscope, equipped with PlanApo objectives (40× 1.0‐NA 1.0 and 60× 1.49‐NA), and controlled by Volocity (Improvision) software. Excitation light was provided by 488‐nm/50‐mW diode laser (Coherent) and 561‐nm/50‐mW diode laser (Cobolt), and fluorescence was detected by EM‐CCD camera (C9100‐50; Hamamatsu Photonics).
Total internal reflection fluorescence (TIRF) microscopy was performed on a setup built around either a Nikon Ti‐E or Ti2 microscope equipped with 60× 1.49‐NA and a 100× 1.49‐NA objective. For most experiments, excitation light was provided by 488‐nm (for GFP) and 561‐nm (for mCherry) DPSS lasers coupled to the TIRF illuminator through an optic fiber. The output from the lasers was controlled by an acousto‐optic tunable filter, and fluorescence was detected with an EM‐CCD camera (Andor iXon DU‐897). Acquisition was controlled by Andor iQ software. Images were sampled at 0.20 Hz with exposure times in the 100–500 ms range.
SDC microscopy was carried out at RT and TIRF microscopy at 37°C.
Live cell imaging
Cells were washed twice and incubated with Ca2+ buffer (10 mM HEPES, pH 7.4, 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 10 mM glucose, and 2 mM CaCl2) before imaging with either a SDC microscope or a TIRF microscope.
PS exposure/lipid scrambling assay
Phosphatidylserine in the outer PM leaflet was detected using annexin V‐Cy3 (Biovision), annexin V‐FITC (BD), or FM1‐43 (Molecular Probes/Life Technologies), and in the inner leaflet by expression of Lact‐C2‐mCherry. Cells were plated on 35‐mm glass‐bottomed dishes at low density (MatTek Corp). Cells were incubated with Ca2+ buffer containing annexin V‐Cy3, annexin V‐FITC, or FM1‐43 at the indicated dilution and stimulated with 2 μM ionomycin. PS exposure was monitored with a SDC microscope at room temperature. 50‐fold dilution for annexin V‐Cy3 and 20‐fold dilution for annexin V‐FITC were used; FM1‐43 was used at 3 μM. Relatively high concentration of annexin was necessary to ensure consistent detection of PS exposure.
Image analysis
All fluorescent images were analyzed off‐line using Image J (NIH). For the analysis of DAG levels in the PM using C1PKC‐mCherry, changes in PM mCherry fluorescence (TIRF miscoscopy) over time were analyzed by manually selecting large areas of the cell footprint. Mean fluorescence intensity values of selected regions were obtained and normalized (F) to average values before stimulation after background subtraction (F0). Background signals correspond to the average fluorescent intensity of the regions where the cells are not present. Changes in annexin V‐Cy3, annexin V‐FITC, Lact‐C2‐mCherry, or FM1‐43 fluorescence over time (SDC microscopy) were analyzed by manually drawing regions at edges of cells, and peak fluorescence intensity was measured. After background subtraction, fluorescence intensities of the regions were obtained and normalized with average values before stimulation. Quantification of fluorescence changes was performed using Excel (Microsoft), and all data are presented as mean and SEM.
Statistical analysis
No statistical method was used to predetermine sample size, and the experiments were not randomized for live cell imaging. Cells were pooled from 1 to 6 independent experiments. Comparisons of data were carried out by either the two‐tailed Student's t‐test or the t‐test with Bonferroni corrections for multiple comparisons as appropriate with Prism 6 (GraphPad software). Dose–response curves were fit using four‐parameter logistic equations to calculate EC50 (GraphPad software).
Author contributions
All authors participated in the design of experiments, data analysis, and interpretation. XB designed and performed all of the liposome tethering and lipid transfer assays. YS designed and performed all of the imaging studies in living cells. XB, YS, and PDC wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Expanded View Figures PDF
Review Process File
Acknowledgements
We thank Yiying Cai for discussion and critical reading of the manuscript, and Frank Wilson, Heather Wheeler, and Louise Lucast for technical assistance. We thank Yale Keck Biotechnology Resource Laboratory for Mass Spectrometry. P.D.C. was supported in part by NIH Grants R37NS036251, DK45735, and DA018343. X.B. was supported by a Human Frontier Science Program long‐term fellowship; Y.S. was supported by fellowships from the Uehara Memorial Foundation and the Japan Society for the Promotion of Science (JSPS), a Grant‐in‐Aid for Young Scientists (A) from JSPS, a Nanyang Assistant Professorship grant, and a Lee Kong Chian School of Medicine Start‐up Grant, Nanyang Technological University.
The EMBO Journal (2018) 37: 219–234
Contributor Information
Yasunori Saheki, Email: yasunori.saheki@ntu.edu.sg.
Pietro De Camilli, Email: pietro.decamilli@yale.edu.
References
- AhYoung AP, Jiang J, Zhang J, Khoi Dang X, Loo JA, Zhou ZH, Egea PF (2015) Conserved SMP domains of the ERMES complex bind phospholipids and mediate tether assembly. Proc Natl Acad Sci USA 112: E3179–E3188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alva V, Lupas AN (2016) The TULIP superfamily of eukaryotic lipid‐binding proteins as a mediator of lipid sensing and transport. Biochem Biophys Acta 1861: 913–923 [DOI] [PubMed] [Google Scholar]
- Antal CE, Callender JA, Kornev AP, Taylor SS, Newton AC (2015) Intramolecular C2 domain‐mediated autoinhibition of protein kinase C betaII. Cell Rep 12: 1252–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KL, Conboy JC (2013) Lipid flip‐flop in binary membranes composed of phosphatidylserine and phosphatidylcholine. J Phys Chem B 117: 15041–15050 [DOI] [PubMed] [Google Scholar]
- Chang CL, Hsieh TS, Yang TT, Rothberg KG, Azizoglu DB, Volk E, Liao JC, Liou J (2013) Feedback regulation of receptor‐induced Ca2+ signaling mediated by E‐Syt1 and Nir2 at endoplasmic reticulum‐plasma membrane junctions. Cell Rep 5: 813–825 [DOI] [PubMed] [Google Scholar]
- Chapman ER, Desai RC, Davis AF, Tornehl CK (1998) Delineation of the oligomerization, AP‐2 binding, and synprint binding region of the C2B domain of synaptotagmin. J Biol Chem 273: 32966–32972 [DOI] [PubMed] [Google Scholar]
- Codazzi F, Teruel MN, Meyer T (2001) Control of astrocyte Ca(2+) oscillations and waves by oscillating translocation and activation of protein kinase C. Curr Biol 11: 1089–1097 [DOI] [PubMed] [Google Scholar]
- Contreras FX, Sanchez‐Magraner L, Alonso A, Goni FM (2010) Transbilayer (flip‐flop) lipid motion and lipid scrambling in membranes. FEBS Lett 584: 1779–1786 [DOI] [PubMed] [Google Scholar]
- Craxton M (2001) Genomic analysis of synaptotagmin genes. Genomics 77: 43–49 [DOI] [PubMed] [Google Scholar]
- Craxton M (2007) Evolutionary genomics of plant genes encoding N‐terminal‐TM‐C2 domain proteins and the similar FAM62 genes and synaptotagmin genes of metazoans. BMC Genom 8: 259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez‐Busnadiego R, Saheki Y, De Camilli P (2015) Three‐dimensional architecture of extended synaptotagmin‐mediated endoplasmic reticulum‐plasma membrane contact sites. Proc Natl Acad Sci USA 112: E2004–E2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez‐Chacon R, Konigstorfer A, Gerber SH, Garcia J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Sudhof TC (2001) Synaptotagmin I functions as a calcium regulator of release probability. Nature 410: 41–49 [DOI] [PubMed] [Google Scholar]
- Friedman JR, Voeltz GK (2011) The ER in 3D: a multifunctional dynamic membrane network. Trends Cell Biol 21: 709–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Kojima T, Aruga J, Niinobe M, Mikoshiba K (1995) Functional diversity of C2 domains of synaptotagmin family. Mutational analysis of inositol high polyphosphate binding domain. J Biol Chem 270: 26523–26527 [DOI] [PubMed] [Google Scholar]
- Gallo A, Vannier C, Galli T (2016) Endoplasmic reticulum‐plasma membrane associations: structures and functions. Annu Rev Cell Dev Biol 32: 279–301 [DOI] [PubMed] [Google Scholar]
- Garcia P, Gupta R, Shah S, Morris AJ, Rudge SA, Scarlata S, Petrova V, McLaughlin S, Rebecchi MJ (1995) The pleckstrin homology domain of phospholipase C‐delta 1 binds with high affinity to phosphatidylinositol 4,5‐bisphosphate in bilayer membranes. Biochemistry 34: 16228–16234 [DOI] [PubMed] [Google Scholar]
- Giordano F, Saheki Y, Idevall‐Hagren O, Colombo SF, Pirruccello M, Milosevic I, Gracheva EO, Bagriantsev SN, Borgese N, De Camilli P (2013) PI(4,5)P(2)‐dependent and Ca(2+)‐regulated ER‐PM interactions mediated by the extended synaptotagmins. Cell 153: 1494–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond GR, Balla T (2015) Polyphosphoinositide binding domains: key to inositol lipid biology. Biochem Biophys Acta 1851: 746–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk JW, Vuist WM, Feijge MA, Reutelingsperger CP, Lindhout T (1997) Collagen but not fibrinogen surfaces induce bleb formation, exposure of phosphatidylserine, and procoagulant activity of adherent platelets: evidence for regulation by protein tyrosine kinase‐dependent Ca2+ responses. Blood 90: 2615–2625 [PubMed] [Google Scholar]
- Hoglinger D, Nadler A, Haberkant P, Kirkpatrick J, Schifferer M, Stein F, Hauke S, Porter FD, Schultz C (2017) Trifunctional lipid probes for comprehensive studies of single lipid species in living cells. Proc Natl Acad Sci USA 114: 1566–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz LF, Hirdes W, Suh BC, Hilgemann DW, Mackie K, Hille B (2005) Phospholipase C in living cells: activation, inhibition, Ca2+ requirement, and regulation of M current. J Gen Physiol 126: 243–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idevall‐Hagren O, Lu A, Xie B, De Camilli P (2015) Triggered Ca2+ influx is required for extended synaptotagmin 1‐induced ER‐plasma membrane tethering. EMBO J 34: 2291–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H, Park J, Lee C (2016) Crystal structure of Mdm12 reveals the architecture and dynamic organization of the ERMES complex. EMBO Rep 17: 1857–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec KO, Alva V, Lupas AN (2010) Homology of SMP domains to the TULIP superfamily of lipid‐binding proteins provides a structural basis for lipid exchange between ER and mitochondria. Bioinformatics 26: 1927–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec KO, Alva V, Lupas AN (2011) Bioinformatics of the TULIP domain superfamily. Biochem Soc Trans 39: 1033–1038 [DOI] [PubMed] [Google Scholar]
- Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P (2009) An ER‐mitochondria tethering complex revealed by a synthetic biology screen. Science 325: 477–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Hong W (2006) Diverse membrane‐associated proteins contain a novel SMP domain. FASEB J 20: 202–206 [DOI] [PubMed] [Google Scholar]
- Lees JA, Messa M, Sun EW, Wheeler H, Torta F, Wenk MR, De Camilli P, Reinisch KM (2017) Lipid transport by TMEM24 at ER‐plasma membrane contacts regulates pulsatile insulin secretion. Science 355: eaah6171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy A, Zheng JY, Lazarowitz SG (2015) Synaptotagmin SYTA forms ER‐plasma membrane junctions that are recruited to plasmodesmata for plant virus movement. Curr Biol 25: 2018–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhermusier T, Chap H, Payrastre B (2011) Platelet membrane phospholipid asymmetry: from the characterization of a scramblase activity to the identification of an essential protein mutated in Scott syndrome. J Thromb Haemost 9: 1883–1891 [DOI] [PubMed] [Google Scholar]
- Liu LK, Choudhary V, Toulmay A, Prinz WA (2017) An inducible ER‐Golgi tether facilitates ceramide transport to alleviate lipotoxicity. J Cell Biol 216: 131–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manford AG, Stefan CJ, Yuan HL, Macgurn JA, Emr SD (2012) ER‐to‐plasma membrane tethering proteins regulate cell signaling and ER morphology. Dev Cell 23: 1129–1140 [DOI] [PubMed] [Google Scholar]
- Martens S, Kozlov MM, McMahon HT (2007) How synaptotagmin promotes membrane fusion. Science 316: 1205–1208 [DOI] [PubMed] [Google Scholar]
- Michelassi F, Liu H, Hu Z, Dittman JS (2017) A C1‐C2 module in munc13 inhibits calcium‐dependent neurotransmitter release. Neuron 95: 577–590 e575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min SW, Chang WP, Sudhof TC (2007) E‐Syts, a family of membranous Ca2+‐sensor proteins with multiple C2 domains. Proc Natl Acad Sci USA 104: 3823–3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AJ, Jacob R (1994) Ionomycin enhances Ca2+ influx by stimulating store‐regulated cation entry and not by a direct action at the plasma membrane. Biochem J 300(Pt 3): 665–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram JF, Wolfbauer G, Vaughan AM, Tang C, Albers JJ (2003) Phospholipid transfer protein interacts with and stabilizes ATP‐binding cassette transporter A1 and enhances cholesterol efflux from cells. J Biol Chem 278: 52379–52385 [DOI] [PubMed] [Google Scholar]
- Perez‐Sancho J, Vanneste S, Lee E, McFarlane HE, Esteban Del Valle A, Valpuesta V, Friml J, Botella MA, Rosado A (2015) The Arabidopsis synaptotagmin1 is enriched in endoplasmic reticulum‐plasma membrane contact sites and confers cellular resistance to mechanical stresses. Plant Physiol 168: 132–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Mistry A, Ammirati MJ, Chrunyk BA, Clark RW, Cong Y, Culp JS, Danley DE, Freeman TB, Geoghegan KF, Griffor MC, Hawrylik SJ, Hayward CM, Hensley P, Hoth LR, Karam GA, Lira ME, Lloyd DB, McGrath KM, Stutzman‐Engwall KJ et al (2007) Crystal structure of cholesteryl ester transfer protein reveals a long tunnel and four bound lipid molecules. Nat Struct Mol Biol 14: 106–113 [DOI] [PubMed] [Google Scholar]
- Reinisch KM, De Camilli P (2016) SMP‐domain proteins at membrane contact sites: structure and function. Biochem Biophys Acta 1861: 924–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheki Y, Bian X, Schauder CM, Sawaki Y, Surma MA, Klose C, Pincet F, Reinisch KM, De Camilli P (2016) Control of plasma membrane lipid homeostasis by the extended synaptotagmins. Nat Cell Biol 18: 504–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheki Y, De Camilli P (2017a) Endoplasmic reticulum‐plasma membrane contact sites. Annu Rev Biochem 86: 659–684 [DOI] [PubMed] [Google Scholar]
- Saheki Y, De Camilli P (2017b) The extended‐synaptotagmins. Biochem Biophys Acta 1864: 1490–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauder CM, Wu X, Saheki Y, Narayanaswamy P, Torta F, Wenk MR, De Camilli P, Reinisch KM (2014) Structure of a lipid‐bound extended synaptotagmin indicates a role in lipid transfer. Nature 510: 552–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclip A, Bacaj T, Giam LR, Sudhof TC (2016) Extended synaptotagmin (ESyt) triple knock‐out mice are viable and fertile without obvious endoplasmic reticulum dysfunction. PLoS One 11: e0158295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh BC, Horowitz LF, Hirdes W, Mackie K, Hille B (2004) Regulation of KCNQ2/KCNQ3 current by G protein cycling: the kinetics of receptor‐mediated signaling by Gq. J Gen Physiol 123: 663–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmay A, Prinz WA (2012) A conserved membrane‐binding domain targets proteins to organelle contact sites. J Cell Sci 125: 49–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay MG, Moss T (2016) Loss of all 3 Extended Synaptotagmins does not affect normal mouse development, viability or fertility. Cell Cycle 15: 2360–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner S, Ogunjimi AA, Wang HR, Rotin D, Sicheri F, Wrana JL, Forman‐Kay JD (2007) Autoinhibition of the HECT‐type ubiquitin ligase Smurf2 through its C2 domain. Cell 130: 651–662 [DOI] [PubMed] [Google Scholar]
- Willars GB, Nahorski SR, Challiss RA (1998) Differential regulation of muscarinic acetylcholine receptor‐sensitive polyphosphoinositide pools and consequences for signaling in human neuroblastoma cells. J Biol Chem 273: 5037–5046 [DOI] [PubMed] [Google Scholar]
- Williamson P, Bevers EM, Smeets EF, Comfurius P, Schlegel RA, Zwaal RF (1995) Continuous analysis of the mechanism of activated transbilayer lipid movement in platelets. Biochemistry 34: 10448–10455 [DOI] [PubMed] [Google Scholar]
- Xu J, Bacaj T, Zhou A, Tomchick DR, Sudhof TC, Rizo J (2014) Structure and Ca(2)(+)‐binding properties of the tandem C(2) domains of E‐Syt2. Structure 22: 269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung T, Gilbert GE, Shi J, Silvius J, Kapus A, Grinstein S (2008) Membrane phosphatidylserine regulates surface charge and protein localization. Science 319: 210–213 [DOI] [PubMed] [Google Scholar]
- Yu H, Liu Y, Gulbranson DR, Paine A, Rathore SS, Shen J (2016) Extended synaptotagmins are Ca2+‐dependent lipid transfer proteins at membrane contact sites. Proc Natl Acad Sci USA 113: 4362–4367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Zhou P, Wang AL, Wu D, Zhao M, Sudhof TC, Brunger AT (2017) The primed SNARE‐complexin‐synaptotagmin complex for neuronal exocytosis. Nature 548: 420–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaal RF, Comfurius P, Bevers EM (2005) Surface exposure of phosphatidylserine in pathological cells. Cell Mol Life Sci 62: 971–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A (2000) FM1‐43 reports plasma membrane phospholipid scrambling in T‐lymphocytes. Biochem J 349: 255–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded View Figures PDF
Review Process File


