Abstract
Introduction
2-D ultrasound shear wave elastography (SWE) could be considered as a new noninvasive tool for monitoring fetal lung development based on evaluation of mechanical properties during pregnancy. Interesting results are available concerning the use of SWE on developing organs, especially on premature infants and animal models. The main objective in this study is to evaluate the feasibility of 2-D SWE in human fetal lungs between 24 and 34 weeks of gestation (WG). The secondary objective is to modellise fetal lung-to-liver elastography ratio (LLE ratio) and to assess variations between normal lung and lung surfactant-enriched after a corticosteroids course indicated for a threatened preterm labour (TPL).
Methods/design
A prospective case-control study will be performed between 24 and 34 WG. Fetal lungs and liver will be explored by SWE into two groups: fetuses of women with an uncomplicated pregnancy (control group) and fetuses of women with a TPL requiring administration of corticosteroids (cases group). LLE ratio will be defined as the value of the lung elasticity divided by the value of the liver elasticity.
Primary judgement criterion is the value of elasticity modulus expressed in kilopascal. Lungs and liver will be explored through three measurements to define the most reproducible regions with the lowest intra- and inter-observer variability. Feasibility will be evaluated by assessing the number of examinations performed and the number of examinations with interpretable results. Intra- and inter-observer reproducibility will be evaluated by means of the intra-class correlation coefficient.
Ethics and dissemination
Approval of the study protocol was obtained from the human ethical research committee (Comité de Protection des Personnes EST II, process number 15/494) and the French National Agency for Medicines and Health Products Safety (process number 2015-A01575-44). All participants will sign a statement of informed consent.
Trial registration number
NCT02870608; Recruiting.
Keywords: Shear wave elastography, elasticity, fetal, lung.
Strengths and limitation of the study.
This study will be the first one to demonstrate applicability of shear wave elastography in human fetal lungs.
Pharmacovigilance data will be collected at birth and 3 months later in order to assess safety of the device.
Different factors will be taken into account to understand variability of measures: acquisition depth, number of measurements and representative values such as median or mean values.
We shall not attempt to define reference value of fetal lung elasticity or to correlate SWE value with respiratory function at birth.
Introduction
Background
Prenatal evaluation of fetal lung maturity (FLM) is still a challenge and different approaches have been reported with invasive biological tools or fetal imaging including ultrasounds and MRI. FLM is mainly correlated with surfactant level and numerous tests have been developed to quantify this production: lecithin/sphingomyelin ratio (L/S), laminar body count, testing for Phosphatidyl glycerol with thin-layer chromatography and estimation of the surfactant/albumin ratio using polarised light. All of these tests have been shown to have good sensitivity and negative predictive values. However, they all have a low positive predictive value and need invasive procedure.1 2 Thus, amniocentesis for FLM became obsolete.3
Lung and liver signal or echogenicity were often compared as ratio to assess FLM in several studies.4–10 Non-invasive assessment of FLM has been first attempted using ultrasonography quantified by grey level histogram.4 11 12 These studies demonstrated sonographic changes associated with lung maturation but weak correlations between echogenicity and respiratory distress syndrome (RDS). Quantitative analysis with specific software has been secondly proposed to improve robustness. Fetal lung texture analysis with this method demonstrated a strong correlation with gestational age, biological test on amniotic fluid and risk of neonatal respiratory morbidity.13 14 Additionally, several studies found a significant association between fetal lung-to-liver signal intensity ratio and estimated gestational age with fetal MRI. Actually, this technique is mainly used to assess fetal lung development and volume, especially in case of congenital diaphragmatic hernia or severe oligohydramnios. Nevertheless, the accuracy of both MRI and ultrasounds for estimating long-term respiratory outcomes remains limited.15–19 These previous techniques propose prenatal evaluation of lung maturity based on imaging features, but prediction of the pulmonary function through prenatal biomechanical properties seems relevant. With this hypothesis, we advanced the concept of ‘prenatal functional imaging’ and shear wave elastography (SWE) could be a promising tool.
SWE is a recent ultrasound technology using acoustic radiation force imaging (ARFI), which enables the assessment of the stiffness of tissues in real time through a colour quantitative elastogram.20 SWE assesses tissue elasticity (E), which is the tendency of tissue to resist deformation with an applied force. In a locally homogeneous and purely elastic medium, we can calculate Young’s modulus (kPa) from the shear wave speed (m.s-1) with the formula: E=3ρ.csw² (ρ being the medium density and csw the shear wave speed). The stiffness at any location of the region of interest (ROI) can be sampled using measurement tools to obtain a quantitative evaluation either in terms of shear wave speed or kPa. A low shear wave speed corresponds to a soft tissue, while a shear wave high speed indicates a stiff tissue. The study of deep organs is possible with SWE because this method does not require any compression-relaxation sequence on the target organ. One of the advantages of SWE over other elastography methods is that the generation of the mechanical impulse is operator-independent. SWE is expanding its range nowadays by its promising role in the examination of various organs (liver, thyroid) and for aiding discrimination of lesion characteristic, especially breast or prostate tumours.21–24
There is to date no publication regarding its use on human fetuses. The Food and Drug Administration (FDA) has so far not yet approved the use of SWE for obstetric clinical applications because of the paucity of data in the literature. Although there is no report about apparent histological changes with shear wave, the absence of other bioeffects could not be discarded and further studies are recommended.25 However, setting parameters respect mechanical and thermal indexes for soft tissues and bone, which are necessary to allow an obstetric examination defined by the FDA.26 Thus, there is a debate concerning the safety of SWE in fetal medicine. Herman et al studied the models and regulatory considerations for transient temperature rise during ARFI, and found that any transient increase in temperature caused by pulse bursts might still be within the safe limits determined by the FDA.27 Other authors showed that transducer heating was below 1°C for the current clinical applications of ARFI. According to experimental studies that simulate the heating of soft tissue during ARFI, they demonstrate that ARFI on soft tissue is safe, provided that thermal index be monitored.28 29
Interesting and reassuring results are available concerning the use of SWE on developing organs, especially on premature infants and animal models. Principal organs explored are the liver, brain and lungs. Quarello et al demonstrated the possibility of using SWE in non-human primate fetuses and its feasibility in exploring fetal organs. They found that elasticity values were related to organs and gestational age.30 Concerning fetal lungs elasticity, measurements of the proximal lungs seem to increase throughout the pregnancy. Concerning the use of SWE on premature infants, results are more numerous and include exploration of the liver or the brain. Alison et al have demonstrated the feasibility and reproducibility of liver stiffness in preterm neonates with intra uterine growth restriction (IUGR) and gestational age at birth between 26 and 31 weeks of gestation (WG).31 Other studies reported the contribution of SWE to the diagnosis of biliary atresia in neonates.32 33 Kim et al described the variation of brain elasticity in different regions in healthy neonates born between 28 and 40 WG.34 Su et al quantitatively evaluated the effect of ARFI in neonatal brain development. The authors found that full-term neonates had significantly higher elasticity values than preterm neonates and there were no reported immediate adverse events.35 Other teams work on the application of this technology to neonates as a supplementary tool to detect early ischaemic brain injury.36
If we describe fetal thorax centred on a four-chamber view of the fetal heart with SWE, the following observations can be made (figure 1)
Figure 1.
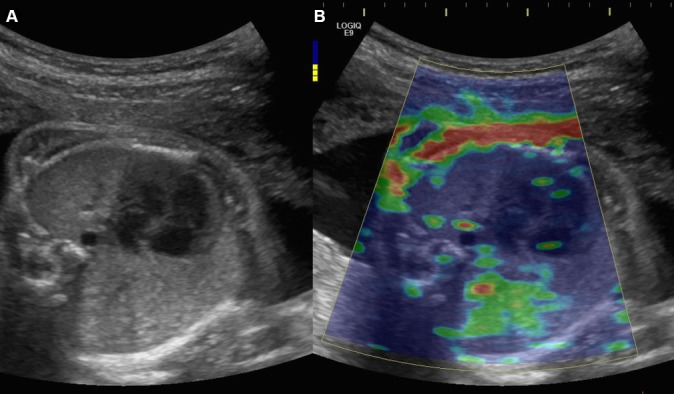
(A) B-mode image of the fetal thorax centred on a four-chamber view of the fetal heart, using an abdominal convex probe 1–6 MHz. (B) Elastogram of the fetal thorax showing a colour-coded elasticity map: blue identifies deformable tissue and red indicates rigid tissues (kPa).
SWE is able to make distinction between fetal soft and stiff tissues. Fetal ribs appear red coloured mapped, whereas proximal lung appears blue coloured.
A homogeneous coloured area can be obtained on the proximal lung with a colour scale ranging from 0 to 48 kPa. A homogeneous ROI is important for the reliability of the results.
SWE can differ according to the acquisition depth. Proximal lung appears with a blue homogenous distribution whereas distal lung appears with green homogenous distribution.
Objectives
The main objective in this study is to evaluate the feasibility of 2-D SWE in human fetal lungs between 24 and 34 WG. The secondary objective is to modellise fetal lung-to-liver elastography ratio (LLE ratio) and to assess variations between normal lung and lung surfactant-enriched after a corticosteroids course indicated for a threatened preterm labour (TPL).
Methods/design
Study design and setting
A prospective case-control study will be performed at the University Hospital of Besançon, (Besançon, France), Department of Obstetrics and Gynaecology. Fetal lungs and liver will be explored by SWE between 24 and 34 WG in two groups: fetuses of women with an uncomplicated pregnancy will be considered as the control group and fetuses of women with a threatened preterm labour requiring administration of corticosteroids will be enrolled as cases. The first eligible patient was enrolled in May 2016 and we plan to enrol for 18 months. End of follow-up of children will be completed approximately 6 months after the last birth.
In this pilot study, corticosteroids generate an effect model interesting for the assessment of LLE ratio variation in the cases. We take advantage of the action of corticosteroids to explore another medium with different supposed biomechanical properties. Indeed, corticosteroids accelerated the development of type two pneumocytes leading to surfactant production. Similarly, there is a significant increase in saturated phosphatidylcholine content in the fetal lung, potentially modifying the propagation of shear waves.37
The control group will be recruited during routine prenatal visits or ultrasounds according to the French ultrasounds schedule (ultrasound screening during the second trimester between 20 and 25 WG and the third trimester between 30 and 35 WG).38 Cases will be enrolled during their hospitalisation for threatened preterm delivery (figure 2). The design, conduction and reporting of this study will follow the norms of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.39
Figure 2.
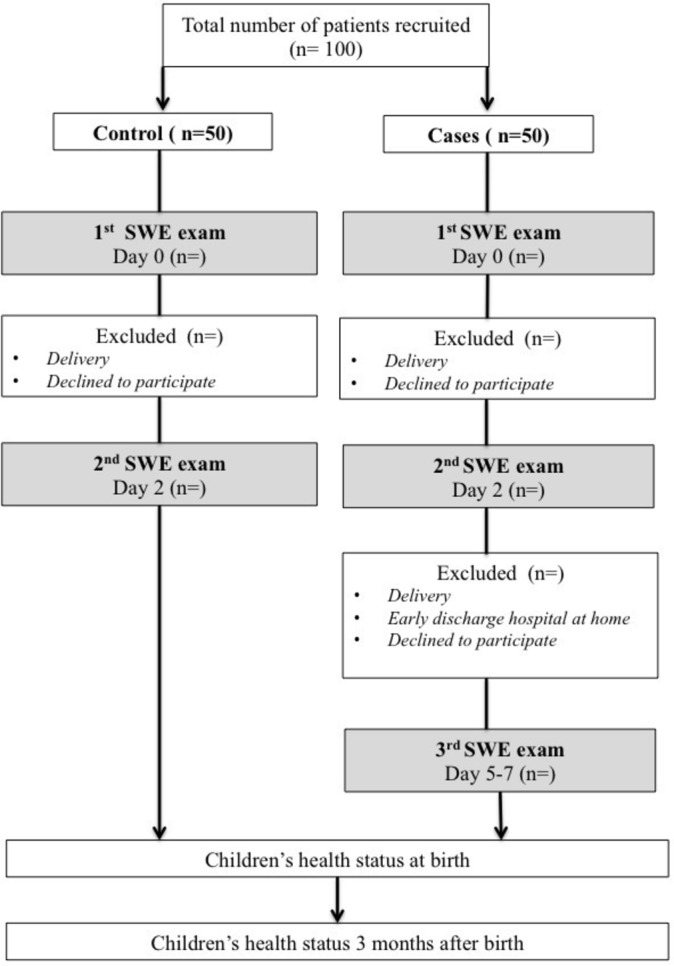
Design of the study.
Ethical considerations
The study respects the ethical standards established in the Declaration of Helsinki.40 Investigators undertake to respect law no. 2004–806 of 9 August 2004 relative to public health policy on clinical trials, made effective by implementing Decree 2006–477 of 26 April 2006.41 Approval of the study protocol was obtained from the human ethical research committee (Comité de Protection des Personnes EST II, process number 15/494) and the French National Agency for Medicines and Health Products Safety (process number 2015-A01575-44). The study is registered on clinicaltrial.gov with the following number: NCT02870608. All participants will sign a statement of informed consent and will be allowed to abandon the study at any time, without negative consequences. All procedures of the study will be confidential. The study will involve the collection and storage of ultrasound images, and study participants will therefore be thoroughly informed. All variables (patient parameters, data items, data elements) will be aggregated into electronic case report forms.
As explained in the background, the FDA has so far not yet approved the use of SWE for obstetric clinical applications because of the paucity of data in the literature. In this study, fetuses will be included from 24 to 34 WG, over the period of organogenesis. In the patient information sheet, it is specified that previous studies explored the liver or brain of premature infants with SWE. Alison et al studied liver stiffness in preterm neonates with IUGR and gestational age at birth between 26 and 31 WG.31 Franchi-Abella et al evaluate the feasibility and diagnostic accuracy of SWE for the assessment of liver stiffness in 96 patients, and seven were preterm newborns between 30 and 35 WG.42 Both studies did not report adverse outcomes and were approved by an institutional review board. Moreover, the consent form mentions conclusions of an experimental study of the biological effects associated with SWE in brains of neonatal mice. Results indicated that using SWE does not cause detectable histological changes. Potential effects on intracellular signalling pathways were detected when the SWE scanning lasted more than 30 min.25 Thus, patients will be informed that our study is focused on fetuses with a gestational age similar to preterm newborn included in the above-mentioned studies. Moreover, exploration with SWE will concern only lung and liver with duration of less than 10 min in respect of biophysical safety indexes: Thermal index Ti≤0.7 and Mechanical index Mi<1,9.
Participants
Cases will be recruited during hospitalisation for a threatened preterm labour requiring the administration of corticosteroids. The complementary inclusion criteria are: pregnant women aged 18 years or more; singleton pregnancy with eutrophic fetus; signature of consent; and affiliation to health insurance scheme (figure 2). Patients will be stratified by gestational age between 24 and 34 WG, with one class every WG (10 classes in total) and five patients per class. After obtaining consent, a SWE examination will be performed before the first intramuscular administration of corticosteroids ‘day 0’ (betamethasone 12 mg). A second SWE examination will be performed within 24 hours after the second intramuscular administration of corticosteroids ‘day 2’. A third SWE will be performed between the fifth and the seventh day following the first administration of corticosteroids ‘day 5–7’, if the women are not early discharged at home.
For the control group, women will be matched by gestational age and will be recruited during their pregnancy monitoring. Information concerning the study will be given as early as the second trimester by obstetricians or midwives during medical visits or ultrasounds. The complementary inclusion criteria are the following: major pregnant women; uncomplicated pregnancy between 24 and 34 WG; singleton pregnancy with eutrophic fetus; signature of consent; and affiliation to health insurance scheme. If they are willing to participate, a first SWE examination will be proposed at ‘day 0’, followed by a second one in 2 days ‘day 2’. Patients who come back for an SWE examination performed outside the current medical visit will receive financial compensation for travel expenses. As well as cases, control patients will be stratified by gestational age between 24 and 34 WG.
Exclusion criteria common to both groups are: fetal lung or liver pathologies; inclusion in another medical study; and patients under legal incapacity.
Variables and measuring technique
Prenatal variables
Primary judgement criterion is the value of kPa (E) (elasticity modulus) expressed in kilopascal (kPa). A Logic E9 system (General Electric Medical System, Milwaukee, WI, USA) with CE certification CE LNE/G-MED [CE0459] will be used for this study, equipped with abdominal convex probe 1–6 MHz (C1-6-D probe). This technique includes a real-time visualisation of a colour quantitative elastogram coupled to a B-mode image. To provide robust shear wave and optimise its detection, Logiq E9 uses innovative techniques. Comb-push ultrasound SWE generates multiple shear wave sources into theROI and uses directional filtering to remove the interference. The TAST technique is able to robustly track shear waves and correct for the sequential tracking delay.43 This technique has the merit of rapid and solid reconstruction of a large elasticity map (elastogram) with the only single acquisition by generating multiple shear waves from multiple unfocused push beams.
The shear wave acquisition measurement protocol is saved in a specific preset that will be used for every patient: 2D-Mode: harmonic imaging central depth=6 cm, frequency=6 MHz, acoustic power 100%; Elastography-Mode: shear wave signal frequency ranges from 50 to 400 kHz, acoustic power of the pushing beam: 100%, Tracking power: 100%, colour scale ranging from 0 to 48 kPa. Biophysical safety indices: Thermal index Ti≤0.7 and Mechanical index Mi<1,9 (table 1).
Table 1.
Variables and data stored for each SWE examination. ROI (region of interest)
| Maternal | Fetal | Technical |
| Age (years) | Presentation:
|
Young’s modulus (kPa) in nine ROI:
|
| Weeks of gestation | Estimated fetal weight (grams) | Distance between probe and target ROI for each measurement (cm) |
| Body mass index (kg/m2) | Placenta:
|
Biophysical safety indices:
|
| Subcutaneous adipose tissue thickness (cm) | Amniotic fluid index (cm) |
Fetal lungs will be voluntarily approached laterally to systematically obtain proximal and distal lungs regarding the distance to the probe. Each measurement has to be performed on a homogeneous elastogram to be valid. Operators will perform two cycles of nine kPa measurements, while systematically repositioning the probe on each target organ.
A cycle includes: three measurements on the proximal lung (anterior ‘P1’, medium ‘P2’ and posterior portion ‘P3’); three measurements on the distal lung (anterior ‘D1’, medium ‘D2’ and posterior portion ‘D3’); and three measurements on three liver segments (IV, V, VI) (figure 3). Implementation of SWE displays 2D images of kPa in the target organ. In real time, the elasticity appears colour-coded, where blue identifies deformable tissue and red indicates rigid tissues. The kPa value at any location will be sampled using a round ROI of 5 mm. The distance between the probe and the target organ will be collected. The average elasticity in the ROI will be automatically recorded by the system in a worksheet. The investigators will adjust the position of the ROI using the B-mode image for guidance and take care to obtain the most homogeneous colour-coded ROI before kPa estimation (figure 4). Technical failure was defined as failure to obtain a homogeneous elastogram in more than 50% in the sampling area. To test the inter-observer variability, a second observer will successively perform measurements on 30 fetuses. All measurements will be carried out directly by ultrasound on the target organ. The second observer will perform measurements just after the first one and will be blinded to the feasibility and results obtained by the first one.
Figure 3.
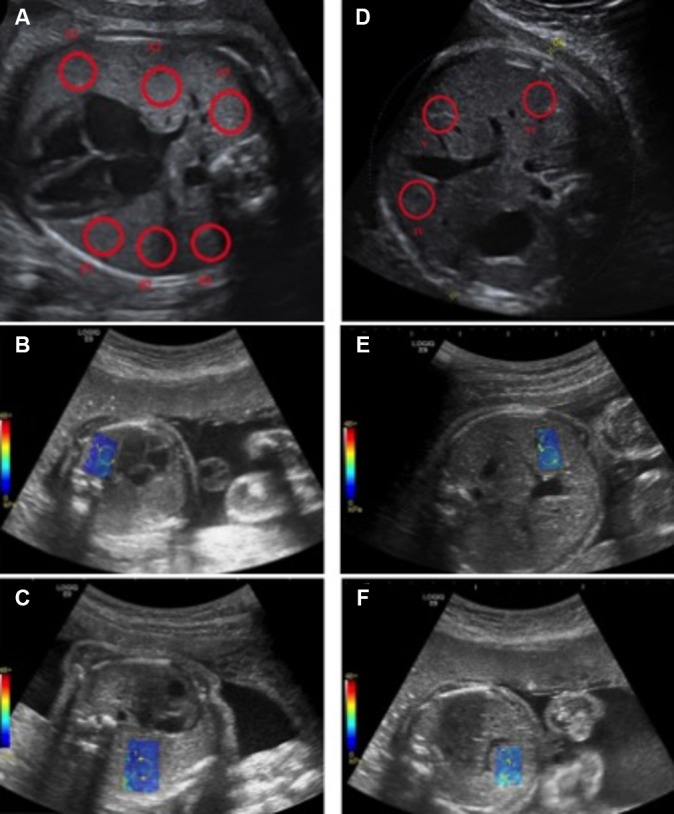
Measurement sites with 2D comb-push SWE using an abdominal convex probe 1–6 MHz (C1-6-D probe). Colour scale ranging from 0 to 48 kPa. Sufficient colour maps covering more than 50% of the sampling area obtained considered as a technical success. ROI are placed on homogeneous elastograms.- (A,B) three measurements on the proximal lung (anterior ‘P1’, medium ‘P2’ and posterior portion ‘P3’). - (A,C) three measurements on the distal lung (anterior ‘D1’, medium ‘D2’ and posterior portion ‘D3’). - (D–F) three measurements on three liver segments (IV, (V, VI). Operator will perform two cycles of nine kPa measurements while systematically repositioning the probe and each target ROI.
Figure 4.
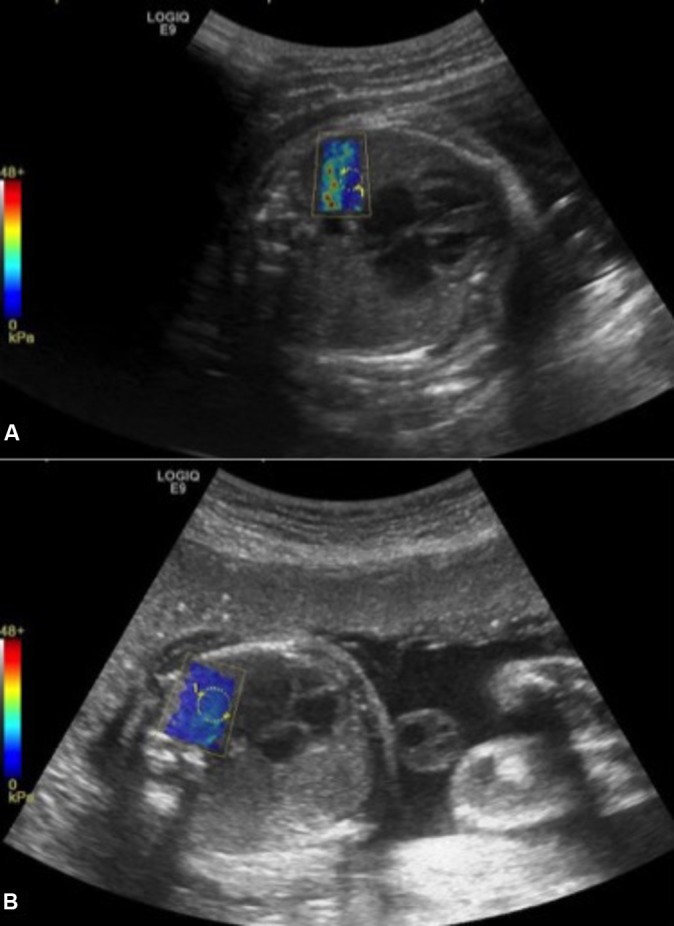
2D comb-push SWE on proximal fetal lung. Colour scale ranging from 0 to 48 kPa. On image (A), elastogram is not homogeneous and is degraded by artefact on the left side due to rib shadowing. By moving the probe to another location, a more homogeneous elastogram (B) is obtained allowing measurement.
Estimation of Fetal Weight Estimation (EFW) will be performed during each examination according to the Hadlock formula based on cephalic circumference (CC), abdominal circumference (AC) and femoral length (FL): log10 EFW=1326 + 0,0107 PC+0,0438 PA+0158 LF+0 00 326 (PA x LF).44
Postnatal variables
The following pharmacovigilance data will be collected at birth and 3 months' later in order to assess safety of the device:
At birth in both groups: term of birth, weight, neonatal transfer, Apgar Score and evaluation of respiratory distress by Silverman Score.45
Three months after birth: medical history, health problems, respiratory diseases or symptoms, liver disease or symptoms and number of hospitalisations since birth.
Limitation of bias
The use of sampling method with five patients per class of gestational age will limit repartition bias between control and cases. Inclusion of any pregnant women attending Besançon University maternity department for the control group, and inclusion of all women with a threatened preterm labour will limit the risk of selection bias. Risk of loss will be limited in the cases group between ‘day 0’ and ‘day 2’ because patients will remain hospitalised for corticosteroids administration. Nevertheless, the risk of loss is most important between ‘day 2’ and ‘day 5–7’ because of possible early discharge at home after the second administration of corticosteroids. For ethical and medical reasons, cases early discharge at home cannot be convened in ‘day 5–7’. Risk of loss will be limited in the control group because of a financial compensation for travel expenses. To limit inter-observer variation, expert sonographers will perform elastography examinations.
Study size
Because of the paucity of data regarding SWE on the human fetal lung, the sample size calculation was based on measurements of fetal lungs in pregnant baboons and extrapolations made by clinicians, with the following assumptions: average expected value of elasticity coefficient for fetal lung will be 2 kPa at 24 WG and 4 kPa at 34 WG. We hypothesised a linear increase of elasticity coefficient during pregnancy in the control group and a decrease in cases exposed to corticosteroids. At ‘day 2’, we therefore expect in the cases a variation of 0.2 kPa (corresponding to 1 week of lung maturation during gestation). Forty-two patients per group will be needed to have 90% power to statistically demonstrate such a difference (0.2 vs 0.057) assuming α risk of 0.05 and SD of 0.2.
Proposed statistical analysis
Technical validation
Feasibility will be evaluated by assessing the number of examinations performed and the number of examinations with interpretable results. All the variables and data that will be stored for each SWE examination are summarised in table 1. Intra- and inter-observer reproducibility will be evaluated by the intra-class correlation coefficient (ICC) with 95% CI. Intra-observer reproducibility will be calculated using the two cycles of measure, inter observer reproducibility between the operators will be calculated by means of the two cycles of measures if the intra-observer is high.46 Only repeatable and reproducible values of each ROI will be considered to modellise the LLE ratio.
Clinical evaluation
Evolution of fetal lung SWE, fetal liver SWE and lung-to-liver-SWE ratio will be assessed by group and overall. Lung-to-liver-SWE ratio will be initially defined as the value of the lung elasticity divided by the value of the liver elasticity.
SWE values before corticosteroids administration will be compared between cases and control groups. If there is a statistically significant difference, a confusional bias will have to be sought between fetal lung elasticity values and threatened preterm labour. All results will be presented as ‘delta’ variation between two measurements in order to limit ‘non-comparability bias’ between both groups. If corticosteroids affect fetal lung elasticity, ‘delta before/after’ will be more important in cases than controls.
Analysis of mean differences between groups will be carried out using a Student’s test or Wilcoxon test according to the distribution of data for quantitative variables. The Shapiro–Wilk test will be used to determine if the data set is well modelled by a normal distribution. The relationship of quantitative variables to each other will be tested using Pearson’s or Spearman’s correlation as appropriate.
Qualitative variables will be expressed as frequencies and quantitative variables will be displayed as the mean ±SD if normally distributed or as the median ±IQR if asymmetrically distributed. Qualitative variables will be analysed using a χ2 test or Fisher test.
For statistical analyses, the level of statistical significance will be set at 5% (P<0.05). Statistical analysis will be performed with statistical software SAS for Windows, version 9.4 and MedCalc software, version 15.
Discussion
2D-SWE could be considered as a new noninvasive tool for monitoring fetal lung development based on the evaluation of mechanical properties during pregnancy. However, standardisation for measuring SWE in the human fetal lung is necessary to demonstrate applicability of the technique, and to understand limiting factors. This pilot study will focus on technical performances before considering clinical applications. In this protocol, SWE will be used after the fetal period of organogenesis, and pharmacovigilance data will be collected at birth and 3 months' later in order to assess the safety of the device.
With the study design, we shall not attempt to define the reference value of fetal lung elasticity during pregnancy or to correlate SWE value with respiratory function at birth. The main objective is to evaluate the feasibility and reproducibility of 2D-SWE in the human fetal lung between 24 and 34 WG and to modellise a new LLE ratio. Indeed, each target organ will be explored through three measurements to define the most relevant and reproducible regions with the lowest intra- and inter-observer variability. The use of the LLE ratio is interesting because it allows comparison between two tissues in which the liver is taken as a reference organ. Several studies indicate that liver signal or echogenicity remain constant through gestation, suggestive of a regular evolution of the tissue characteristics.47 48 Thus, we will compare the evolution of SWE values between two organs subjected to the same technical variations, especially for depth. Both lung and liver tissue density increase during pregnancy and we expect a linear increase of SWE values of these organs. In case of variations of lung SWE values after a course of corticosteroids, LLE ratio should be modified. If we suppose that liver elasticity will be constant between two separate examinations of 48 hours, the LLE ratio will decrease if corticosteroids lead to attenuation of shear wave speed. Conversely, if lung elasticity increases after a course of corticosteroids, the LLE ratio will also increase. Finally, the LLE ratio will be used to increase the reliability of the results and to standardise the study.
One advantage of this protocol is to evaluate SWE in three tissues with different supposed biomechanical properties: normal lung, lung with increase of surfactant synthesis after corticosteroids course and liver. Corticosteroids generate an effect model interesting for the assessment of LLE’s ratio variation in the cases because of a significant increase of phosphatidylcholine content in fetal lungs. This effect is mediated by an activation of choline-phosphate cytidylyltransferase.37 Given the placental transfer and time to induce the cellular mechanism of transcription, an artificial delay of 24 hours seems to be necessary to produce surfactant in pneumocytes type 2. The benefit of corticosteroid administration is greatest at 2 to 7 days after the initial dose.49 50 Two approaches can be proposed after a complete course of corticosteroids. On the one hand, the SWE of the fetal lung could increase because corticosteroids accelerate FLM, and we could expect an advanced ripening by one WG. On the other hand, an increase in phospholipids content in fetal lung after corticosteroids could increase viscosity and lead to dispersion of shear wave speed and attenuation.
Different factors will be taken into account in this study to understand the variability of measures: acquisition depth, number of measurements and representative values such as median or mean values. Shear wave velocity can be different according to the acquisition depth and this parameter is gradually underestimated with increasing depth. Effect is greatest in stiff tissues but little effect is seen in a soft target.51–53 This phenomenon might be explained by a damping of the acoustic push pulse that generates the shear waves by both increased attenuation and increased stiffness of the target.54 Thus, we expected low variation between proximal and distal lung because they may be considered as soft tissues. There are also debates about the acquisition number during SWE and the designers of the device did not provided advices. The number of measurements (NMs) reported in the recent literature on various organs is inconsistent (commonly seen are 3, 4 and 5 NMs).55 56 Quarello et al reported 3 NMs on fetal lungs in pregnant baboons, and ICC for and inter-observer variability were better for an average value of three measurements.57 For Alison et al, 9 NMs were performed on liver in preterm neonates with intra uterine growth restriction (three measurements from three different liver segments). Measurements showed high reproducibility on average values (ICC=0.94–0.98 for intra-operator, 0.86 for inter-operator).31 One objective of our protocol is to determine the NMs necessary to evaluate fetal lung SWE with a good inter- and intra-observer reproducibility. Thus, each value will be analysed to determine if mean lung and liver SWE can be achieved through only one valid measurement or more, and if its performance is equivalent to two or three valid measurements.
To sum up, we hope that the results of this study will contribute to clarify the applicability of SWE on the human fetal lung between 24 and 34 WG. SWE could be a new tool for the reliable prediction of lung development through biomechanical properties. This study will be the first one to propose a protocol of measurement underlying limiting factors.
Supplementary Material
Footnotes
Contributors: All the authors contributed to the conception and design of the study. NM, SA and RR provided the idea for the research or article, created the hypothesis and wrote the original proposal. NM, CV, LP and RR significantly contributed to writing the paper. NM, CV, GB and LP wrote this protocol paper. All authors read and approved the final manuscript.
Funding: This study is supported by University Hospital of Besançon, APICHU Réf: API/2015/60.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The Human Research Ethics Committee (Comité de Protection des Personnes EST II) process number 15/494.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bhagwanani SG, Fahmy D, Turnbull AC. Prediction of neonatal respiratory distress by estimation of amniotic-fluid lecithin. Lancet 1972;1:159–62. [DOI] [PubMed] [Google Scholar]
- 2.Field NT, Gilbert WM. Current status of amniotic fluid tests of fetal maturity. Clin Obstet Gynecol 1997;40:366–86. 10.1097/00003081-199706000-00012 [DOI] [PubMed] [Google Scholar]
- 3.Varner S, Sherman C, Lewis D, et al. . Amniocentesis for fetal lung maturity: will it become obsolete? Rev Obstet Gynecol 2013;6:126–34. [PMC free article] [PubMed] [Google Scholar]
- 4.Maeda K, Utsu M, Yamamoto N, et al. . Echogenicity of fetal lung and liver quantified by the grey-level histogram width. Ultrasound Med Biol 1999;25:201–8. 10.1016/S0301-5629(98)00160-4 [DOI] [PubMed] [Google Scholar]
- 5.Duncan KR, Gowland PA, Moore RJ, et al. . Assessment of fetal lung growth in utero with echo-planar MR imaging. Radiology 1999;210:197–200. 10.1148/radiology.210.1.r99ja42197 [DOI] [PubMed] [Google Scholar]
- 6.Moshiri M, Mannelli L, Richardson ML, et al. . Fetal lung maturity assessment with MRI fetal lung-to-liver signal-intensity ratio. AJR Am J Roentgenol 2013;201:1386–90. 10.2214/AJR.12.9679 [DOI] [PubMed] [Google Scholar]
- 7.Oka Y, Rahman M, Sasakura C, et al. . Prenatal diagnosis of fetal respiratory function: evaluation of fetal lung maturity using lung-to-liver signal intensity ratio at magnetic resonance imaging. Prenat Diagn 2014;34:1289–94. 10.1002/pd.4469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorincour G, Bach-Segura P, Ferry-Juquin M, et al. . Lung signal on fetal MRI: normal values and usefulness for congenital diaphragmatic hernia. J Radiol 2009;90:53–8. [DOI] [PubMed] [Google Scholar]
- 9.Beck AP, Araujo Júnior E, Leslie AT, et al. . Assessment of fetal lung maturity by ultrasound: objective study using gray-scale histogram. J Matern Fetal Neonatal Med 2015;28:617–22. 10.3109/14767058.2014.927862 [DOI] [PubMed] [Google Scholar]
- 10.Fried AM, Loh FK, Umer MA, et al. . Echogenicity of fetal lung: relation to fetal age and maturity. AJR Am J Roentgenol 1985;145:591–4. 10.2214/ajr.145.3.591 [DOI] [PubMed] [Google Scholar]
- 11.Serizawa M, Maeda K. Noninvasive fetal lung maturity prediction based on ultrasonic gray level histogram width. Ultrasound Med Biol 2010;36:1998–2003. 10.1016/j.ultrasmedbio.2010.08.011 [DOI] [PubMed] [Google Scholar]
- 12.Prakash KN, Ramakrishnan AG, Suresh S, et al. . Fetal lung maturity analysis using ultrasound image features. IEEE Trans Inf Technol Biomed 2002;6:38–45. [DOI] [PubMed] [Google Scholar]
- 13.Bonet-Carne E, Palacio M, Cobo T, et al. . Quantitative ultrasound texture analysis of fetal lungs to predict neonatal respiratory morbidity. Ultrasound Obstet Gynecol 2015;45:427–33. 10.1002/uog.13441 [DOI] [PubMed] [Google Scholar]
- 14.Palacio M, Bonet-Carne E, Cobo T, et al. . Prediction of neonatal respiratory morbidity by quantitative ultrasound lung texture analysis: a multicenter study. Am J Obstet Gynecol 2017;217 10.1016/j.ajog.2017.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cannie M, Jani J, Meersschaert J, et al. . Prenatal prediction of survival in isolated diaphragmatic hernia using observed to expected total fetal lung volume determined by magnetic resonance imaging based on either gestational age or fetal body volume. Ultrasound Obstet Gynecol 2008;32:633–9. 10.1002/uog.6139 [DOI] [PubMed] [Google Scholar]
- 16.Kastenholz KE, Weis M, Hagelstein C, et al. . Correlation of observed-to-expected MRI fetal lung volume and ultrasound lung-to-head ratio at different gestational times in fetuses with congenital diaphragmatic hernia. AJR Am J Roentgenol 2016;206:856–66. 10.2214/AJR.15.15018 [DOI] [PubMed] [Google Scholar]
- 17.Kehl S, Becker L, Eckert S, et al. . Prediction of mortality and the need for neonatal extracorporeal membrane oxygenation therapy by 3-dimensional sonography and magnetic resonance imaging in fetuses with congenital diaphragmatic hernias. J Ultrasound Med 2013;32:981–8. 10.7863/ultra.32.6.981 [DOI] [PubMed] [Google Scholar]
- 18.Strizek B, Cos Sanchez T, Khalifé J, et al. . Impact of operator experience on the variability of fetal lung volume estimation by 3D-ultrasound (VOCAL) and magnetic resonance imaging in fetuses with congenital diaphragmatic hernia. J Matern Fetal Neonatal Med 2015;28:858–64. 10.3109/14767058.2014.935760 [DOI] [PubMed] [Google Scholar]
- 19.Rubesova E. Why do we need more data on MR volumetric measurements of the fetal lung? Pediatr Radiol 2016;46:167–71. 10.1007/s00247-015-3521-7 [DOI] [PubMed] [Google Scholar]
- 20.Bercoff J, Pernot M, Tanter M, et al. . Monitoring thermally-induced lesions with supersonic shear imaging. Ultrason Imaging 2004;26:71–84. 10.1177/016173460402600201 [DOI] [PubMed] [Google Scholar]
- 21.Erkan M, Canberk S, Kilicoglu GZ, et al. . Avoidance of unnecessary fine-needle aspiration with the use of the Thyroid Imaging Reporting Data System classification and strain elastography based on The Bethesda System for Reporting Thyroid Cytopathology. Mol Clin Oncol 2016;5:625–30. 10.3892/mco.2016.1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rouvière O, Melodelima C, Hoang Dinh A, et al. . Stiffness of benign and malignant prostate tissue measured by shear-wave elastography: a preliminary study. Eur Radiol 2017;27:1858–66. 10.1007/s00330-016-4534-9 [DOI] [PubMed] [Google Scholar]
- 23.Denis M, Gregory A, Bayat M, et al. . Correlating tumor stiffness with Immunohistochemical subtypes of breast cancers: prognostic value of comb-push ultrasound shear elastography for differentiating luminal subtypes. PLoS One 2016;11:e0165003 10.1371/journal.pone.0165003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menten R, Leonard A, Clapuyt P, et al. . Transient elastography in patients with cystic fibrosis. Pediatr Radiol 2010;40:1231–5. 10.1007/s00247-009-1531-z [DOI] [PubMed] [Google Scholar]
- 25.Li C, Zhang C, Li J, et al. . An experimental study of the potential biological effects associated with 2-D shear wave elastography on the neonatal brain. Ultrasound Med Biol 2016;42:1551–9. 10.1016/j.ultrasmedbio.2016.02.018 [DOI] [PubMed] [Google Scholar]
- 26.Fowlkes JB. Bioeffects committee of the American institute of ultrasound in medicine. American institute of ultrasound in medicine consensus report on potential bioeffects of diagnostic ultrasound: executive summary. J Ultrasound Med 2008;27:503–15. [DOI] [PubMed] [Google Scholar]
- 27.Herman BA, Harris GR. Models and regulatory considerations for transient temperature rise during diagnostic ultrasound pulses. Ultrasound Med Biol 2002;28:1217–24. 10.1016/S0301-5629(02)00558-6 [DOI] [PubMed] [Google Scholar]
- 28.Palmeri ML, Frinkley KD, Nightingale KR. Experimental studies of the thermal effects associated with radiation force imaging of soft tissue. Ultrason Imaging 2004;26:100–14. 10.1177/016173460402600203 [DOI] [PubMed] [Google Scholar]
- 29.Karaman E. Response to ‘Safety of elastography applied to the placenta: Be careful with ultrasound radiation force’. J Obstet Gynaecol Res 2017;43:1510 10.1111/jog.13382 [DOI] [PubMed] [Google Scholar]
- 30.Quarello E, Lacoste R, Mancini J, et al. . Feasibility and reproducibility of ShearWave(TM) elastography of fetal baboon organs. Prenat Diagn 2015;35:1112–6. 10.1002/pd.4655 [DOI] [PubMed] [Google Scholar]
- 31.Alison M, Biran V, Tanase A, et al. . Quantitative shear-wave elastography of the lver in preterm neonates with intra-uterine growth restriction. PLoS One 2015;10:e0143220 10.1371/journal.pone.0143220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanquinet S, Rougemont AL, Courvoisier D, et al. . Acoustic radiation force impulse (ARFI) elastography for the noninvasive diagnosis of liver fibrosis in children. Pediatr Radiol 2013;43:545–51. 10.1007/s00247-012-2595-8 [DOI] [PubMed] [Google Scholar]
- 33.Hanquinet S, Courvoisier DS, Rougemont AL, et al. . Contribution of acoustic radiation force impulse (ARFI) elastography to the ultrasound diagnosis of biliary atresia. Pediatr Radiol 2015;45:1489–95. 10.1007/s00247-015-3352-6 [DOI] [PubMed] [Google Scholar]
- 34.Kim HG, Park MS, Lee JD, et al. . Ultrasound elastography of the neonatal brain: preliminary study. J Ultrasound Med 2017;36:1313–9. 10.7863/ultra.16.06079 [DOI] [PubMed] [Google Scholar]
- 35.Su Y, Ma J, Du L, et al. . Application of acoustic radiation force impulse imaging (ARFI) in quantitative evaluation of neonatal brain development. Clin Exp Obstet Gynecol 2015;42:797–800. [PubMed] [Google Scholar]
- 36.Bailey C, Huisman T, de Jong RM, et al. . Contrast-enhanced ultrasound and elastography Imaging of the neonatal brain: a review. J Neuroimaging 2017;27:437–41. 10.1111/jon.12443 [DOI] [PubMed] [Google Scholar]
- 37.Post M, Barsoumian A, Smith BT. The cellular mechanism of glucocorticoid acceleration of fetal lung maturation. Fibroblast-pneumonocyte factor stimulates choline-phosphate cytidylyltransferase activity. J Biol Chem 1986;261:2179–84. [PubMed] [Google Scholar]
- 38.Rapport de la Conférence Nationale en Echographie Obstetricale et Fœtale. L’echographie de dépistage prénatal CNEOF. 14 juillet, 2016. available at http://www.cfef.org/archives/bricabrac/cneof/rapportcneof2016.pdf
- 39.Noah N. The STROBE initiative: strengthening the reporting of observational studies in epidemiology (STROBE). Epidemiol Infect 2008;136:865 10.1017/S0950268808000733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 41.Maillols-Perroy A-C, Tillet Y. The adoption of Jardé law modifies the legal framework of clinical research in France. Therapie Avr 2012;67:77–87. [DOI] [PubMed] [Google Scholar]
- 42.Franchi-Abella S, Corno L, Gonzales E, et al. . Feasibility and diagnostic accuracy of supersonic shear-wave elastography for the assessment of liver stiffness and liver fibrosis in children: a pilot study of 96 patients. Radiology 2016;278:554–62. 10.1148/radiol.2015142815 [DOI] [PubMed] [Google Scholar]
- 43.Song P, Macdonald M, Behler R, et al. . Two-dimensional shear-wave elastography on conventional ultrasound scanners with time-aligned sequential tracking (TAST) and comb-push ultrasound shear elastography (CUSE). IEEE Trans Ultrason Ferroelectr Freq Control 2015;62:290–302. 10.1109/TUFFC.2014.006628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hadlock FP, Harrist RB, Carpenter RJ, et al. . Sonographic estimation of fetal weight. The value of femur length in addition to head and abdomen measurements. Radiology 1984;150:535–40. 10.1148/radiology.150.2.6691115 [DOI] [PubMed] [Google Scholar]
- 45.Silverman WA, Andersen DH. A controlled clinical trial of effects of water mist on obstructive respiratory signs, death rate and necropsy findings among premature infants. Pediatrics 1956;17:1–10. [PubMed] [Google Scholar]
- 46.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–10. [PubMed] [Google Scholar]
- 47.Dubinsky TJ, Moshiri M, Adams Waldorf K, et al. . Increased fetal lung T2 signal is not due to increasing surfactant concentration: an in vitro T2 mapping analysis. Prenat Diagn 2017;37:211–4. 10.1002/pd.4987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mills M, Winter TC, Kennedy AM, et al. . Determination of fetal lung maturity using magnetic resonance imaging signal intensity measurements. Ultrasound Q 2014;30:61–7. 10.1097/RUQ.0000000000000054 [DOI] [PubMed] [Google Scholar]
- 49.Mulder EJ, Derks JB, Visser GH. Antenatal corticosteroid therapy and fetal behaviour: a randomised study of the effects of betamethasone and dexamethasone. Br J Obstet Gynaecol 1997;104:1239–47. 10.1111/j.1471-0528.1997.tb10969.x [DOI] [PubMed] [Google Scholar]
- 50.Loehle M, Schwab M, Kadner S, et al. . Dose-response effects of betamethasone on maturation of the fetal sheep lung. Am J Obstet Gynecol 2010;202:e1–7. 10.1016/j.ajog.2009.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shin NY, Kim MJ, Lee MJ, et al. . Transient elastography and sonography for prediction of liver fibrosis in infants with biliary atresia. J Ultrasound Med 2014;33:853–64. 10.7863/ultra.33.5.853 [DOI] [PubMed] [Google Scholar]
- 52.Tang A, Cloutier G, Szeverenyi NM, et al. . Ultrasound elastography and MR elastography for assessing liver fibrosis: Part 1, Principles and Techniques. Am J Roentgenol 2015;205:22–32. 10.2214/AJR.15.14552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carlsen JF, Pedersen MR, Ewertsen C, et al. . A comparative study of strain and shear-wave elastography in an elasticity phantom. Am J Roentgenol 2015;204:236–242. 10.2214/AJR.14.13076 [DOI] [PubMed] [Google Scholar]
- 54.Tozaki M, Saito M, Joo C, et al. . Ultrasonographic tissue quantification of the breast using acoustic radiation force impulse technology: phantom study and clinical application. Jpn J Radiol 2011;29:598–603. 10.1007/s11604-011-0591-9 [DOI] [PubMed] [Google Scholar]
- 55.Ling W, Lu Q, Quan J, et al. . Assessment of impact factors on shear wave based liver stiffness measurement. Eur J Radiol 2013;82:335–41. 10.1016/j.ejrad.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 56.Grenier N, Gennisson JL, Cornelis F, et al. . Renal ultrasound elastography. Diagn Interv Imaging 2013;94:545–50. 10.1016/j.diii.2013.02.003 [DOI] [PubMed] [Google Scholar]
- 57.Quarello E, Lacoste R, Mancini J, et al. . Shear wave elastography of fetal lungs in pregnant baboons. Diagn Interv Imaging 2016;97:605–10. 10.1016/j.diii.2015.11.019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


