Abstract
Objective
We compared the incidence and risk of chronic kidney disease (CKD) between subjects with new-onset migraine and matched controls without migraine in this large-scale retrospective cohort study.
Design
Population-based cohort study.
Setting
8880 subjects with migraine and 503 070 subjects without migraine were enrolled between January 1, 2000 and December 31, 2013, all diagnosed to be without kidney disease. All the participants were registered in the National Health Insurance Research Database.
Participants
Finally, data from 7156 subjects with migraine and 7156 propensity-score-matched control subjects were analysed.
Primary outcome measure
We used Cox proportional hazards regression to estimate adjusted HRs for incident CKD; subgroup analyses were performed to assess the interactive effects of migraine with demographics, comorbidities and long-term medications.
Results
The incidence of CKD was higher in the migraine group than in the control group. The risk of developing CKD was significantly higher in subjects with migraine than without migraine (P=0.031). Subjects with migraine aged <65 years (age 40–64 (adjusted HR (aHR) 1.35; 95% CI 1.05 to 1.73); age <40 (aHR 1.55; 95% CI 1.02 to 2.36)), with ≥1 comorbid diseases (1–2 diseases (aHR 1.30; 95% CI 1.01 to 1.68); ≥3 diseases (aHR 1.45; 95% CI 1.01 to 2.07)), and not receiving anti-migraine agents (aHR 1.26; 95% CI 1.04 to 1.54) were at a higher risk of developing CKD compared with the control subjects. The interaction between migraine and comorbidities was not significant; age, male gender and long-term use of non-steroidal anti-inflammatory drugs (NSAIDs) were independent risk factors for CKD in subjects with migraine.
Conclusion
Migraine may be an independent risk factor for CKD. Young subjects with migraine, and those with comorbid conditions or without medical control, are likely to be at higher risk for CKD. Ageing, male sex and NSAIDs tend to have an association with CKD in subjects with migraine.
Keywords: chronic kidney disease, national health insurance research database, anti-migraine agents, propensity score, migraine
Strengths and limitations of this study.
This is the first population-based retrospective cohort study to examine the significant association between migraine and subsequent developing chronic kidney disease (CKD) in our study.
Subjects with migraine aged <65 years, with ≥1 comorbid disease, and not receiving anti-migraine agents were associated with a higher risk of developing CKD compared with the control subjects.
Our results suggest that migraine may be a significant and independent risk factor for CKD. Moreover, older age and long-term use of non-steroidal anti-inflammatory drugs are likely to be independent risk factors of CKD for migraine subjects.
We indeed tried to minimise certain confoundings, commonly seen in registry-based studies, including the use of a propensity-score matching, considering the effects of medical surveillance bias by taking clinical visits number into account, and tried to validate the diagnoses of both migraine and CKD.
We are unable to obtain detailed clinical information (including laboratory data, experimental tests and pathology results) from the National Health Insurance programme.
Introduction
Headache is a non-specific symptom frequently encountered in subjects with chronic kidney disease (CKD).1 2 Although new-onset migraine is more prevalent among younger subjects than among older subjects, the cumulative incidence of migraine in subjects over 55 years is still high and around 20%–34%.3 4 Migraine is often accompanied by additional symptoms such as nausea, dizziness or seeing flashing lights or blind spots, which may cause several mental and physical problems.5 Growing evidence suggests that migraine is not a localised problem; it involves peripheral and central neuronal excitation and vascular disturbances6 7 that could lead to chronic hypertension and kidney disease.7 8 Recent studies suggest that the physical decline and disability associated with CKD influence mortality more than multimorbidity.9
The brain and kidneys show similar anatomical and functional microvascular regulation and possess similar cardiometabolic risk factors.10–12 Episodic migraine disorder involving sensory sensitivity has widespread influence on the central nervous system and related organs; changes in the synchronisation of cortical activity may be an explanation of its pathophysiology.13 Recent migraine research has focused on small molecules, calcitonin gene-related peptide (CGRP), substance P and endothelin.6 14 In fact, the interaction between these neuropeptides and CKD is mediated by vasoafferent nerve activation, given the fact that the initial insult could activate renal sympathoexcitation.8 15
In subjects with chronic migraine, several factors could trigger CKD, such as blood pressure fluctuations, coexistent medical conditions and the overuse of symptomatic medications5; moreover, about 48% of the subjects receiving haemodialysis complain of migraine. Most subjects were treated with a combination of analgesics (81%), while 19% of the subjects used a single analgesic to treat this discomfort.16
Although some researchers have found that several subjects with CKD suffer from frequent migraines, very few studies have reported a causal relationship between the two.17 With the increasing prevalence of migraine and its poor clinical outcome in subjects with CKD, identifying the relationship between migraine and CKD is crucial to prevent the development and progression of CKD. We conducted a nationwide, large-scale, population-based study to determine whether migraine is a contributing factor to CKD.
Materials and methods
Data source
The data for the present study were retrieved from the Taiwan National Health Insurance Research Database (NHIRD), which covers >99% of the Taiwanese population of approximately 23 million. Longitudinal Health Insurance Database 2005 (LHID2005) (containing 1 million patients randomly selected from the NHIRD in 2005 and longitudinally linked with NHIRD from 1996 to 2013) was accessed under the permission of the National Health Insurance for research. The comprehensive healthcare information maintained in the LHID2005 included the date of birth, sex, area of residence, medications, medical procedures and diagnostic codes. The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes were used to identify the diseases. These diagnostic codes have been shown to have high accuracy and validity.18–21 This study was approved after a full ethical review by the Institutional Review Board (IRB) of the Changhua Christian Hospital (approval number 150925), and the IRB waived the need for consent. Those data were accessed anonymously.
Study population
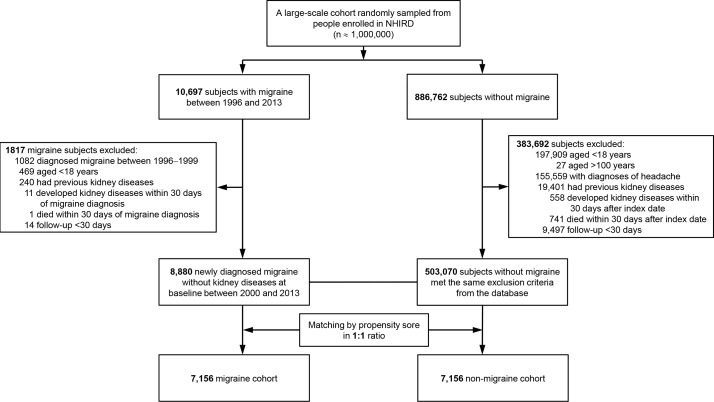
We used a 4-year look-back (1996–1999) period to identify subjects with new-onset migraine by excluding pre-existing migraine diagnosis. Migraine was defined as at least three records of a migraine diagnostic code made by a neurology specialist within 1 year (A-code A229 and ICD-9-CM codes 346.x, online supplementary table S1).22 We defined various diseases, for example, migraine, CKD or comorbid conditions, using diagnostic codes (online supplementary table S1). These diagnostic codes were from ambulatory visit records and principal diagnosis. Migraine was diagnosed by neurologists to have at least three records, but CKD and comorbid conditions were diagnosed by any physician to have at least three records. Subjects who were diagnosed with migraine during the look-back period were excluded from this study. We identified 897 459 subjects including 10 697 subjects with newly diagnosed migraine and 886 762 subjects without migraine from the NHIRD (figure 1) between January 1, 1996 and December 31, 2013. The migraine cohort comprised all the participants who visited a neurologist at least three times presenting with headaches diagnosed as migraine, and the index date was defined as the first date of the migraine diagnosis. In addition, the control subjects were also required to have three visits in the index year. Since migraines are often misdiagnosed as other types of headache, we excluded 155 559 subjects with various types of headache from the control cohort. Among the subjects who were diagnosed with headache, 146 156 subjects were diagnosed with unspecified headache (ICD-9-CM 784.0) and 9403 subjects were diagnosed with tension-type headache (ICD-9-CM 307.81). Other exclusion criteria were as follows: (1) diagnosis of kidney disease before the index date, (2) age <18 or >100 years, (3) follow-up period <30 days, (4) development of kidney disease within 30 days of the migraine diagnosis and (5) death within 30 days of the migraine diagnosis. We identified 8880 subjects with newly diagnosed migraine without a history of acute kidney injury, CKD or end-stage renal disease between January 1, 2000 and December 31, 2013; one propensity-score-matched subject was selected for each subject with migraine. We used the nearest-neighbour algorithm with a calliper of 0.1 SD units to construct matched pairs, with the assumption that the proportion of 1.0 is perfect.23 24 The index date for the control participants was randomly assigned on the basis of the diagnosis date for the subjects from the migraine group. The final sample included the migraine and non-migraine/control groups containing 7156 subjects, respectively.
Figure 1.
Flowchart of subject selection for the study cohort. A total of 8880 migraine subjects without a history of acute kidney injury, chronic kidney diseases or end-stage renal disease were identified in the National Health Insurance Research Database (NHIRD) from 2000 to 2013. One propensity-score-matched patient was selected from the NHIRD for each subject with migraine.
bmjopen-2017-018483supp001.pdf (60.4KB, pdf)
Validation of the CKD diagnosis using ICD-9-CM codes
To evaluate the accuracy of the CKD diagnostic codes in the NHIRD, we studied two groups of subjects, including 9551 subjects with CKD diagnosis codes and 1247 subjects without CKD diagnosis, at the Changhua Christian Hospital from January 2013 to December 2015. We validated these codes using the standard definition of CKD (estimated glomerular filtration rate, <60 mL min−1 per 1.73 m2) and electronic medical records from the outpatient, emergency and inpatient departments. Similar to the numbers reported in previous studies,25 26 the sensitivity, specificity, positive predictive value and negative predictive value of using the ICD-9-CM codes for CKD diagnosis were 24.8% (1179/4760), 98.9% (5970/6038), 94.6% (1179/1247) and 62.5% (5970/9551), respectively.
Outcome measures and relevant variables
Our primary goal was to investigate the association between migraine and the risk of incident CKD after adjusting for age, sex and other confounding factors. The outcomes and comorbidities were identified by examining the A-codes and ICD-9-CM codes (online supplementary table S1). The primary outcome was incident CKD as defined by the A-codes and ICD-9-CM codes (at least three records of CKD diagnostic codes, as previously described).27 28 Both the migraine and non-migraine cohorts were followed from the index date to the date of the first CKD occurrence, the date they withdrew from the insurance system or the end of 2013. Major comorbid diseases diagnosed at least twice before the index date were defined as baseline comorbidities based on the claims data. The comorbid conditions included hypertension, diabetes mellitus, hyperlipidaemia, coronary artery disease (CAD), congestive heart failure (CHF), stroke and chronic obstructive pulmonary disease (COPD). The Charlson Comorbidity Index (CCI) scores were used to quantify the severity of the baseline comorbidities.29 Additionally, the long-term medications, according to Anatomical Therapeutic Chemical (ATC) codes defined by WHO, thought to be associated with renal outcomes, including anti-diabetic agents, diuretics, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (ACEIs/ARBs), beta-blockers, non-steroidal anti-inflammatory drugs (NSAIDs), analgesic drugs other than NSAIDs, statins and anti-migraine agents, were also recorded (online supplementary table S2).
bmjopen-2017-018483supp002.pdf (85.7KB, pdf)
Statistical analysis
The clinical characteristics in the migraine and non-migraine cohorts were summarised using proportions and mean±SD values. χ2 tests and t-tests were used to compare the distributions of discrete and continuous variables, respectively. Standardised difference (StD) scores were intuitive indexes that measure the effect size between two groups. Cox’s proportional-hazard models with competing risks of death were used to estimate the relative risk of developing CKD in subjects with migraine compared with the non-migraine cohort. The baseline characteristic variables, including age, sex, monthly income, comorbid conditions, CCI scores, visit frequency, number of serum creatinine test and long-term medications, were used as covariates for adjustment in the multivariate Cox’s analysis. To improve the reliability of our results, we used three different adjusted models, including covariate adjustment using the propensity scores, all clinical variables and a full model comprising all the clinical variables as well as prescribed medication as time-dependent covariates. The propensity scores were calculated using multivariate logistic regression to predict the probability of migraine occurrence (online supplementary table S3). The cumulative incidence of CKD was calculated using the Kaplan-Meier method, and the indices were compared in the migraine and non-migraine groups using log-rank tests. Additionally, the cumulative incidence functions for the Fine and Grey competing risks model (CKD or withdraw and death without CKD), one kind of proportional-hazards model for subdistribution analysis, were also used.30 There are five situations to be withdrawn from the NHI programme, including (1) convert the insured unit, (2) change the insured identity, (3) loss of insurance (loss of nationality of the Republic of China, households move out of foreign countries, expiration of expiry of expatriates), (4) death and (5) missing for 6 months or confirm missing. Death is defined as no medical record after withdrawal from the NHI programme between 1996 and 2013.31 Subgroup analyses were used to differentiate the risk for CKD in subjects with migraine compared with the control cohort among the various subpopulations. Furthermore, the subtypes of migraine and anti-migraine agents were also analysed while determining the risk of CKD in subjects with migraine. All the statistical analyses were performed using the SAS 9.4 software (SAS Institute, Cary, NC, USA). Results with two-tailed P values <0.05 were considered statistically significant.
bmjopen-2017-018483supp003.pdf (233.4KB, pdf)
Results
Characteristics of the study population
The flowchart in figure 1 shows the selection process, while table 1 shows the characteristics of the study population. A total of 511 950 participants were enrolled in the study, including 8880 subjects with incident migraine and 503 070 subjects without migraine. Compared with the non-migraine group, the migraine group included a higher number of women, number of individuals with a middle-range income (monthly income, NT$15 840–25 000), number of subjects more likely to visit for an illness, severity in the CCI scores, proportions of chronic diseases except diabetes mellitus and CHF, and medications except anti-diabetic agents (table 1). After propensity-score matching, all characteristics except medication for migraine are balanced between the migraine and non-migraine groups. There is more use of selective serotonin reuptake inhibitors, serotonin–norepinephrine reuptake inhibitors, propranolol and flunarizine in the subjects with migraine.
Table 1.
Demographics and clinical characteristics of the study subjects before and after propensity score matching
| Variables * | Before propensity-score matching | Propensity-score matched | StD‡ | |||
| Non-migraine (n=503 070) | Migraine (n=8880) | P value† | Non-migraine (n=7156) | Migraine (n=7156) | ||
| Age (SD), years | 42.2 (16.6) | 42.1 (14.5) | 0.876 | 40.8 (14.1) | 40.8 (14.1) | 0.001 |
| Male, n (%) | 274 757 (54.6) | 2192 (24.7) | <0.001 | 1780 (24.9) | 1780 (24.9) | 0.000 |
| Monthly income, NTD, n (%) | ||||||
| <15 840 | 234 301 (46.6) | 4033 (45.4) | 0.031 | 3164 (44.2) | 3225 (45.1) | 0.017 |
| 15 840–25 000 | 153 457 (30.5) | 2979 (33.6) | <0.001 | 2266 (31.7) | 2343 (32.7) | 0.023 |
| >25 000 | 115 312 (22.9) | 1868 (21.0) | <0.001 | 1726 (24.1) | 1588 (22.2) | 0.046 |
| Clinic visit frequency, visits per year (SD) | 13.5 (12.2) | 26.3 (19.2) | <0.001 | 18.8 (12.4) | 24.3 (17.8) | 0.361 |
| 0, n (%) | 6168 (1.2) | 18 (0.2) | <0.001 | 13 (0.18) | 14 (0.2) | 0.003 |
| 1–5, n (%) | 105 443 (21.0) | 370 (4.2) | <0.001 | 335 (4.68) | 341 (4.8) | 0.004 |
| 6–10, n (%) | 132 280 (26.3) | 1100 (12.4) | <0.001 | 1016 (14.2) | 1004 (14.0) | 0.005 |
| >10, n (%) | 259 186 (51.5) | 7392 (83.2) | <0.001 | 5792 (80.94) | 5797 (81.0) | 0.002 |
| CCIS (SD) | 1.1 (1.8) | 2.0 (2.1) | <0.001 | 1.3 (1.7) | 1.7 (1.8) | 0.177 |
| 0, n (%) | 269 349 (53.5) | 2360 (26.6) | <0.001 | 2964 (41.4) | 2218 (31.0) | 0.218 |
| 1, n (%) | 104 891 (20.9) | 2215 (24.9) | <0.001 | 1802 (25.2) | 1946 (27.2) | 0.046 |
| 2, n (%) | 52 287 (10.4) | 1639 (18.5) | <0.001 | 1096 (15.3) | 1295 (18.1) | 0.075 |
| ≥3, n (%) | 76 543 (15.2) | 2666 (30.0) | <0.001 | 1294 (18.1) | 1697 (23.7) | 0.139 |
| No of serum creatinine test | 2.6 (5.5) | 5.5 (9.4) | <0.001 | 4.0 (5.9) | 4.0 (5.4) | 0.011 |
| Comorbid conditions, n (%) | ||||||
| Hypertension | 61 933 (12.3) | 1488 (16.8) | <0.001 | 831 (11.6) | 960 (13.4) | 0.055 |
| Diabetes mellitus | 27 982 (5.6) | 403 (4.5) | <0.001 | 210 (2.9) | 297 (4.2) | 0.066 |
| Hyperlipidaemia | 35 314 (7.0) | 832 (9.4) | <0.001 | 482 (6.7) | 555 (7.8) | 0.039 |
| CAD | 43 242 (8.6) | 1483 (16.7) | <0.001 | 726 (10.2) | 819 (11.4) | 0.042 |
| CHF | 6056 (1.2) | 86 (1.0) | 0.044 | 43 (0.6) | 57 (0.8) | 0.023 |
| Stroke | 13 574 (2.7) | 694 (7.8) | <0.001 | 149 (2.1) | 249 (3.5) | 0.085 |
| COPD | 33 947 (6.8) | 694 (7.8) | <0.001 | 505 (7.1) | 471 (6.6) | 0.018 |
| PAOD | 1932 (0.4) | 43 (0.5) | 0.131 | 22 (0.3) | 31 (0.4) | 0.021 |
| Urinary calculi | 5129 (1.0) | 172 (1.9) | <0.001 | 85 (1.2) | 92 (1.3) | 0.009 |
| Medications, n (%) | ||||||
| Anti-diabetic agents | 22 619 (4.5) | 299 (3.4) | <0.001 | 137 (1.9) | 225 (3.1) | 0.078 |
| Diuretics | 24 248 (4.8) | 487 (5.5) | 0.004 | 229 (3.2) | 324 (4.5) | 0.069 |
| ACEIs/ARBs | 37 240 (7.4) | 736 (8.3) | 0.002 | 362 (5.1) | 495 (6.9) | 0.078 |
| Statins | 17 566 (3.5) | 375 (4.2) | <0.001 | 166 (2.3) | 248 (3.5) | 0.068 |
| Anti-migraine agents§ | 43 228 (8.6) | 4026 (45.3) | <0.001 | 1036 (14.5) | 2877 (40.2) | 0.603 |
| Acute medicines | ||||||
| NSAIDs | 82 105 (16.3) | 4084 (46.0) | <0.001 | 2630 (36.8) | 2702 (37.8) | 0.021 |
| Analgesic drugs other than NSAIDs¶ | 36 143 (7.2) | 2215 (24.9) | <0.001 | 1063 (14.9) | 1215 (17.0) | 0.058 |
| Preventive medicines | ||||||
| Beta-blockers | 43 027 (8.6) | 2289 (25.8) | <0.001 | 1118 (15.6) | 1183 (16.5) | 0.025 |
| SSRI | 7490 (1.5) | 733 (8.3) | <0.001 | 208 (2.9) | 481 (6.7) | 0.179 |
| Propranolol | 14 956 (3.0) | 1538 (17.3) | <0.001 | 504 (7.0) | 975 (13.6) | 0.217 |
| SNRI | 7321 (1.5) | 804 (9.1) | <0.001 | 156 (2.2) | 523 (7.3) | 0.243 |
| Flunarizine | 4029 (0.8) | 1250 (4.1) | <0.001 | 74 (1.0) | 856 (12.0) | 0.455 |
*Variables are expressed as mean±SD or n (%).
†Two-sided t-test or χ2 test between the migraine and non-migraine cohorts.
‡StD, standardised difference of greater than 0.1 is considered important imbalance.
§Acute medicines include ergot alkaloids and selective serotonin agonists, preventive medicines include beta-blockers, antiepileptics (topiramate and valproate), tricyclic antidepressants, selective serotonin reuptake inhibitors, serotonin–norepinephrine reuptake inhibitors and other anti-migraine drugs (http://www.migraine.org.uk/ and Silberstein SD, et al. Evidence-based guideline update: Pharmacologic treatment for episodic migraine prevention in adults. Neurology 2012;78:1337–1345).
¶Includes aspirin, acetaminophen and cyclooxygenase-2 inhibitors.
ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; CAD, coronary artery disease; CCIS, Charlson Comorbidity Index score; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; NSAIDs, non-steroidal anti-inflammatory drugs; NTD, new Taiwan dollars; PAOD, peripheral artery occlusive disease; SNRI, serotonin–norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitors; StD, standardised difference.
Risk of incident CKD
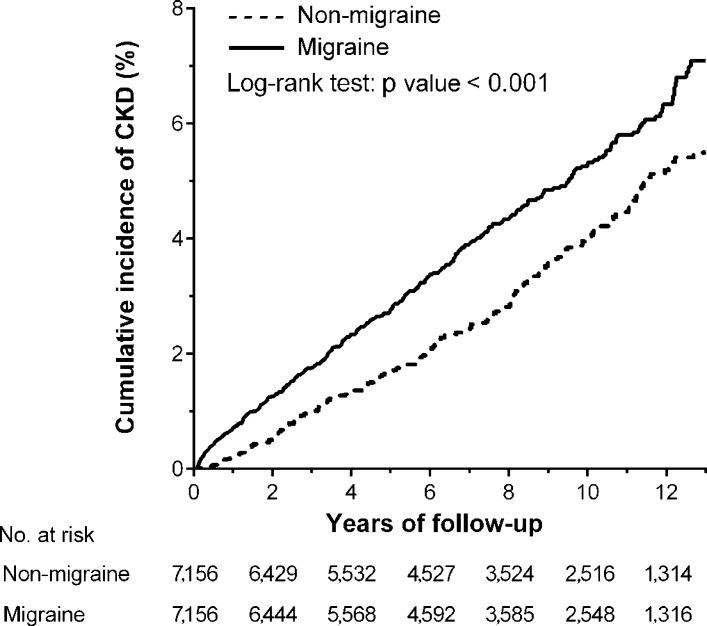
During the follow-up period, the proportion of subjects with incident CKD (4.01% vs 3.14%, P=0.004) and the incidence rate of CKD (5.57 vs 4.14 per 1000 person-years) (table 2) were significantly higher in the migraine cohort than in the non-migraine cohort. The Kaplan-Meier analysis showed that the cumulative incidence of CKD was significantly higher in subjects with migraine than subjects without migraine (log-rank test, P<0.001) (figure 2). The cumulative incidence function before and after propensity-score matching also showed that incident CKD was significantly higher in subjects with migraine (online supplementary figure S1). Four models were used to adjust the risk for incident CKD in the migraine cohort compared with that in the control cohort (table 2). First, after propensity-score matching, the risk of developing CKD was significantly higher in subjects with migraine (crude HR, 1.37; 95% CI 1.16 to 1.62; P<0.001). Second, after the adjustment for the propensity score, the incidence of CKD remained higher in the migraine cohort than that in the non-migraine cohort (adjusted HR (aHR) 1.25; 95% CI 1.06 to 1.49; P=0.010). Third, after adjusting for all the confounding variables listed in table 1, the risk of CKD was similarly higher in the migraine cohort than that in the non-migraine cohort (aHR 1.22; 95% CI 1.02 to 1.46; P=0.032). Finally, in the full adjustment model, the increased risk for CKD in the migraine group remained significant (aHR 1.22; 95% CI 1.02 to 1.47; P=0.031). To assess the reliability of our results, we also performed a sensitivity analysis for the risk of incident CKD before propensity-score matching and found that the association between migraine and CKD remained consistent (online supplementary table S4, figure S1).
Table 2.
Incidence and risk of CKD in subjects with migraine and matched participants
| Events | PY | Incidence* | Model 1† | Model 2‡ | Model 3§ | Model 4¶ | |||||
| cHR (95% CI) | P value | aHR (95% CI) | P value | aHR (95% CI) | P value | aHR (95% CI) | P value | ||||
| Control cohort | 234 | 56 573.4 | 4.14 (3.61–4.67) | 1 (reference) | − | 1 (reference) | – | 1 (reference) | − | 1 (reference) | – |
| Migraine cohort | 309 | 55 463.2 | 5.57 (4.95–6.19) | 1.37 (1.16 to 1.62) | <0.001 | 1.25 (1.06 to 1.49) | 0.010 | 1.22 (1.02 to 1.46) | 0.032 | 1.22 (1.02 to 1.47) | 0.031 |
*Incidence rate, per 1000 person-years.
†Model 1: cHR for propensity-score-matched data.
‡Model 2: adjusted for propensity score.
§Model 3: adjusted for all variables listed in table 1.
¶Model 4: adjusted for all variables listed in table 1, where medications were considered time-dependent covariates.
aHR, adjusted HR; cHR, crude HR; CKD, chronic kidney disease; PY, person-years.
Figure 2.
Kaplan-Meier analysis of the cumulative incidence of chronic kidney disease (CKD) among subjects with (solid line) and without (dashed line) migraine.
bmjopen-2017-018483supp004.jpg (69.3KB, jpg)
Subgroup analysis of CKD risk by age, sex, comorbid conditions and anti-migraine agents between subjects with migraine and without migraine
Table 3 shows that the HR for subsequent CKD was significantly higher in subjects with migraine of both sexes compared with that in the control subjects. The HR of CKD following migraine was higher in younger (<40 years) and middle-aged (40–64 years) subjects, but not in elderly subjects (≥65 years). Additionally, subjects with migraine with other comorbid conditions (1–2 and ≥3 comorbid diseases) had higher risk of developing CKD than those without any comorbid conditions. However, the interaction between number of comorbidity and migraine was not significant (Pinteraction=0.733). It seemed that there is no obvious correlation between developing CKD and concomitant chronic diseases in subjects with migraine. On the other hand, subjects with migraine who were not prescribed any anti-migraine agents were found to have a significantly increased risk of CKD (aHR 1.26; 95% CI 1.04 to 1.54; P=0.022; table 3).
Table 3.
Subgroup analyses of risk for CKD in subjects with migraine and control cohorts
| Subgroup | Subjects without migraine | Subjects with migraine | Compared with the control cohort | |||||||
| n | Events | n | Events | aHR (95% CI)* | P value | Pinteraction | aHR (95% CI)† | P value | Pinteraction | |
| Sex | 0.904 | 0.942 | ||||||||
| Female | 5376 | 143 | 5376 | 188 | 1.38 (1.11 to 1.72) | 0.004 | 1.27 (1.02 to 1.59) | 0.034 | ||
| Male | 1780 | 91 | 1780 | 121 | 1.41 (1.07 to 1.84) | 0.014 | 1.34 (1.01 to 1.79) | 0.045 | ||
| Age, years | 0.437 | 0.069 | ||||||||
| <40 | 4483 | 62 | 4475 | 89 | 1.44 (0.97 to 2.15) | 0.073 | 1.55 (1.02 to 2.36) | 0.040 | ||
| 40–64 | 2202 | 106 | 2207 | 145 | 1.42 (1.13 to 1.79) | 0.002 | 1.35 (1.05 to 1.73) | 0.020 | ||
| ≥65 | 471 | 66 | 474 | 75 | 1.14 (0.82 to 1.59) | 0.443 | 1.05 (0.75 to 1.45) | 0.787 | ||
| Comorbid conditions | 0.387 | 0.733 | ||||||||
| 0 | 5302 | 80 | 5042 | 89 | 1.21 (0.89 to 1.64) | 0.231 | 1.29 (0.92 to 1.79) | 0.135 | ||
| 1–2 | 1549 | 107 | 1739 | 139 | 1.34 (1.09 to 1.65) | 0.005 | 1.30 (1.01 to 1.68) | 0.042 | ||
| ≥3 | 305 | 47 | 375 | 81 | 1.45 (1.02 to 2.08) | 0.040 | 1.45 (1.01 to 2.07) | 0.044 | ||
| Anti-migraine agents | 0.326 | 0.921 | ||||||||
| No | 6120 | 175 | 4279 | 123 | 1.32 (1.09 to 1.59) | 0.004 | 1.26 (1.04 to 1.54) | 0.022 | ||
| Yes | 1036 | 59 | 2877 | 186 | 1.48 (0.95 to 2.30) | 0.084 | 1.26 (0.94 to 1.68) | 0.124 | ||
*Adjusted for the propensity score.
†Adjusted for all variables listed in table 1, where medications were considered time-dependent covariates.
aHR, adjusted HR; CKD, chronic kidney disease.
Influence of migraine and other comorbid conditions on incident CKD
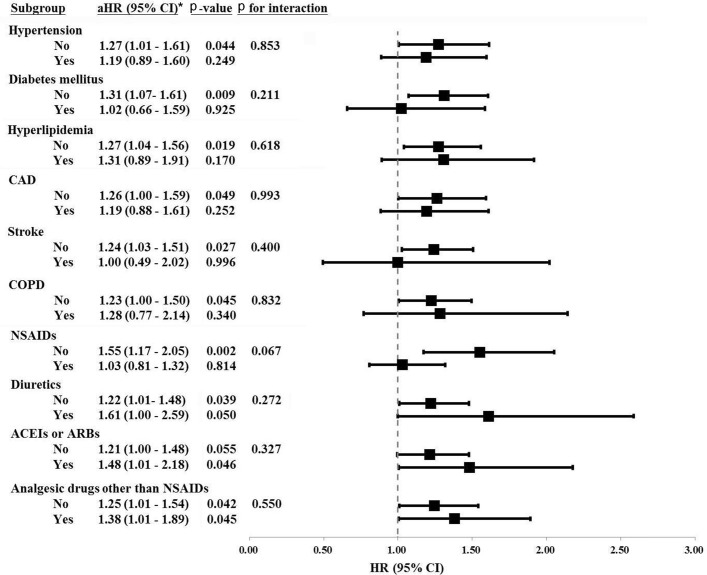
Figure 3 shows that only subjects with migraine without pre-existing hypertension (aHR 1.27; 95% CI 1.01 to 1.61; P=0.044), diabetes mellitus (aHR 1.31; 95% CI 1.07 to 1.61; P=0.009), hyperlipidaemia (aHR 1.27; 95% CI 1.04 to 1.56; P=0.019), CAD (aHR 1.26; 95% CI 1.00 to 1.59; P=0.049), stroke (aHR 1.24; 95% CI 1.03 to 1.51; P=0.027), COPD (aHR 1.23; 95% CI 1.00 to 1.50; P=0.045), NSAIDs (aHR 1.55; 95% CI 1.17 to 2.05; P=0.002) or diuretics (aHR 1.22; 95% CI 1.01 to 1.48; P=0.039) had a higher risk of CKD. We also found no obvious interaction of developing CKD between migraine and concomitant chronic diseases, including hypertension, diabetes mellitus, hyperlipidaemia, CAD, stroke and COPD (figure 3, P>0.05, all). Regarding the long-term used medications and risk for CKD, we found no significant interactions between migraine and diuretics (P=0.272), ACEIs/ARBs (P=0.327) and analgesic drugs other than NSAIDs (P=0.550). Whether subjects with migraine under long-term medication (eg, diuretics, ACEIs/ARBs and analgesic drugs other than NSAIDs) or not was not associated with incident CKD (figure 3).
Figure 3.
Subgroup analysis for the association of migraine with incident chronic kidney disease. ACEI, angiotensin-converting enzyme inhibitor; aHR, adjusted HR; ARBs, angiotensin II receptor blockers; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; NSAIDs, non-steroidal anti-inflammatory drugs. *Adjusted for all variables listed in table 1, where medications were considered time-dependent covariates.
Association for incident CKD in subjects with migraine
In contrast to the comparison between individuals from the migraine and non-migraine cohorts, older subjects in the migraine cohort showed higher risks for incident CKD (40–64 years: aHR 2.24, 95% CI 1.66 to 3.00, P<0.001; and ≥65 years: aHR 3.23, 95% CI 2.23 to 4.66, P<0.001; table 4). CKD events predominantly occurred in male subjects with migraine (aHR 1.92, 95% CI 1.59 to 2.33, P<0.001). The risks of CKD were similar in subjects suffering from unspecified type of migraine, migraine without aura and migraine with aura. The association of incident CKD was significantly high in subjects with migraine using propranolol (aHR 1.28, 95% CI 1.01 to 1.62, P=0.039) and NSAIDs (aHR 1.26, 95% CI 1.02 to 1.56, P=0.029) as anti-migraine agents.
Table 4.
Risk factors of CKD in subjects with migraine
| Variables | Subjects with migraine | |
| aHR* (95% CI) | P value | |
| Age, years | ||
| <40 | 1 (reference) | – |
| 40–64 | 2.24 (1.66 to 3.00) | <0.001 |
| ≥65 | 3.23 (2.23 to 4.66) | <0.001 |
| Men | 1.92 (1.59 to 2.33) | <0.001 |
| Type of migraine | ||
| Unspecified | 1 (reference) | – |
| Without aura | 1.13 (0.68 to 1.87) | 0.640 |
| With aura | 0.67 (0.43 to 1.04) | 0.071 |
| Anti-migraine agents | ||
| Acute medicines | ||
| NSAIDs | 1.26 (1.02 to 1.56) | 0.029 |
| Ergot alkaloids | 1.02 (0.80 to 1.30) | 0.888 |
| Selective serotonin agonists | 0.43 (0.16 to 1.16) | 0.097 |
| Preventive medicines | ||
| Topiramate | 1.09 (0.65 to 1.83) | 0.754 |
| Valproate | 1.10 (0.73 to 1.67) | 0.651 |
| Tricyclic antidepressants | 1.12 (0.88 to 1.42) | 0.356 |
| Flunarizine | 1.00 (0.77 to 1.29) | 0.977 |
| Propranolol | 1.28 (1.01 to 1.62) | 0.039 |
| Selective serotonin reuptake inhibitors | 1.21 (0.91 to 1.61) | 0.182 |
| SNRI | 1.12 (0.81 to 1.55) | 0.506 |
*Adjusted for all variables listed in table 1 and for the type of migraine, where medications were considered time-dependent covariates.
aHR, adjusted HR; CKD, chronic kidney disease; NSAIDs, non-steroidal anti-inflammatory drugs; SNRI, serotonin–norepinephrine reuptake inhibitors.
Discussion
We demonstrated, for the first time, an association between migraine and subsequent CKD independent of demographics, comorbid conditions and long-term medications. The migraine cohort had a 1.22-fold higher risk of subsequent CKD than the non-migraine cohort in the full adjustment model (table 2). The subgroup analysis revealed that younger subjects with migraine had a higher risk of CKD than elderly subjects, compared with the control cohort. Subjects with migraine who were not prescribed any anti-migraine agents had a significant higher risk of CKD compared with the control cohort. However, among the subjects with migraine, older subjects had higher subsequent CKD than younger subjects. Moreover, subjects with migraine who regularly used symptomatic relief agents such as NSAIDs and prophylaxis such as propranolol were prone to have incident CKD.
The risk of CKD in elderly subjects with migraine was not significantly higher than that in subjects without migraine. However, younger subjects with migraine were more prone to have CKD compared with the control cohort. We also did a combined subgroup analysis and cumulative incidence function of CKD among different age groups. Within the migraine or non-migraine cohorts, older subjects are prone to have a higher risk of subsequent CKD than younger subjects (online supplementary figure S2). Compared with the non-migraine cohort, the subjects with migraine before propensity-score matching had a relatively middle-range income. One possible explanation is that long-term physical illness, as well as mental and psychosocial stress, may lead to the dysregulation of central and peripheral neuropeptides in the subjects.6 7 In addition, neuronal hyperexcitability, neurogenic extravasation, vasoactive secretion and cortical spreading depression are especially high in young subjects.32 While the duration of each migraine attack is usually between 15 and 30 min, the injury from the brain to the kidney may be accumulated.
bmjopen-2017-018483supp006.jpg (188.2KB, jpg)
We observed a different relationship between the age and risk of CKD in subjects with migraine and in all the participants. Compared with the non-migraine cohort, the CKD risk was not significantly higher in elderly subjects with migraine (table 3). In figure 3, the subgroup analysis revealed that migraine was associated with risk of CKD after studying the interaction with a single disease. However, table 3 shows that migraine subjects with comorbidities (1–2 or ≥3 diseases) had more risk of CKD compared with the control cohort. Besides, there was a substantially high association with CKD in the elderly within the migraine cohort (table 4). The probability of developing secondary headache increases steadily with age. Secondary headache includes headache related to the side effects from drugs, diseases such as temporal arteritis, trigeminal neuralgia, sleep apnoea, postherpetic neuralgia, subarachnoid haemorrhage and intracerebral haemorrhage.5 In addition, we investigated the effects of anti-migraine agents on the risk of CKD in subjects with migraine. Our results revealed that some symptomatic relief agents for migraine, including propranolol and NSAIDs, had higher associated risk of incident CKD (table 4). NSAIDs are known to be strongly associated with CKD due to the inhibition of cyclo-oxygenase activity,33 but whether anti-migraine agents are independent risk factors of CKD remains under debate.34–36 Although topiramate administration was reported to have an associated risk of urinary calculi,22 we did not find a significant risk of incident CKD in subjects with migraine.
Several factors underlying the pathophysiology of migraine have been reported, including oxidative stress37 and neuropeptides affecting neurogenic vasodilatation, plasma protein extravasation, and peripheral and central sensitisation6; these factors may subsequently lead to kidney diseases. The oxidative stress-relative pathway is recognised as a risk factor for both migraine and CKD.37 38 Some well-known small molecules, such as the somatostatin receptor,6 39 vasoactive molecules (endothelin)6 40 and neuropeptides (eg, CGRP, substance P, neuropeptide Y or vasoactive intestinal peptide),6 8 15 41–43 were recently reported to be correlated with migraine as well as underlying different mechanisms of CKD. Our results support previous studies and the proposed hypothesis that patients suffering from migraine are at a higher risk of kidney diseases. Therefore, aggressive management involving regular follow-up of kidney function and a CKD education programme is warranted in these subjects.
Several limitations of this study should be addressed. First, some information such as history of smoking, body mass index, blood pressure measurements, estimated glomerular filtration rate and proteinuria were not available in the NHIRD. Thus, we were unable to determine the CKD severity in this study. Second, a prospective survey questionnaire for determining the severity of migraine and the quality of life of subjects was lacking. We were unable to incorporate these variables into the baseline or adjusted analysis models to improve the reliability and validity of the association between migraine and CKD. Third, analgesic and statin use were more frequent among migraine users. The bias contributing to events of CKD due to medication use may be underestimated. Fourth, the fact that subjects not receiving anti-migraine agents had an increased risk of developing CKD compared with control subjects is paradoxical. Most importantly, there is no record of over-the-counter medicines, and those young people suffering from migraine without regular anti-migraine agents may have additional use of painkiller with non-official prescription. Fifth, those who had a migraine diagnosis before 1996 and in the absence of migraine diagnostic codes between 1996 and 1999 could be mistakenly identified as subjects with new-onset migraine. Sixth, our data exclusively came from administrative coding, which may have limited the reliability of results. Seventh, as migraine is a particularly difficult diagnosis, it is lacking validation or justification of ICD-9-CM codes for accurate migraine diagnosis.44
In conclusion, we found a significant association between migraine and subsequent CKD in this study. Younger subjects with migraine were more likely to develop incident CKD compared with the patients in the non-migraine cohort. Age and some anti-migraine agents (symptomatic relief agents such as NSAIDs and prophylaxis such as propranolol) were found to be independent risk factors for CKD within the migraine cohort. As migraine predominantly affects young and middle-aged adults, the early detection of CKD and prevention of CKD progression in their later life are important issues in these subjects.
bmjopen-2017-018483supp005.pdf (154.5KB, pdf)
Supplementary Material
Acknowledgments
The authors thank the Internal Medicine Research Center, Changhua Christian Hospital, Changhua, Taiwan, for their help with the statistical analysis. This study was also conducted on behalf of the 105-CCH-IRP-093 Project from the Changhua Christian Hospital Research Foundation. This study was also supported by the Taiwan Ministry of Science and Technology (MOST 106-2314-B-075A-003).
Footnotes
S-CW and C-LW contributed equally.
Contributors: SCW, CLW, CTK, PFC, CCC and DCT contributed to writing the manuscript draft. CLW, SCW and CTK collected the data. CLW, SCW, MJW, CCC and DCT contributed to analysing the data. CCC and DCT contributed to study design and interpretation. CCC and DCT contributed to critically revising the manuscript for important intellectual content. CLW and CTK carried out advice on the statistical analyses. CLW and CCC assisted with obtaining of funding. CLW, SCW, CTK, PFC, CCC and DCT provided administrative, technical and/or material support. CCC and DCT supervised the study.
Funding: This study was conducted on behalf of the 105-CCH-IRP-093 Project from the Changhua Christian Hospital Research Foundation.
Competing interests: None declared.
Ethics approval: This study was approved after a full ethical review by the Institutional Review Board (IRB) of the Changhua Christian Hospital (approval number 150925), and the IRB waived the need for consent. Those data were accessed anonymously.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Sandler DP, Smith JC, Weinberg CR, et al. Analgesic use and chronic renal disease. N Engl J Med 1989;320:1238–43. 10.1056/NEJM198905113201903 [DOI] [PubMed] [Google Scholar]
- 2. Krishnan AV, Kiernan MC. Neurological complications of chronic kidney disease. Nat Rev Neurol 2009;5:542–51. 10.1038/nrneurol.2009.138 [DOI] [PubMed] [Google Scholar]
- 3. Schwaiger J, Kiechl S, Seppi K, et al. Prevalence of primary headaches and cranial neuralgias in men and women aged 55–94 years (Bruneck Study). Cephalalgia 2009;29:179–87. 10.1111/j.1468-2982.2008.01705.x [DOI] [PubMed] [Google Scholar]
- 4. Pascual J, Berciano J. Experience in the diagnosis of headaches that start in elderly people. J Neurol Neurosurg Psychiatry 1994;57:1255–7. 10.1136/jnnp.57.10.1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hershey LA, Bednarczyk EM. Treatment of headache in the elderly. Curr Treat Options Neurol 2013;15:56–62. 10.1007/s11940-012-0205-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Just S, Arndt K, Weiser T, et al. Pathophysiology of migraine: a role for neuropeptides. Drug Discov Today 2006;3:327–33. 10.1016/j.ddmec.2006.07.002 [DOI] [Google Scholar]
- 7. Wang Z, Martorell BC, Wälchli T, et al. Calcitonin gene-related peptide (CGRP) receptors are important to maintain cerebrovascular reactivity in chronic hypertension. PLoS One 2015;10:e0123697 10.1371/journal.pone.0123697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim J, Padanilam BJ. Renal denervation prevents long-term sequelae of ischemic renal injury. Kidney Int 2015;87:350–8. 10.1038/ki.2014.300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Landi F, Liperoti R, Russo A, et al. Disability, more than multimorbidity, was predictive of mortality among older persons aged 80 years and older. J Clin Epidemiol 2010;63:752–9. 10.1016/j.jclinepi.2009.09.007 [DOI] [PubMed] [Google Scholar]
- 10. Wu CL, Tsai CC, Kor CT, et al. Stroke and risks of development and progression of kidney diseases and end-stage renal disease: a nationwide population-based cohort study. PLoS One 2016;11:e0158533 10.1371/journal.pone.0158533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mogi M, Horiuchi M. Clinical interaction between brain and kidney in small vessel disease. Cardiol Res Pract 2011;2011:1–5. 10.4061/2011/306189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Toyoda K, Ninomiya T. Stroke and cerebrovascular diseases in patients with chronic kidney disease. Lancet Neurol 2014;13:823–33. 10.1016/S1474-4422(14)70026-2 [DOI] [PubMed] [Google Scholar]
- 13. Goadsby P. Pathophysiology of migraine. Ann Indian Acad Neurol 2012;15:15–22. 10.4103/0972-2327.99993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sinha G. Migraine mAbs crowd into late-stage trials. Nat Biotechnol 2015;33:676–7. 10.1038/nbt0715-676c [DOI] [PubMed] [Google Scholar]
- 15. Kim J, Padanilam BJ. Renal nerves drive interstitial fibrogenesis in obstructive nephropathy. J Am Soc Nephrol 2013;24:229–42. 10.1681/ASN.2012070678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Antoniazzi AL, Bigal ME, Bordini CA, et al. Headache and hemodialysis: a prospective study. Headache 2003;43:99–102. 10.1046/j.1526-4610.2003.03025.x [DOI] [PubMed] [Google Scholar]
- 17. Davidovits M, Eidlitz Markus T. Headache in pediatric and adolescent patients with chronic kidney disease, with and without hemodialysis: a comparative cohort study. Cephalalgia 2017;1:033310241771923 10.1177/0333102417719235 [DOI] [PubMed] [Google Scholar]
- 18. Cheng CL, Kao YH, Lin SJ, et al. Validation of the national health insurance research database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf 2011;20:236–42. 10.1002/pds.2087 [DOI] [PubMed] [Google Scholar]
- 19. Cheng CL, Lee CH, Chen PS, et al. Validation of acute myocardial infarction cases in the national health insurance research database in Taiwan. J Epidemiol 2014;24:500–7. 10.2188/jea.JE20140076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hsu TW, Liu JS, Hung SC, et al. Renoprotective effect of renin–angiotensin–aldosterone system blockade in patients with predialysis advanced chronic kidney disease, hypertension, and anemia. JAMA Intern Med 2014;174:347–54. 10.1001/jamainternmed.2013.12700 [DOI] [PubMed] [Google Scholar]
- 21. Yu YB, Gau JP, Liu CY, et al. A nation-wide analysis of venous thromboembolism in 497,180 cancer patients with the development and validation of a risk-stratification scoring system. Thromb Haemost 2012;108:225–35. 10.1160/TH12-01-0010 [DOI] [PubMed] [Google Scholar]
- 22. Tsai MJ, Chen YT, Ou SM, et al. Increased risk of urinary calculi in patients with migraine: a nationwide cohort study. Cephalalgia 2015;35:652–61. 10.1177/0333102414553825 [DOI] [PubMed] [Google Scholar]
- 23. Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med 2008;27:2037–49. 10.1002/sim.3150 [DOI] [PubMed] [Google Scholar]
- 24. Kuo KL, Hung SC, Liu JS, et al. Add-on Protective effect of pentoxifylline in advanced chronic kidney disease treated with renin–angiotensin–aldosterone system blockade—a nationwide database analysis. Sci Rep 2015;5:17150 10.1038/srep17150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin MY, Chiu YW, Chang JS, et al. Association of prescribed Chinese herbal medicine use with risk of end-stage renal disease in patients with chronic kidney disease. Kidney Int 2015;88:1365–73. 10.1038/ki.2015.226 [DOI] [PubMed] [Google Scholar]
- 26. Winkelmayer WC, Schneeweiss S, Mogun H, et al. Identification of individuals with CKD from Medicare claims data: a validation study. Am J Kidney Dis 2005;46:225–32. 10.1053/j.ajkd.2005.04.029 [DOI] [PubMed] [Google Scholar]
- 27. Yu TM, Lin CL, Chang SN, et al. Increased risk of stroke in patients with chronic kidney disease after recurrent hypoglycemia. Neurology 2014;83:686–94. 10.1212/WNL.0000000000000711 [DOI] [PubMed] [Google Scholar]
- 28. Huang ST, Lin CL, Yu TM, et al. Nonapnea sleep disorders and incident chronic kidney disease: a population-based retrospective cohort study. Medicine 2015;94:e429 10.1097/MD.0000000000000429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 30. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 31. Wu CY, Chen YJ, Ho HJ, et al. Association between nucleoside analogues and risk of hepatitis B virus–related hepatocellular carcinoma recurrence following liver resection. JAMA 2012;308:1906–13. [DOI] [PubMed] [Google Scholar]
- 32. Longoni M, Ferrarese C. Inflammation and excitotoxicity: role in migraine pathogenesis. Neurol Sci 2006;27(Suppl 2):s107–s110. 10.1007/s10072-006-0582-2 [DOI] [PubMed] [Google Scholar]
- 33. Hsu CC, Wang H, Hsu YH, et al. Use of nonsteroidal anti-inflammatory drugs and risk of chronic kidney disease in subjects with hypertension: nationwide longitudinal cohort study. Hypertension 2015;66:524–33. 10.1161/HYPERTENSIONAHA.114.05105 [DOI] [PubMed] [Google Scholar]
- 34. Olesen J, Göbel H. The International Classification of Headache Disorders. 8 3rd edn (Beta version): International Headache Society, 2016. https://www.ichd-3.org/8-headache-attributed-to-a-substance-or-its-withdrawal/ [Google Scholar]
- 35. Kobuchi S, Tanaka R, Funai A, et al. Involvement of renal sympathetic nerve overactivation in the progression of chronic kidney disease in rats. J Cardiovasc Pharmacol 2014;63:9–15. 10.1097/FJC.0000000000000016 [DOI] [PubMed] [Google Scholar]
- 36. Nagler EV, Webster AC, Vanholder R, et al. Antidepressants for depression in stage 3–5 chronic kidney disease: a systematic review of pharmacokinetics, efficacy and safety with recommendations by European renal best practice (ERBP). Nephrol Dial Transplant 2012;27:3736–45. 10.1093/ndt/gfs295 [DOI] [PubMed] [Google Scholar]
- 37. Bernecker C, Ragginer C, Fauler G, et al. Oxidative stress is associated with migraine and migraine-related metabolic risk in females. Eur J Neurol 2011;18:1233–9. 10.1111/j.1468-1331.2011.03414.x [DOI] [PubMed] [Google Scholar]
- 38. Chen YH, Hung SC, Tarng DC. Length polymorphism in heme oxygenase-1 and cardiovascular events and mortality in hemodialysis patients. Clin J Am Soc Nephrol 2013;8:1756–63. 10.2215/CJN.01110113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bhandari S, Watson N, Long E, et al. Expression of somatostatin and somatostatin receptor subtypes 1–5 in human normal and diseased kidney. J Histochem Cytochem 2008;56:733–43. 10.1369/jhc.2008.950998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Komers R, Plotkin H. Dual inhibition of renin–angiotensin–aldosterone system and endothelin-1 in treatment of chronic kidney disease. Am J Physiol Regul Integr Comp Physiol 2016;310:R877–R884. 10.1152/ajpregu.00425.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen CF, Chen LW, Chien CT, et al. Renal function of substance P in rats chronically exposed to hypoxia. Aviat Space Environ Med 1997;68:705–9. [PubMed] [Google Scholar]
- 42. Sucajtys-Szulc E, Karbowska J, Kochan Z, et al. Up-regulation of NPY gene expression in hypothalamus of rats with experimental chronic renal failure. Biochim Biophys Acta 2007;1772:26–31. 10.1016/j.bbadis.2006.08.008 [DOI] [PubMed] [Google Scholar]
- 43. Arnaout MA, Hamzeh YS, Ajlouni KM. Prolactin responses to vasoactive intestinal polypeptide and thyrotropin releasing hormone in chronic renal failure. Acta Endocrinol 1991;125:651–6. 10.1530/acta.0.1250651 [DOI] [PubMed] [Google Scholar]
- 44. Manuel DG, Rosella LC, Stukel TA. Importance of accurately identifying disease in studies using electronic health records. BMJ 2010;341:c4226 10.1136/bmj.c4226 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-018483supp001.pdf (60.4KB, pdf)
bmjopen-2017-018483supp002.pdf (85.7KB, pdf)
bmjopen-2017-018483supp003.pdf (233.4KB, pdf)
bmjopen-2017-018483supp004.jpg (69.3KB, jpg)
bmjopen-2017-018483supp006.jpg (188.2KB, jpg)
bmjopen-2017-018483supp005.pdf (154.5KB, pdf)