Abstract
Introduction
Piecemeal endoscopic resection (ER) for esophageal high-grade intraepithelial neoplasia (HGIN) or early squamous cell carcinoma (ESCC) is usually performed with the ER-cap technique. This requires submucosal lifting and multiple snares. Multiband mucosectomy (MBM) uses a modified variceal-band ligator without submucosal lifting. In high-risk areas where ESCC is common and limited endoscopic expertise is available, MBM might be a better applicable ER-technique.
Aim
To compare MBM to ER-cap for piecemeal ER of esophageal ESCC.
Methods
Patients with mucosal HGIN/ESCC (≥2≤6 cm, max 2/3 of circumference) were included. Lesions were delineated after 1.25% Lugol staining, followed by randomisation to MBM or ER-cap and piecemeal resection. Endpoints: procedure-time, procedure-costs, complete endoscopic resection, adverse events, absence of HGIN/ESCC at 3 and 12 months follow-up.
Results
In 84 patients (59 male, mean age 60 yrs) ER was performed with MBM (n=42) or ER-cap (n=42). There was no difference in baseline characteristics. Endoscopic complete resection was achieved in all lesions. Procedure time was significantly shorter with MBM (11 vs. 22 minutes, p<0.0001). One perforation was seen after ER-cap and treated conservatively. Total costs of disposables was less for MBM (€200 vs. €251, p=0.04). At 3 and 12 months follow-up none of the patients demonstrated HGIN/ESCC at the resection site.
Conclusion
Piecemeal ER of esophageal ESCC with MBM is faster and cheaper compared to ER-cap. Both techniques are highly effective and safe. MBM may have significant advantages over the ER-cap technique, especially in countries where ESCC is extremely common but endoscopic expertise and resources are limited.
INTRODUCTION
Endoscopic resection is the treatment of choice for early esophageal squamous cell neoplasia that is confined to the mucosa, such as high-grade intraepithelial neoplasia (HGIN) and early stages of esophageal squamous cell carcinoma (ESCC) 1. ESCC is the 6th most common cause of cancer related death world-wide, and has a particularly high incidence in certain high-risk areas, such as Central Asia, East Africa, Iran and China 2,3. Nearly half of the worldwide ESCC cases occur in China where ESCC is the fourth leading cause of cancer-related death. ESCC reaches a remarkable incidence of 1/1000 in certain densely populated areas, comprising over 100 million people4.
ESCC is usually diagnosed at a late stage and then has a poor prognosis due to a relative thin esophageal wall and a rich lymphatic network, which contributes to metastatic spread of the disease at an early stage5,6. The chance of lymph node metastasis depends on the penetration depth and the differentiation of the lesion7. Timely detection of early stages of ESCC, when the lesion is still confined to the superficial mucosal layer, is of obvious clinical importance. In high-incidence areas in China, high volume screening programs are currently being conducted, in which over 150,000 subjects per year undergo endoscopy with Lugol’s staining. Areas suspicious for neoplasia will appear as unstained lesions (USLs) in the brown iodine-stained normal squamous esophagus. In approximately 5% of screened subjects in China endoscopic therapy is warranted yet limited endoscopic expertise and resources are available. For the adequate execution of esophageal screening programs in these high-risk areas, a low complexity, low cost treatment modality would have many advantages.
The most widely used technique for endoscopic resection of early squamous cell neoplasia in the esophagus is the ER-cap technique. With this technique lesions up to 2 cm in diameter can be removed en-bloc, while larger lesions require removal in multiple pieces (i.e. piece-meal resection). The ER-cap procedure is, however, technically demanding, especially during piecemeal resections where submucosal lifting and positioning of a new electrosurgical snare in the cap is required for every separate resection1.
An alternative ER technique is the multi-band mucosectomy (MBM) technique, using a modified variceal-band ligator 8. MBM does not require submucosal lifting, positioning of the snare is much easier and the same snare can be used for all resections. Previous studies in Barrett’s esophagus have shown that MBM is safe and effective for removal of mucosal neoplasia, with shorter procedure times and lower costs compared to ER-cap9–11. A recent feasibility study on the use of MBM for esophageal squamous neoplasia, performed in a tertiary care unit in China, suggested that MBM is safe, effective, fast and has a durable treatment effect 12.
We therefore hypothesized that MBM may have significant advantages over the ER-cap technique, especially in countries where endoscopic expertise and resources are limited.
The aim of this study was to prospectively compare MBM vs ER-cap for piece-meal ER of early mucosal squamous cell neoplasia of the esophagus, and assess the effectiveness, safety and durability of the treatment effect. The secondary purpose of this study was to investigate ease of application and costs, which may be of practical importance for the implementation of high-volume screening programs in areas with a high risk of esophageal squamous neoplasia.
PATIENTS AND METHODS
Setting
All endoscopic procedures were performed at the Cancer Institute and Hospital, Chinese Academy of Medical Sciences, Beijing, or the Feicheng People’s Hospital, Feicheng, People’s Republic China. The departments of endoscopy and pathology in these hospitals are tertiary referral centres with extensive experience in the detection and treatment of early neoplasia in the esophagus, and all endoscopies were performed by highly experienced endoscopists (BW, JB, GW).
The study was approved by the medical ethical committees of the two hospitals and informed consent was obtained from all patients.
Patients
Patients enrolled in a screening program for ESCC were eligible for participation in this study if they met all of the following inclusion criteria:
Age 18–85 years;
Histologically proven HGIN or ESCC in a lesion in the squamous esophagus;
Lesion consistent with Paris Classification 13 type 0-IIa, 0-IIb or 0-IIc, or a combination of these;
Lesion and surrounding Lugol’s unstained lesions (USLs) (i.e. the treatment area; TA) measuring ≥ 2 cm and ≤ 6 cm and encompassing ≤ 2/3 of the circumference. Circumferential extent was measured as hours in a clock. Percentage of circumferential extent was calculated as 100%/12 (8.3%) per hour;
No signs of submucosal invasion or metastatic disease, as assessed by EUS and CT-scan;
Written informed consent.
Patients were not eligible if they met any of the following exclusion criteria:
Any other neoplastic lesion in the squamous esophagus visible with white light endoscopy or visible with Lugol’s staining containing HGIN/ESCC that could not be included in the maximum size TA;
Prior endoscopic treatment <3 cm from the TA, prior radiation therapy, or prior esophageal surgery other than uncomplicated fundoplication;
Any uncontrolled bleeding risk (e.g. due to coagulopathy, esophageal varices, etc.).
Baseline qualifying endoscopy
Patients underwent a baseline qualifying endoscopy four weeks prior to enrollment. During that endoscopy, the esophagus was first assessed with white light endoscopy and lesions were classified according to the Paris Classification of superficial neoplastic lesions of the esophagus13. Subsequently, the esophagus was inspected with narrow band imaging (NBI), and after chromoendoscopy with Lugol’s staining (1.25%), the location and extent of the neoplastic epithelium was recorded. Endoscopic ultrasound and computed tomography (CT)-scan were performed on all patients to exlude metastatic disease.
Endoscopic resection procedures
All procedures were performed under deep sedation using a combination of Propofol and Alfentanyl. The esophagus was first inspected with white light endoscopy, followed by NBI and chromoendoscopy with Lugol’s staining (1.25%). The treatment area according to the baseline qualifying endoscopy was identified and delineated by placing electrocoagulation markers 1–2 mm outside all margins of the treatment area. After delineation, the endoscope was removed and the patient was randomized to either MBM or ER-cap (see below) and timing of the procedure was started.
After resection of the treatment area, the wound was inspected and evaluated for complete removal of all the coagulation markers. Lugol’s staining was repeated to assess if residual USLs at the resection margins of the wound were present. Any remaining USLs were then further resected with the same technique. In case additional resections were considered to be contraindicated (e.g. due to increased risk of stenosis or too small residual tissue to justify resection), remaining USLs were treated with argon plasma coagulation (APC, 40–60 Watt forced coagulation, ERBE Vio 300D, Erbe Elektromedizin GmbH, Tübingen, Germany). Resected specimens were retrieved from the stomach using a foreign body retrieval basket (Roth net, US Endoscopy, Mentor OH, USA).
During the procedure, time was recorded at the initial introduction of the endoscope, the start of the resection procedure after randomization, and the final removal of the endoscope (after retrieval of all specimens and treatment of any complications).
Multiband-mucosectomy procedure
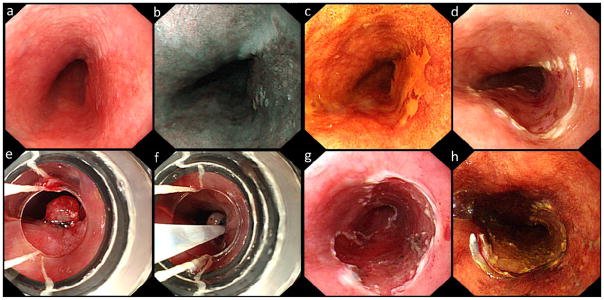
For cases randomized to MBM, the single-use Duette™ Multi-Band Mucosectomy kit (Cook Medical, Limerick, Ireland) was assembled on the endoscope. This kit consists of 6 rubber bands on a transparent cap (inner Ø 9 mm), releasing wires attached to a specially designed releasing handle and a 7 Fr hexagonal braided polypectomy snare that can be reused for multiple resections. After assembling the MBM kit on the endoscope, the endoscope was re-introduced and the delineated treatment area identified. Next, the treatment area was sucked into the cap and a rubber band was released, thus creating a pseudo-polyp. The hexagonal snare was positioned and closed underneath the rubber band of the pseudo-polyp which was then resected using 80 W pure coagulation current (ERBE VIO 300D, Erbe Elektromedizin GmbH, Tübingen, Germany). If multiple resections were required to remove the treatment area, pseudo-polyp formation and resection were repeated with the same kit until the treatment area was endoscopically removed. If more than 6 resections were required, an additional MBM kit was used (figure 1).
Figure 1.
white light (a) and narrow band imaging (NBI, b) image of an early lesion, visible at the 2–5 o’clock position. The lesion is clearly visible after Lugol’s staining (c). Image after demarcation (d) and the view through the MBM-cap with a pseudopolyp created by a rubber band (e) and with a snare (f). Image after resection (g) and Lugol’s staining after resection (h).
Endoscopic resection-cap procedure
For ER-cap procedures, an ER-cap kit was used, comprising a hard, oblique cap (inner Ø 13.8 mm), a spraying catheter, an injection needle and a crescent shaped snare (Olympus Inc., Tokyo, Japan). After randomization, the ER-cap was placed on the tip of the endoscope and the endoscope introduced. The delineated lesion was identified and subsequently lifted by diluted adrenaline (1:100.000 NaCl 0.9%) to achieve adequate submucosal lifting. In case of a non-lifting sign, the procedure was aborted. After submucosal lifting, the lesion was sucked in the cap to assess resectability, followed by placement of the electrosurgical snare in the rim of the cap. Then, the lesion was again sucked into the cap until a full “red-out” was reached, after which the snare was closed. The thus created pseudopolyp was subsequently resected using 80 W pure coagulation current (ERBE VIO 300D, Erbe Elektromedizin GmbH, Tübingen, Germany) under direct vision. In case of piecemeal resections, the submucosal injection, lifting, pre-suction and placement of a new snare was repeated for every resection.
Post-procedural care
Post-procedural management was conducted according to standard guidelines. Generally, patients were admitted for clinical observation for 24 hours. Patients used a clear liquid diet for the day of the endoscopic resection, followed by a soft diet the day thereafter. Oral analgesics (Lidocaine oral suspension) were given on demand.
Histological evaluation
Resection specimens were pinned down on cork with the luminal side up. In case of piece-meal resection, the treatment area was reconstructed after staining with Lugol’s solution. Maximum and minimum diameters of the reconstructed area were measured after pinning the specimens down on cork. Specimens were subsequently fixed in formalin for 24 hours, then cut in 2-mm sections and embedded in paraffin.
Five micron sections were cut and mounted on glass slides and stained with haematoxylin and eosin. All slides were evaluated independently by two expert gastro-intestinal pathologists (LX, NL) for the following parameters:
Worst grade of squamous neoplasia (no intraepithelial neoplasia; low grade intraeptithelial neoplasia (LGIN); moderate grade intraepithelial neoplasia (MGIN); high grade intraepithelial neoplasia (HGIN); invasive esophageal squamous cell cancer).
In case of invasive cancer: depth of tumor invasion (m2, m3, or sm), grade of differentiation, presence of lymphatic or vascular invasion;
Presence of neoplasia at the deep (vertical) resection margin.
The maximum thickness of resection specimens (in μm);
The maximum thickness of the resected submucosal layer (in μm).
Follow up endoscopy
All patients were scheduled for a follow up endoscopy three and twelve months after the ER procedure. The resection scar was identified and inspected using white light endoscopy and Lugol’s staining to assess the presence of USLs. USLs within 1 cm of the ER scar were considered residual (at 3 month FU) or recurrent (> 3 month FU) lesions, while those >1 cm from the scar were considered new lesions. Biopsies were obtained with standard biopsy forceps from the resection scar and any residual USLs. All biopsies were evaluated independently by two expert gastro-intestinal pathologists (LX, NL).
In case of a USL within 1cm of the ER scar, or HGIN/ESCC detected in any lesion during a follow up endoscopy, the lesion was managed according to the endoscopist’s preference by APC (40–60 Watt forced coagulation), radiofrequency ablation (RFA, Covedien, GI Solutions, Sunnyvale, USA) or by additional endoscopic resection. An extra 3 month follow up visit was scheduled after this retreatment.
Study endpoints
The purpose of this study was to assess the safety, effectiveness, and cost of MBM, compared to ER-cap. The hypothesis was that both techniques would demonstrate a comparable performance. However, due to the limited number of disposables required for MBM and the relative ease of application of the technique, procedure time and costs might be significantly less, compared to ER-cap.
Primary endpoints for this study therefore were:
procedure time, defined as the time between randomization and the end of the ER-procedure, including removal of specimens and treatment of complications;
procedure costs, defined as the costs of disposables used during the resection procedure.
Secondary endpoints were:
Endoscopically complete removal of neoplasia defined as the complete removal of the delineated treatment area including all electrocoagulation markers;
Complete histological remission of neoplasia, defined as absence of HGIN/ESCC within 1 cm of the ER-scar at the 3 month follow-up visit.
Complete histological remission of neoplasia, defined as absence of USLs containing HGIN/ESCC within 1 cm of the ER-scar at the final follow-up visit.
Number and severity of acute (during procedure), early (0–48 hrs) and delayed (>48 hrs) clinically relevant complications, graded as ‘mild’ (unplanned hospital admission, hospitalization <3 days, hemoglobin drop <3g/dL, no transfusion), ‘moderate’ (4–10 days hospitalization, <4 units blood transfusion, need for repeat endoscopic intervention), ‘severe’ (hospitalization >10 days, ICU admission, need for surgery, > 4 units blood transfusion) or ‘fatal’ (death attributable to procedure)14;
maximum diameter and thickness of the specimens and thickness of the submucosa;
presence of USLs > 1cm from the ER scar containing HGIN/ESCC during follow-up.
Sample size, randomization and statistical analysis
Based on previous studies in Barrett’s esophagus, MBM was hypothesized to show an equally effective performance compared to ER-cap for piecemeal resection of ESCC11. Since MBM is technically less complex and requires fewer disposables, a significant reduction in procedure time and costs was anticipated. To study this clinically important benefit of MBM over ER-cap, the sample size was calculated to require a total of 84 patients, with a power of 80% and a significance level of 0.05. This sample size would detect any significant reduction in costs (>20%) and/or duration (>30%) of the procedure.
Patients that were considered eligible for inclusion were randomized to either one of the resection techniques after delineation of the lesion. For the randomization procedure, a set of serially numbered, opaque, sealed envelopes containing an even number of ER-cap and MBM lots was used. The randomization was noted on the case record form and the randomization lot was placed with the patient files.
Statistical analysis was performed with SPSS 18.02 software for Windows (IBM Nederland, Amsterdam, The Netherlands). For variables with a normal distribution the mean (±SD) was used and for variables with a skewed distribution the median (IQR) was used. Where appropriate, the student t test and the Mann-Whitney test were used. All tests were two-tailed and a p<0.05 was considered as statistically significant.
RESULTS
Patients
A total of 84 patients were included: 59 men and 25 women, with a mean age of 60 years (SD ± 7 years). The worst histology prior to the ER-procedure was HGIN (n=61) or ESCC (n=23). No signs of submucosal involvement or lymph node metastasis were found upon EUS and/or CT.
The baseline characteristics did not differ significantly between the groups (table 1).
Table 1.
Patient and lesion characteristics (n=84)
| MBM (n=42) | ER-cap (n=42) | p-value | |
|---|---|---|---|
|
| |||
| Male : female | 28 : 14 | 31 : 11 | 0.48 |
| Mean age at time of MBM procedure (years) | 60 (SD ±7) | 59 (SD ±8) | 0.56 |
| Worst histology prior to ER: | |||
| HGIN | 32 (76%) | 29 (69%) | 0.47 |
| ESCC | 10 (24%) | 13 (31%) | |
| Paris classification: | |||
| 0-Iia | 2 | 2 | 0.67 |
| 0-IIb | 40 | 39 | |
| 0-IIc | 0 | 0 | |
| 0-IIa+c | 0 | 1 | |
| Median length of treatment area (cm) | 5 (IQR: 4–5) | 5 (IQR: 4–5) | 0.79 |
| Median circumferential extent (%) | 42 % (IQR: 25–50) | 33% (IQR: 25–50) | 0.47 |
The majority of the lesions were classified as Paris classification 0-IIb. Median length of the treatment areas (TA) was 5 cm (IQR: 4–5) with a median circumferential extent of 41% (IQR: 25–50%) (table 1).
Endoscopic procedures
Endoscopic resection was performed in all 84 patients. In the MBM group, a median of 5 resections (IQR 4–6) was required to remove the lesion, versus 4 (IQR 3–5) in the ER-cap group (P=NS). Endoscopic complete resection was achieved in all 84 patients (100%). Two MBM patients were treated with additional APC for small residual USLs (1–5% of the TA) at the TA margin which were not large enough to justify additional resections, compared to 3 ER-cap patients.
The procedure time to perform the endoscopic resection was significantly less for MBM (11 minutes, IQR 7–16) compared to ER-cap (22 minutes, IQR: 11–32). The reduction in procedure time was 50%, with a median reduction of 8 minutes (95% CI 3–13; P<0.0001).
Complications
One perforation occurred during an ER-cap procedure in a patient with a lesion of 5 cm and 33% of the circumference. The procedure was completed, the perforation was closed with clips and the patient was treated conservatively. The patient was admitted for observation after an esophageal contrast X-ray and discharged the next day. Follow up showed a completely healed esophagus without stenosis or residual/recurrent neoplasia.
During the endoscopic resection procedure mild bleeding was observed in one patient treated with MBM, and in 5 patients in the ER-cap group. None of these bleeding episodes were considered clinically significant, since all bleedings were effectively treated within the same endoscopic session using conventional hemostasic techniques. Early (0–48 hrs) or delayed (>48 hrs) bleedings did not occur, and no patients required blood transfusion.
During follow-up, none of the patients showed symptoms of stenosis. At 3-month follow up 9 patients in the MBM group demonstrated a relative stenosis of the esophagus, which still allowed passage of the gastroscope, versus 8 patients in the ER-cap group. None of these stenoses required intervention. No other acute, early or late complications occurred, and no patients required unplanned prolonged hospitalization.
Costs of disposables
The costs of the disposables were significantly less for the MBM procedures compared to the ER-cap procedure (€200 (IQR 200–400) vs €251 (IQR 210–300) respectively; p=0.04). This resulted in a median reduction of € 51 (95% CI 30–72) with the use of MBM.
Resection specimens and histology
Histological assessment of the resection specimens in the MBM group showed ESCC in 23 cases (55%), HGIN in 16 (38%), MGIN in 2 (5%) and LGIN in 1 case (2%). In the ER-cap group, the resection specimens showed ESCC in 21 cases (50%), HGIN in 17 (41%), MGIN in 2 (5%) and LGIN in 2 cases (5%).
The median maximum dimensions of the individual MBM resection specimens were 18x12 mm (IQR 15–20 and 10–15) compared to 20x15 mm(IQR 15–21 and 11–20) for ER-cap resection specimens (p=NS). MBM resection specimens had a median thickness of 2050 μm (IQR 1500–2675) and a submucosal thickness of 900 μm (IQR 600–1150), versus 1725 μm (IQR 1375–2188) and 800 μm (IQR 488–1063) for ER-cap resection specimens (p=NS and p=NS respectively). Details of the histologic findings are summarized in table 2.
Table 2.
Endoscopic procedures and histologic characteristics
| MBM | ER-cap | p-value | |
|---|---|---|---|
|
| |||
| Endoscopic complete removal of lesions | 42/42 (100%) | 42/42 (100%) | 1 |
| Median number of resections | 5 (IQR: 4–6) | 4 (3–5) | 0.69 |
| Median MBM resection time* | 11 minutes (IQR: 7–16) | 22 (IQR: 12–31) | <0.0001 |
| Complications | |||
| clinically not relevant bleeding during ER | 1 | 5 | |
| acute (during ER) | 1 moderate (perforation, treated conservatively) | 0.93 | |
| early (0–48 hrs) | |||
| delayed (>48 hrs) | 9 (mild stenoses, clinically not relevant) | 8 (mild stenoses, clinically not relevant) | |
| Costs of disposables (€) | 200 (IQR: 200–400) | 251 (IQR: 210–300) | 0.04 |
|
| |||
| Median max. dimensions of individual resection specimens | 18 x 12 mm (IQR: 15–21/10–16) | 20 x 15 (IQR: 15–21/11–20) | 0.07 |
| Median max. thickness of resected lesions** | 2050 μm (IQR: 1460–2332) | 1725 (IQR: 1375–2188) | 0.06 |
| Median max. submucosal thickness** | 900 μm (IQR: 521–1207) | 800 (IQR: 488–1063) | 0.27 |
| Worst histology of ER specimens: | 0.62 | ||
| LGIN | 1 (2%) | 2 (5%) | |
| MGIN | 2 (5%) | 2 (5%) | |
| HGIN | 16 (38%) | 17 (40%) | |
| ESCC | 23 (55%) | 21 (50%) | |
| Max. penetration depth of ESCC: | 0.89 | ||
| m2 | 12 | 9 | |
| m3 | 7 | 8 | |
| sm | 5 | 4 | |
| Max. differentiation grade of ESCC: | 0.56 | ||
| G1 | 3 | 6 | |
| G2 | 10 | 13 | |
| G3 | 11 | 2 | |
Time between introduction of endoscope with assembled MBM or ER-cap kit and end of the ER procedure, inclusive of removal of resection specimens and treatment of complications
Maximum thickness of the reconstructed lesion
NS: not significant; IQR: interquartile range; LGIN: low grade intraepithelial neoplasia; MGIN: moderate grade intraepithelial neoplasia; HGIN: high grade intraepithelial neoplasia; ESCC: esophageal squamous cell carcinoma
Follow up procedures
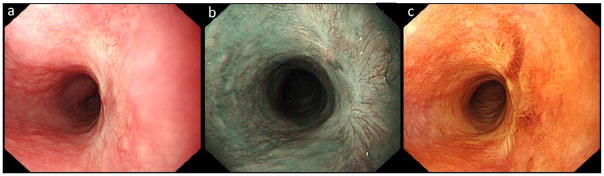
Endoscopic follow up was performed in 77 patients (92%). Three patients were lost to follow up and 4 patients were referred for surgery because of deep submucosal invasion +/− poorly differentiated carcinoma. Median follow up duration was 15 months (IQR 13–20) and the median number of follow up visits was 3 (IQR 3–4). At three months follow up, all ER wounds had completely healed (figure 2). Complete histological remission of HGIN/ESCC was seen in all patients. Biopsies of the scar revealed LGIN in 14 cases and MGIN in 1. In 55 patients, a USL was found elsewhere in the esophagus, showing normal squamous epithelium in 39 patients, LGIN in 12, MGIN in 3 and HGIN in 1 case.
Figure 2.
white light (a), narrow band imaging (NBI, b) and Lugol’s staining (c) image of the resection scar at the 2–5 o’clock position, seen at three months follow-up visit.
At the last follow up visit, complete histological remission of HGIN/ESCC at the site of, and within 1cm of, the ER scar was shown for all 77 patients, of whom 12 showed LGIN.
Biopsies from the surrounding mucosa > 1 cm from the ER scar showed normal squamous mucosa in 68 and LGIN in 9 cases. No statistical differences in histological outcome were found between the two treatment groups (table 3).
Table 3.
Follow up (n=77)
| MBM (n=38) | ER-cap (n=39) | p-value | |
|---|---|---|---|
|
| |||
| Median follow up | 15 months (IQR 13–17) | 15 months (IQR 12–21) | 0.54 |
|
| |||
| Median number of follow up visits | 2 (IQR 2–3) | 2 (IQR 2–3) | 0.78 |
|
| |||
| Worst histology of scar at 3 month follow up: | 0.44 | ||
| normal squamous | 32 | 30 | |
| LGIN | 6 | 8 | |
| MGIN | 0 | 1 | |
| Worst histology of scar at last follow up: | 0.63 | ||
| normal squamous | 31 | 34 | |
| LGIN | 7 | 5 | |
|
| |||
| Worst histology of USLs >1cm from scar at 3 month follow up | (n=26) | (n=29) | 0.39 |
| normal squamous | 21 | 18 | |
| LGIN | 3 | 9 | |
| MGIN | 1 | 2 | |
| HGIN | 1 | 0 | |
| Worst histology of (USL) >1cm from scar at last follow up: | 0.61 | ||
| normal squamous | 33 | 35 | |
| LGIN | 5 | 4 | |
NS: not significant; IQR: interquartile range; LGIN: low grade intraepithelial neoplasia; MGIN: moderate grade intraepithelial neoplasia; HGIN: high grade intraepithelial neoplasia; ESCC: esophageal squamous cell carcinoma
DISCUSSION
This is the first randomized trail comparing multiband mucosectomy (MBM) with the ER-cap technique for endoscopic resection of early squamous neoplasia of the esophagus. For treatment of early neoplasia in Barrett’s esophagus, previous studies have demonstrated that MBM is faster and cheaper than ER-cap, with a comparable performance in terms of effectiveness and safety. A recent feasibility study on MBM for esophageal squamous cell carcinoma (ESCC) showed promising results, with fast procedure times, a low number of complications and a durable treatment effect12. In the current study an endoscopically complete resection was achieved in all patients. This is in concordance with the randomized study comparing MBM with ER-cap for early Barrett’s neoplasia in which a success rate of 100% for MBM was reported11. During follow up, none of the patients showed signs of residual or recurrent disease at the site of the initial lesion, confirming the results of the uncontrolled feasibility series on MBM for ESCC.
With the observed low rate of complications, this study again shows the safety of endoscopic resection for early esophageal neoplasia, both during the procedure and long term. Minor bleeding may occur during the either of the procedures but is generally easily managed endoscopically without significantly prolonging the procedure or clinical consequences afterwards. One perforation occurred in the ER-cap group, while in the previous uncontrolled study, one perforation was observed after an MBM procedure. This may reflect the relative vulnerability of the esophageal wall in ESCC patients. In Barrett’s esophagus, due to the inherent state of inflammation and metaplasia, the mucosal layer is thicker and perforations after ER-cap or MBM procedures are extremely rare.
Wide spread endoscopic resection of esophageal lesions may result in symptomatic stenosis especially when the resection encompasses more than 75% of the circumference. Although we did observe relative narrowing during endoscopic follow-up in our patients, none of these where symptomatic or required dilatation. This may be a result of the per-protocol avoidance of resections >2/3 of the circumference. However, in our opinion it is unlikely that the stenosis rate after MBM will be higher than that of the ER-cap technique: it is the extent of the required resection that is the most determining factor for esophageal scarring. MBM may allow a more targeted resection since the size of the resection can be controlled after the mucosa has been captured by the rubber band. For instances in which a significant proportion of the captured mucosa is outside the electrocoagulation marks, the created pseudopolyp can be reduced in size by gently pushing the rubber band with the edge of the transparent cap.
This study was conducted in Chinese high-risk areas, where a large patient load imposes a significant burden on the available endoscopic capacity and expertise. Costs, procedure time and endoscopic complexity are relevant parameters in this context. Endoscopic resection with MBM showed a significant reduction both in procedure-time and in the cost of disposables. Although not formally assessed, we anticipate that the reduction in procedure time had an even larger impact on the total procedure costs than the reduction in cost of the disposables alone. In our opinion, the reduced procedure time reflects the lower technical complexity of the MBM technique. The ER-cap technique requires repetitive submucosal lifting and placement of the thin snare in the ridge of the ER-cap is not always easy, especially for less experienced endoscopists. Based on experience in an ER/RFA training program in the Netherlands we have found the MBM technique to be much more easily implemented by non-expert endoscopists than the ER-cap technique15. A limitation of our study in this respect is the fact that all procedures were performed by expert endoscopists. We observed a significant discrepancy between the histological pre-ER biopsy diagnosis and the histological diagnosis based on the ER specimens: 23 versus 45 lesions with ESCC and 0 versus 9 lesions with submucosal invasion, despite the absence of signs of submucosal invasion during EUS or on CT. This histological upstaging shows the importance of ER as a diagnostic as well as a therapeutic technique. It also demonstrates an advantage of ER over ablative therapies for USLs with a biopsy diagnosis of HGIN or ESCC, and especially for large USLs, where biopsy sampling error may be significant.
Interestingly, no statistical difference in size of the resection specimens was observed between the MBM and ER-cap groups, in contrast to previous data from resections of Barrett’s esophagus. The ER-cap used in this study was a hard oblique cap with a diameter of 12 mm. The ER-cap technique can also be performed using larger diameter caps; for example the larger 16mm diameter soft oblique cap can obtain larger and possibly deeper resection specimens. Thus, in the case of a lesion with a 15–20mm diameter, such a larger ER-cap may allow for an en-bloc resection by the ER-cap technique, whereas the MBM technique would require a piecemeal resection. For larger lesions, however, both ER-cap and MBM require piecemeal resection and in terms of size and depth the standard ER-cap and the MBM do not differ in performance. In our opinion the large ER-cap may be less suited for piecemeal resection due to a higher risk of complications caused by overlap of resections.
To avoid these drawbacks of piecemeal resection, endoscopic submucosal dissection (ESD) may be a valuable alternative. During ESD, the lesion is lifted from the submucosa by fluid injection, followed by en-bloc dissection through the submucosal layer. En-bloc resection by ESD is associated with a lower risk of local recurrence, compared to piecemeal resection16–18, but given the low local recurrence rate with piecemeal resection and a strict follow-up regimen, the high complexity and long learning curve of ESD may not justify its implementation in general endoscopic practice. MBM has the large advantage of being relatively simple, fast and cheap, which is of pivotal importance in large volume centers with limited resources. Despite the histological upside of ESD, the technique will not be suited for the purpose of large screening and treatment programs in areas with an extremely high incidence of ESCC.
Endoscopic resection with multiband mucosectomy offers a safe, effective and durable therapeutic approach for early esophageal squamous cell carcinoma. Compared to the now standard ER-cap technique, MBM is faster, cheaper and arguably less complex. In a high risk population where ESCC is extremely common, yet resources may be limited, MBM is an attractive alternative to ER-cap.
Acknowledgments
The authors would like to acknowledge Huaying Xun, Guixiang Yu, Fenghuan Ju, Yongqiang Xie, Li Sun, Liang Zuo, Haiyan Wang, and Hai Wang from the Departments of Endoscopy, Pathology, and Anesthesiology, Cancer Institute and Hospital, Chinese Academy of Medical Sciences, Beijing, P.R. China, and Ruixue Zhou, Deli Zhao, Peng Ji, Shengyong Liang from the Feicheng People’s Hospital, Feicheng, Shandong Province, P.R. China, for their valuable contributions to this study.
Abbreveations
- ER
Endoscopic resection
- ESCC
Esophageal squamous cell carcinoma
- MBM
MultiBand Mucosectomy
- LGIN
low grade intraepithelial neoplasia
- MGIN
medium grade intraepithelial neoplasia
- HGIN
high grade intraepithelial neoplasia
- APC
argon plasma coagulation
- USL
unstained lesion
- TA
treatment area
- IQR
interquartile range
References
- 1.Inoue H, Takeshita K, Hori H, Muraoka Y, Yoneshima H, Endo M. Endoscopic mucosal resection with a cap-fitted panendoscope for esophagus, stomach, and colon mucosal lesions. Gastrointest Endosc. 1993;39(1):58–62. doi: 10.1016/s0016-5107(93)70012-7. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 4.Lu X-J, Chen Z-F, Guo C-L, et al. Endoscopic survey of esophageal cancer in a high-risk area of China. World J Gastroenterol. 2004;10(20):2931–2935. doi: 10.3748/wjg.v10.i20.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Araki K, Ohno S, Egashira A, Saeki H, Kawaguchi H, Sugimachi K. Pathologic features of superficial esophageal squamous cell carcinoma with lymph node and distal metastasis. Cancer. 2002;94(2):570–575. doi: 10.1002/cncr.10190. [DOI] [PubMed] [Google Scholar]
- 6.Bollschweiler E, Baldus SE, Schröder W, et al. High rate of lymph-node metastasis in submucosal esophageal squamous-cell carcinomas and adenocarcinomas. Endoscopy. 2006;38(2):149–156. doi: 10.1055/s-2006-924993. [DOI] [PubMed] [Google Scholar]
- 7.Dawsey SM, Lewin KJ, Wang GQ, et al. Squamous esophageal histology and subsequent risk of squamous cell carcinoma of the esophagus. A prospective follow-up study from Linxian, China. Cancer. 1994;74(6):1686–1692. doi: 10.1002/1097-0142(19940915)74:6<1686::aid-cncr2820740608>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 8.Soehendra N, Seewald S, Groth S, et al. Use of modified multiband ligator facilitates circumferential EMR in Barrett’s esophagus (with video) Gastrointest Endosc. 2006;63(6):847–852. doi: 10.1016/j.gie.2005.06.052. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez Herrero L, Pouw RE, van Vilsteren FGI, et al. Safety and efficacy of multiband mucosectomy in 1060 resections in Barrett’s esophagus. Endoscopy. 2011;43(3):177–183. doi: 10.1055/s-0030-1256095. [DOI] [PubMed] [Google Scholar]
- 10.Peters FP, Kara MA, Curvers WL, et al. Multiband mucosectomy for endoscopic resection of Barrett’s esophagus: feasibility study with matched historical controls. Eur J Gastroenterol Hepatol. 2007;19(4):311–315. doi: 10.1097/MEG.0b013e328080ca90. [DOI] [PubMed] [Google Scholar]
- 11.Pouw RE, van Vilsteren FGI, Peters FP, et al. Randomized trial on endoscopic resection-cap versus multiband mucosectomy for piecemeal endoscopic resection of early Barrett’s neoplasia. Gastrointest Endosc. 2011;74(1):35–43. doi: 10.1016/j.gie.2011.03.1243. [DOI] [PubMed] [Google Scholar]
- 12.Zhang YM, Boerwinkel DF, He S, et al. Prospective feasibility study on the use of multiband mucosectomy for endoscopic resection of early squamous neoplasia in the esophagus. Endoscopy. 2013;45(3):167–173. doi: 10.1055/s-0032-1326011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58(6 Suppl):S3–43. doi: 10.1016/s0016-5107(03)02159-x. [DOI] [PubMed] [Google Scholar]
- 14.Pouw RE, Seewald S, Gondrie JJ, et al. Stepwise radical endoscopic resection for eradication of Barrett’s oesophagus with early neoplasia in a cohort of 169 patients. Gut. 2010;59(9):1169–1177. doi: 10.1136/gut.2010.210229. [DOI] [PubMed] [Google Scholar]
- 15.Van Vilsteren FGI, Pouw RE, Herrero LA, et al. Learning to perform endoscopic resection of esophageal neoplasia is associated with significant complications even within a structured training program. Endoscopy. 2012;44(1):4–12. doi: 10.1055/s-0031-1291384. [DOI] [PubMed] [Google Scholar]
- 16.Repici A, Hassan C, Carlino A, et al. Endoscopic submucosal dissection in patients with early esophageal squamous cell carcinoma: results from a prospective Western series. Gastrointest Endosc. 2010;71(4):715–721. doi: 10.1016/j.gie.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Ishihara R, Iishi H, Takeuchi Y, et al. Local recurrence of large squamous-cell carcinoma of the esophagus after endoscopic resection. Gastrointest Endosc. 2008;67(6):799–804. doi: 10.1016/j.gie.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Esaki M, Matsumoto T, Hirakawa K, et al. Risk factors for local recurrence of superficial esophageal cancer after treatment by endoscopic mucosal resection. Endoscopy. 2007;39(1):41–45. doi: 10.1055/s-2006-945143. [DOI] [PubMed] [Google Scholar]